Abstract
Two groups of calves were subjected to dietary stress by withholding of food beginning 1 or 14 days after inoculation with 1010 CFU of Escherichia coli O157:H7. Following treatment, neither group had a significant increase in fecal shedding of E. coli O157:H7. A third group of calves had food withheld for 48 h prior to inoculation with 107 CFU of E. coli O157:H7. These calves were more susceptible to infection and shed significantly more E. coli O157:H7 organisms than calves maintained on a normal diet.
Escherichia coli O157:H7 was first associated with human disease during investigations of two outbreaks of hemorrhagic colitis in 1982 (23). Traceback studies support an epidemiological link between human disease and the consumption of undercooked ground beef in 40% of outbreaks in the United States (6). The association between E. coli O157:H7 and ground beef has been supported by field surveys which have identified E. coli O157:H7 in 0.3 to 2.2% of healthy beef and dairy cattle (1, 8, 20). In cattle, the organism appears to be confined to the gastrointestinal tract and is shed in feces (4, 9, 20). During slaughter and processing, meat surfaces may become contaminated by ingesta or feces on the hide (13).
Reducing the levels of E. coli O157:H7 organisms that enter slaughter plants would require two interrelated strategies: (i) reducing the number of cattle shedding E. coli O157:H7 and (ii) reducing the magnitude of shedding (CFU/gram) by those animals infected with the organism. Both strategies may require identification of E. coli O157:H7 reservoirs and vectors as well as management practices which facilitate transmission of the organism to cattle or affect the level of shedding. Because of the persistent, albeit low, levels of E. coli O157:H7 infection in many herds, cattle have been considered by some to be a reservoir for the organism (3, 16, 24). During some on-farm surveys, E. coli O157:H7 has also been isolated from the feces of deer, sheep, dogs, goats, and a horse, as well as from water troughs, fly trap samples, and bird droppings (7, 15, 18, 19, 21, 22).
There is evidence that suggests that management practices can affect the level of E. coli O157:H7 shedding by cattle. Three decades ago, Brownlie and Grau demonstrated that the incidence and numbers of E. coli and Salmonella sp. organisms in the rumens and feces of cattle and sheep increased after dietary and/or transportation stress (5, 10). When cattle are transported, they may experience food deprivation when food is not available or is refused. During the 1994–1995 USDA-APHIS National Animal Health Monitoring System’s Cattle on Feed Evaluation, fecal samples from 100 feedlots were tested for E. coli O157:H7. Cattle which had recently arrived at feedlots had a prevalence of 3.01%, compared to 1.08% for cattle which had been on feed the longest (2).
The rumens of well-fed cattle represent a hostile environment to coliforms (14). In well-fed animals, the metabolic activities of rumen anaerobes produce concentrations (>100 mM) of volatile fatty acids and pHs of 6.0 to 6.8 which suppress coliform populations (17). When food is withheld from cattle for 24 to 48 h, reduced levels of substrates for anaerobes result in a decrease in rumen volatile fatty acid concentrations (<50 mM) and an increase in rumen pHs (>7.0) (17). In vitro studies have shown that E. coli isolates, including serotype O157:H7, are more inhibited by rumen fluid collected from well-fed cattle than from cattle fasted for 24 to 48 h (17).
In spite of an understanding of the dynamics of rumen coliform populations, the relationship between coliform populations in the rumen and feces is unclear. Coliforms that survive the rumen pass into the lower gut, where the environment is presumably less harsh. Although total coliform levels in the feces can fluctuate, the effects of dietary stress on transient coliform populations passing from the rumen and on resident coliform populations present in the lower gut are unknown. Assessing the effects of dietary stress on fecal shedding is confounded by the observation that in well-fed ruminants, there can be a 1,000-fold variation in daily fecal coliform shedding by a single animal (11).
In this study, we tested the effect of dietary stress on fecal shedding of E. coli O157:H7 in experimentally inoculated 3- to 4-month-old weaned calves. On the farm, calves in this age group may experience dietary stress due to weaning and changes in housing conditions. Weaned calves, in some on-farm surveys, had higher prevalences of shedding than preweaned calves or adults (12, 25) and may be important factors in the spread of the organism within farms.
