Abstract
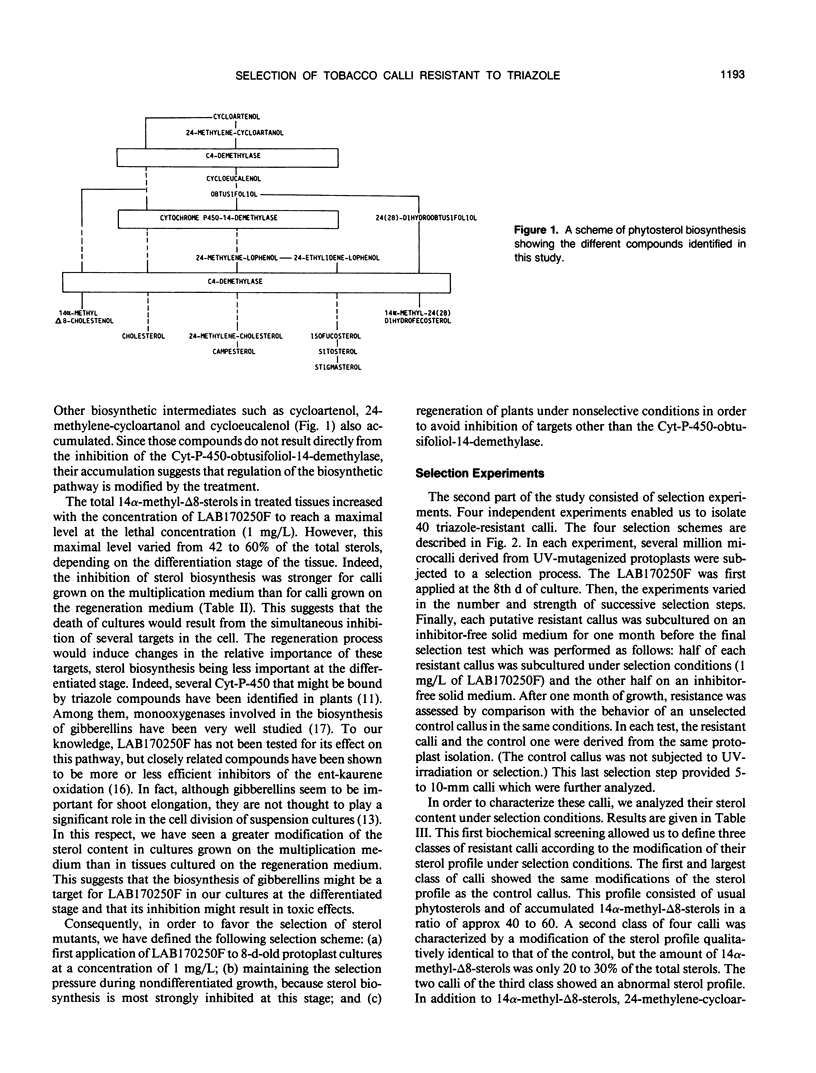
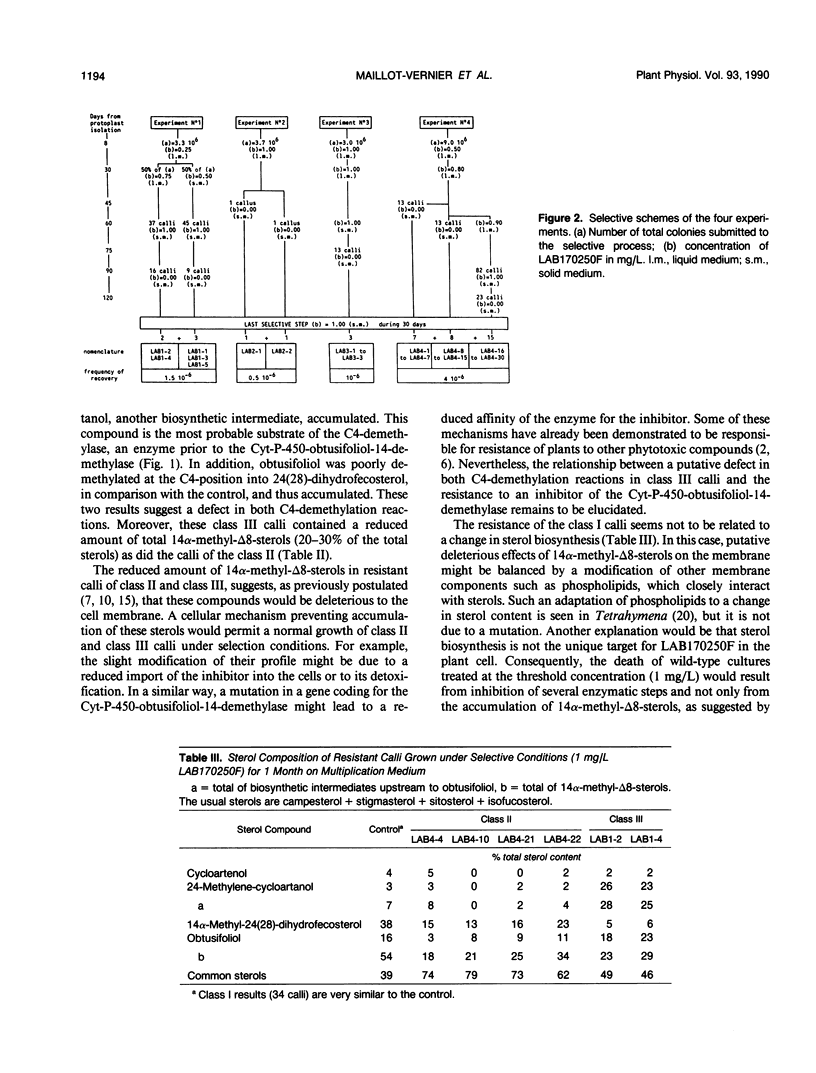
The selection of biochemical mutants has been undertaken in order to elucidate regulatory and functional aspects of sterol biosynthesis in plants. 2-(4-Chlorophenyl)-3-phenyl-1-(1H-1,2,4- triazol-1-yl)-2,3-oxidopropane (LAB170250F), an experimental fungicide of the triazole family, was used as a selective agent. Indeed, this compound is a strong inhibitor of the cytochrome-P-450-obtusifoliol-14-demethylase in sterol biosynthesis. The selection strategy consisted of screening large populations of microcalli derived from ultraviolet-mutagenized protoplasts of Nicotiana tabacum L. cv Xanthi for resistance to a lethal concentration of LAB170250F. The best selective conditions were first determined, i.e. strength of the selection pressure as well as the time and duration of its application in the developmental process from protoplast to whole plant. Selection experiments resulted in the recovery of 40 resistant calli. These calli were divided into three classes according to the modification of their sterol content in response to LAB170250F. Some of these calli might be impaired in sterol biosynthesis, but most have a sterol profile identical to that of the control calli. This suggests that the toxic properties of LAB170250F are due to the parallel inhibition of sterol biosynthesis and of at least one additional unidentified target in the plant cell.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourgin J. P., Goujaud J., Missonier C., Pethe C. Valine-Resistance, a Potential Marker in Plant Cell Genetics. I. Distinction between Two Types of Valine-Resistant Tobacco Mutants Isolated from Protoplast-Derived Cells. Genetics. 