Abstract
Background
Postoperative pain is one of the most common complications after surgery. In order to detect early and intervene in time for moderate to severe postoperative pain, it is necessary to identify risk factors and construct clinical prediction models. This study aimed to identify significant risk factors and establish a better-performing model to predict moderate to severe acute postoperative pain after orthopedic surgery under general anesthesia.
Methods
Patients who underwent orthopedic surgery under general anesthesia were divided into patients with moderate to severe pain group (group P) and patients without moderate to severe pain group (group N) based on VAS scores. The features selected by Lasso regression were processed by the random forest and multivariate logistic regression models to predict pain outcomes. The classification performance of the two models was evaluated through the testing set. The area under the curves (AUC), the accuracy of the classifiers, and the classification error rate for both classifiers were calculated, the better-performing model was used to predict moderate to severe acute postoperative pain after orthopedic surgery under general anesthesia.
Results
A total of 327 patients were enrolled in this study (228 in the training set and 99 in the testing set). The incidence of moderate to severe postoperative pain was 41.3%. The random forest model revealed a classification error rate of 25.2% and an AUC of 0.810 in the testing set. The multivariate logistic regression model revealed a classification error rate of 31.3% and an AUC of 0.764 in the testing set. The random forest model was chosen for predicting clinical outcomes in this study. The risk factors with the greatest and second contribution were immobilization and duration of surgery, respectively.
Conclusions
The random forest model can be used to predict moderate to severe acute postoperative pain after orthopedic surgery under general anesthesia, which is of potential clinical application value.
Keywords: Random forest model, Logistic regression model, Machine learning, Pain prediction, Analgesia
Background
Postoperative pain is one of the most common complications after surgery, the incidence of moderate to severe postoperative pain varies from 25 to 66% according to the previous reports [1, 2]. The consequences of suboptimal postoperative pain control include negative effects on postoperative recovery, increased incidence of respiratory and circulatory complications, increased length of hospital stay and healthcare costs, as well as an increased risk of transition to chronic pain or neuropathic pain [3, 4]. With the advances in modern medicine, postoperative pain remains a challenge, hence improving pain control is an international initiative promoted by multiple health organizations including WHO [5].
Orthopedic surgeries are considered to be some of the most painful procedures that have a variety of options for postoperative analgesia ranging from surgeon provided (e.g., local anesthesia) to more intensive techniques (e.g., nerve blockade or patient-controlled epidural analgesia) requiring care from an acute pain service [6]. According to the author’s clinical experience and some research reports, compared with patients receiving spinal anesthesia or regional anesthesia, acute postoperative pain is more severe in patients undergoing orthopedic surgery under general anesthesia [7, 8].
The ability to identify and focus care on patients at higher risk of moderate to severe postoperative pain would improve analgesia and patient satisfaction. The construction of a reliable postoperative pain prediction model based on risk factors can be applied in the early identification of orthopedic patients with a high risk of moderate to severe postoperative pain, which is vital in taking timely interventions to prevent pain from worsening.
Random forest algorithms can build a machine learning model based on sample data and be used to make predictions, and its performance advantages are mainly due to ensemble learning [9]. The previous studies demonstrated that the logistic regression model had limited performance in predicting acute postoperative pain [8, 10], while there have been no reports of using random forests to predict postoperative pain to the best of our knowledge.
Accordingly, we constructed machine learning models to predict moderate to severe acute postoperative pain of orthopedic patients under general anesthesia by identifying the risk factors. In addition, we evaluated the efficiency of the random forest algorithm-based prediction model by comparing it with the multivariate logistic regression-based model.
Materials and methods
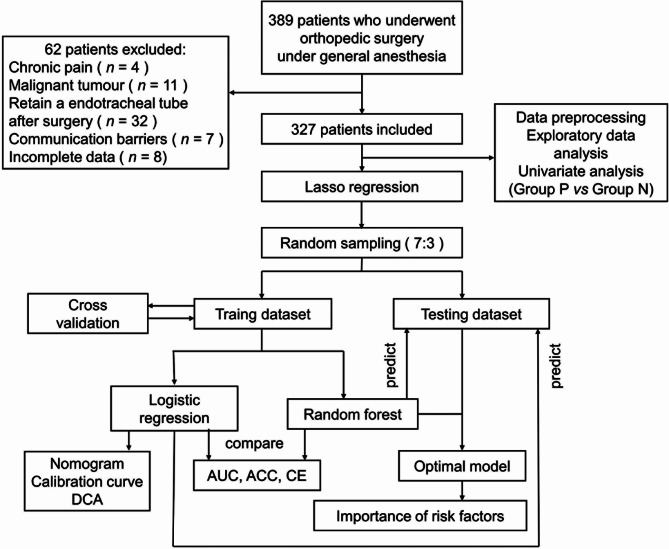
This retrospective observational cohort study was conducted following the Declaration of Helsinki (as revised in October 2013). The study was approved and monitored by the Ethics Committee of Shanxi Bethune Hospital (Third Hospital of Shanxi Medical University). Because of the retrospective nature of the study and the patient’s identity information has been concealed, the requirement for informed consent was waived by the Ethics Committee of Shanxi Bethune Hospital. We present the following article in accordance with the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) reporting checklist [11]. The procedure of establishing moderate to severe acute postoperative pain prediction models in this study is shown in Fig. 1.