Calves were purchased from local farms and were housed in accordance with the guidelines of the American Association for Laboratory Animal Care. Calves were housed individually in climate-controlled BL-2 containment barns. Some calves within a treatment group had nose-to-nose contact. The calves were allowed to acclimate to their new environment for 2 weeks prior to experimentation. The calves were fed twice daily with both pelleted feed (16% protein, 2.5% fat, 8.0% fiber) and alfalfa hay cubes (15% protein, 1.5% fat, 25% fiber) in amounts equal to 1% of their body weights. All calves had free access to water throughout the experiments. Calves remained healthy following inoculation with E. coli O157:H7. The calves were subjected to dietary stress by withholding of food for 2 days (beginning with the first morning meal on day 1 (0 h) followed by a feeding of one-half the daily ration on the morning of day 3, after which food was withheld for another 48 h until the afternoon of day 5). All waste was sterilized by heat in the National Animal Disease Center sewage treatment facility.
Dietary stress in recently inoculated calves.
In the first experiment, five experimental and eight control weaned 3- to 4-month-old calves were divided into two groups, with each group having control calves. The calves were inoculated by stomach tube with 1010 CFU of E. coli O157:H7 strain 3081, as previously described (9). All calves were fed after inoculation. Food was subsequently withheld from the diet-stressed calves according to the dietary stress protocol. Fresh fecal samples were collected daily from the calves beginning one morning prior to and for 7 days postinoculation (p.i.). The samples were immediately processed. One-gram samples were diluted in phosphate-buffered saline and then plated on MacConkey agar (for total coliform counts) or TKASMAC (sorbitol MacConkey containing kanamycin [100 μg/ml; Sigma], ampicillin [100 μg/ml; Sigma], and tellurite [2.5 μg/ml; Sigma]) and incubated for 16 h at 37°C (9). Enrichment cultures were prepared by adding 10 g of feces to 100 ml of Trypticase soy broth (BBL) containing 0.15% (wt/vol) no. 3 bile salts (Difco) and tellurite (2.5 μg/ml; Sigma). Cultures were incubated for 16 h at 37°C, diluted in phosphate-buffered saline, and then plated on TKASMAC as described above. The sensitivity of the direct plating assay is 50 CFU/g (9).
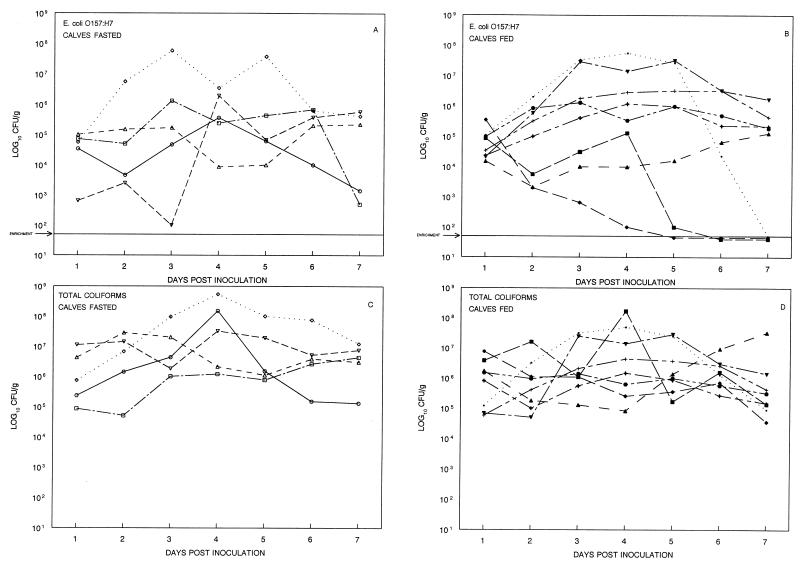
In previous studies, shedding of E. coli O157:H7 strain 3081 by experimentally inoculated preweaned calves and adult cattle that were well fed reached a peak during the first week p.i. Shedding followed a downward trend over time until levels were undetectable at 7 to 27 weeks p.i. for preweaned calves and 2 to 14 weeks p.i. for adults (9). Within each age group, there was wide variation among individuals in the magnitude of shedding. The peak level of shedding for preweaned calves was 4.0 × 105 to 1.6 × 109 CFU/g; for adults the level of shedding was 1.2 × 105 to 1.0 × 107 CFU/g. Occasionally, E. coli O157:H7 shedding by an animal varied 1,000-fold over several days (9). In the present study, total coliform and E. coli O157:H7 shedding of the well-fed and diet-stressed groups for days 1 to 7 p.i. were compared by repeated-measures analysis of variance (SAS Institute). Shedding of coliforms and E. coli O157:H7 varied widely among animals of both groups, as did the day of peak shedding (Fig. 1). During days 1 to 7, the peak level of shedding of total coliforms was 4.3 × 106 to 1.5 × 108 CFU/g for the fasted calves and 1.5 × 106 to 1.7 × 108 CFU/g for the well-fed controls. Although the ranges of peak shedding were similar for both groups, the fasted calves shed more total coliforms than the well-fed controls (P < 0.04). The peak level of shedding of E. coli O157:H7 was 2.2 × 105 to 5.8 × 107 CFU/g for the fasted calves and 1.3 × 105 to 5.6 × 107 CFU/g for the well-fed controls. The difference in E. coli O157:H7 shedding between the fasted and well-fed calves was not significant (P < 0.91). In a previous study of experimentally inoculated calves (6 to 8 weeks old), fecal shedding of E. coli O157:H7 was variable when food was withheld (4).
