Abstract
Hepatitis delta virus (HDV) has been detected in the minor salivary gland (MSG) tissue of Sjögren’s disease (SjD) patients in the absence of a hepatitis B virus (HBV) coinfection. Previous research has shown that HDV antigen (HDAg) expression can trigger an SjD-like phenotype in vivo, demonstrating a potential cause-and-effect relationship. We hypothesize that if HDV plays a role in the development of SjD, then HDV profiles may be correlated with disease manifestations. This retrospective study characterized HDV in a cohort of 48 SjD MSG samples collected between 2014 and 2021. Analyses of HDAg expression, including cell type and subcellular localization, in situ hybridization of HDV RNA, and comparative analyses with associated SjD and viral hepatitis clinical features, were conducted. HDAg was detected in MSG acinar, ductal, myoepithelial, and adipose cells and localized with the nuclei, cytoplasm, and mitochondria. In situ hybridization detected HDV genomic RNA localization in the MSG nuclei. A significant negative correlation was found between HDAg intensity and focal lymphocytic inflammation and in patients with both anti-SSA/Ro-52 and anti-SSA/Ro-60. In analyzing autoimmune disease comorbidities with SjD, it was found that SjD patients diagnosed with autoimmune thyroiditis and/or hypothyroidism were significantly more represented in the high HDAg intensity group compared to the negative and moderate HDAg intensity groups. No significant associations were detected between MSG-localized HDAg and liver enzymes or an evident HBV coinfection. This study has further confirmed that there is a nonhepatic reservoir for chronic HDV persistence in SjD-affected salivary gland tissue in a third independent SjD patient cohort. In addition, this study describes the unique colocalization of HDAg with mitochondria. The detection of HDV antigen and sequence within SjD-affected salivary gland tissue, and in the absence of an evident current or past HBV coinfection, warrants further investigation.
Keywords: Sjogren’s syndrome, Sjogren syndrome, hepatitis d virus, hepatitis delta virus, hepatitis b virus, non-communicable chronic disease
Introduction
Sjögren’s disease (SjD) is a noncommunicable, chronic autoimmune disease afflicting ≥0.01% of the population in the United States (Maciel et al. 2017; Izmirly et al. 2019). SjD is classified by the detection of decreased tear and/or saliva production, development of autoantibodies (anti-SSA/Ro, anti-SSB/La), and focal lymphocytic inflammation within the salivary gland tissue and in connection with significant extraglandular manifestations (Shiboski et al. 2017). The etiology of this female-predominant disease is attributed to a combination of genetic susceptibility factors, altered hormone profiles, and/or chronic pathogen exposure (Igoe and Scofield 2013; Liu et al. 2016; Vivino et al. 2019; Maslinska and Kostyra-Grabczak 2022). Previously, hepatitis delta virus (HDV) was detected in SjD salivary gland tissue and shown to trigger an SjD-like phenotype in vivo (Weller et al. 2016). This current retrospective study builds upon the discovery of HDV in SjD patients and aims to characterize the clinical and in situ features of HDV within an Utahn SjD cohort.
Hepatitis delta virus is a rare infectious disease estimated to affect 12 to 72 million people worldwide (Stockdale et al. 2018; Chen et al. 2019; Miao et al. 2020). As a member of the Kolmioviridae family, HDV is the only viral satellite RNA (sat-RNA) known to infect humans (Appendix Fig. 1). This ~1,700-nt circular, single-stranded sat-RNA produces 2 antigens: the small HDAg (S-HDAg) and large HDAg (L-HDAg) from a single open reading frame (Casey 2006). A helper virus, such as hepatitis B virus (HBV), is required for transmission and packaging, and the tissue tropism of HDV is dictated by the tropism of the helper virus (Yan et al. 2012). Once inside the cell, HDV uses the S-HDAg and host cellular proteins for replication and can persist in the absence of an active helper virus coinfection (Chang et al. 2005). The L-HDAg facilitates the packaging of the ribonucleoprotein complex into HBV (Casey 2006). Coinfection with HBV is associated with enhanced symptoms of viral hepatitis, increased risk of liver decompensation, and hepatocellular carcinoma (Urban et al. 2021). Since the discovery of HDV in the 1970s (Rizzetto et al. 1977), this unique pathogen has been extensively studied in the context of viral hepatitis. However, the recent discovery of HDV infection in nonhepatic tissue and its association with the development of exocrine gland dysfunction suggest that there may be more to this sat-RNA than previously understood (Weller et al. 2016).
In a previous study, HDV sequence and antigen were detected in minor salivary gland (MSG) tissue of SjD patients (Weller et al. 2016). In these cases, a current or prior HBV infection was not detected. Furthermore, expression of HDV antigens in a murine model triggered the development of a complete SjD-like phenotype in vivo. Specifically, expression of S-HDAg led to the development of autoantibodies (anti-SSA/Ro and anti-SSB/La) and decreased stimulated saliva flow in the mice. Mice expressing both S-HDAg and L-HDAg developed significant focal inflammation in their salivary gland tissue and developed anti-SSA/Ro antibodies. These findings suggest a potential cause-and-effect relationship between HDV antigen expression and the development of the SjD phenotype.
The presence and impact of HDV localized outside of hepatic tissue has not been well-defined. The aim of this study was to characterize the presence and impact of HDV in MSG tissue of patients diagnosed with SjD. In this study, we performed a retrospective analysis of an SjD cohort to evaluate HDV antigen and sequence localization in MSG biopsies. We then used the HDV profiles in comparative analyses with SjD classification criteria, autoimmune disease comorbidities, liver enzymes, and viral hepatitis tests. Our findings show that HDV localized within multiple salivary gland cell types and had a unique subcellular localization pattern. This study provides essential information on HDV colocalization within SjD MSG tissue and identifies SjD clinical parameters that significantly correlate with HDV antigen expression levels.