Fig. 1.
The procedure of establishing moderate to severe acute postoperative pain prediction models in this study
Sample selection
Patients who underwent orthopedic surgery under general anesthesia in Shanxi Bethune Hospital from January 2020 to June 2020, were included in the study. The demographic and perioperative characteristics were extracted from the Electronic Medical Record (EMR) database.
Inclusion criteria
The inclusion criteria for this study were as follows: (1) Patients between the age of 18 and 100. (2) Patients underwent orthopedic surgery under general anesthesia. Children and adolescents were not included in this study because they are in the stage of growth and development, and their physiological characteristics are more complex, so they are not suitable for study together with adults.
Exclusion criteria
The exclusion criteria for this study were as follows: (1) Patients with chronic pain (which included musculoskeletal pain disorders, peripheral neuropathy, and migraines), (2) Patients with malignant tumors. Because long-term chronic pain such as tumors is prone to special conditions such as hyperalgesia and neuropathic pain, resulting in inaccurate pain scores. (3) Patients retain an endotracheal tube after surgery, (4) Patients with cognitive dysfunction or who cannot communicate normally. Due to the difficulty of self-assessing pain scores in these two groups. (5) Outpatient surgeries, (6) Incomplete clinical data. Because the medical records in these two groups may miss key data.
Pain scoring methods and diagnosis of moderate to severe postoperative pain
The primary outcome was pain scores at rest on postoperative day one (POD1) using a visual analogue scale (VAS), with 0 representing no pain and 10 representing the most intense pain. Moderate to severe pain was defined as a VAS score of 4 or greater, which has been previously identified as a value at which patients request additional analgesias, become unsatisfied with pain control, and have interference with functional activity [12]. The VAS score was self-assessed by the patients based on his or her pain level under the guidance of an anesthesiologist or anesthesia nurse, and recorded by the anesthesiologist. Patients and staff were blinded to this study.
Variables
Demographic variables were defined and analyzed as follows: sex, age, and body mass index (BMI), which have been shown to be associated with postoperative pain in many studies [13–15]. Perioperative variables including physical status score based on the American Society of Anesthesiologists physical status classification (ASA score), which was routinely included in anesthesia-related studies [16]. surgical score, type of surgery (open surgery vs. endoscopic surgery), surgical site (spinal area, joint, limb bones, muscles and soft tissues), blood loss during surgery, intraoperative blood transfusion, indwelling urinary catheters, indwelling drains, tourniquet during surgery, and arteriovenous catheterization were included to reflect the degree of tissue damage and intensity of noxious stimulation [17, 18]. Multimodal analgesia methods contained patient-controlled intravenous analgesia (PCIA) pumps, peripheral nerve blockade, and preemptive analgesia were included, which may be beneficial in reducing the incidence of acute postoperative pain [19, 20]. Variables including history of surgery or anesthesia, history of depression or anxiety, preoperative VAS score, immobilization, secondary surgery in a short period (within a month), and timing of surgery (emergency surgery vs. elective surgery) may reflect special medical history related to postoperative pain [21–23]. Furthermore, the duration of surgery, time from withdrawal of medicine to awake, consumption of sufentanil, remifentanil, propofol, sevoflurane and rocuronium were included to assess the impact of drug dosage, time and other factors on outcomes [24, 25]. In particular, “surgical score” is a scoring system developed by the National Health Commission of the PRC according to the difficulty and risk of surgery, with a score ranging from 1 to 4, the higher scores indicating greater surgical difficulty. And “arteriovenous catheterization” refers to puncture catheterization for the purpose of invasive blood pressure measurement or infusion through the central venous.