1985 Feb;109(2):393–407. doi: 10.1093/genetics/109.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang T. Y., Telakowski C., Heuvel W. V., Alberts A. W., Vagelos P. R. Isolation and partial characterization of a cholesterol-requiring mutant of Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1977 Mar;74(3):832–836. doi: 10.1073/pnas.74.3.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl C. E., Dahl J. S., Bloch K. Effects of cycloartenol and lanosterol on artificial and natural membranes. Biochem Biophys Res Commun. 1980 Jan 15;92(1):221–228. doi: 10.1016/0006-291x(80)91542-9. [DOI] [PubMed] [Google Scholar]
- Grandbastien M. A., Bourgin J. P., Caboche M. Valine-Resistance, a Potential Marker in Plant Cell Genetics. II. Optimization of Uv Mutagenesis and Selection of Valine-Resistant Colonies Derived from Tobacco Mesophyll Protoplasts. Genetics. 1985 Feb;109(2):409–425. doi: 10.1093/genetics/109.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nes W. R., Sekula B. C., Nes W. D., Adler J. H. The functional importance of structural features of ergosterol in yeast. J Biol Chem. 1978 Sep 10;253(17):6218–6225. [PubMed] [Google Scholar]
- Rahier A., Taton M. The 14 alpha-demethylation of obtusifoliol by a cytochrome P-450 monooxygenase from higher plants' microsomes. Biochem Biophys Res Commun. 1986 Nov 14;140(3):1064–1072. doi: 10.1016/0006-291x(86)90743-6. [DOI] [PubMed] [Google Scholar]
- Umeki S., Nozawa Y. Effects of sterol manipulation on microsomal desaturase activities in Tetrahymena: with regard to thermal acclimation. Biochem Biophys Res Commun. 1983 May 31;113(1):96–101. doi: 10.1016/0006-291x(83)90436-9. [DOI] [PubMed] [Google Scholar]


