Abstract
Background
Abdominal aortic aneurysms (AAAs) can result in high mortality upon rupture but are usually undiagnosed because of the absence of symptoms in the early stage. Ultrasound screening is regarded as an impactful way to prevent the AAA-related death but cannot be performed efficiently; therefore, a target population, especially in Asia, for this procedure is lacking. Additionally, although dyslipidaemia and atherosclerosis are associated with AAA. However, it remains undetermined whether the non-high-density lipoprotein-cholesterol to high-density lipoprotein-cholesterol ratio (NHHR) is associated with AAA. Therefore, this study was aimed at examining whether NHHR is associated with AAA.
Method
A total of 9559 participants who underwent AAA screening at Guangdong Provincial People’s Hospital and through screening in two communities in Dongguan, from June 2019 to June 2021 joined in this screening program. The diagnosis of AAA was confirmed by the ultrasound examination of the abdominal aorta rather than any known or suspected AAA. Clinical and laboratory data of participants were collected. The participants were separated into a normal group and an AAA group according to the abdominal aortic status. To eliminate confounding factors, a propensity score matching (PSM) approach was utilized. The independent relationship between NHHR and AAA was assessed through the utilization of multivariable logistic regression analysis. In addition, internal consistency was evaluated through subgroup analysis, which controlled for significant risk factors.
Results
Of all the participants, 219 (2.29%) participants were diagnosed with AAA. A significant elevation in NHHR was identified in the AAA group when contrasted with that in the normal group (P < 0.001). As demonstrated by the results of the multivariable logistic regression analysis, AAA was independently associated with NHHR before (odds ratio [OR], 1.440, P < 0.001) and after PSM (OR, 1.515, P < 0.001). Significant extension was observed in the areas under the receiver operating characteristic curves (AUROCs) of NHHR compared to those of single lipid parameters before and after PSM. An accordant association between NHHR and AAA in different subgroups was demonstrated by subgroup analysis.
Conclusion
In the Chinese population, there is an independent association between NHHR and AAA. NHHR might be propitious to distinguish individuals with high risk of AAA.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12944-023-01939-4.
Keywords: Non-HDL-C/HDL-C ratio, Abdominal aortic aneurysm, Ultrasond screening, Chinese population
Introduction
Abdominal aortic aneurysm (AAA) is an irreversible and parlous disease carrying a mortality rate of 67–94%, and commonly, symptoms do not manifest prior to rupture [1]. Four screening programs, which were based on randomized controlled trials from 1991 to 2004, all indicated a decline in AAA-related mortality [2–5]. Ultrasound screening is regarded as an impactful way to prevent AAA-related death and AAA rupture [6]. However, studies have reported that in Western Europe and America, the prevalence of AAA has decreased to 1.3–1.7%, which influences the effectiveness and cost- effectiveness of screening [7–9]. Therefore, parameters that could identify individuals at increased risk of AAA, should be explored to enhance the AAA prevalence in the more targeted screening for AAA [10].
AAA is an aortic dilatation of over 3 cm inside the abdominal area [11]. Patients with AAAs usually suffer from atherosclerosis simultaneously, and the relevance between peripheral atherosclerosis or coronary heart disease (CAD) and AAA has been proposed in numerous studies [12–14]. According to a 7-year prospective study, atherosclerosis risk factors were strongly connected with AAA prevalence [15]. Lately, there has been an increased focus on elucidating the importance of non-traditional lipid indicators, such as non-high-density lipoprotein cholesterol (non-HDL-C) and the non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol (non-HDL-C/HDL-C) ratio (NHHR) [16, 17], in predicting atherosclerotic cardiovascular disease [18–20]. As a novel lipid parameter, NHHR, which consists of atherogenic and antiatherogenic lipid particles, has been considered as a diagnostic marker for many dyslipidemia-related diseases, for example diabetes mellitus [21–23], metabolic syndrome [18] and carotid atherosclerosis [19, 20]. However, studies appraising the association between NHHR and AAA are limited. The present study was aimed at exploring whether NHHR was associated with AAA, and increasing the prevalence for AAA in the screening.
Methods
Study population
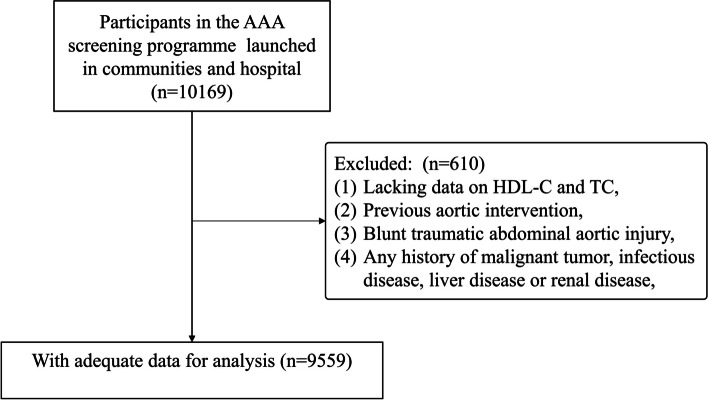
The study population comprised 10,169 Chinese adults who underwent AAA screening at Guangdong Provincial People’s Hospital or through screening programs in two communities in Dongguan, China, from June 2019 to June 2021. The diagnosis of AAA was confirmed by ultrasound examination of the abdominal aorta, rather than any known or suspected AAA. In this study, the participant selection standard encompassed (1) any history of malignant tumor, infectious disease, liver disease or renal disease, (2) blunt traumatic abdominal aortic injury, (3) previous aortic intervention, and (4) a lack of data on HDL-C and total cholesterol (TC) levels. Figure 1 depicts the flowchart for the participant selection standards.
Fig. 1.
Patient selection process. AAA, abdominal aortic aneurysm; TC, total cholesterol; HDL-c, high-density lipoprotein cholesterol
Definition
In this study, AAA was defined as having an abdominal aortic diameter (AAD) greater than 30 mm [11]. Non-HDL-C was established through computing the numerical difference between TC (mmol/L) and HDL-C (mmol/L) [24].
Measurement and data collection
All study participants had an ultrasound scan, which is recommended for AAA screening in the latest guidelines [25, 26]. Not only does ultrasound scanning exhibit high sensitivity (94%-100%) but it also demonstrates high specificity (98%-100%) in the detection of AAA [27–31]. Radiologists who were recruited for the screening all satisfied the undermentioned requirements: 1) Over 5 years of radiological experience should be possessed 2) abdominal aorta ultrasound scans should have been conducted at a minimum frequency of once per month over the past 12 months. Before performing measurements, radiologists must learn the measurement standards for AAA screening and adhere to these requirements. The ultrasound scans were performed in a plane perpendicular to the aortic longitudinal axis. Regarding the setting of the caliper, radiologists were required to measure the abdominal aortic diameter with an outer-to-outer (OTO) measurement, which was defined as measuring from outer anterior wall to the outer posterior wall. In addition, the measurement was started from the diaphragm, and ended at the bifurcation of the aorta. The maximal abdominal aortic diameter was defined as the largest diameter from the lowest renal artery to the aortic bifurcation in the transverse plane and the longitudinal plane [32–34].
