Abstract
The capacity of a tissue to continuously alter its phenotype lies at the heart of how an animal is able to quickly adapt to changes in environmental stimuli. Within tissues, differentiated cells are rigid and play a limited role in adapting to new environments; however, differentiated cells are replenished by stem cells that are defined by their phenotypic plasticity. Here we demonstrate that a Wnt-responsive stem cell niche in the junctional epithelium is responsible for the capability of this tissue to quickly adapt to changes in the physical consistency of a diet. Mechanical input from chewing is required to both establish and maintain this niche. Since the junctional epithelium directly attaches to the tooth surface via hemidesmosomes, a soft diet requires minimal mastication, and consequently, lower distortional strains are produced in the tissue. This reduced strain state is accompanied by reduced mitotic activity in both stem cells and their progeny, leading to tissue atrophy. The atrophied junctional epithelium exhibits suboptimal barrier functions, allowing the ingression of bacteria into the underlying connective tissues, which in turn trigger inflammation and mild alveolar bone loss. These data link the mechanics of chewing to the biology of tooth-supporting tissues, revealing how a stem cell niche is responsible for the remarkable adaptability of the junctional epithelium to different diets.
Keywords: Wnt, stem cell, gingiva, mechanosensitive, proliferation, plasticity
Introduction
Teeth are the sole transmucosal organ in the mammalian body and, as a consequence, require a unique epithelial barrier. The junctional epithelium (JE) fulfills this function, serving as a continuously renewing epithelial seal that, by virtue of its direct attachment to the tooth surface, curtails microbial ingression and thus maintains periodontal health. The JE contains a Wnt-responsive stem cell niche, which serves 2 critical functions: first, the niche replaces cells that are lost during normal day-to-day living, and second, it generates cells that, after injury, contribute to repair and regeneration of the JE (Yuan, Chen, Gauer, et al. 2020; Yuan, Chen, Van Brunt, et al. 2020).
Stem cell maintenance depends on both chemical and physical niche signals (Vining and Mooney 2017). Wnt proteins are among the best-studied niche signals. Secreted Wnt proteins operate over short distances, binding to receptors expressed on adjacent stem cells and driving Wnt-dependent transcriptional programs. Wnt-responsive cells can be identified by Axin2, and genetic tracing studies confirm that Axin2-positive cells function as stem cells in multiple tissues (Clevers et al. 2014).
Physical stimuli also shape stem cell niches. In epithelial tissues, cell–cell adhesions and changes in cell density demonstrably affect stem cell fates (Liang et al. 2017). These and other studies point to mechanical forces as being critically involved in stem cell niche development (Chacon-Martinez et al. 2018).
Here, our objective was to understand how physical and chemical forces together shape the JE and its stem cell niche. One method to alter mechanical forces experienced by the JE is to change the physical consistency of the diet. Since the JE is attached via hemidesmosomes to the tooth surface, mastication-induced physiological tooth movement would be anticipated to produce stresses and strains in the JE, just as it does in the underlying periodontal ligament (PDL). The magnitude of stresses and strains would be proportional to the hardness of the diet (Ustriyana et al. 2022). Using this model, we investigated whether Wnt-responsive stem cells were sensitive to mastication-related strain states and if JE barrier functions were affected by changes in mechanical forces, thus potentially reconciling an extensive historic literature equating a soft diet with periodontal disease in animals and humans (Baer 1956).
Method and Materials
Please see the Appendix for information on lineage tracing, timepoints for molecular and cellular analyses, finite element methods, and quantification.
Animals
Experimental protocols were approved by the Stanford Committee on Animal Research (#13146) and performed in accordance with ARRIVE (Animal Research: Reporting of In Vivo Experiments) 2.0 guidelines. Animals were maintained under a light/dark cycle of 12 h. Males and females were employed in equal numbers (for distribution, see Appendix Table 2) and housed in groups not exceeding 5/cage.
Diets
Hard chow diet consisted of Teklad Global Rodent Diet (#2918; Envigo); the soft diet consisted of Nutra-Gel Diet Grain-Based Formula (S4798-TRAY; Bio-Serv). A nutritional comparison provided by the manufacturers ensured that hard and soft diets contained similar profiles of amino acids, fatty acids, carbohydrates, minerals, and vitamins. Mice were randomly assigned to soft versus hard diet groups and provided food ad libitum. Weight gain was monitored as a measure of feeding pattern and control of energy intake (Appendix Fig. 1).