FIG. 1.
Fecal shedding (day 1 to day 7 p.i.) of E. coli O157:H7 and total coliforms by calves maintained on a dietary stress regimen and calves that were well fed. Calves were inoculated with 1010 CFU of E. coli O157:H7 on day 0. Calves on the dietary stress regimen had food withheld for 48 h beginning on day 1, were fed a one-half ration on day 3, and then were fasted for 48 h until day 5. (A) E. coli O157:H7 recovered from diet-stressed calves. (B) E. coli O157:H7 recovered from well-fed calves. (C) Total coliforms recovered from diet-stressed calves. (D) Total coliforms recovered from well-fed calves.
The absence of an increase in shedding of E. coli O157:H7 by the diet-stressed group may have been the result of inhibitory effects of inoculation into a well-fed rumen. The significant increase in total coliform shedding by the fasted calves is consistent with the results reported by Brownlie and Grau (5) and suggests that indigenous coliforms are most likely to proliferate during dietary stress.
Dietary stress 2 weeks p.i.
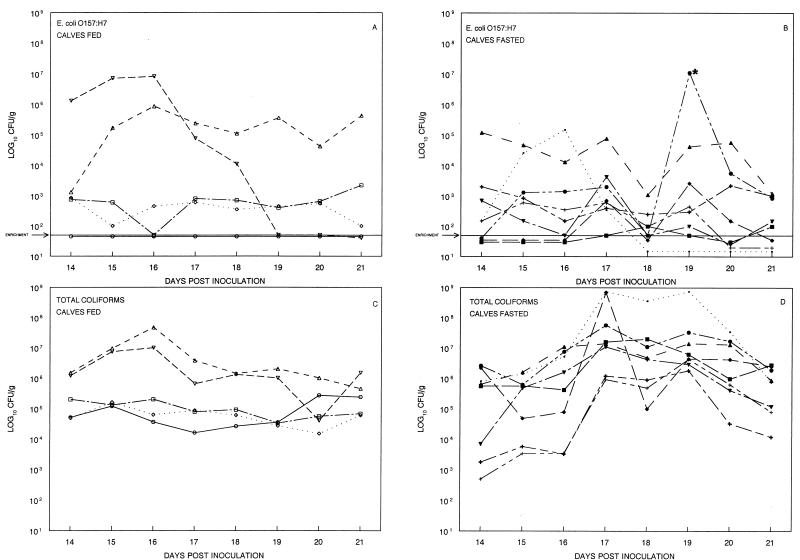
Further experiments were conducted to determine if dietary stress would cause an increase in E. coli O157:H7 shedding in calves colonized with the organism for 2 weeks. On the morning of day 14 p.i., the inoculated control calves from the previous experiment were placed on the dietary stress regimen. The group that had been on the dietary stress regimen for days 1 to 5 p.i. continued to be fed the normal daily ration; however, these calves were not considered to be concurrent controls. The feces of both groups were monitored for 7 days thereafter (Fig. 2). For most calves, the differences in shedding of E. coli O157:H7 organisms from day 14 to day 21 were similar to the individual variations seen in preweaned calves and adults that are well fed (9). However, calf 1 had a greater-than-100,000-fold increase in E. coli O157:H7 shedding (Fig. 2B). On the day of peak shedding (day 19), E. coli O157:H7 became the predominant coliform (1.1 × 107 CFU/g of 3.3 × 107 CFU/g [total coliforms]) and exceeded what had previously been the highest E. coli O157:H7 shedding value (1.3 × 106 CFU/g) observed during the first week p.i. Prior to dietary stress, the E. coli O157:H7 daily shedding pattern of calf 1 was similar to other members of the group, and its response to dietary stress could not be predicted. The day following peak shedding, the calf’s E. coli O157:H7 counts had declined to less than 1% of total coliforms. The shedding by calf 1 suggests that dietary stress may lead to increased shedding of E. coli O157:H7; however, we think that suppressive ecological factors such as the presence of competing organisms, bacteriocins, lysis by phage, and predation by protozoans may have a role in limiting the increase.