Methods
Materials and methods are reported in the Appendix.
Results
SjD Patient Demographics and Clinical Characterization
This study investigated the presence and impact of HDV in the MSG tissue of patients with a Utahn SjD cohort. A retrospective analysis was conducted on 48 archived MSG biopsies from SjD patients (Table 1). The study cohort consisted of 91.7% females and 8.3% males with an average age of 49.9 ± 13.2 y at SjD diagnosis. Lymphocytic focus scores ≥1 foci/4mm2 area were observed in 68.8% of the biopsies analyzed. Antinuclear antibodies (ANA) were positive in 48.4% of the patients, anti-SSA/Ro in 27.3%, anti-SSB/La in 2.1%, and rheumatoid factor (RF) in 15.2%. No evidence of hypocomplementemia was found in the cohort, but abnormal (high) C3 and C4 levels were detected in 9.1% and 18.2% of the patients, respectively (Appendix Table 1).
Table 1.
Sjögren’s Syndrome Patient Demographics and Characterization of Clinical Features.
| Characteristic | Sjögren’s Syndrome (n = 48) |
|---|---|
| Sex | |
| Female | 44 (91.7) |
| Male | 4 (8.3) |
| Race | |
| Asian | 0 (0.0) |
| American Indian or Alaskan Native | 0 (0.0) |
| Black or African American | 0 (0.0) |
| Native Hawaiian or Pacific Islander | 0 (0.0) |
| White | 45 (93.8) |
| Other | 3 (6.3) |
| Ethnicity | |
| Hispanic/Latino | 5 (10.4) |
| Not Hispanic/Latino | 43 (89.6) |
| Age, mean ± SD, y | 49.9 ± 13.2 |
| Age group | |
| 0–19 | 0 (0.0) |
| 20–39 | 10 (20.8) |
| 40–59 | 25 (52.1) |
| 60+ | 13 (27.1) |
| Foci/4 mm² | 1.5 ± 1.9 |
| ≥1 | 33 (68.8) |
| <1 | 15 (31.3) |
| Autoantibodies | |
| ANA (+) | 15/31 (48.4) |
| Anti-SSA (+) | 12/44 (27.3) |
| Anti-SSB (+) | 1/43 (2.1) |
| RF (+) | 5/33 (15.2) |
Values are presented as number (%) unless otherwise indicated.
ANA, antinuclear antibody; RF, rheumatoid factor.
HDAg Detected in SjD Labial MSG Tissue
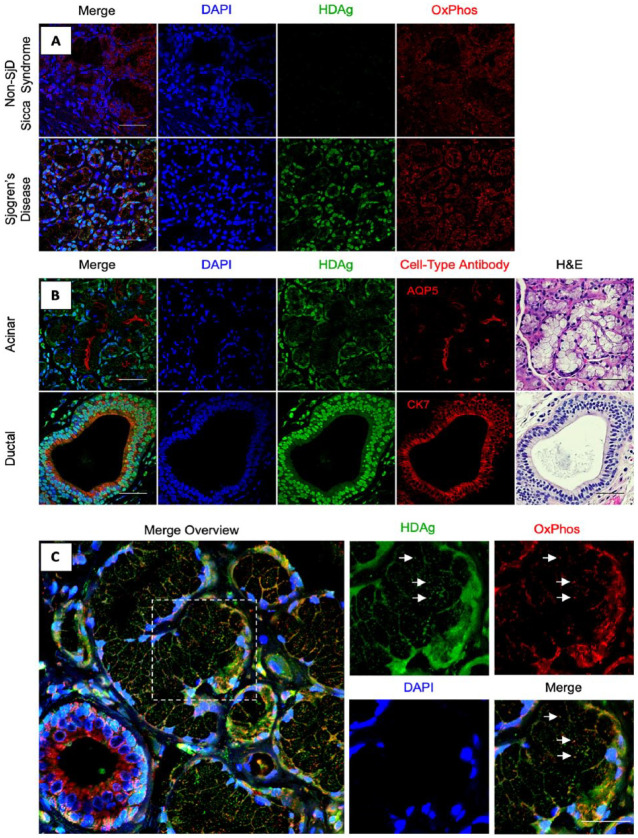
Immunohistochemical analysis was performed to assess the presence of HDAg in SjD and non-SjD sicca syndrome MSG tissue. As shown in Figure 1A, MSG tissues were analyzed for HDAg presence and localization using counterstaining for nuclei (DAPI) and mitochondria (OxPhos antibody cocktail). The analysis revealed elevated levels of HDV antigen in SjD MSG tissue compared to the non-SjD sicca syndrome MSG tissue.
Figure 1.
Cellular and subcellular localization of hepatitis delta virus antigen (HDAg) in Sjögren’s disease (SjD) labial minor salivary glands. (A) Localization of HDAg in non-SjD Sicca syndrome control relative to SjD patient HDAg profile. n = 4, scale bars = 50 μm. (B) Cellular localization patterns of HDAg were identified through immunohistochemical staining of formalin-fixed, paraffin-embedded (FFPE) minor labial salivary gland sections. Cell nuclei were stained with DAPI (blue); anti-HDAg antibody was used for immunostaining of HDAg (green). Antibodies for AQP5 and CK7 were used for identification of acinar and ductal cells, respectively (red). n = 4, scale bars = 50 μm. (C) Subcellular colocalization of HDAg with mitochondria was observed (white arrows). Cell nuclei were stained with DAPI (blue); anti-HDAg antibody was used for immunostaining of HDAg (green). OxPhos Human Antibody Cocktail was used for identification of mitochondria (red). n = 48, scale bars = 25 μm.