Results
A Wnt-Responsive JE Stem Cell Niche Forms with the Onset of Mastication
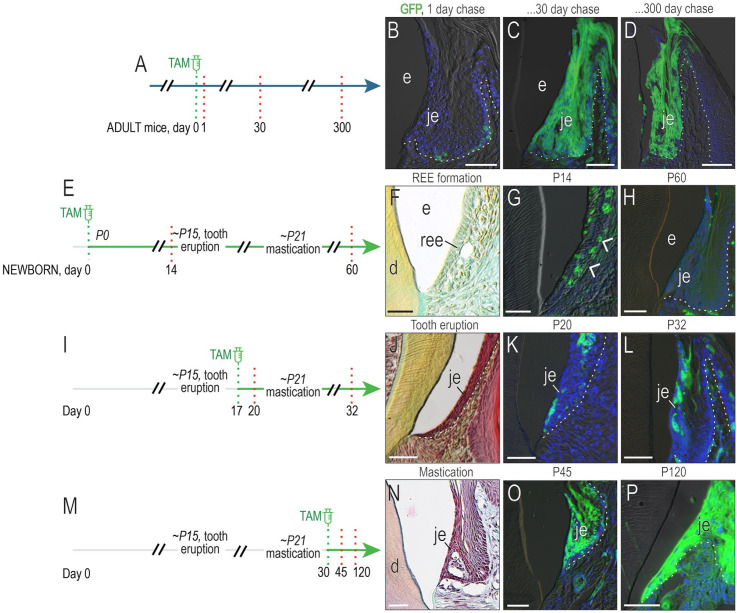
A stem cell niche is responsible for maintaining the JE. We showed this using Axin2CreERT2/+;R26RmTmG/+ mice in which tamoxifen exposure induces Axin2-expressing, Wnt-responsive cells to express green fluorescent protein (GFP) (Fig. 1A). Within 24 h, a subset of JE cells at the basal lamina were GFP+ (Fig. 1B). Despite a turnover rate of ~4 d (Yuan, Chen, Gauer, et al. 2020), GFP+ cells persisted in the JE 1 mo after tamoxifen labeling (Fig. 1C). GFP+ cells were still present 300 d later (Fig. 1D).
Figure 1.
Masticatory forces are associated with establishment of a Wnt-responsive junctional epithelium (JE) stem cell niche. (A) Schematic of experimental design, where tamoxifen was delivered to adult Axin2CreERT2/+;R26RmTmG/+ mice, thus inducing a recombination event in which Axin2-expressing, Wnt-responsive cells are permanently labeled with green fluorescent protein (GFP). (B) Twenty-four hours after tamoxifen delivery, a few GFP+ cells were labeled in the JE. (C) Thirty days after tamoxifen delivery, GFP+ cells filled the JE. (D) Three hundred days after tamoxifen, progeny of the original Wnt-responsive, GFP+ cells persisted in the JE. (E) Schematic of experimental design, where newborn Axin2CreERT2/+;R26RmTmG/+ pups received tamoxifen via nursing; 14 d later, (F) the reduced enamel epithelium was evident around unerupted teeth. (G) GFP+ cells in the reduced enamel epithelium (white arrowheads). (H) Sixty days after tamoxifen, the mature JE was devoid of GFP+ cells. (I) Schematic of experimental design, where shortly after tooth eruption (e.g., at P17), Axin2CreERT2/+;R26RmTmG/+ pups received tamoxifen. (J) At P20, shortly before mastication of hard food began, (K) a few GFP+ cells were detected in the JE. (L) At P32, a subset of GFP+ JE cells persisted. (M) Schematic of experimental design, where tamoxifen was delivered to Axin2CreERT2/+;R26RmTmG/+ pups on P30, after mastication had begun. (N) At this time, the JE achieved its adult morphology. (O) At P45, the JE was fully populated by GFP+ cells. (P) At 120 d, GFP+ cells persisted. d, dentin; e, enamel; ree, reduced enamel epithelium. Scale bars: 50 µm. White dotted lines indicate the boundary between epithelium and underlying connective tissue.
To determine when this Wnt-responsive stem cell population becomes established, we examined P14 Axin2CreERT2/+;R26RmTmG/+ pups whose teeth had yet to erupt. The JE forms from the reduced enamel epithelium (REE; Fig. 1E, F). A subset of postsecretory ameloblasts in the REE was GFP+ (Fig. 1G), but when the JE was reexamined at P60, the GFP+ population was no longer detectable (Fig. 1H).
Tamoxifen was then delivered to pups around the time of tooth eruption (Fig. 1I). Shortly thereafter, the JE was populated by a few Wnt-responsive cells; 12 d later, descendants of the Wnt-responsive population persisted (Fig. 1J–L). By P30, when mice were receiving nutrition solely by mastication of a hard diet, lineage-tracing analyses demonstrated the presence of a GFP+ population (Fig. 1M–O) that persisted well into middle age (Fig. 1P). Collectively, these lineage-tracing data demonstrate that a Wnt-responsive stem cell niche is established only after tooth eruption.