FIG. 2.
Fecal shedding (day 14 to day 21 p.i.) of E. coli O157:H7 and total coliforms by calves maintained on a dietary stress regimen and calves that were well fed. Calves were inoculated with 1010 CFU of E. coli O157:H7 on day 0. Calves that were well fed on day 1 to day 14 p.i. were subjected to dietary stress by having food withheld for 48 h beginning on day 15; they were fed a one-half ration on day 17 and then were fasted for 48 h until day 19, when the normal diet was resumed. Calves that had been subjected to dietary stress on day 1 to day 5 p.i. were well fed from day 14 to day 21 p.i. The asterisk indicates calf 1, which is described in the text. The sensitivity of the enrichment culture is <50 CFU/g. (A) E. coli O157:H7 recovered from well-fed calves. (B) E. coli O157:H7 recovered from diet-stressed calves. (C) Total coliforms recovered from well-fed calves. (D) Total coliforms recovered from diet-stressed calves.
Dietary stress prior to inoculation.
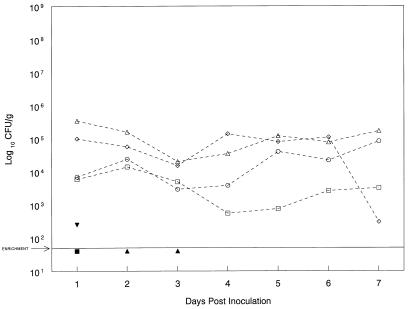
In further experiments to determine the effects of dietary stress upon susceptibility and shedding, a different regimen was used. Calves were fasted for 2 days prior to inoculation with 107 CFU of E. coli O157:H7. We chose 107 CFU as a minimally effective dose since previous experiments had demonstrated that only 2 of 5 well-fed adults inoculated with this dose had detectable shedding (<5.0 × 101 CFU/g) for 1 or 2 days p.i. (9). In this experiment, we used two groups of four 3- to 4-month-old weaned calves. In each group, two calves were well fed and two were fasted for 2 days prior to inoculation. Only three of the four well-fed calves shed at detectable levels, the longest for 3 days (Fig. 3). In contrast, the fasted calves inoculated with 107 CFU had significantly greater shedding (P = 0.001), with a peak of 1.4 × 104 to 3.5 × 105 CFU/g during the first week p.i. When E. coli O157:H7 shedding (day 1 to day 7) of the fasted calves inoculated with 107 CFU was compared with the shedding (day 1 to day 7) of well-fed calves (from the first experiment) inoculated with 1010 CFU, there was no difference (P = 0.73).
FIG. 3.
Fecal shedding (day 1 to day 7) of E. coli O157:H7 by calves that were well fed (solid symbols) or fasted (open symbols) for 48 h prior to inoculation with 107 CFU of E. coli O157:H7 on day 0. E. coli O157:H7 organisms were recovered from well-fed calves on day 1 (two of four calves), day 2 (one of four), and day 3 (one of four). The sensitivity of the enrichment culture is <50 CFU/g.
The increased susceptibility to infection by the fasted calves is consistent with in vitro studies in which rumen fluid from fasted cattle allowed unrestricted growth of E. coli O157:H7 (17). This suggests that interventions during the time period when calves and adults may experience dietary stress may limit the spread of E. coli O157:H7. In anticipation of periods of dietary stress, application of treatments which can maintain rumen function might decrease susceptibility. Calves and adults could also be treated with agents such as bacteriocins or inoculated with select microbes to competitively exclude E. coli O157:H7.