Feature selection
The pre-processed data were randomly split into training and testing sets. In the training set, demographic and perioperative characteristics above were selected as candidate risk factors because of previous reports and clinical experiences. After univariate analysis, the Lasso regression model was applied to screen the optimized variables by running cyclic coordinate descent. Age, duration of surgery, blood loss during surgery, time from withdrawal of medicine to awake, sufentanil consumption, remifentanil consumption, propofol consumption, sevoflurane consumption, and rocuronium consumption were entered into the Lasso regression procedure as continuous variables. ASA score, timing of surgery, type of surgery, indwelling urinary catheters, arteriovenous catheterization, secondary surgery in a short period, immobilization, intraoperative blood transfusion, and tourniquet during surgery were entered as dichotomous variables. Lasso regression was generated using the glmnet package in R, the optimal lambda value was determined by 10-fold cross-validation. Lasso regression can force the coefficients of redundant variables to 0 and thus directly exclude them. The retained variables were selected as the input variables of the random forest models and multivariate logistic regression models.
Random forest modeling
The mlr3 package based on R was applied for random forest model construction and hyperparameter tuning. The data was resampled by using the bootstrapping/bagging method. The variation range of the hyperparameter space were pre-set as: “num.trees” [300 ~ 1000], “mtry” [2 ~ 5], “min.node.size” [2 ~ 10], and “max.depth” [2 ~ 10]. AutoTuner functions of the mlr3 package were used for the grid search and automatic tuning of hyperparameters, the cross-validation technique was used to tune the number of estimators in the classifier, and all training was conducted with 10-fold cross-validation to prevent overfitting. All the indicators included in the risk prediction model were analyzed based on the mean decrease in accuracy and the mean decrease in the Gini coefficient.
Logistic regression modeling
The mlr3 package was applied for logistic regression model construction and hyperparameter tuning. The training set was conducted with 10-fold cross-validation to improve predictive performance and prevent overfitting. Independent risk factors were identified using a multivariate logistic regression model that entered variables selected in Lasso analysis, and odds ratio (OR) along with 95% confidence interval (CI) were calculated. The nomograms were applied to visualize the prediction model, the calibration curves were applied to visualize Hosmer-Lemeshow goodness-of-fit test, and the decision curves were used to determine clinical benefit.
Evaluation of machine learning models
The confusion matrixes, the accuracy of the classifiers (“classif.acc”), the classification error rate (“classif.ce”) and the area under the receiver operating characteristic curve (AUC) were analyzed to evaluate the performance and clinical usefulness of the random forest classifier and the logistic regression classifier by comparing the predicted results with the true results. Given that the incidence of positive events in this study was 41.3%, the threshold of the ROC curve was set to 0.4 instead of the default 0.5.
Statistical analysis
Statistical analyses were performed using the RStudio software (version 2022.12.0-353), which runs R software (version 4.1.3; http://www.Rproject.org). Descriptive statistics were computed for all variables. These included means and standard deviations (SD) for continuous variables that conform to normal distributions, median and interquartile range for continuous variables that do not conform to normal distributions, and frequencies for categorical factors. Comparisons of the distribution of demographic variables and clinical characteristics were performed using the two-tailed t-test (or the Mann-Whitney test as appropriate) for continuous variables and the chi-square test (or the Fisher exact test as appropriate) for categorical variables. P values of 0.05 or lower were considered statistically significant.
Results
Patient characteristics
A total of 327 patients were enrolled in this study, The rate of moderate to severe acute postoperative pain among all enrolled patients was 41.3%. After univariate analysis, fourteen characteristics were retained for subsequent Lasso analysis. The demographic and perioperative characteristics of all enrolled patients are shown in Table 1.
Table 1.