The JE Stem Cell Niche Degenerates in Response to a Soft Diet
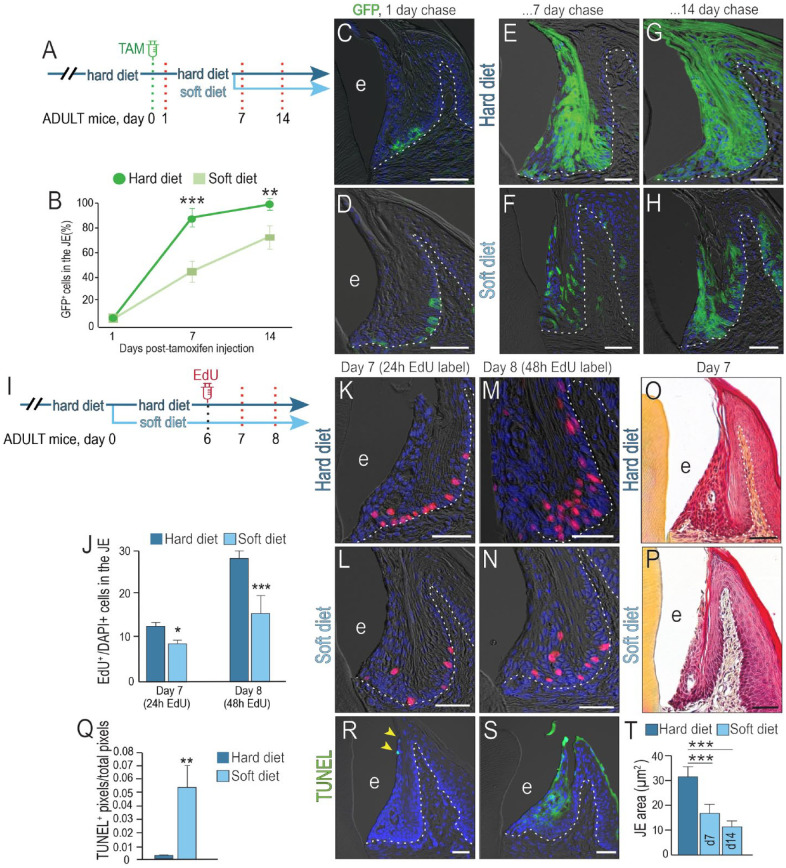
To test if the Wnt-responsive JE stem cell niche was sensitive to masticatory forces, adult mice were assigned to groups whose diets were nutritionally equivalent but differed in hardness, with the hard chow diet requiring significantly greater bite force to process compared to a soft diet (Ginot et al. 2018). No significant differences in body weight were observed between the 2 cohorts (Appendix Fig. 1), supporting the conclusion that the diets were comparable in terms of nutritional value.
To compare JE stem cell niche activity in the 2 groups, a single dose of tamoxifen was delivered to Axin2CreERT2/+;R26RmTmG/+ mice (Fig. 2A), and the distribution of Wnt-responsive, GFP+ cells was evaluated 24 h later. At this starting timepoint, the distribution and number of GFP+ cells were equivalent between the hard and soft diet cohorts (Fig. 2B–D). Thereafter, animals were fed their respective diets for the next 7 d. In the hard diet group, the initial GFP+, Wnt-responsive population had expanded to nearly fill the JE (Fig. 2E); in the soft diet group, significantly fewer GFP+ cells were present (Fig. 2F; quantified in 2B).
Figure 2.
Masticatory forces are required to maintain both the Wnt-responsive stem cell niche and mitotic activity in the junctional epithelium (JE). (A) Schematic of lineage-tracing experiments in adult Axin2CreERT2/+;R26RmTmG/+ mice. The day of tamoxifen delivery was counted as day 0. After 1 d, mice were randomly assigned to either a hard diet or a soft diet cohort and maintained on this diet for either 7 or 14 d. (B) Quantification of green fluorescent protein (GFP)+ cells in the JE of the hard and soft diet cohorts at the timepoints indicated, followed by a Student’s t test, where 2 asterisks represent a P value ≤0.01, and 3 asterisks indicate a P value ≤0.001 (n = 4). Evaluations of the number and distribution of GFP+ cells were performed 1 d after tamoxifen delivery to the (C) hard and (D) soft diet groups. The same analyses were performed (E, F) 7 d and (G, H) 14 d following tamoxifen delivery for hard and soft diet groups. (I) Schematic of a mitotic activity experiment in adult mice, where EdU was delivered 6 d after animals had been switched to a soft diet. The control group was maintained on a hard diet for the duration of the experiment. The distribution and number of EdU+ cells were determined after 24 h (e.g., day 7 of the experiment) and after 48 h (e.g., day 8 of the experiment). (J) Quantification of EdU+ cells in the JE, normalized to the total number of DAPI+ cells in the JE at the timepoints indicated. A Student’s t test was performed, where 1 asterisk represents a P value ≤0.05, and 3 asterisks indicate a P value ≤0.001 (n = 4). Evaluation of the number of EdU+ cells was performed 24 h after EdU delivery to the (K) hard and (L) soft diet groups. Forty-eight hours later, labeling identified both EdU+, mitotically active cells and their progeny in the JE of animals on a (M) hard and (N) soft diet. Pentachrome staining of the JE after animals were maintained for 7 d on a (O) hard and (P) soft diet. (Q) Quantification of terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) activity; 2 asterisks represent a P value ≤0.05. TUNEL staining (R) in the hard diet group (yellow arrows) and (S) in the soft diet group. (T) Quantification of JE area determined by ImageJ; 3 asterisks indicate a P value ≤0.001. Abbreviations: as noted in Figure 1. Scale bars: 50 µm. White dotted lines indicate the boundary between epithelium and underlying connective tissue.
Another analysis was performed 14 d after labeling the Wnt-responsive stem cell population (Fig. 2G, H). These data demonstrated that progeny of the Wnt-responsive population were sensitive to changes in mastication-related forces and divided at a significantly slower rate in the soft diet cohort.