Although an inoculum of greater than 107 CFU of in vitro-grown E. coli O157:H7 is required to obtain shedding of greater than 105 CFU/g in well-fed calves (9), we have observed shedding at this level in a well-fed calf inoculated with 105 CFU of E. coli O157:H7 derived from feces (data not shown). It is reasonable to expect that a smaller inoculum of feces-derived E. coli O157:H7 would cause shedding in fasted calves and adults. Zhao et al. (25) have reported that of 31 positive E. coli O157:H7 fecal samples collected from farms, 16 had 103 to 105 CFU/g while 15 were positive by enrichment culture only. The inoculation dose of 1010 CFU used in this study and studies by Brown et al. (4) would require 105 g of feces containing 105 CFU/g. Diet-stressed calves and adults would likely be more susceptible than well-fed animals to infection and shedding of E. coli O157:H7 after ingesting smaller amounts of feces contaminated with the organism.
Consistent with previous observations, dietary stress of calves can cause increased shedding of coliforms. In some infected animals, shedding of E. coli O157:H7 can also increase. Diet-stressed calves are more susceptible to infection by E. coli O157:H7 than are well-fed calves. During marketing, when uninfected calves are comingled with calves infected with E. coli O157:H7, dietary stress could result in an increase in the prevalence of calves shedding E. coli O157:H7.
Acknowledgments
We thank Norman Lyon, Robert Morgan, Deb Lebo, and Caryn Hurd for expert technical assistance and Grace Liu for statistical analysis.
REFERENCES
- 1.Anonymous. Escherichia coli O157:H7 issues and ramifications. Fort Collins, Colo: USDA:APHIS:VS Centers for Epidemiology and Animal Health; 1994. [Google Scholar]
- 2.Anonymous. Escherichia coli O157:H7 shedding by feedlot cattle. Fort Collins, Colo: USDA:APHIS:VS National Animal Health Monitoring System; 1995. [Google Scholar]
- 3.Borczyk A A, Karmali M A, Lior H, Duncan L M. Bovine reservoir for verotoxin-producing Escherichia coli O157:H7. Lancet. 1987;i:98. doi: 10.1016/s0140-6736(87)91928-3. [DOI] [PubMed] [Google Scholar]
- 4.Brown C A, Harmon B G, Zhoa T, Doyle M P. Experimental Escherichia coli O157:H7 carriage in calves. Appl Environ Microbiol. 1997;63:27–32. doi: 10.1128/aem.63.1.27-32.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brownlie L E, Grau F H. Effect of food intake on growth and survival of salmonellas and Escherichia coli in the bovine rumen. J Gen Microbiol. 1967;46:125–134. doi: 10.1099/00221287-46-1-125. [DOI] [PubMed] [Google Scholar]
- 6.Cannon M, Thomas H, Sellers W, Bates M, Blake P, Stetler H, Toomey K, Fowler J, Halford S, Young G, Hall S, Erwin P, Boaz V, Swinger G. Outbreak of Escherichia coli O157:H7 infection—Georgia and Tennessee, June 1995. Morbid Mortal Weekly Rep. 1996;45:249–251. [PubMed] [Google Scholar]
- 7.Chapman P A, Siddons C A. Sheep as a potential source of verocytotoxin-producing Escherichia coli O157. Vet Rec. 1996;138:23–24. [PubMed] [Google Scholar]
- 8.Clarke R C, Read S C, McEwen S A, Lynch J, Schoonderwoerd M, Lior H, Gyles C L. Isolation of verocytotoxin-producing Escherichia coli from animals and food products. In: Todd E C D, MacKenzie J M, editors. Escherichia coli O157:H7 and other verotoxigenic E. coli in foods. Ottawa, Ontario, Canada: Polyscience Publications; 1991. pp. 121–129. [Google Scholar]
- 9.Cray W C, Jr, Moon H W. Experimental infection of calves and adult cattle with Escherichia coli O157:H7. Appl Environ Microbiol. 1995;61:1586–1590. doi: 10.1128/aem.61.4.1586-1590.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grau F H, Brownlie L E, Roberts E A. Effect of some preslaughter treatments on the Salmonella population in the bovine rumen and faeces. J Appl Bacteriol. 1968;31:157–163. doi: 10.1111/j.1365-2672.1968.tb00353.x. [DOI] [PubMed] [Google Scholar]