The cellular localization of HDAg in labial MSG tissues was evaluated using immunohistochemical staining of aquaporin 5 (AQP5) and cytokeratin 7 (CK7) to detect acinar and ductal cells, respectively. Results showed that HDAg was present in both acinar and ductal cells of the labial MSG tissue (Fig. 1B). Labial MSG tissues comprise seromucous acini with a predominance of mucous acini (Appendix Fig. 2A). HDAg was detected in mucous acini within MSG tissue (Appendix Fig. 2A–E). HDAg was also found to colocalize with the nuclear region of adipocyte cells within the connective tissue (Appendix Fig. 2F–J) and with myoepithelial cells (Appendix Fig. 2K–O). No evidence of HDAg expression was observed in lymphocytic foci (data not shown).
The labial MSG tissues were analyzed to determine the subcellular localization patterns of HDAg, including subcellular localization of HDAg in the nucleus and mitochondria of the MSG tissues (Fig. 1C). The results showed that HDAg was present in the nucleus of both acinar and ductal cells and colocalized with the mitochondria (Fig. 1B, C). HDAg in adipocytes presented with a nuclear localization pattern (Appendix Fig. 2F–J).
Nuclear Localized HDV Sequence Detected in SjD MSG Tissue
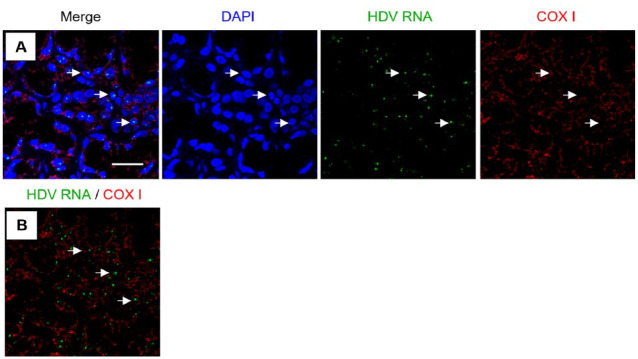
In situ hybridization was performed to detect HDV genomic and anti–genomic RNA sequence using custom RNAScope probes. Results, shown in Figure 2, revealed that HDV genomic RNA was localized within the cell nuclei (Fig. 2A). HDV RNA did not colocalize with mitochondrial RNA, as determined by an RNAscope probe detecting COX I RNA (Fig. 2B). The anti–genomic HDV sequence was not detected in the labial MSG tissues analyzed (data not shown). Lower copy number of antigenomic sequences compared to genomic sequences (1:15) present during the HDV replication cycle may limit detectability (Chen et al. 1986). Previously, nuclear localization of the HDV sequence has been linked to active replication of the viral genome (Tseng and Lai 2009).
Figure 2.
Subcellular colocalization of hepatitis delta virus (HDV) genomic RNA with nuclei in Sjögren’s disease (SjD) minor salivary gland (MSG) biopsies. (A) Merged overview of MSG tissue with cell nuclei (blue), HDV RNA (green), and mitochondrial transcript COX I RNA (red). (B) Merged overview of in situ hybridization of probes for HDV RNA and COX I demonstrating lack of colocalization between HDV RNA and mitochondrial localized RNA. White arrows indicate examples of colocalization of HDV RNA with cell nuclei; n = 14, scale bar = 25 μm.
HDAg Association with Clinical Features of SjD
In this study, labial MSG biopsies from SjD patients were analyzed for the presence and intensity of HDAg. Comparisons were made between HDAg intensity and various clinical parameters of SjD classification, including focal lymphocytic inflammation, autoantibody profile, and patient demographics. HDAg intensity was classified into 3 categories: high, moderate, and negative intensity as outlined in the Methods section and Appendix Figure 3. There were no significant differences in HDAg intensity based on race or ethnicity (Table 2). However, a significant difference was observed in the median age of patients in each HDAg intensity group. The median age of the high HDAg intensity group was 49.5 ± 10.4, the moderate intensity group was 42.1 ± 13.3, and the negative group was 57.2 ± 12.1 (P = 0.004) (Table 2). In addition, a significant difference was observed between HDAg intensity groupings and age groups, with higher HDAg intensity in younger age groups (age 20–39) and lower HDAg intensity in the 60+ age group (P = 0.03) (Table 2).
Table 2.
Patient Demographics and Sjögren’s Syndrome Diagnostic Data Stratified by Hepatitis Delta Virus Antigen Intensity Level.
| Characteristic | Negative (n = 17) | Moderate HDAg (n = 15) | High HDAg (n = 16) | P |
|---|---|---|---|---|
| Sex | 0.83 | |||
| Male | 1 (5.9) | 1 (6.7) | 2 (12.5) | |
| Female | 16 (94.1) | 14 (93.3) | 14 (87.5) | |
| Race | NS | |||
| American Indian or Alaskan Native | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Asian | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Black or African American | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Native Hawaiian or Pacific Islander | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| White | 15 (88.2) | 14 (93.3) | 16 (100.0) | |
| Other | 2 (11.8) | 1 (6.7) | 0 (0.0) | |
| Ethnicity | 0.60 | |||
| Hispanic/Latino | 3 (17.6) | 1 (6.7) | 1 (6.3) | |
| Not Hispanic/Latino | 14 (82.4) | 14 (93.3) | 15 (93.8) | |
| Age, mean ± SD, y | 57.2 ± 12.1 | 42.1 ± 13.3 | 49.5 ± 10.4 | 0.004 |
| Age group | 0.03 | |||
| 0–19 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| 20–39 | 0 (0.0) | 6 (40.0) | 5 (31.3) | |
| 40–59 | 10 (58.8) | 7 (46.7) | 8 (50.0) | |
| 60+ | 7 (41.2) | 2 (13.3) | 3 (18.8) | |
| Foci/4 mm2, mean ± SD | 2.1 ± 2.5 | 1.5 ± 2.0 | 1.0 ± 0.8 | 0.18 |
| ≥1 | 12 (70.6) | 8 (53.3) | 10 (62.5) | 0.66 |
| <1 | 5 (29.4) | 7 (46.7) | 6 (37.5) | |
| Autoantibodies | ||||
| Anti-SSA (+) | 5/16 (31.3) | 4/13 (30.8) | 3/15 (20.0) | 0.76 |
| Anti-SSB (+) | 0/15 (0.0) | 0/13 (0.0) | 1/15 (6.7) | 1 |
| RF (+) | 2/11 (18.2) | 2/9 (22.2) | 2/13 (15.4) | 1 |
| ANA (+) | 5/11 (45.5) | 4/9 (44.4) | 6/11 (54.5) | 1 |
| Adipose tissue (% of area) | ||||
| ≥10% | 5 (29.4) | 1 (6.7) | 5 (31.3) | 0.20 |
| <10% | 12 (70.6) | 14 (93.3) | 11 (68.8) |
Values are presented as number (%) unless otherwise indicated.