EdU labeling confirmed that mastication-related forces altered cellular activity in the JE. Twenty-four hours after EdU delivery (Fig. 2I), the distribution of cells in S phase (e.g., EdU+ cells) was assessed (Fig. 2J). In both cohorts, labeled cells were located along the basal lamina (compare Fig. 2K, L), but even relative to the overall area of the JE, there were significantly more EdU+ cells in S phase in the hard diet group (Fig. 2J).
Tissues were reassessed 48 h after EdU delivery. Since EdU was only given once (Fig. 2I), an increase in cell number at this timepoint reflected cell division in the initial EdU+ population. The number of labeled JE cells in the hard diet cohort increased nearly 2.5-fold (Fig. 2J, M), whereas in the soft diet group, EdU-labeled JE cells increased less than 2-fold (Fig. 2J, N). In alignment with these data, the area of the JE was significantly smaller in the soft diet cohort (Fig. 2O, P).
A soft diet also led to increased programmed cell death in the JE (Fig. 2Q). Normally, terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) activity is restricted to the tip of the JE (yellow arrows, Fig. 2R). In the soft diet group, TUNEL activity was widespread (Fig. 2S). In combination, reduced mitosis and increased apoptosis in the soft diet cohort culminated in JE atrophy (Fig. 2T). These effects of mastication-related physical forces on the JE were even more pronounced if a soft food diet was initiated after weaning (Appendix Fig. 2).
Establishing a Link between Chewing Mechanics and Biological Responses in the JE
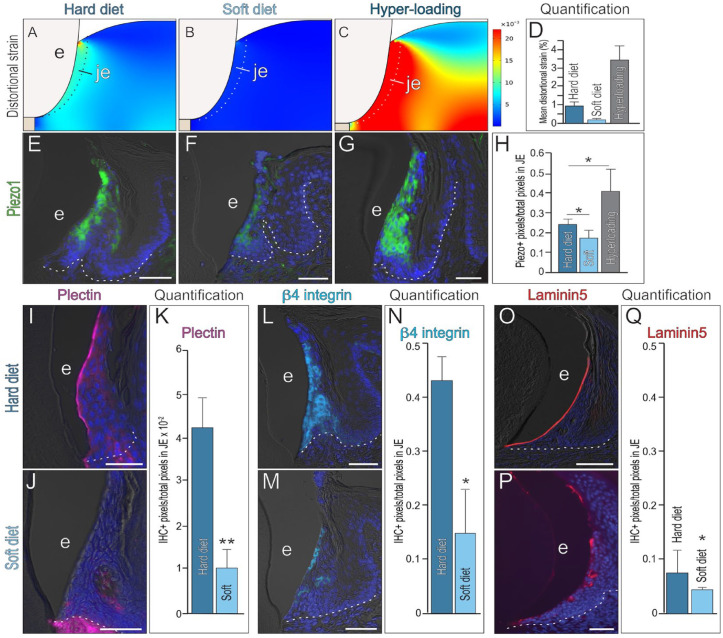
To gain mechanistic insights into the link between a soft diet and JE responses, we considered the various ways diet could affect the soft tissues. A nutritional difference had been eliminated as a variable, which led us to consider mechanical forces required to process food. Masticatory forces induce the physiologic movement of teeth within their sockets, made possible by the PDL. Like the ligament, the JE is also directly attached to the tooth; therefore, masticatory forces that produce tooth movement would also create strains in the JE. Values of Young’s elastic modulus and Poisson’s ratio were assigned to the JE (Appendix Table 1) along with bite forces. Masticating a soft diet produced distortional strains that were 10 times lower than those produced when masticating a hard diet (Fig. 3A, B). If masticatory forces were elevated by combining a hard diet with hyperloading, then distortional strains were 3 times larger than the hard diet cohort (Fig. 3C).
Figure 3.
The junctional epithelium (JE) and its stem cell niche are mechanoresponsive. Distortional strains in the JE produced by masticatory loading associated with (A) a hard diet (B), a soft diet, and (C) hyperloading in combination with a hard diet. (D) Quantification of peak distortional strains in the JE of the 3 loading scenarios. Expression of the mechanosensitive protein Piezo1 in the JE of mice maintained on (E) a hard diet, (F) a soft diet for 4 wk, or (G) a hard diet coupled with hyperloading for 1 d. (H) Quantification of Piezo1+ pixels/total pixels in the JE. One asterisk represents a P value ≤0.05 (n = 3). Representative image of quantitative immunohistochemistry (qIHC) localization of the hemidesmosomal protein Plectin in the JE of the (I) hard diet versus (J) soft diet cohort, quantified in (K). Two asterisks represent a P value ≤0.05 (n = 3). Representative image of qIHC localization of the hemidesmosomal protein β4 integrin in the JE of the (L) hard diet versus (M) soft diet cohort, quantified in (N). One asterisk represents a P value ≤0.05 (n = 3) Representative image of qIHC localization of the hemidesmosomal protein Laminin5 in the JE of the (O) hard diet versus (P) soft diet cohort, quantified in (Q). One asterisk represents a P value ≤0.05 (n = 3) Abbreviations as indicated in Figure 1; scale bars: 50 µm. White dotted lines indicate the boundary between epithelium and underlying connective tissue.