Participants were gauged for weight and height with them donning lightweight attire and standing barefoot. The participants' information pertaining to their health, including age, smoking history, and previous medical conditions, was self-reported by the participants and documented by the researchers. Smoking was regarded as a binary variable denoting whether individuals had ever smoked (yes/no) in the past. Prior to blood sample extraction, participants were required to undergo an 8-h fasting period. Uric acid (UA), serum creatinine (Cr), low-density lipoprotein cholesterol (LDL-C), HDL-C, TC and TG were tested with the Hitachi 7600 machine (Kyowa, Japan). Conventional lipid parameters, comprising TC, TG, HDL-C, and LDL-C, could be reliably detected using enzymatic methods. HbA1c was tested with an HLC-723 G7 (Tosoh, Japan).
Demographics, clinical characteristics, laboratory and ultrasound scan findings were recorded by 2 researchers independently.
Propensity score matching analysis
To strengthen the repeatability of the study, propensity score matching (PSM) was applied to eliminate probable confounders and selection bias of this retrospective review. The characteristics used to calculate the propensity score were age, BMI, sex, smoking, hypertension, diabetes mellitus (DM), coronary artery disease (CAD), peripheral artery disease (PAD), stroke, prior usage of angiotensin system inhibitors, beta-blockers, statins, and metformin. One to-one nearest-neighbor matching was implemented using a 0.2 caliper. After matching, 2 groups of 219 subjects were identified. Standardized mean differences (SMDs) were utilized to estimate the difference between the 2 matched groups. Commonly, it is acceptable to obtain a maximum SMD of 0.10 or even 0.15.
Statistical analysis
Participant characteristics were considered based on the presence or absence of AAA. Continuous variables are presented, with the mean and standard deviation (SD) directed to the data that conforms to a normal distribution, and with medians along with the interquartile range (IQR) when dealing with data that does not follow a normal distribution. Student's t-test was utilized to conduct the comparisons on data demonstrating a normal distribution, while the Mann–Whitney U test was employed for data that did not adhere to normal distribution. Furthermore, in the presentation of categorical variables, they are depicted either in terms of relative frequencies (percentages). Subsequently, the comparison of categorical variables involved the application of either the chi-square test or Fisher's exact test. For the sake of estimating the association between NHHR and AAA, NHHR was categorized into tertiles [low (< 2.50 mmol/L), middle (2.50–3.51 mmol/L), high (> 3.51 mmol/L)]. An assessment of the independent association between NHHR and AAA was performed through the implementation of logistic regression analysis. As a consequence, this analysis yielded odds ratios (ORs) along with their corresponding 95% confidence intervals (CIs). Initially, univariate logistic regression analysis was conducted on all the collected variables. Multivariable logistic regression analysis was employed for investigating factors independently linked to the disease, employing variables with a P < 0.05 in the univariate analysis. Subsequently, three main models were constructed for adjusting the covariate, namely, Model 1, unadjusted; Model 2, adjusted for age, BMI and sex; and Model 3, adjusted for age, BMI, sex, smoking, hypertension, DM, CAD, PAD, stroke, levels of alanine aminotransferase(ALT), aspartate aminotransferase(AST), uric acid, blood urea nitrogen (BUN), Cr, TG, TC, LDL-C, HBA1C and fasting glucose, and use of angiotensin system inhibitors, beta-blockers, statins and metformin.
In the subgroup analysis, NHHR was probed to determine whether it was associated with AAA in the several subgroups, which included age, sex, smoking, hypertension, CAD and previous statin use. In every subgroup, the multiple stepwise logistic regression was implemented.
The diagnostic performance of the variables in predicting AAA was assessed utilizing receiver operating characteristic (ROC) curves. Subsequently, for the purpose of quantifying and comparing results of the analysis, the area under the curve (AUC) along with its paired 95% CI was computed. In addition, the Youden index (YI) was used to determine a cutoff value for NHHR. Based on this cutoff value, the division of participants into two groups was carried out with the objective of exploring the connection between NHHR and AAA.
Results
Baseline characteristics
This study comprised 9559 participants, of whom 6,144 were male (64.3%), and the average age was 70.3 ± 0.1 years. The total number of 97.7% (9340) and 2.3% (219) of the participants were distributed in the normal group and AAA group severally (details in Supplementary Table 1). Before matching, the AAA group commonly had a larger percentage of comorbidities (except for diabetes mellitus). There was no substantial difference in comorbidities between the normal and AAA groups, after matching. In the AAA group, NHHR exhibited relatively elevated values compared to the normal group, HDL-C levels demonstrated a decrease in the AAA group compared to the normal group. The baseline characteristics concerning the participants, which were separated by the abdominal aorta status, are displayed in Table 1.
Table 1.
Baseline characteristics Stratified by the groups with and without AAAa
| Variables | Unmatched population | Matched population | ||||||
|---|---|---|---|---|---|---|---|---|
| Normal (n = 9340) | AAA (n = 219) | SMD | P† | Normal (n = 219) | AAA (n = 219) | SMD | P† | |