ANA, antinuclear antibody; HDAg, hepatitis delta virus antigen; ns, not significant; RF, rheumatoid factor.
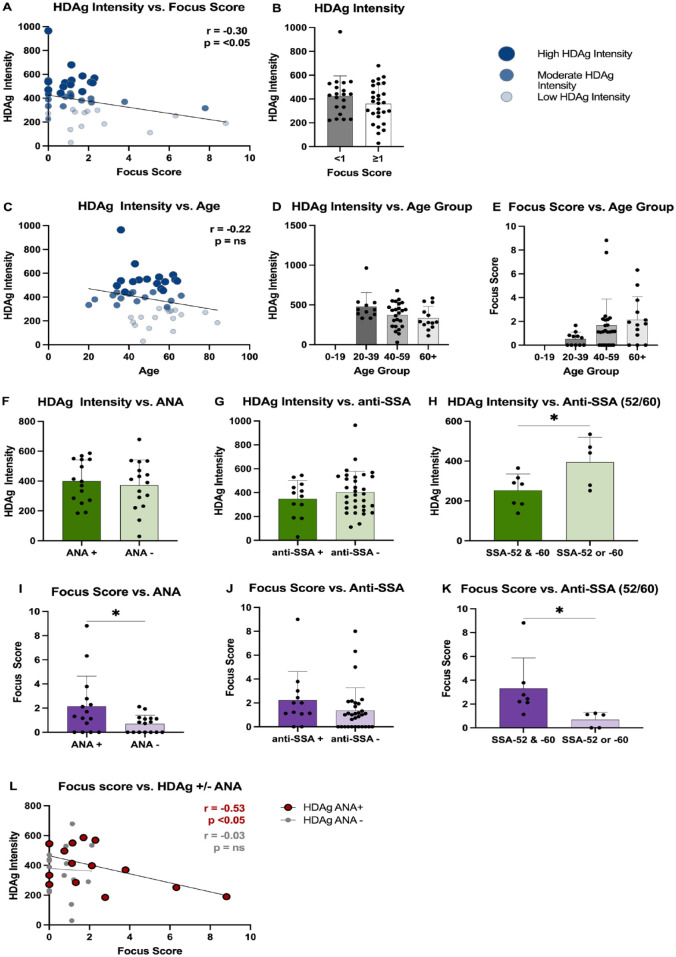
The analysis of HDAg intensity in MSG tissues from SjD patients in the Utahn cohort revealed multiple significant correlations with demographics and clinical features. A negative correlation was observed between HDAg intensity and focus score (r = −0.30, P < 0.05) (Fig. 3A). No significant difference was found in HDAg intensity between biopsies with focus scores of <1 or ≥1 foci/4 mm2 (Fig. 3B). A negative trend was also observed between HDAg intensity and age, with HDAg intensity steadily decreasing with increasing age (Fig. 3C, D). An increasing trend was seen between focus score and age groups, although this was not statistically significant (Fig. 3E). These findings highlight the potential impact of HDAg expression on SjD pathogenesis and suggest a relationship between HDAg intensity, age, and focus score in this patient population.
Figure 3.
Hepatitis delta virus antigen (HDAg) intensity comparisons and clinical characteristics correlation analysis. (A) Pearson correlation analysis between HDAg fluorescence intensity of minor salivary gland (MSG) biopsy samples and biopsy focus score, n = 48. (B) Welch’s t test comparison between HDAg fluorescence intensity and focus score grouped by focus score ≥1 and <1, n = 48. (C) Pearson correlation between HDAg fluorescence intensity of MSG biopsy samples and age, n = 48. Age groups of biopsied patients were then compared with (D) HDAg intensity and (E) focus score, n = 48. Patients’ biopsies’ HDAg intensity was compared against (F) antinuclear antibody testing data (n = 15) and (G) anti-SSA antibody (n = 12). (H) Patients with SSA-52 and SSA-60 and patients with either SSA-52 or SSA-60 were compared by HDAg intensity (n = 12). Patients’ biopsies’ focus scores were compared against (I) antinuclear antibody testing data (n = 15) and (J) anti-SSA antibody (n = 12). (K) Patients with SSA52 and SSA-60 and patients with either SSA-52 or SSA-60 were compared by focus score (n = 12). (L) Patients’ biopsies were grouped according to antinuclear antibody (ANA) test status and plotted according to HDAg intensity and focus score. Statistics were performed in GraphPad Prism, (A, C, L) Pearson correlation, (B, F–K) Welch’s t test, (D, E) one-way analysis of variance with multiple comparison test. Bar graph data are shown as mean ± SD; *P < 0.05.