The patterns of mean distortional strain (Fig. 3D) were mirrored by patterns of the mechanosensitive protein Piezo1 (Wang et al. 2020). Compared to the hard diet cohort, Piezo1 expression was significantly reduced in the JE of the soft diet group (Fig. 3E, F, quantified in 3H). In the hyperloading scenario, Piezo1 expression was increased relative to the hard diet control group (Fig. 3E, G, quantified in 3H). These data established a correlation between distortional strain patterns and Piezo1 expression patterns in the JE.
The JE attaches to the tooth surface via a mechanosensitive hemidesmosomal protein, Plectin; Plectin, in turn, interacts with hemidesmosomal protein β4 integrin, and integrins interact with hemidesmosomal laminins. In the hard diet group, Plectin expression was limited to JE cells facing the enamel surface; in the soft diet group, Plectin was absent (Fig. 3I–K). β4 integrin expression in the hard diet group overlapped with Plectin at the JE edge; internal JE cells also expressed β4 integrin (Fig. 3L); in the soft diet group, β4 integrin expression was significantly reduced throughout the JE (Fig. 3M, N). Laminin5 is expressed by enamel-facing JE cells in the hard diet group (Fig. 3O); in the soft diet group, only patchy, weak Laminin5 expression was observed (Fig. 3P, Q). Together, these data demonstrated that mastication of a soft diet produced lower distortional strains in the JE, which were reflected in loss of mechanosensitive Piezo1 and Plectin expression, and downregulation of other hemidesmosomal proteins in the JE.
A Soft Diet Compromises JE Barrier Functions, Leading to Periodontal Attachment Loss
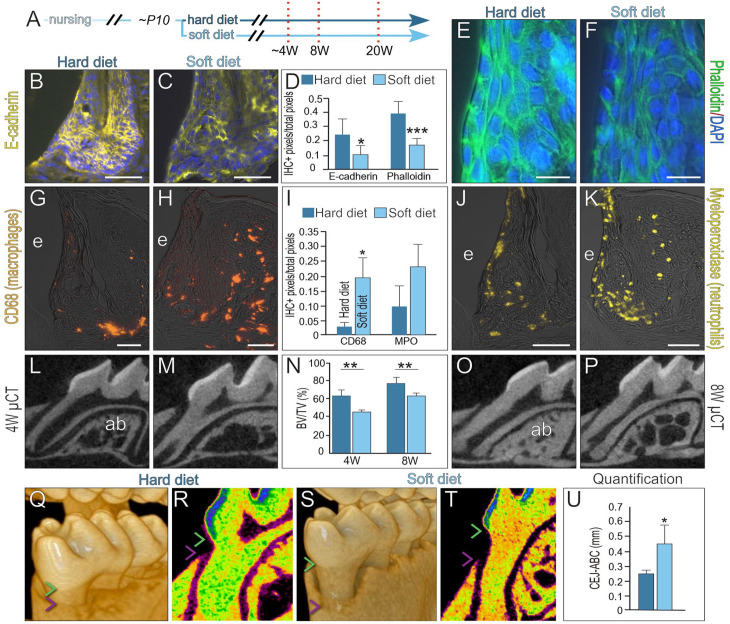
Commensurate with weakening in the attachment apparatus, E-cadherin expression (Fig. 4A–D) and phalloidin staining (Fig. 4D–F) were reduced in the JEs of the soft diet cohort. After 8 wk on a soft diet, greater microbial ingression was observed, as indicated by significantly more CD68+ macrophages in connective tissues (Fig. 4G–I). Myeloperoxidase+ neutrophils were also more abundant in the connective tissues of the soft diet group (Fig. 4I–K). Alveolar bone density was reduced in the soft diet group, as shown by micro–computed tomography (µCT) quantification at 4 wk (compare Fig. 4L–N) and 8 wk (Fig. 4N–P).
Figure 4.
Compromised junctional epithelium (JE) barrier functions are associated with mild inflammation and alveolar bone loss. (A) Schematic of experimental design, where prior to weaning at P10, mice were provided either a hard or a soft diet. The JE was analyzed after 4, 8, and 20 wk. Representative image of immunohistochemistry (IHC) localization of E-cadherin in the (B) hard and (C) soft diet groups. (D) Quantification of E-cadherin and phalloidin staining. One asterisk represents a P value ≤0.05, and 3 asterisks indicate a P value ≤0.001. Representative image of phalloidin staining in the (E) hard and (F) soft diet groups. After 8 wk on a hard or soft diet, representative image of IHC localization of the macrophage marker CD68 in the (G) hard and (H) soft diet groups, quantified in (I). One asterisk represents a P value ≤0.05 (n = 4). Quantitative IHC localization of the neutrophil marker myeloperoxidase in the (J) hard and (K) soft diet groups. Two-dimensional micro–computed tomography (µCT) imaging of the alveolar bone around the first molar after 4 wk on a (L) hard versus (M) soft diet. (N) Quantification of bone volume/total volume in alveolar bone. Two asterisks indicate a P value ≤0.01 (n = 4). Two-dimensional µCT imaging of alveolar bone around the first molar after 8 wk on a (O) hard versus (P) soft diet. After 20 wk, volumetric µCT imaging was used to measure the distance between the cementoenamel junction (CEJ; green arrowhead) and the alveolar bone crest (ABC; purple arrowhead) in the (Q) hard diet versus (S) soft diet groups. (R, T) Relative density color-mapping of the CEJ and ABC indicated on a representative 2-dimensional µCT density scan and (U) quantification. One asterisk represents a P value ≤0.05. Abbreviations as indicated in Figure 1, and ab, alveolar bone. Scale bars: 50 µm.