| Age (years) | 70.3 ± 0.1 | 72.6 ± 0.4 | 0.345 | < 0.001 | 72.6 ± 0.5 | 72.6 ± 0.4 | 0.004 | 0.946 |
| BMI (Kg/m2) | 23.9 ± 0.03 | 23.8 ± 0.2 | 0.043 | 0.507 | 24.0 ± 0.2 | 23.8 ± 0.2 | 0.098 | 0.583 |
| Sex (male) | 63.7 (5949) | 89.0 (195) | 0.625 | < 0.001 | 92.2 (202) | 89.0 (195) | 0.110 | 0.251 |
| Smoking, % | 23.7 (2216) | 40.2 (88) | 0.358 | < 0.001 | 37.4 (82) | 40.2 (88) | 0.056 | 0.556 |
| Hypertension, % | 54.0 (5047) | 66.7 (146) | 0.260 | < 0.001 | 66.2 (145) | 66.7(146) | 0.010 | 0.919 |
| Diabetes mellitus, % | 21.6 (2013) | 18.7 (41) | 0.071 | 0.313 | 15.1 (33) | 18.7 (41) | 0.097 | 0.308 |
| Coronary artery disease, % | 38.8 (3627) | 56.2 (3750) | 0.352 | < 0.001 | 53.0 (116) | 56.2 (123) | 0.064 | 0.502 |
| Peripheral artery disease, % | 3.3(312) | 3.7(8) | 0.017 | 0.799 | 5.0 (11) | 3.7 (8) | 0.067 | 0.482 |
| Stroke, % | 5.4(503) | 10.0(22) | 0.175 | 0.003 | 9.6 (21) | 10.0 (22) | 0.015 | 0.872 |
| Maximal abdominal aortic diameter (mm) | 19.0 (17.0–20.5) | 36.0 (32.0–47.0) | 2.317 | < 0.001 | 20.0 (18.0–21.0) | 36.0 (32.0–47.0) | 2.303 | < 0.001 |
| ALT (U/L) | 20.0 (14.7–27.7) | 18.0 (14.0–25.0) | 0.060 | 0.003 | 21.0 (16.0–27.4) | 18.0 (14.0–25.0) | 0.214 | < 0.001 |
| AST (U/L) | 22.0 (18.3–28.0) | 21.0 (18.7–27.5) | 0.015 | 0.407 | 22.9 (19.0–28.4) | 21.0 (18.7–27.5) | 0.087 | 0.069 |
| UA (mmol/L) | 398.0 (327.9–460.0) | 422.0 (368.0–504.3) | 0.332 | < 0.001 | 402.0 (351.3–483.3) | 422.0 (368.0–504.3) | 0.234 | 0.006 |
| CR (μmol/L) | 81.0 (67.0–96.8) | 94.9 (77.7–118.3) | 0.328 | < 0.001 | 89.2 (75.1–105.0) | 94.9 (77.7–118.3) | 0.154 | 0.010 |
| BUN (mmol/L) | 5.5 (4.5–6.9) | 6.2 (5.2–8.2) | 0.320 | < 0.001 | 5.9 (4.6–7.3) | 6.2 (5.2–8.2) | 0.141 | 0.106 |
| TG (mmol/L) | 1.4 (1.0–1.9) | 1.4 (1.0–1.7) | 0.087 | 0.912 | 1.4 (1.0–1.9) | 1.4 (1.0–1.7) | 0.039 | 0.245 |
| TC (mmol/L) | 4.6 (3.8–5.4) | 4.6 (3.7–5.3) | 0.076 | 0.265 | 4.1 (3.5–5.2) | 4.6 (3.7–5.3) | 0.216 | 0.034 |
| LDL- C (mmol/L) | 2.8 (2.2–3.5) | 2.9 (2.3–3.5) | 0.060 | 0.250 | 2.6 (2.0–3.3) | 2.9 (2.3–3.5) | 0.293 | 0.002 |
| HDL-C (mmol/L) | 1.1 (1.0–1.4) | 1.0 (0.9–1.2) | 0.102 | < 0.001 | 1.1 (0.9–1.3) | 1.0 (0.9–1.2) | 0.206 | 0.049 |
| non-HDL-C (mmol/L) | 3.4 (2.7–4.2) | 3.6 (2.8–4.2) | 0.102 | 0.114 | 3.1 (2.5–3.9) | 3.6 (2.8–4.2) | 0.343 | 0.001 |
| non-HDL-C / HDL-C ratio | 2.9 (2.2–3.8) | 3.5 (2.9–4.3) | 0.457 | < 0.001 | 3.0 (2.3–3.8) | 3.5 (2.9–4.3) | 0.449 | < 0.001 |
| Fasting glucose (mmol/L) | 5.3 (4.7–6.3) | 5.2 (4.5–6.6) | 0.088 | 0.109 | 5.3 (4.6–6.2) | 5.2 (4.5–6.6) | 0.107 | 0.197 |
| HBA1C (mmol/L) | 6.1 (5.7–6.3) | 6.0 (5.6–6.2) | 0.273 | < 0.001 | 6.1 (5.8–6.2) | 6.0 (5.6–6.2) | 0.236 | 0.021 |
| Medication use | ||||||||
| Angiotensin system inhibitors, % | 47.5 (4439) | 56.6 (124) | 0.183 | 0.008 | 54.3 (119) | 56.6 (124) | 0.046 | 0.631 |
| Beta-blockers, % | 42.1 (3932) | 62.6 (137) | 0.418 | < 0.001 | 58.0 (127) | 62.6 (137) | 0.093 | 0.329 |
| Statins, % | 51.0 (4762) | 67.1 (147) | 0.332 | < 0.001 | 65.8 (144) | 147.0 (67.1) | 0.029 | 0.761 |
| Metformin, % | 9.6 (895) | 7.8 (17) | 0.065 | 0.365 | 7.8 (17) | 7.8 (17) | < 0.001 | 1.000 |
Abbreviations: AAA Abdominal aortic aneurysm, BMI Body mass index, ALT Alanine aminotransferase, AST aspartate aminotransferase, UA Uric acide, Cr Creatinine, BUN Blood urea nitrogen, TG Triglyceride, TC Total cholesterol, LDL-C Low-density lipoprotein cholesterol, HDL-C High-density lipoprotein cholesterol, non-HDL-C Non-high-density lipoprotein cholesterol, non-HDL-C/HDL-C ratio Non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio, HbA1c Hemoglobin A1c, SMD Standardized mean difference
aValues are given as mean + standard deviation, number (percentage), or median (quartiles 1 through 3). †P-values were derived from Mann–Whitney U-tests for continuous variables, and Chi-square tests for categorical variables
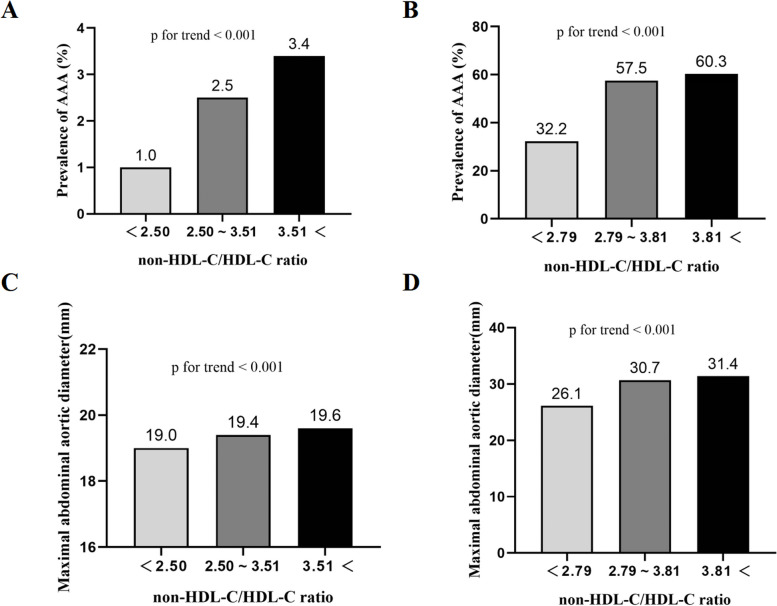
To enhance clinical utility, three groups were formed among the participants (low NHHR group: NHHR < 2.50; medium NHHR group: 2.50 ≤ NHHR ≤ 3.51; high NHHR group: NHHR > 3.51) after dividing NHHR into tertiles. The high NHHR group exhibited a distinct increase in the prevalence of AAA in comparison to the medium NHHR group and low NHHR group (1.0% versus 2.5% versus 3.4%; P<0.001) (Fig. 2A). The high NHHR group showed a remarkably greater maximum AAD in contrast to both the low NHHR and medium NHHR groups. (19.0 versus 19.4 versus 19.6; P<0.001) (Fig. 2B).
Fig. 2.
The prevalence of AAA according to non-HDL-c/HDL-c ratio tertiles before (A) and after PSM (B). Maximal abdominal aortic aneurysm according to non-HDL-c/HDL-c ratio tertiles before (C) and after PSM (D). AAA, abdominal aortic aneurysm; non-HDL-c/HDL-c ratio, non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio; PSM, propensity score matching
Univariate and multivariable logistic regression analysis
As demonstrated by the findings of univariate logistic regression analysis, NHHR exhibited a substantial association with AAA (OR, 1.391; P < 0.001). Other significant parameters comprised age, sex, smoking, hypertension, CAD, stroke, and levels of UA, Cr, BUN, HDL-C and HBAC1. To confirm that no multicollinearity existed among all variables, not only tolerance, but also the variance inflation factor (VIF) were assessed before conducting the multivariable logistic regression analysis with these significant factors (Supplement. Table 2). In this analysis, NHHR was still linked to the prevalence of AAA (OR, 1.440; P < 0.001) (Table 2). After adjusting for confounders with stepwise multivariable logistic regression analysis, when considering the low NHHR group as a reference, it was observed that the high NHHR group exhibited the strongest association with AAA. (OR, 4.231; 95% CI, (2.754–6.500); P < 0.001) (Table 3).
Table 2.