- 11.Grau F H, Brownlie L E, Smith M G. Effects of food intake on numbers of salmonellae and Escherichia coli in rumen and feces of sheep. J Appl Bacteriol. 1969;32:112–117. doi: 10.1111/j.1365-2672.1969.tb02195.x. [DOI] [PubMed] [Google Scholar]
- 12.Hancock D D, Besser T E, Kinsel M L, Tarr P I, Rice D H, Paros M G. The prevalence of Escherichia coli O157:H7 in dairy and beef cattle in Washington State. Epidemiol Infect. 1994;113:199–207. doi: 10.1017/s0950268800051633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hardin M D, Acuff G R, Lucia L M, Oman J S, Savell J W. Comparison of methods for decontamination of beef carcass surfaces. J Food Prot. 1995;58:368–374. doi: 10.4315/0362-028X-58.4.368. [DOI] [PubMed] [Google Scholar]
- 14.Hollowell C A, Wolin M J. Basis for the exclusion of Escherichia coli from the rumen ecosystem. Appl Microbiol. 1965;13:918–924. doi: 10.1128/am.13.6.918-924.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kudva I T, Hatfield P G, Hovde C J. Escherichia coli O157:H7 in microbial flora of sheep. J Clin Microbiol. 1996;34:431–433. doi: 10.1128/jcm.34.2.431-433.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orskov F, Orskov I, Villar J A. Cattle as reservoir of verotoxin-producing Escherichia coli O157:H7. Lancet. 1987;ii:276. doi: 10.1016/s0140-6736(87)90860-9. [DOI] [PubMed] [Google Scholar]
- 17.Rasmussen M A, Cray W C, Jr, Casey T A, Whipp S C. Rumen contents as a reservoir of enterohemorrhagic Escherichia coli. FEMS Microbiol Lett. 1993;114:79–84. doi: 10.1016/0378-1097(93)90145-r. [DOI] [PubMed] [Google Scholar]
- 18.Rice D H, Hancock D D. 76th Annual Meeting of the Conference of Research Workers in Animal Diseases, Chicago, Ill. 13 to 14 November 1995. 1995. Non-bovine sources of Escherichia coli O157:H7, abstr. 66. [Google Scholar]
- 19.Rice D H, Hancock D D, Besser T E. Verotoxigenic E. coli O157 colonisation of wild deer and range. Vet Rec. 1995;137:524. doi: 10.1136/vr.137.20.524. [DOI] [PubMed] [Google Scholar]
- 20.Riley L W, Remis R S, Helgerson S D, McGee H B, Wells J G, Davis B R, Helbert R J, Olcott E S, Johnson L M, Hargrett N T, Blake P A, Cohen M L. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N Engl J Med. 1983;308:681–685. doi: 10.1056/NEJM198303243081203. [DOI] [PubMed] [Google Scholar]
- 21.Shukla R, Slack R, George A, Cheasty T, Rowe B, Scutter J. Escherichia coli O157 infection associated with a farm visitor centre. Commun Dis Rep CDR Rev. 1995;5:R86–R90. [PubMed] [Google Scholar]
- 22.Trevena W B, Hooper R S, Wray C, Willshaw G A, Cheasty T, Domingue G. Verocytotoxin-producing Escherichia coli O157 associated with companion animals. Vet Rec. 1996;138:400. [PubMed] [Google Scholar]
- 23.Wells J G, Davis B R, Wachsmuth I K, Riley L W, Remis R S, Sokolow R, Morris G K. Laboratory investigation of hemorrhagic colitis outbreaks associated with a rare Escherichia coli serotype. J Clin Microbiol. 1983;18:512–520. doi: 10.1128/jcm.18.3.512-520.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wells J G, Shipman L D, Greene K D, Sowers E G, Green J H, Cameron D N, Downes F P, Martin M L, Griffin P M, Ostroff S M, Potter M E, Tauxe R V, Wachsmuth I K. Isolation of Escherichia coli serotype O157:H7 and other Shiga-like-toxin-producing E. coli from dairy cattle. J Clin Microbiol. 1991;29:985–989. doi: 10.1128/jcm.29.5.985-989.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao T, Doyle M P, Shere J, Garber L. Prevalence of enterohemorrhagic Escherichia coli O157:H7 in a survey of dairy herds. Appl Environ Microbiol. 1995;61:1290–1293. doi: 10.1128/aem.61.4.1290-1293.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]