After 20 wk on a soft diet, volumetric µCT rendering demonstrated significant apical migration of the alveolar bone crest (Fig. 4Q, S), with no changes in cusp height (Appendix Fig. 4). Thermal density mapping (Fig. 4R, T) and quantification of the distance from the cementoenamel junction to the alveolar bone crest showed a significant amount of alveolar bone loss in the soft diet cohort (Fig. 4U).
Discussion
Over the course of mammalian evolution, teeth and their supporting structures have adapted to a remarkably broad range of diets and environments (Gaengler 2000). The early hominid dentition and jaw skeleton was suited for chewing raw foods that required extensive mastication (Organ et al. 2011), but as humans gained control of fire, cooking led to a softer diet (Carmody and Wrangham 2009), and this softer diet was accompanied by widespread changes in the size of the digestive tract, the brain, and, most notably, the teeth and jaws (Berthaume et al. 2020).
Presumably, soft tissues supporting the teeth would have also adapted to changes in masticatory activity. Soft tissues are usually not preserved in the fossil record, but support for an interdependency between dental soft and hard tissues can be found in cases where diets can be precisely monitored (Cave 2014). Veterinary studies have established a correlation between consumption of a soft diet and an increase in diseases of the oral soft tissues, including periodontitis (Watson 1994; Gorrel 1998). Of particular note has been the repeated observation that oral health status in companion animals dramatically improves when a soft diet is replaced with a hard diet (Brown and Park 1968). Here, we provide a molecular framework for understanding how a soft diet affects periodontal health.
Masticatory Forces Are Required to Establish and Maintain the JE Stem Cell Niche
Dietary effects on health are frequently viewed through a nutritional lens, with a tendency to lose sight of physical actions exerted by food on tissues of the oral cavity (Pelzer 1940). By providing animals with 2 kinds of diets that were equivalent in nutritional value but differed in hardness, we could explore the relationship between the physical character of food and plasticity of the JE stem cell niche.
We demonstrated that the Wnt-responsive JE stem cell niche is established commensurate with mastication. When teeth are in the process of erupting, Wnt-responsive cells and their progeny transiently exist in the JE, but a long-lived Wnt-responsive niche is established in the JE only after mastication begins (Fig. 1). Other stem cell populations are known to respond to mechanical stimuli (Maul et al. 2011), and mechanical stimuli can activate Wnt signaling (Farge 2003), but to our knowledge, this is the first stem cell niche in the oral cavity that has been shown to require mechanical input for its establishment.
Maintenance of the Wnt-responsive JE stem cell niche also required mechanical input. Absent mechanical input, the JE atrophies (Fig. 2), much like muscle mass deteriorates when loading is suspended. An association between consumption of a soft diet and periodontal tissue atrophy was noted in the early 1940s, when vigorous mastication was considered a means to ensure gingival health (Pelzer 1940; Baer 1956). Data from animal studies supported this interpretation, showing a downward extension of the soft tissue attachment and greater inflammation in cases where a soft diet was consumed (Burwasser and Hill 1939).
Linking the Mechanics of Mastication with the JE’s Biological Response
Mechanistic insights into the link between masticatory forces and JE atrophy were gained by modeling the strains produced in the JE, which were reflected in the dynamic expression patterns of the mechanosensitive protein, Piezo1 (Fig. 3). Piezo1 encodes for a calcium ion channel, which has been shown to respond within milliseconds to a defined in vitro mechanical stimulus (Liao et al. 2021). In this study, we measured not activity but expression of the protein itself, and a change in expression level typically takes much longer (on the order of minutes to hours) to detect. We observed a significant change in Piezo1 expression levels between the hard and soft diet cohorts (Fig. 3).
How is Piezo1 expression linked to subsequent biological responses? Biomechanical principles dictate that distortional strain levels are highest at sites where cells are adherent to a rigid substrate (e.g., the tooth surface), which corresponds to sites where Piezo1 expression is highest (Fig. 3). This Piezo1+ domain, however, is distinct from the basal lamina, where cells proliferate and the Wnt-responsive niche exists (Fig. 2). How does one reconcile these spatially separate domains? At present, our understanding is guided by a growing literature showing that physical cues from the microenvironment influence stem cell specification (Discher et al. 2005; Engler et al. 2006; Discher et al. 2009). Our working hypothesis is that stem cells in the JE are tuned to strain states that maintain their self-renewal and proliferation (e.g., Fig. 2), while the differentiation of stem cell progeny appears to be tuned to different strain states (Fig. 3). Together, these data suggest that differences in JE matrix stiffness control different aspects of JE cell behavior.
Ongoing studies are focused on understanding the causal link between strain states, mechanosensitivity, and ultimately JE biological responses. Such work has direct clinical implications: disease processes alter the physical microenvironment and, in doing so, can potentially reduce or eliminate cues that stem cells require for their maintenance or differentiation. Consequently, a logical starting point for regenerative approaches is to characterize the mechanosensitivity of the stem cells to matrix elasticity. Our work presented here represents such a step toward rebuilding the JE after disease or damage has compromised its barrier functions.