Autoantibody profiles were also evaluated in relation to HDAg intensity. No significant differences were observed between HDAg intensity and the presence of ANA or anti-SSA (Fig. 3F–G). However, patients positive for either anti-SSA/Ro-52 or anti-SSA/Ro-60 alone exhibited elevated HDAg intensity levels compared to patients who were positive for both anti-SSA/Ro-52 and anti-SSA/Ro-60 (P < 0.05) (Fig. 3H). These findings suggest that HDAg intensity may be influenced by certain autoantibody profiles in SjD patients.
Autoantibody profiles were also evaluated in relation to focal inflammation. Patients who tested positive for ANA had a significantly higher focus score compared to patients negative for ANA (P < 0.05) (Fig. 3I). No difference in focus score was observed between patients who tested positive or negative for anti-SSA (anti-SSA/Ro-52 or anti-SSA/Ro-Ro-60) (Fig. 3J). However, patients positive for both anti-SSA/Ro-52 and anti-SSA/Ro-60 had a significantly higher focus score compared to patients positive for either anti-SSA/Ro-52 or anti-SSA/Ro-60 alone (P < 0.05) (Fig. 3K). In SjD patients positive for ANA, HDAg intensity negatively correlated with focus score (Fig. 3L). No correlation was observed between HDAg intensity and focus score in ANA-negative patients. In summary, HDAg intensity negatively correlated with focus score and was decreased in patients positive for both anti-SSA/Ro-52 and anti-SSA/Ro-60.
Hepatitis delta virus antigen was detected in the nuclei of adipose tissue within labial MSG tissue from patients with SjD (Appendix Fig. 2F–J). The presence of HDV in adipose tissue was evaluated alongside demographic and clinical data from the cohort of 48 SjD patients. No significant correlations were found between the percent area of adipose tissue and focus score, age, sex, or autoantibody profile (Appendix Fig. 4).
Comorbidities in HDV+ SjD Patients
Evaluation of autoimmune and infectious disease comorbidities was performed with our defined Utahn SjD cohort (Appendix Table 2). Of the autoimmune diseases evaluated, SjD patients diagnosed with autoimmune thyroiditis and/or hypothyroidism were significantly more represented in the high HDAg intensity group relative to negative and moderate HDAg intensity (P < 0.006). Within the Utahn SjD cohort analyzed, 14.6% (7/48) of SjD patients and 37.5% (6/16) of SjD patients with high HDAg intensity had a diagnosis of autoimmune thyroiditis or hypothyroidism (Appendix Table 2). In addition, there was an increasing trend of myositis in the high HDAg intensity group, although this trend was not statistically significant. Other comorbidities, including lupus, rheumatoid arthritis, multiple sclerosis, and ankylosing spondylitis, did not show significant trends based on HDAg intensity group. No SjD patient in the Utah SjD cohort analyzed had been diagnosed with hepatitis C virus (HCV) or hepatitis B virus (HBV) as determined by the presence of associated International Classification of Diseases (ICD) codes.
Absence of HBV Coinfection or Viral Hepatitis
Hepatitis delta virus has been extensively studied in association with an HBV coinfection. Therefore, liver enzymes, HBV lab test results, and HBV ICD codes were analyzed for potential current or prior HBV coinfection and undiagnosed liver disease in the SjD patient cohort. Results showed that 12%, 31%, and 29% of the SjD patients had abnormal levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP), respectively (Appendix Table 3). However, there were no significant correlations between abnormal AST, ALT, ALP, or AST/ALT ratio and HDAg intensity (Appendix Fig. 5). Similarly, there were no significant correlations between HDAg intensity and abnormal levels of bilirubin, albumin, or protein. Of the 20 patients tested for HBsAg or HBcAb, none had a positive result, indicating absence of documented HBV coinfections in this study population.
Discussion
This study demonstrates the presence of HDV antigen and genomic sequence in SjD patients’ MSG tissue, primarily in acinar and ductal cells, as well as myoepithelial cells and adipocytes. HDAg localized with the nuclei, cytoplasm, and mitochondria. The study is the first to detect and locate HDV RNA in human MSG tissue, confirming HDV’s presence in nonhepatic tissues. The presence of HDV antigen and sequence in SjD-affected MSG, which has been shown to trigger the SjD phenotype in vivo (Weller et al. 2016), further supports that a viral mechanism may be involved in the development of SjD.
A comparative analysis identified several clinical features that correlated with HDAg expression in patients with SjD. The intensity of HDAg expression in the affected MSG tissue was found to be negatively correlated with the presence of focal inflammation. This may be attributed to either a targeted clearance of HDV in patients with elevated focal inflammation or a limited role of HDV in the inflammatory component of SjD. Due to the lack of detectible anti-HDV antibodies noted prior (Weller et al. 2016), we hypothesize that the mechanisms of HDV in SjD development may be independent or upstream from the focal inflammation. This latter possibility is supported by the lack of detectable antibodies to HDAg in HDV-positive SjD patients (Weller et al. 2016). It is also possible that changes in HDAg expression during the development and progression of SjD may contribute to the higher levels of HDAg in younger SjD patients, as increased MSG inflammation has been noted in older SjD patients (Bouma et al. 2015). However, it is not yet clear if the inflammation associated with SjD is responsible for the decreased HDAg detection observed in older patients with more inflammation.