Univariate and multivariable logistic regression analysis of baseline variables and prevalence of AAA in the matched and unmatched populations
| Unmatched population | Matched population | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| Variables | OR | P | OR | P | OR | P | OR | P |
| Age | 1.046 | < 0.001 | 1.056 (1.035–1.077) | < 0.001 | 1.000 | 0.965 | ||
| BMI | 0.986 | 0.544 | 0.963 | 0.304 | ||||
| Sex | 4.631 | < 0.001 | 3.968 (2.555–6.162) | < 0.001 | 0.684 | 0.253 | ||
| Smoking | 2.160 | < 0.001 | 1.697 (1.264–2.279) | 0.001 | 1.122 | 0.556 | ||
| Hypertension | 1.701 | < 0.001 | 1.392 (1.032–1.877) | 0.030 | 1.021 | 0.919 | ||
| Diabetes mellitus | 0.838 | 0.314 | 1.298 | 0.308 | ||||
| Coronary artery disease | 2.018 | < 0.001 | 1.138 | 0.502 | ||||
| Peripheral artery disease | 1.097 | 0.799 | 0.717 | 0.483 | ||||
| Stroke | 1.962 | 0.003 | 1.515 (0.953–2.407) | 0.079 | 1.053 | 0.872 | ||
| ALT | 0.992 | 0.105 | 0.988 | 0.034 | 0.985 (0.974–0.997) | 0.012 | ||
| AST | 1.000 | 0.881 | 0.998 | 0.394 | ||||
| UA | 1.003 | < 0.001 | 1.002 | 0.016 | 1.002 (1.000–1.004) | 0.018 | ||
| Cr | 1.003 | < 0.001 | 1.002 | 0.126 | ||||
| BUN | 1.077 | < 0.001 | 1.056 (1.025–1.088) | < 0.001 | 1.035 | 0.150 | ||
| TG | 0.915 | 0.269 | 1.053 | 0.686 | ||||
| TC | 0.938 | 0.277 | 1.218 | 0.025 | ||||
| LDL-c | 1.062 | 0.392 | 1.404 | 0.003 | ||||
| HDL-c | 0.210 | < 0.001 | 0.507 | 0.033 | ||||
| non-HDL-c | 1.092 | 0.15 | 1.416 | < 0.001 | ||||
| non-HDL-c/HDL-c ratio | 1.391 | < 0.001 | 1.440 (1.301–1.593) | < 0.001 | 1.520 | < 0.001 | 1.515(1.269–1.809) | < 0.001 |
| Fasting glucose | 0.961 | 0.221 | 0.954 | 0.268 | ||||
| HBA1C | 0.733 | < 0.001 | 0.638 (0.530–0.767) | < 0.001 | 0.752 | 0.017 | ||
| Medication use | ||||||||
| Angiotensin system inhibitors | 1.441 | 0.008 | 1.097 | 0.631 | ||||
| Beta-blockers | 2.297 | < 0.001 | 2.198 (1.647–2.934) | < 0.001 | 1.210 | 0.329 | ||
| Statins | 1.963 | < 0.001 | 1.063 | 0.761 | ||||
| metformin | 0.794 | 0.366 | 1.000 | 1.000 | ||||
Abbreviations: AAA Abdominal aortic aneurysm, BMI Body mass index, ALT Alanine aminotransferase, AST aspartate aminotransferase, UA Uric acide, Cr Creatinine, BUN Blood urea nitrogen, TG Triglyceride, TC Total cholesterol, LDL-c Low-density lipoprotein cholesterol, HDL-c High-density lipoprotein cholesterol, non-HDL-c non-high-density lipoprotein cholesterol, non-HDL-c/HDL-c ratio Non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio, HbA1c Hemoglobin A1c
Table 3.
Associations between the prevalence of AAA and the non-HDL-c/HDL-c ratio as continuous variables and in tertiles
| Variables | No. of participants (AAA patients) | Unadjusted OR (95%CI) | P value | Model 1a OR (95%CI) | P value | Model 2b OR (95%CI) | P value |
|---|---|---|---|---|---|---|---|
| non-HDL-c / HDL-c ratio | 9559 (219) | 1.391(1.269–1.526) | 0.001 | 1.417(1.287–1.56) | 0.001 | 1.584(1.411–1.778) | <0.001 |
| non-HDL-c / HDL-c ratio | |||||||
| <2.50 | 3187 (32) | 1.000 | 1.000 | 1.000 | |||
| 2.50~3.51 | 3186 (79) | 2.507(1.658–3.791) | <0.001 | 2.566(1.693–3.888) | <0.001 | 2.633 (1.725–4.019) | <0.001 |
| >3.51 | 3186 (108) | 3.459(2.325–5.148) | <0.001 | 3.604(2.416–5.375) | <0.001 | 4.231 (2.754–6.500) | <0.001 |
*a Model 1 adjusted for age, body mass index and sex
*b Model 2 adjusted for age, body mass index, sex, smoking, hypertension, diabetes mellitus, coronary artery disease, peripheral artery disease, stroke, levels of alanine aminotransferase, aspartate aminotransferase, uric acid, blood urea nitrogen, serum creatinine, triglyceride, total cholesterol, low-density lipoprotein cholesterol, fasting glucose and hemoglobin A1c, and use of angiotensin system inhibitors, beta-blockers, statins and metformin
Propensity score matching analysis
One-to-one nearest-neighbor matching was utilized to eliminate the possible confounding factors accordingly. Two groups, each consisting of 219 participants, were formed. After matching, NHHR in the AAA group still exceeded that in the normal group. In the meanwhile, the AAA group exhibited a reduced HDL-C level in comparison to the normal group. (Table 1). Furthermore, the high NHHR group possessed a considerably highest prevalence of AAA (32.2% versus 57.5% versus 60.3%; P<0.01) and maximal AAD (26.1 versus 30.7 versus 31.4; P<0.01) than the low NHHR group (Fig. 2C, D). Subsequently, logistic regression analyses, both univariate and multivariable, were conducted in the matched cohort. (Table 2). NHHR, which was revealed by the univariate logistic regression analysis, was in association with the prevalence of AAA (odds ratio [OR], 1.520; P < 0.001). Moreover, NHHR, which was proved by the multivariable logistic regression analysis, might be linked to AAA prevalence independently (OR, 1.515; P < 0.001).
Subgroups analyses
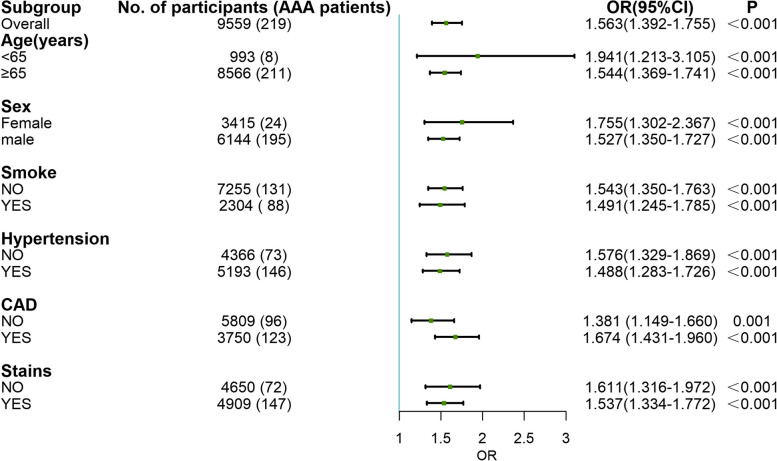
To validate the internal stability of the study, stratified analyses to probe the odds of AAA with changes in NHHR in different subgroups were performed. In consequence, NHHR remained substantially tied to the prevalence of AAA when considering all stratified subgroups (P < 0.001), which was comprised of age, sex, smoking, hypertension, CAD and previous statin use (Fig. 3).