Hard Tissue Changes in the Periodontium Can Be Traced Back to a Dysfunctional JE
Alveolar bone has been called “the servant of the teeth” (Brodie 1938) because around functionless teeth, alveolar bone atrophies. We observed a similar effect when mice were fed a soft diet: alveolar bone became significantly more porous (Fig. 4). In addition, we observed an apical displacement of crestal bone height (Fig. 4). These changes align with descriptions of disuse atrophy of the periodontium (Cohn 1965), with 2 exceptions: in our case, there was a significant weakening of the JE attachment (Fig. 3), and there was an increase in neutrophil and macrophage invasion into the connective tissues.
It is widely agreed that a disruption in JE barrier functions leads to periodontitis (Schroeder and Listgarten 1997), but how this barrier function becomes compromised in patients is not entirely clear. Once disrupted, however, bacteria then play a key role in disease progression. A current animal model of periodontitis attempts to mimic these steps by physically detaching the JE from the tooth surface via ligature placement, which subsequently accumulates bacteria and leads to acute inflammation and rapid, severe alveolar bone loss (Marchesan et al. 2018). By avoiding physical detachment of the JE and instead reducing a trophic stimulus (e.g., masticatory loading), we were able to observe gradual JE atrophy coupled with a weakening of its hemidesmosomal attachment, which culminated in inflammation and alveolar bone loss (Figs. 2–4). These changes occurred without the introduction of nonnative bacterial species or injection of bacterial by-products. Taken together, our experiments demonstrate that JE barrier function depends on stem cell niche function. Since the stem cell niche is fundamental to JE maintenance and repair (Yuan, Chen, Gauer, et al. 2020; Yuan, Chen, Van Brunt, et al. 2020), physical signals received by the niche ultimately play a critical role in maintaining the periodontium.
Conclusions
Multiple barrier features in the JE are compromised as a result of an animal being maintained on a soft diet, beginning with a reduction in tissue turnover, as demonstrated by reduced cell proliferation and increased cell death (Fig. 2). Together with a reduction in the number of JE stem cell progeny, the JE atrophies. Over time, the atrophied JE weakens in its attachment to the tooth surface, indicated by reduced Laminin5, Plectin, and β4 integrin expression. Weaker E-cadherin expression and decreased phalloidin staining together indicate enhanced permeability of the JE in the soft diet group, along with an increase in immunosurveillance cells, including CD68+ macrophages and Myeloperoxidase+ neutrophils throughout the JE. What remains to be demonstrated is a direct, causal link between the physical and biological stimuli shown here and the events that collectively compromise the barrier functions of the JE.
Author Contributions
X. Yuan, contributed to conception and design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; B. Liu, contributed to conception and design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; P. Cuevas, J. Brunski, F. Aellos, J. Petersen, T. Koehne, S. Bröer, R. Grüber, A. LeBlanc, X. Zhang, Q. Xu, contributed to data acquisition, analysis, and interpretation, critically revised the manuscript; J.A. Helms, contributed to conception and design, data analysis and interpretation, drafted and critically revised the manuscript. All authors gave their final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, sj-docx-1-jdr-10.1177_00220345231185288 for Linking the Mechanics of Chewing to Biology of the Junctional Epithelium by X. Yuan, B. Liu, P. Cuevas, J. Brunski, F. Aellos, J. Petersen, T. Koehne, S. Bröer, R. Grüber, A. LeBlanc, X. Zhang, Q. Xu and J.A. Helms in Journal of Dental Research
Footnotes
A supplemental appendix to this article is available online.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Institutes of Health (NIH) 1R01DE031270-01A1 to J.A. Helms and R00DE028585 to X. Yuan.