HDAg colocalization with mitochondria has not previously been reported. However, a previous study by Casaca et al. (2011) found that HDAg interacts with multiple proteins that originate in the mitochondria, including ND2, ND4, COX I, COX II, ATP8, and a mitochondrial-localized protein (NDUFB7), in a yeast 2-hybrid screening. This suggests that HDAg may localize to the mitochondria in order to interact with these proteins. Antimitochondrial antibodies (AMAs) have been reported in a subset of SjD patients, occurring in 1.7% to 13% of SjD patients and most frequently in patients with primary biliary cirrhosis (PBC) (Selmi et al. 2012; Scofield et al. 2018). There have also been reports of an increased prevalence of AMAs in PBC (Rizzetto 2020). However, there is currently no evidence of a direct association between the presence of AMAs and HDV. The potential impact of HDAg colocalization with mitochondria on cellular respiration requires further investigation.
Patients with elevated HDV antigen expression were found to have an increased incidence of autoimmune thyroiditis and hypothyroidism (Appendix Table 2). This is consistent with previous research indicating a higher prevalence of autoimmune thyroid disease (AITD) in SjD patients (Jara et al. 2007; Baldini et al. 2018). Caramaschi et al. (2013) found that SjD patients with thyroid disease had a less severe clinical phenotype of SjD. Our findings of lower foci scores and autoantibody profiles in patients with elevated HDV antigen expression are in line with this observation. Lu et al. (2013) also reported an increased risk of hypothyroidism in younger (20–44 y old) SjD patients. A similar trend was observed in our analysis, with higher HDAg intensity in younger age groups. It remains unclear whether SjD and AITD share a common pathogenesis and whether HDV plays a role. Forty percent of evaluated patients had at least 1 additional autoimmune disease. While this is in line with other studies noting 23% to 40% of SjD patients reporting extraglandular diseases (Malladi et al. 2012; Mariette and Criswell 2018), this high prevalence may be attributed to the limited use of MSG biopsies in SjD diagnosis and potential increased utilization of MSG biopsies during the differential diagnosis process in patients with polyautoimmunity. Further large-scale studies are needed to better understand the relationship between HDV and SjD, as well as the potential link to other autoimmune diseases and shared etiologies.
Multiple limitations must be considered in this study. First, this retrospective study relied on physician-based diagnosis of SjD, rather than a prospective study that relied on established SjD classification criteria (Shiboski et al. 2017). Relying on physician-based diagnoses may result in incomplete testing and diagnoses across the study cohort. Second, the small size of the study may have affected the strength of the associations observed between the HDV profile and in situ and clinical features. Larger prospective clinical studies are needed to establish associations between salivary gland-localized HDV and the SjD phenotype. To support such large-scale evaluations of HDV in the SjD patient population, it would be necessary to increase MSG biopsy usage for SjD diagnosis and validate systemic markers for the HDV+/HBV– profile.
It is not clear how SjD patients are acquiring HDV in the absence of detectable HBV coinfection. Traditionally, HDV is thought to require HBV for packaging and transmission, but it can replicate and persist in the absence of active HBV coinfection by using host cellular machinery (Chang et al. 2005). In this retrospective study, as well as in a previous cohort study (Weller et al. 2016), patients with detectable HDAg expression in MSG did not have evidence of current or past HBV coinfection (Appendix Tables 3 and 4, Appendix Fig. 5). Multiple studies have investigated the potential role of HBV as a trigger for SjD and have found a similar or lower incidence of HBV in SjD compared to the general population (Ram et al. 2008; Marcos et al. 2009; Chen et al. 2012). A recent study detected HDV in a small number of HBV–/HCV+ patients (Chemin et al. 2021). However, none of the SjD patients in our current study had evidence of current or past HCV infection as determined by ICD for HCV diagnosis (Appendix Table 3). Further research is needed to understand alternative mechanisms of HDV localization to MSG tissue in the absence of a detectable current or past HBV coinfection.
Our understanding of HDV is rapidly advancing. Recently, HDV was classified as a member of the Kolmioviridae family based on the discovery of >10 new HDV-like sequences isolated from various animal species, in the absence of readily detectable HBV or other Hepadnaviridae coinfections (Wille et al. 2018; Chang et al. 2019; Hetzel et al. 2019; Iwamoto et al. 2021). In addition, HDV has been shown to package into non-HBV particles in vitro, including vesiculovirus, flavivirus, hepacivirus, arenavirus, and orthohantavirus (Perez-Vargas et al. 2019; Szirovicza et al. 2020). While there are only a few reported cases of detected HDV without evident HBV coinfections in humans (Weller et al. 2016; Chemin et al. 2021; Rodgers et al. 2022), these recent findings suggest that HDV and HDV-like sequences may be able to package into non-HBV particles and shift HDV tropism to nonhepatic tissues.
In summary, further research is needed to fully understand the role of HDV in SjD. It is currently unclear how HDV mechanistically contributes to the development of the SjD phenotype. Larger studies are necessary to determine how HDV is localizing and persisting in SjD salivary gland tissue and how SjD patients are acquiring the unique HDV+/HBV– profile.
Author Contributions
M.C. Hesterman, S.V. Furrer, contributed to conception and design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; B.S. Fallon, contributed to conception and design, data interpretation, drafted and critically revised manuscript; M.L. Weller, contributed to conception and design, data acquisition and analysis, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, sj-docx-1-jdr-10.1177_00220345231186394 for Analysis of Hepatitis D Virus in Minor Salivary Gland of Sjögren’s Disease by M.C. Hesterman, S.V. Furrer, B.S. Fallon and M.L. Weller in Journal of Dental Research
Acknowledgments
MSG biopsies and accompanying pathology reports and electronic medical record data requests were obtained from the University of Utah’s UHealth system, UHealth’s dermatopathology and surgical pathology departments, and the Data Science Services, respectively. We thank S. Shankar, E. Diggins, B. Acosta, and R. Hill for diligent review of this manuscript.