The demographic and perioperative characteristics of all enrolled patients
| Variables | Total patients (n = 327) |
Group P (n = 135) |
Group N (n = 192) |
t / χ2 / Z | P-value |
|---|---|---|---|---|---|
| Demographics | |||||
| Sex | 0.3378 | 0.5611 | |||
| Male | 201 | 86 | 115 | ||
| Female | 126 | 49 | 77 | ||
| Age, years | 51.63 ± 14.38 | 48.47 ± 14.14 | 53.84 ± 14.16 | 3.3781 | 0.0008 |
| BMI, kg/m2 | 24.66 ± 3.92 | 24.27 ± 4.12 | 24.93 ± 3.76 | 1.4772 | 0.1408 |
| Perioperatives | |||||
| ASA score | 8.8704 | 0.0029 | |||
| I or II | 218 | 77 | 141 | ||
| III or IV | 109 | 58 | 51 | ||
| Surgical score | 0.55496 | 0.4563 | |||
| I or II | 35 | 17 | 18 | ||
| III or IV | 292 | 118 | 174 | ||
| Timing of surgery | 11.634 | 0.0006 | |||
| emergency surgery | 62 | 38 | 24 | ||
| elective surgery | 265 | 97 | 168 | ||
| Type of surgery | 4.7037 | 0.0301 | |||
| open surgery | 289 | 126 | 163 | ||
| endoscopic surgery | 38 | 9 | 29 | ||
| Surgical site | 6.5915 | 0.0861 | |||
| spinal area | 130 | 59 | 71 | ||
| joint | 51 | 24 | 27 | ||
| limb bones | 90 | 27 | 63 | ||
| muscles and soft tissues | 56 | 25 | 31 | ||
| History of surgery or anesthesia | 0.5876 | 0.4434 | |||
| yes | 86 | 32 | 54 | ||
| no | 241 | 103 | 138 | ||
| Preoperative VAS score | 0 (0-0) | 0 (1-0) | 0 (0-0) | -0.8729 | 0.3827 |
| History of depression or anxiety | 2.3131 | 0.1283 | |||
| yes | 12 | 8 | 4 | ||
| no | 315 | 127 | 188 | ||
| Indwelling urinary catheters | 14.806 | 0.0001 | |||
| yes | 227 | 110 | 117 | ||
| no | 100 | 25 | 75 | ||
| Indwelling drains | 2.2149 | 0.1367 | |||
| yes | 300 | 128 | 172 | ||
| no | 27 | 7 | 20 | ||
| PCIA | 2.444 | 0.118 | |||
| yes | 272 | 118 | 154 | ||
| no | 55 | 17 | 38 | ||
| Nerve blockade | 0.7377 | 0.3904 | |||
| yes | 49 | 17 | 32 | ||
| no | 278 | 118 | 160 | ||
| Arteriovenous catheterization | 14.299 | 0.0002 | |||
| yes | 80 | 48 | 32 | ||
| no | 247 | 87 | 160 | ||
| Secondary surgery in a short period | 4.9972 | 0.0253 | |||
| yes | 19 | 13 | 6 | ||
| no | 308 | 122 | 186 | ||
| Duration of surgery, minutes | 124 (170-80) | 135 (185-104.5) | 110 (159.25-72) | 11.0483 | 7.286e-05 |
| Blood loss during surgery, ml | 150 (400-100) | 200 (475-100) | 150 (300-57.5) | 11.1878 | 9.011e-05 |
| Time from withdrawal of medicine to awake, minutes | 15.77 ± 3.76 | 15.10 ± 3.81 | 16.24 ± 3.66 | 2.7464 | 0.006 |
| Preemptive analgesia | 0.21917 | 0.6397 | |||
| yes | 243 | 98 | 145 | ||
| no | 84 | 37 | 47 | ||
| Immobilization | 29.615 | 5.27e-08 | |||
| yes | 104 | 66 | 38 | ||
| no | 223 | 69 | 154 | ||
| Intraoperative blood transfusion | 10.794 | 0.0010 | |||
| yes | 95 | 53 | 42 | ||
| no | 232 | 82 | 150 | ||
| Sufentanil consumption, µg | 45 (50-40) | 50 (60-40) | 40 (50-35) | 11.8357 | 0.0003 |
| Remifentanil consumption, mg | 1.2 (1.5-0.8) | 1.2 (1.7-1) | 1 (1.5-0.7) | -3.2160 | 0.0013 |
| Propofol consumption, mg | 700 (875-475) | 700 (900-500) | 575 (850-400) | -3.0314 | 0.0024 |
| Sevoflurane consumption, ml | 30 (40-15) | 30 (45-22.5) | 25 (40-15) | -3.7777 | 0.0002 |
| Rocuronium consumption, mg | 90 (120-65) | 100 (125-70) | 80 (120-57.5) | -2.9820 | 0.0029 |
| Tourniquet during surgery | 52.177 | 5.071e-13 | |||
| yes | 65 | 53 | 12 | ||
| no | 262 | 82 | 180 | ||
Filtered features for machine learning model establishing
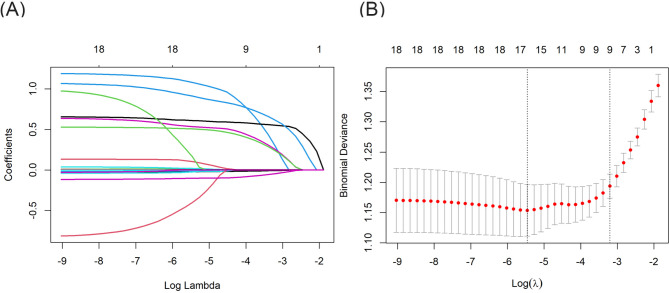
Using the Lasso regression model, eighteen characteristics were tested for their ability to predict the clinical outcomes and to avoid overfitting. The Lasso coefficient profiles of features and the optimal penalization coefficient lambda+1se are shown in Fig. 2. The feature selection results revealed that nine variables, including age, indwelling urinary catheters, arteriovenous catheterization, secondary surgery in a short period of time, duration of surgery, blood loss during surgery, immobilization, time from withdrawal of medicine to awake and tourniquet during surgery, could be used to predict moderate to severe acute postoperative pain (Table 2).