ORCID iDs: X. Yuan  https://orcid.org/0000-0002-8063-9431
https://orcid.org/0000-0002-8063-9431
S. Bröer  https://orcid.org/0000-0002-7969-6586
https://orcid.org/0000-0002-7969-6586
Q. Xu  https://orcid.org/0000-0003-0484-0878
https://orcid.org/0000-0003-0484-0878
J.A. Helms  https://orcid.org/0000-0002-0463-396X
https://orcid.org/0000-0002-0463-396X
References
- Baer PN. 1956. The relation of the physical character of the diet to the periodontium and periodontal disease; a collective review. Oral Surg Oral Med Oral Pathol. 9(8):839–844. [DOI] [PubMed] [Google Scholar]
- Berthaume MA, Lazzari V, Guy F. 2020. The landscape of tooth shape: over 20 years of dental topography in primates. Evol Anthropol. 29(5):245–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie AG. 1938. Dental science and dental art. Philadelphia (PA): Lea & Febiger. [Google Scholar]
- Brown MG, Park JF. 1968. Control of dental calculus in experimental beagles. Lab Anim Care. 18(5):527–535. [PubMed] [Google Scholar]
- Burwasser P, Hill TJ. 1939. The effect of hard and soft diets on the gingival tissues of dogs. J Dent Res. 18(4):389–393. [Google Scholar]
- Carmody RN, Wrangham RW. 2009. The energetic significance of cooking. J Hum Evol. 57(4):379–391. [DOI] [PubMed] [Google Scholar]
- Cave N. 2014. The nutritional management of dental disease. The World Small Animal Veterinary Association World Congress Proceedings; Massey University, Palmerston North, New Zealand: [accessed 2023 Jun 14]. https://www.vin.com/apputil/content/defaultadv1.aspx?id=7054877&pid=12886 [Google Scholar]
- Chacon-Martinez CA, Koester J, Wickstrom SA. 2018. Signaling in the stem cell niche: regulating cell fate, function and plasticity. Development. 145(15):dev165399. [DOI] [PubMed] [Google Scholar]
- Clevers H, Loh KM, Nusse R. 2014. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science. 346(6205):1248012. [DOI] [PubMed] [Google Scholar]
- Cohn SA. 1965. Disuse atrophy of the periodontium in mice. Arch Oral Biol. 10(6):909–919. [DOI] [PubMed] [Google Scholar]
- Discher DE, Janmey P, Wang YL. 2005. Tissue cells feel and respond to the stiffness of their substrate. Science. 310(5751):1139–1143. [DOI] [PubMed] [Google Scholar]
- Discher DE, Mooney DJ, Zandstra PW. 2009. Growth factors, matrices, and forces combine and control stem cells. Science. 324(5935):1673–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. 2006. Matrix elasticity directs stem cell lineage specification. Cell. 126(4):677–689. [DOI] [PubMed] [Google Scholar]
- Farge E. 2003. Mechanical induction of twist in the drosophila foregut/stomodeal primordium. Curr Biol. 13(16):1365–1377. [DOI] [PubMed] [Google Scholar]
- Gaengler P. 2000. Evolution of tooth attachment in lower vertebrates to tetrapods. Cambridge: Cambridge University Press. [Google Scholar]
- Ginot S, Herrel A, Claude J, Hautier L. 2018. Skull size and biomechanics are good estimators of in vivo bite force in murid rodents. Anat Rec (Hoboken). 301(2):256–266. [DOI] [PubMed] [Google Scholar]
- Gorrel C. 1998. Periodontal disease and diet in domestic pets. J Nutr. 128(12 Suppl):2712S–2714S. [DOI] [PubMed] [Google Scholar]
- Liang J, Balachandra S, Ngo S, O’Brien LE. 2017. Feedback regulation of steady-state epithelial turnover and organ size. Nature. 548(7669):588–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Hsiao MY, Xiang G, Zhong P. 2021. Optimal pulse length of insonification for piezo1 activation and intracellular calcium response. Sci Rep. 11(1):709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesan J, Girnary MS, Jing L, Miao MZ, Zhang S, Sun L, Morelli T, Schoenfisch MH, Inohara N, Offenbacher S, et al. 2018. An experimental murine model to study periodontitis. Nat Protoc. 13(10):2247–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maul TM, Chew DW, Nieponice A, Vorp DA. 2011. Mechanical stimuli differentially control stem cell behavior: morphology, proliferation, and differentiation. Biomech Model Mechanobiol. 10(6):939–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organ C, Nunn CL, Machanda Z, Wrangham RW. 2011. Phylogenetic rate shifts in feeding time during the evolution of homo. Proc Natl Acad Sci U S A. 108(35):14555–14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelzer R. 1940. A study of the local oral effect of diet on the periodontal tissues and the gingival capillary structures. J Am Dent Assoc. 27(1):13–25. [Google Scholar]
- Schroeder HE, Listgarten MA. 1997. The gingival tissues: the architecture of periodontal protection. Periodontol 2000. 13:91–120. [DOI] [PubMed] [Google Scholar]
- Ustriyana P, He R, Srirangapatanam S, Chang J, Arman ST, Sidhu S, Wang B, Kang M, Ho SP. 2022. Food hardness can regulate orthodontic tooth movement in mice. J Periodontal Res. 57(2):269–283. [DOI] [PubMed] [Google Scholar]
- Vining KH, Mooney DJ. 2017. Mechanical forces direct stem cell behaviour in development and regeneration. Nat Rev Mol Cell Biol. 18(12):728–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, You X, Lotinun S, Zhang L, Wu N, Zou W. 2020. Mechanical sensing protein piezo1 regulates bone homeostasis via osteoblast-osteoclast crosstalk. Nat Commun. 11(1):282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson AD. 1994. Diet and periodontal disease in dogs and cats. Aust Vet J. 71(10):313–318. [DOI] [PubMed] [Google Scholar]
- Yuan X, Chen J, Gauer J, Xu Q, Van Brunt LA, Helms JA. 2020. The junctional epithelium is maintained by a stem cell population. J Dent Res. 100(2):209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Chen J, Van Brunt LA, Grauer J, Xu Q, Pei X, Wang L, Zhao Y, Helms JA. 2020. Formation and regeneration of a Wnt-responsive junctional epithelium. J Clin Periodontol. 47(12):1476–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-jdr-10.1177_00220345231185288 for Linking the Mechanics of Chewing to Biology of the Junctional Epithelium by X. Yuan, B. Liu, P. Cuevas, J. Brunski, F. Aellos, J. Petersen, T. Koehne, S. Bröer, R. Grüber, A. LeBlanc, X. Zhang, Q. Xu and J.A. Helms in Journal of Dental Research