Fig. 3.
Multiple logistic regression analysis of the non-HDL-c/HDL-c ratio and risk for the prevalence of AAA in subgroups. The adjusted variables were age, BMI, sex, hypertension, diabetes mellitus, coronary artery disease, peripheral artery disease, stroke, levels of alanine aminotransferase, aspartate aminotransferase, uric acid, blood urea nitrogen, serum creatinine, triglyceride, total cholesterol, low-density lipoprotein cholesterol, fasting glucose and hemoglobin A1c, and use of angiotensin system inhibitors, beta-blockers, statins and metformin. OR, odds ratio
Receiver operating characteristic curve analysis
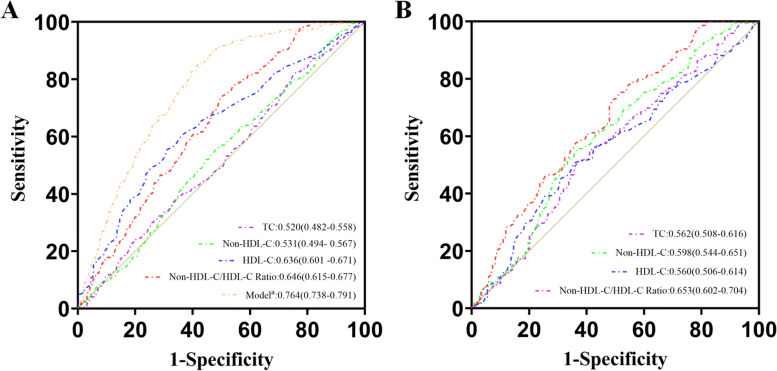
With the intention of calculating the predictive accuracy for NHHR, the ROC curve analysis was employed in this study. A comparison of HDL-C (AUC, 0.636; 95% CI, 0.601–0.671), non-HDL-C (AUC, 0.531; 95% CI, 0.494–0.567), TC (AUC, 0.520; 95% CI, 0.482–0.558) and NHHR (AUC, 0.646; 95% CI, 0.615–0.677) indicated that NHHR had the best predictive value. In addition, to enhance the diagnostic efficiency of NHHR, it was combined with the latest guideline-recommended risk determinants of AAA, which was comprised of age, gender, smoking and CAD, to form the Model a [35]. As a result, a favorable predictive performance was exhibited by the Model a (AUC, 0.764; 95% CI, 0.738–0.790) (Fig. 4A). Following PSM, ROC curve analysis was implemented, without certain confounding factors. Once more, the superior predictive value for AAA was demonstrated by NHHR (AUC, 0.653; 95% CI, 0.602–0.704) in comparison with non-HDL-C (AUC, 0.598; 95% CI, 0.544–0.651), TC (AUC, 0.562; 95% CI, 0.508–0.616) and HDL-C (AUC, 0.560; 95% CI, 0.506–0.614) as well (Fig. 4B). According to the YI, NHHR held the cutoff values, which were 2.83 before PSM and 2.75 after PSM. Based on these cutoff values, NHHR was divided into two groups [before PSM: low (< 2.83), high (> 2.83); after PSM: low (< 2.75), high (> 2.75)]. In contrast to the low NHHR group, both before and after PSM, the high NHHR group demonstrated a higher prevalence of AAA (Supplement Figure 1).
Fig. 4.
Receiver operating characteristic (ROC) curves of HDL-c, non-HDL-c and the non-HDL-c/HDL-c ratio for predicting the prevalence of AAA before (A) and after PSM (B). Model a was comprised of age, sex, smoking, hypertension, coronary artery disease and non-HDL-c/HDL-c ratio. HDL-c, high-density lipoprotein cholesterol; non-HDL-c, non-high-density lipoprotein cholesterol; non-HDL-c/HDL-c ratio, non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio; PSM, propensity score matching
Discussion
NHHR is a satisfactory diagnostic biomarker for AAA according to this study. As a result, AAA was found to be strongly associated with a high NHHR, which played a more important role than traditional lipid parameters in AAA screening among a Chinese population.
Among the numerous atherogenic lipid parameters presented, NHHR integrated all atherogenic cholesterols, including very low density lipoprotein cholesterol (VLDL-C), LDL-C, intermediate density lipoprotein cholesterol (IDL-C) and lipoprotein (a), in addition to HDL-C, which is an anti-atherogenic factor [24, 36, 37]. Lately, numerous researches has demonstrated a connection between NHHR and various dyslipidemia-related diseases, such as metabolic syndrome [18], liver disease [38], coronary atherosclerosis [39] and carotid atherosclerosis [20]. Moreover, dyslipidemia, especially the atherogenic dyslipidemia, affects the formation and progression of AAA [15, 40]. Iribarren and his colleagues considered that, when the levels of cholesterol exceed 240 mg/dl, it was in significant association with AAA (OR:2.82) [41]. As per the literature suggests,, an association was observed between the presence of AAA and HDL-C levels (MD, -0.15 mmol/L) [42]. Yasuhiko K et al. found that, in contrast to subjects in the lowest quintile of plasma lipoprotein(a), the individuals in the highest quintile exhibited a remarkable elevation on the subject of prevalence of AAA. (HR:1.57; 95% CI:1.19–2.08) through follow-up [43]. Nevertheless, there is a paucity of recent studies that have focused on the correlation between AAA and NHHR, which includes various atherogenic and antiatherogenic lipid particles. This study corroborated previous studies validated the correlation between HDL-C with AAA, as well as suggested NHHR could have a significant association with the prevalence of AAA.
However, the potential mechanism leading to NHHR induced prevalence of AAA was not fully expounded, and the damage of the atherogenic lipid particles to the aortic wall was only partly revealed. While it was documented that there is a notable association between AAA and atherosclerosis [15], it's an oversimplification to regard AAA merely as a upshot of advanced atherosclerosis [44]. The pathophysiological process is complicated and elusive and comprises three pivotal factors: proteolysis, smooth muscle cell apoptosis and inflammation [45]. A cohort study demonstrated a link between elevated LDL-C concentrations and matrix metalloproteinase-9 (MMP-9) allele [46]; in addition, the cholesterol metabolite, hydroxycholesterol (27-OHC), could increase MMP9 at the mRNA level [47]. Yin J et al. reported that cholesterol oxides might be able to trigger apoptosis in vascular smooth muscle cells based on animal experiments [48]. The intracellular redox system and activation of proinflammatory genes seemed to be changed by Lp(a), which led to the chronic inflammation in the aortic wall by means of its oxidized phospholipid content [49, 50]. Similarly, studies revealed that LDL-C could induce inflammation as well [51], and that modified LDL could lead to the NLRP3 inflammasome priming and activation in macrophages [52], of which affect formation of AAA [53]. Non-HDL-C, that is abundant and included more constituents than other lipoprotein particles, comprised all the morbific lipoproteins mentioned above. Apart from these effects, NHHR is adjusted by HDL-C, which exerts anti-inflammatory effects [54].
This study also verified that the association of NHHR with AAA existed in different age, sex, smoking, hypertension and CAD conditions, although are were all the risk factors of AAA [35]. The diagnostic value of NHHR is enhanced by its universality, especially in the widespread AAA screening. Owing to intact AAAs, which are commonly asymptomatic, an AAA screening program with ultrasonography demonstrated timely diagnosis of AAAs and reduced AAA-related mortality [55]. Although the US Preventive Services Task Force have advocated a single AAA ultrasound screening for male individuals between 65 and 75 years old that have a smoking history [56], AAA screening is prone to trigger overdiagnosis. Therefore, NHHR is anticipated to assist in identifying high-risk AAA individuals, while improving screening diagnostic accuracy, thus preventing overdiagnosis during AAA ultrasound screening.