Footnotes
A supplemental appendix to this article is available online.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by funding from the National Institute of Health, the National Institute of Dental and Craniofacial Research (NIDCR R00/DE021745), the University of Utah School of Dentistry, the Undergraduate Research Opportunities Program, and the Summer Program for Undergraduate Research at the University of Utah.
ORCID iDs: B.S. Fallon  https://orcid.org/0000-0001-8308-418X
https://orcid.org/0000-0001-8308-418X
M.L. Weller  https://orcid.org/0000-0002-6646-1403
https://orcid.org/0000-0002-6646-1403
References
- Baldini C, Ferro F, Mosca M, Fallahi P, Antonelli A. 2018. The association of Sjögren syndrome and autoimmune thyroid disorders. Front Endocrinol (Lausanne). 9:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouma HR, Bootsma H, van Nimwegen JF, Haacke EA, Spijkervet FK, Vissink A, Kroese FGM. 2015. Aging and immunopathology in primary Sjögren’s syndrome. Curr Aging Sci. 8(2):202–213. [DOI] [PubMed] [Google Scholar]
- Caramaschi P, Biasi D, Caimmi C, Scambi C, Pieropan S, Barausse G, Adami S. 2013. The co-occurrence of Hashimoto thyroiditis in primary Sjogren’s syndrome defines a subset of patients with milder clinical phenotype. Rheumatol Int. 33(5):1271–1275. [DOI] [PubMed] [Google Scholar]
- Casaca A, Fardilha M, da Cruz e Silva E, Cunha C. 2011. The heterogeneous ribonuclear protein C interacts with the hepatitis delta virus small antigen. Virol J. 8:358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey JL. editor. 2006. Hepatitis delta virus. Current topics in microbiology and immunology. Berlin Heidelberg: Springer-Verlag; [accessed 2019 Sep 13]. https://www.springer.com/us/book/9783540298014. [Google Scholar]
- Chang J, Gudima SO, Tarn C, Nie X, Taylor JM. 2005. Development of a novel system to study hepatitis delta virus genome replication. J Virol. 79(13):8182–8188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W-S, Pettersson JH-O, Le Lay C, Shi M, Lo N, Wille M, Eden J-S, Holmes EC. 2019. Novel hepatitis D-like agents in vertebrates and invertebrates. Virus Evol. 5(2):vez021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemin I, Pujol FH, Scholtès C, Loureiro CL, Amirache F, Levrero M, Zoulim F, Pérez-Vargas J, Cosset F-L. 2021. Preliminary evidence for hepatitis delta virus exposure in patients who are apparently not infected with hepatitis B virus. Hepatology. 73(2):861–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H-Y, Shen D-T, Ji D-Z, Han P-C, Zhang W-M, Ma J-F, Chen W-S, Goyal H, Pan S, Xu H-G. 2019. Prevalence and burden of hepatitis D virus infection in the global population: a systematic review and meta-analysis. Gut. 68(3):512–521. [DOI] [PubMed] [Google Scholar]
- Chen M-H, Hsiao L-T, Chen M-H, Tsai C-Y, Huang Y-H, Chou C-T. 2012. Clinical significance of chronic hepatitis B virus infection in patients with primary Sjögren’s syndrome. Clin Rheumatol. 31(2):309–315. [DOI] [PubMed] [Google Scholar]
- Chen PJ, Kalpana G, Goldberg J, Mason W, Werner B, Gerin J, Taylor J. 1986. Structure and replication of the genome of the hepatitis delta virus. Proc Natl Acad Sci U S A. 83(22):8774–8778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetzel U, Szirovicza L, Smura T, Prähauser B, Vapalahti O, Kipar A, Hepojoki J. 2019. Identification of a novel deltavirus in Boa constrictors. mBio. 10(2):e00014-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igoe A, Scofield RH. 2013. Autoimmunity and infection in Sjögren’s syndrome. Curr Opin Rheumatol. 25(4):480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto M, Shibata Y, Kawasaki J, Kojima S, Li Y-T, Iwami S, Muramatsu M, Wu H-L, Wada K, Tomonaga K, et al. 2021. Identification of novel avian and mammalian deltaviruses provides new insights into deltavirus evolution. Virus Evol. 7(1):veab003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izmirly PM, Buyon JP, Wan I, Belmont HM, Sahl S, Salmon JE, Askanase A, Bathon JM, Geraldino-Pardilla L, Ali Y, et al. 2019. The incidence and prevalence of adult primary Sjögren’s syndrome in New York county. Arthritis Care Res (Hoboken). 71(7):949–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jara LJ, Navarro C, Brito-Zerón M, del P, García-Carrasco M, Escárcega RO, Ramos-Casals M. 2007. Thyroid disease in Sjögren’s syndrome. Clin Rheumatol. 26(10):1601–1606. [DOI] [PubMed] [Google Scholar]
- Liu K, Kurien BT, Zimmerman SL, Kaufman KM, Taft DH, Kottyan LC, Lazaro S, Weaver CA, Ice JA, Adler AJ, et al. 2016. X chromosome dose and sex bias in autoimmune diseases: increased prevalence of 47,XXX in systemic lupus erythematosus and Sjögren’s syndrome. Arthritis Rheumatol. 68(5):1290–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M-C, Yin W-Y, Tsai T-Y, Koo M, Lai N-S. 2013. Increased risk of primary Sjögren’s syndrome in female patients with thyroid disorders: a longitudinal population-based study in Taiwan. PLoS One. 8(10):e77210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciel G, Crowson CS, Matteson EL, Cornec D. 2017. Prevalence of primary Sjögren’s syndrome in a US population-based cohort. Arthritis Care Res (Hoboken). 69(10):1612–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malladi AS, Sack KE, Shiboski S, Shiboski C, Baer AN, Banushree R, Dong Y, Helin P, Kirkham BW, Li M, et al. 2012. Primary Sjögren’s syndrome as a systemic disease: a study of participants enrolled in an international Sjögren’s syndrome registry. Arthritis Care Res (Hoboken). 64(6):911–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcos M, Alvarez F, Brito-Zerón P, Bove A, Perez-De-Lis M, Diaz-Lagares C, Sanchez-Tapias J-M, Ramos-Casals M. 2009. Chronic hepatitis B virus infection in Sjögren’s syndrome. Prevalence and clinical significance in 603 patients. Autoimmun Rev. 8(7):616–620. [DOI] [PubMed] [Google Scholar]