Strengths and limitations
There are a few limitations to be acknowledged in the current research. First, due to its cross-sectional nature, this study might be affected by selection bias.. However, to minimize potential bias in the study, both PSM and multivariable logistic regression analyses were employed. Additionally, this study could not establish causative links. Second, only once was the lipid profile evaluated and noted. A lack of reduplicated measurement of the lipid profile could lead to the influence of acute stress and occasionality. Third, although we have already supplemented some of the patient's medication information, there was no detailed information about previous use of drugs, including specific lipid-lowering medications, dosing frequency, and duration of medication use. Therefore the influence of drugs, such as stains, could not be adjusted accurately. Finally, this study was only consisted of 219 AAA patients. However, this was a persistent study with durative AAA screening in the hospital and communities. In prospective research, we intend to prioritize exploring the diagnostic significance and prognostic assessment of NHHR.
Conclusion
In addition to the traditionally pivotal lipid parameters, NHHR was in association with AAA independently. The association existed in different age, sex, smoking, hypertension and coronary arterial disease conditions. Clinicians could utilize NHHR to assist in identifying high-risk AAA individuals, and improve efficiency of screening, thus preventing overdiagnosis during AAA ultrasound screening.
Supplementary Information
Additional file 1: Supplementary Figure 1.
Additional file 2: Supplementary Figure 2.
Additional file 3: Supplementary Figure 3.
Additional file 4: Supplementary Table 1. Baseline characteristics of all the participants.
Additional file 5: Supplementary Table 2. Multicollinearity test of variable in multivariable logistic regression analysis.
Acknowledgements
The authors acknowledge the expert language assistance of Dr. Jiahui Li.
Abbreviations
- AAA
Abdominal aortic aneurysms
- CAD
Coronary heart disease
- Non-HDL-C
Non-high-density lipoprotein cholesterol
- AUROC
Areas under the receiver operating characteristic curves
- Non-HDL-C/HDL-C
Non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol
- NHHR
Non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio
- TC
Total cholesterol
- AAD
Abdominal aortic diameter
- LDL-C
Low-density lipoprotein cholesterol
- SMD
Standardized mean differences
- IQR
Interquartile range
- ORs
Odds ratios
- CIs
Confidence intervals
- BUN
Blood urea nitrogen
- PSM
Propensity score matching
- ROC
Receiver operating characteristic
- AUC
Area under the curve
- VLDL-C
Very low density lipoprotein cholesterol
- IDL-C
Intermediate density lipoprotein cholesterol
- MMP-9
Matrix metalloproteinase-9
Authors’ contributions
L.J.F and F.Y.Q proposed the design of the study and provided comprehensive guidance throughout the entire process; L.W.H. finished the principal manuscript text, L.S.Y. was in charge of collecting data, L.W. preside over data quality monitoring, L.J.T., Z.T. and Y.F. contributed to analyzing and interpreting the data, and L.Y. and H.W.H. engaged in the proofreading the manuscript. The submitted version was approved by all the authors. Personal responsibility for their individual contributions has been committed to by the authors.
Funding
Financial backing for the research, writing, and publication of this article was recognized by the author(s), including the National Natural Science Foundation of China (82200519); Natural Science Foundation of Guangdong Province, China (2022A1515010897) and Medical Scientific Research Foundation of Guangdong Province, China (A2021348). The investigation's structure, data gathering, analysis, and the interpretation of results were not influenced by the funding entities.
Availability of data and materials
Provided there is a valid and reasonable request made for the information derived from this research, it can be obtained from the primary or corresponding author.
Declarations
Ethics approval and consent to participate
Authorization for the research was furnished by the Ethics Committee of Guangdong Provincial People's Hospital (Approval No. GDREC2018215H(R3)). Owing to its retrospective nature, informed consent was waived.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wenhui Lin and Songyuan Luo Coequal contributions was made by these authors, who jointly hold the first authorship.
Contributor Information
Yingqing Feng, Email: fengyingqing2022@163.com.
Jianfang Luo, Email: jianfangluo@sina.com.
References
- 1.Reimerink JJ, van der Laan MJ, Koelemay MJ, Balm R, Legemate DA. Systematic review and meta-analysis of population-based mortality from ruptured abdominal aortic aneurysm. Br J Surg. 2013;100:1405–1413. doi: 10.1002/bjs.9235. [DOI] [PubMed] [Google Scholar]
- 2.Lindholt JS, Sorensen J, Sogaard R, Henneberg EW. Long-term benefit and cost-effectiveness analysis of screening for abdominal aortic aneurysms from a randomized controlled trial. Br J Surg. 2010;97:826–834. doi: 10.1002/bjs.7001. [DOI] [PubMed] [Google Scholar]
- 3.Ashton HA, Gao L, Kim LG, Druce PS, Thompson SG, Scott RA. Fifteen-year follow-up of a randomized clinical trial of ultrasonographic screening for abdominal aortic aneurysms. Br J Surg. 2007;94:696–701. doi: 10.1002/bjs.5780. [DOI] [PubMed] [Google Scholar]
- 4.Thompson SG, Ashton HA, Gao L, Buxton MJ, Scott RA. Multicentre Aneurysm Screening Study G: final follow-up of the Multicentre Aneurysm Screening Study (MASS) randomized trial of abdominal aortic aneurysm screening. Br J Surg. 2012;99:1649–1656. doi: 10.1002/bjs.8897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCaul KA, Lawrence-Brown M, Dickinson JA, Norman PE. Long-term outcomes of the western Australian trial of screening for abdominal aortic aneurysms: secondary analysis of a randomized clinical trial. JAMA Intern Med. 2016;176:1761–1767. doi: 10.1001/jamainternmed.2016.6633. [DOI] [PubMed] [Google Scholar]
- 6.Sakalihasan N, Michel JB, Katsargyris A, Kuivaniemi H, Defraigne JO, Nchimi A, Powell JT, Yoshimura K, Hultgren R. Abdominal aortic aneurysms. Nat Rev Dis Primers. 2018;4:34. doi: 10.1038/s41572-018-0030-7. [DOI] [PubMed] [Google Scholar]
- 7.Persson SE, Boman K, Wanhainen A, Carlberg B, Arnerlov C. Decreasing prevalence of abdominal aortic aneurysm and changes in cardiovascular risk factors. J Vasc Surg. 2017;65:651–658. doi: 10.1016/j.jvs.2016.08.091. [DOI] [PubMed] [Google Scholar]
- 8.Conway AM, Malkawi AH, Hinchliffe RJ, Holt PJ, Murray S, Thompson MM, Loftus IM. First-year results of a national abdominal aortic aneurysm screening programme in a single centre. Br J Surg. 2012;99:73–77. doi: 10.1002/bjs.7685. [DOI] [PubMed] [Google Scholar]
- 9.Oliver-Williams C, Sweeting MJ, Turton G, Parkin D, Cooper D, Rodd C, Thompson SG, Earnshaw JJ. Gloucestershire, Swindon Abdominal Aortic Aneurysm Screening P: lessons learned about prevalence and growth rates of abdominal aortic aneurysms from a 25-year ultrasound population screening programme. Br J Surg. 2018;105:68–74. doi: 10.1002/bjs.10715. [DOI] [PubMed] [Google Scholar]
- 10.de Boer AR, Vaartjes I, van Dis I, van Herwaarden JA, Nathoe HM, Ruigrok YM, Bots ML, Visseren FLJ. group U-Ss: screening for abdominal aortic aneurysm in patients with clinically manifest vascular disease. Eur J Prev Cardiol. 2022;29:1170–1176. doi: 10.1093/eurjpc/zwaa014. [DOI] [PubMed] [Google Scholar]
- 11.Schanzer A, Oderich GS. Management of abdominal aortic aneurysms. N Engl J Med. 2021;385:1690–1698. doi: 10.1056/NEJMcp2108504. [DOI] [PubMed] [Google Scholar]
- 12.Golledge J, Muller J, Daugherty A, Norman P. Abdominal aortic aneurysm: pathogenesis and implications for management. Arterioscler Thromb Vasc Biol. 2006;26:2605–2613. doi: 10.1161/01.ATV.0000245819.32762.cb. [DOI] [PubMed] [Google Scholar]
- 13.Cornuz J, Sidoti Pinto C, Tevaearai H, Egger M. Risk factors for asymptomatic abdominal aortic aneurysm: systematic review and meta-analysis of population-based screening studies. Eur J Public Health. 2004;14:343–349. doi: 10.1093/eurpub/14.4.343. [DOI] [PubMed] [Google Scholar]
- 14.Golledge J, Norman PE. Atherosclerosis and abdominal aortic aneurysm: cause, response, or common risk factors? Arterioscler Thromb Vasc Biol. 2010;30:1075–1077. doi: 10.1161/ATVBAHA.110.206573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forsdahl SH, Singh K, Solberg S, Jacobsen BK. Risk factors for abdominal aortic aneurysms: a 7-year prospective study: the Tromso Study, 1994–2001. Circulation. 2009;119:2202–2208. doi: 10.1161/CIRCULATIONAHA.108.817619. [DOI] [PubMed] [Google Scholar]
- 16.Sheng G, Liu D, Kuang M, Zhong Y, Zhang S, Zou Y. Utility of non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio in evaluating incident diabetes risk. Diabetes Metab Syndr Obes. 2022;15:1677–1686. doi: 10.2147/DMSO.S355980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhen R, Ban J, Jia Z, Liu Y, Li Z, Chen S. The relationship between non-HDL-C /HDL-C Ratio (NHHR) and vitamin D in type 2 diabetes mellitus. Diabetes Metab Syndr Obes. 2023;16:2661–2673. doi: 10.2147/DMSO.S414673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim SW, Jee JH, Kim HJ, Jin SM, Suh S, Bae JC, Kim SW, Chung JH, Min YK, Lee MS, et al. Non-HDL-cholesterol/HDL-cholesterol is a better predictor of metabolic syndrome and insulin resistance than apolipoprotein B/apolipoprotein A1. Int J Cardiol. 2013;168:2678–2683. doi: 10.1016/j.ijcard.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Zhang Z, Xia B, Wang L, Zhang H, Zhu Y, Liu C, Song B. Relationship between the non-HDLc-to-HDLc ratio and carotid plaques in a high stroke risk population: a cross-sectional study in China. Lipids Health Dis. 2020;19:168. doi: 10.1186/s12944-020-01344-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masson W, Epstein T, Huerin M, Lobo M, Molinero G, Siniawski D. Association between non-HDL-C/HDL-C ratio and carotid atherosclerosis in postmenopausal middle-aged women. Climacteric. 2019;22:518–522. doi: 10.1080/13697137.2019.1631787. [DOI] [PubMed] [Google Scholar]
- 21.Zhang N, Hu X, Zhang Q, Bai P, Cai M, Zeng TS, Zhang JY, Tian SH, Min J, Huang HT, et al. Non-high-density lipoprotein cholesterol: High-density lipoprotein cholesterol ratio is an independent risk factor for diabetes mellitus: results from a population-based cohort study. J Diabetes. 2018;10:708–714. doi: 10.1111/1753-0407.12650. [DOI] [PubMed] [Google Scholar]
- 22.Lin D, Qi Y, Huang C, Wu M, Wang C, Li F, Yang C, Yan L, Ren M, Sun K. Associations of lipid parameters with insulin resistance and diabetes: a population-based study. Clin Nutr. 2018;37:1423–1429. doi: 10.1016/j.clnu.2017.06.018. [DOI] [PubMed] [Google Scholar]
- 23.Du T, Yuan G, Zhang M, Zhou X, Sun X, Yu X. Clinical usefulness of lipid ratios, visceral adiposity indicators, and the triglycerides and glucose index as risk markers of insulin resistance. Cardiovasc Diabetol. 2014;13:146. doi: 10.1186/s12933-014-0146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hodkinson A, Tsimpida D, Kontopantelis E, Rutter MK, Mamas MA, Panagioti M. Comparative effectiveness of statins on non-high density lipoprotein cholesterol in people with diabetes and at risk of cardiovascular disease: systematic review and network meta-analysis. BMJ. 2022;376:e067731. doi: 10.1136/bmj-2021-067731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Writing Committee M, Isselbacher EM, Preventza O, Hamilton Black Iii J, Augoustides JG, Beck AW, Bolen MA, Braverman AC, Bray BE, Brown-Zimmerman MM, et al. ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;2022(80):e223–e393. doi: 10.1016/j.jacc.2022.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD, Eggebrecht H, Evangelista A, Falk V, Frank H, Gaemperli O, et al. ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC) Eur Heart J. 2014;35:2873–2926. doi: 10.1093/eurheartj/ehu281. [DOI] [PubMed] [Google Scholar]
- 27.Guirguis-Blake JM, Beil TL, Senger CA, Coppola EL. Primary care screening for abdominal aortic aneurysm: updated evidence report and systematic review for the US preventive services task force. JAMA. 2019;322:2219–2238. doi: 10.1001/jama.2019.17021. [DOI] [PubMed] [Google Scholar]
- 28.Ballard DJ. Selective screening for abdominal aortic aneurysms with physical examination and ultrasound. Arch Intern Med. 1989;149(1463):1466. doi: 10.1001/archinte.149.6.1463. [DOI] [PubMed] [Google Scholar]
- 29.Lindholt JS, Vammen S, Juul S, Henneberg EW, Fasting H. The validity of ultrasonographic scanning as screening method for abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 1999;17:472–475. doi: 10.1053/ejvs.1999.0835. [DOI] [PubMed] [Google Scholar]
- 30.Costantino TG, Bruno EC, Handly N, Dean AJ. Accuracy of emergency medicine ultrasound in the evaluation of abdominal aortic aneurysm. J Emerg Med. 2005;29:455–460. doi: 10.1016/j.jemermed.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 31.Tayal VS, Graf CD, Gibbs MA. Prospective study of accuracy and outcome of emergency ultrasound for abdominal aortic aneurysm over two years. Acad Emerg Med. 2003;10:867–871. doi: 10.1197/aemj.10.8.867. [DOI] [PubMed] [Google Scholar]
- 32.Stackelberg O, Wolk A, Eliasson K, Hellberg A, Bersztel A, Larsson SC, Orsini N, Wanhainen A, Bjorck M. Lifestyle and Risk of Screening-Detected Abdominal Aortic Aneurysm in Men. J Am Heart Assoc. 2017;6:e004725. [DOI] [PMC free article] [PubMed]