- Mariette X, Criswell LA. 2018. Primary Sjögren’s syndrome. N Engl J Med. 378(10):931–939. [DOI] [PubMed] [Google Scholar]
- Maslinska M, Kostyra-Grabczak K. 2022. The role of virus infections in Sjögren’s syndrome. Front Immunol. 13:823659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Z, Zhang S, Ou X, Li S, Ma Z, Wang W, Peppelenbosch MP, Liu J, Pan Q. 2020. Estimating the global prevalence, disease progression, and clinical outcome of hepatitis delta virus infection. J Infect Dis. 221(10):1677–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Vargas J, Amirache F, Boson B, Mialon C, Freitas N, Sureau C, Fusil F, Cosset F-L. 2019. Enveloped viruses distinct from HBV induce dissemination of hepatitis D virus in vivo. Nat Commun. 10(1):2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ram M, Anaya J-M, Barzilai O, Izhaky D, Porat Katz B-S, Blank M, Shoenfeld Y. 2008. The putative protective role of hepatitis B virus (HBV) infection from autoimmune disorders. Autoimmun Rev. 7(8):621–625. [DOI] [PubMed] [Google Scholar]
- Rizzetto M. 2020. The discovery of the hepatitis D virus: three princes of serendip and the recognition of autoantibodies to liver-kidney microsomes. Clin Liver Dis (Hoboken). 16(Suppl 1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzetto M, Canese MG, Aricò S, Crivelli O, Trepo C, Bonino F, Verme G. 1977. Immunofluorescence detection of new antigen-antibody system (δ/anti-δ) associated to hepatitis B virus in liver and in serum of HBsAg carriers. Gut. 18(12):997–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers MA, Biondi MJ, Olivo A, Lark C, Hadji A, Duong PT, Dung NTT, Elaborot AS, Mbanya D, Alkhazashvili M, et al. 2022. Evidence of past hepatitis C and D co-infection in hepatitis B seronegative individuals. The international liver congress. European Association for the Study of the Liver; [accessed 2022 Aug 28]. https://natap.org/2022/EASL/EASL_55.htm. [Google Scholar]
- Scofield RH, Fayyaz A, Kurien BT, Koelsch KA. 2018. Prognostic value of Sjögren’s syndrome autoantibodies. J Lab Precis Med. 3:10.21037/jlpm.2018.08.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmi C, Meroni PL, Gershwin ME. 2012. Primary biliary cirrhosis and Sjögren’s syndrome: autoimmune epithelitis. J Autoimmun. 39(1–2):34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiboski CH, Shiboski SC, Seror R, Criswell LA, Labetoulle M, Lietman TM, Rasmussen A, Scofield H, Vitali C, Bowman SJ, et al. 2017. 2016. American College of Rheumatology/European League against rheumatism classification criteria for primary Sjögren’s syndrome: a consensus and data-driven methodology involving three international patient cohorts. Ann Rheum Dis. 76(1):9–16. [DOI] [PubMed] [Google Scholar]
- Stockdale AJ, Mitambo C, Everett D, Geretti AM, Gordon MA. 2018. Epidemiology of hepatitis B, C and D in Malawi: systematic review. BMC Infect Dis. 18(1):516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szirovicza L, Hetzel U, Kipar A, Martinez-Sobrido L, Vapalahti O, Hepojoki J. 2020. Snake deltavirus utilizes envelope proteins of different viruses to generate infectious particles. mBio. 11(2):e03250-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng C-H, Lai MMC. 2009. Hepatitis delta virus RNA replication. Viruses. 1(3):818–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban S, Neumann-Haefelin C, Lampertico P. 2021. Hepatitis D virus in 2021: virology, immunology and new treatment approaches for a difficult-to-treat disease. Gut. 70(9):1782–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivino FB, Bunya VY, Massaro-Giordano G, Johr CR, Giattino SL, Schorpion A, Shafer B, Peck A, Sivils K, Rasmussen A, et al. 2019. Sjogren’s syndrome: an update on disease pathogenesis, clinical manifestations and treatment. Clin Immunol. 203:81–121. [DOI] [PubMed] [Google Scholar]
- Weller ML, Gardener MR, Bogus ZC, Smith MA, Astorri E, Michael DG, Michael DA, Zheng C, Burbelo PD, Lai Z, et al. 2016. Hepatitis delta virus detected in salivary glands of Sjögren’s syndrome patients and recapitulates a Sjögren’s syndrome-like phenotype in vivo. Pathog Immun. 1(1):12–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wille M, Netter HJ, Littlejohn M, Yuen L, Shi M, Eden J-S, Klaassen M, Holmes EC, Hurt AC. 2018. A divergent hepatitis D-like agent in birds. Viruses. 10(12):720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z, Huang Y, Qi Y, Peng B, Wang H, et al. 2012. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife. 1:e00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-jdr-10.1177_00220345231186394 for Analysis of Hepatitis D Virus in Minor Salivary Gland of Sjögren’s Disease by M.C. Hesterman, S.V. Furrer, B.S. Fallon and M.L. Weller in Journal of Dental Research