- 33.Sprouse LR, 2nd, Meier GH, 3rd, Parent FN, DeMasi RJ, Glickman MH, Barber GA. Is ultrasound more accurate than axial computed tomography for determination of maximal abdominal aortic aneurysm diameter? Eur J Vasc Endovasc Surg. 2004;28:28–35. doi: 10.1016/j.ejvs.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 34.Ashton HA, Buxton MJ, Day NE, Kim LG, Marteau TM, Scott RA, Thompson SG, Walker NM. Multicentre Aneurysm Screening Study G: The Multicentre Aneurysm Screening Study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: a randomised controlled trial. Lancet. 2002;360:1531–1539. doi: 10.1016/S0140-6736(02)11522-4. [DOI] [PubMed] [Google Scholar]
- 35.Isselbacher EM, Preventza O, Hamilton Black J, Jr, Augoustides JG, Beck AW, Bolen MA, Braverman AC, Bray BE, Brown-Zimmerman MM, Chen EP, et al. ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2022;146:e334–e482. doi: 10.1161/CIR.0000000000001106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blaha MJ, Blumenthal RS, Brinton EA, Jacobson TA. National Lipid Association Taskforce on Non HDLC: The importance of non-HDL cholesterol reporting in lipid management. J Clin Lipidol. 2008;2:267–273. doi: 10.1016/j.jacl.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 37.Virani SS, Catellier DJ, Pompeii LA, Nambi V, Hoogeveen RC, Wasserman BA, Coresh J, Mosley TH, Otvos JD, Sharrett AR, et al. Relation of cholesterol and lipoprotein parameters with carotid artery plaque characteristics: the Atherosclerosis Risk in Communities (ARIC) carotid MRI study. Atherosclerosis. 2011;219:596–602. doi: 10.1016/j.atherosclerosis.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang S, Zhong J, Ye M, Miao L, Lu G, Xu C, Xue Z, Zhou X. Association between the non-HDL-cholesterol to HDL-cholesterol ratio and non-alcoholic fatty liver disease in Chinese children and adolescents: a large single-center cross-sectional study. Lipids Health Dis. 2020;19:242. doi: 10.1186/s12944-020-01421-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li T, Yuan D, Wang P, Jia S, Zhang C, Zhu P, Song Y, Tang X, Zhao X, Gao Z, et al. Associations of lipid measures with total occlusion in patients with established coronary artery disease: a cross-sectional study. Lipids Health Dis. 2022;21:118. doi: 10.1186/s12944-022-01733-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anagnostakos J, Lal BK. Abdominal aortic aneurysms. Prog Cardiovasc Dis. 2021;65:34–43. doi: 10.1016/j.pcad.2021.03.009. [DOI] [PubMed] [Google Scholar]
- 41.Iribarren C, Darbinian JA, Go AS, Fireman BH, Lee CD, Grey DP. Traditional and novel risk factors for clinically diagnosed abdominal aortic aneurysm: the Kaiser multiphasic health checkup cohort study. Ann Epidemiol. 2007;17:669–678. doi: 10.1016/j.annepidem.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 42.Takagi H, Manabe H, Kawai N, Goto SN, Umemoto T. Serum high-density and low-density lipoprotein cholesterol is associated with abdominal aortic aneurysm presence: a systematic review and meta-analysis. Int Angiol. 2010;29:371–375. [PubMed] [Google Scholar]
- 43.Kubota Y, Folsom AR, Ballantyne CM, Tang W. Lipoprotein(a) and abdominal aortic aneurysm risk: the Atherosclerosis Risk in Communities study. Atherosclerosis. 2018;268:63–67. doi: 10.1016/j.atherosclerosis.2017.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nordon I, Brar R, Taylor J, Hinchliffe R, Loftus IM, Thompson MM. Evidence from cross-sectional imaging indicates abdominal but not thoracic aortic aneurysms are local manifestations of a systemic dilating diathesis. J Vasc Surg. 2009;50:171–176 e171. doi: 10.1016/j.jvs.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 45.Davis FM, Daugherty A, Lu HS. Updates of recent aortic aneurysm research. Arterioscler Thromb Vasc Biol. 2019;39:e83–e90. doi: 10.1161/ATVBAHA.119.312000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mazzotti DR, Singulane CC, Ota VK, Rodrigues TP, Furuya TK, de Souza FJ, Cordeiro BG, de Oliveira Amaral CM, Chen ES, Jacomini A, et al. Association of APOE, GCPII and MMP9 polymorphisms with common diseases and lipid levels in an older adult/elderly cohort. Gene. 2014;535:370–375. doi: 10.1016/j.gene.2013.11.040. [DOI] [PubMed] [Google Scholar]
- 47.Shen Z, Zhu D, Liu J, Chen J, Liu Y, Hu C, Li Z, Li Y. 27-Hydroxycholesterol induces invasion and migration of breast cancer cells by increasing MMP9 and generating EMT through activation of STAT-3. Environ Toxicol Pharmacol. 2017;51:1–8. doi: 10.1016/j.etap.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 48.Yin J, Chaufour X, McLachlan C, McGuire M, White G, King N, Hambly B. Apoptosis of vascular smooth muscle cells induced by cholesterol and its oxides in vitro and in vivo. Atherosclerosis. 2000;148:365–374. doi: 10.1016/S0021-9150(99)00286-5. [DOI] [PubMed] [Google Scholar]
- 49.Taleb A, Witztum JL, Tsimikas S. Oxidized phospholipids on apoB-100-containing lipoproteins: a biomarker predicting cardiovascular disease and cardiovascular events. Biomark Med. 2011;5:673–694. doi: 10.2217/bmm.11.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ugovsek S, Sebestjen M. Lipoprotein(a)-The Crossroads of Atherosclerosis, Atherothrombosis and Inflammation. Biomolecules. 2021;12:26. [DOI] [PMC free article] [PubMed]
- 51.Jukema RA, Ahmed TAN, Tardif JC. Does low-density lipoprotein cholesterol induce inflammation? If so, does it matter? Current insights and future perspectives for novel therapies. BMC Med. 2019;17:197. doi: 10.1186/s12916-019-1433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jia Y, Zhang L, Liu Z, Mao C, Ma Z, Li W, Yu F, Wang Y, Huang Y, Zhang W, et al. Targeting macrophage TFEB-14-3-3 epsilon Interface by naringenin inhibits abdominal aortic aneurysm. Cell Discov. 2022;8:21. doi: 10.1038/s41421-021-00363-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kajani S, Curley S, McGillicuddy FC. Unravelling HDL-looking beyond the cholesterol surface to the quality within. Int J Mol Sci. 2018;19:1971. [DOI] [PMC free article] [PubMed]
- 55.Johansson M, Zahl PH, Siersma V, Jorgensen KJ, Marklund B, Brodersen J. Benefits and harms of screening men for abdominal aortic aneurysm in Sweden: a registry-based cohort study. Lancet. 2018;391:2441–2447. doi: 10.1016/S0140-6736(18)31031-6. [DOI] [PubMed] [Google Scholar]
- 56.Force USPST. Owens DK, Davidson KW, Krist AH, Barry MJ, Cabana M, Caughey AB, Doubeni CA, Epling JW, Jr, Kubik M, et al. Screening for Abdominal Aortic Aneurysm: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:2211–2218. doi: 10.1001/jama.2019.18928. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Figure 1.
Additional file 2: Supplementary Figure 2.
Additional file 3: Supplementary Figure 3.
Additional file 4: Supplementary Table 1. Baseline characteristics of all the participants.
Additional file 5: Supplementary Table 2. Multicollinearity test of variable in multivariable logistic regression analysis.
Data Availability Statement
Provided there is a valid and reasonable request made for the information derived from this research, it can be obtained from the primary or corresponding author.