Fig. 2.
(A) Lasso coefficient profiles of all candidate features. (B) The tuning parameter λ (lambda) selection in the Lasso models used 10-fold cross-validation by minimum criteria
Table 2.
The characteristics of all enrolled patients(Training set vs. Testing set)
| Variables | Total patients (n = 327) |
Training set (n = 228) |
Testing set (n = 99) |
t / χ2 / Z | P-value |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, years | 51.63 ± 14.38 | 52.32 ± 13.74 | 50.03 ± 15.71 | -1.2564 | 0.2107 |
| Perioperatives | |||||
| Indwelling urinary catheters | 0.9726 | 0.324 | |||
| yes | 227 | 154 | 73 | ||
| no | 100 | 74 | 26 | ||
| Arteriovenous catheterization | 0.84331 | 0.3585 | |||
| yes | 80 | 52 | 28 | ||
| no | 247 | 176 | 71 | ||
| Secondary surgery in a short period | 0.016849 | 0.8967 | |||
| yes | 19 | 14 | 5 | ||
| no | 308 | 214 | 94 | ||
| Duration of surgery, minutes | 124 (170-80) | 124 (166.25-81.5) | 113 (170-79) | 2.1046 | 0.673 |
| Blood loss during surgery, ml | 150 (400-100) | 150 (400-80) | 200 (400-100) | 1.3283 | 0.572 |
| Time from withdrawal of medicine to awake, minutes | 15.77 ± 3.76 | 15.82 ± 3.67 | 15.66 ± 3.97 | -0.34078 | 0.7337 |
| Immobilization | 0.60667 | 0.436 | |||
| yes | 104 | 69 | 35 | ||
| no | 223 | 159 | 64 | ||
| Tourniquet during surgery | 4.2322 | 0.03966 | |||
| yes | 65 | 38 | 27 | ||
| no | 262 | 190 | 72 | ||
| Moderate to severe postoperative pain | 0.11243 | 0.7374 | |||
| yes | 135 | 96 | 39 | ||
| no | 192 | 132 | 60 | ||
Random forest algorithm-based prediction model
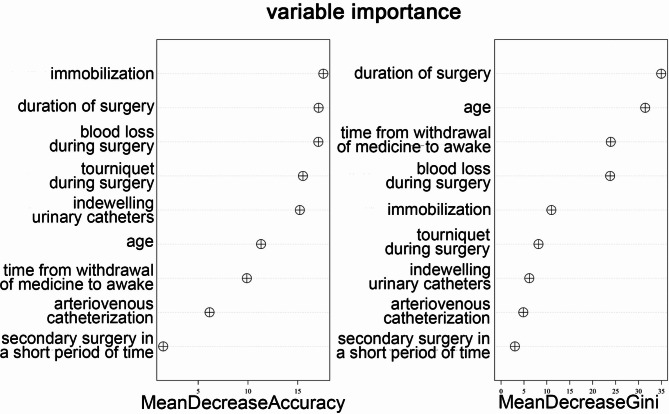
A risk prediction model was constructed based on confirmed characteristics selected by the Lasso algorithm. The number of decision trees was set at 500, the “mtry” parameter was set at 3, the “min.node.size” parameter was set at 5, and the “max.depth” parameter was set at 6 according to the cross-validation algorithm and AutoTuner function. As shown in Fig. 3, the mean decrease in accuracy and mean decrease in Gini for all indicators entered in the random forest model were analyzed. The mean decrease in accuracy showed that immobilization was the highest, followed by duration of surgery, blood loss during surgery, tourniquet during surgery, indwelling urinary catheters, etc. It refers to the degree of decrease in accuracy without the presence of this risk factor in the random forest model, which is equivalent to the classification contribution.
Fig. 3.
Importance of risk factors in the prediction model constructed by random forest
Logistic regression algorithm-based prediction model
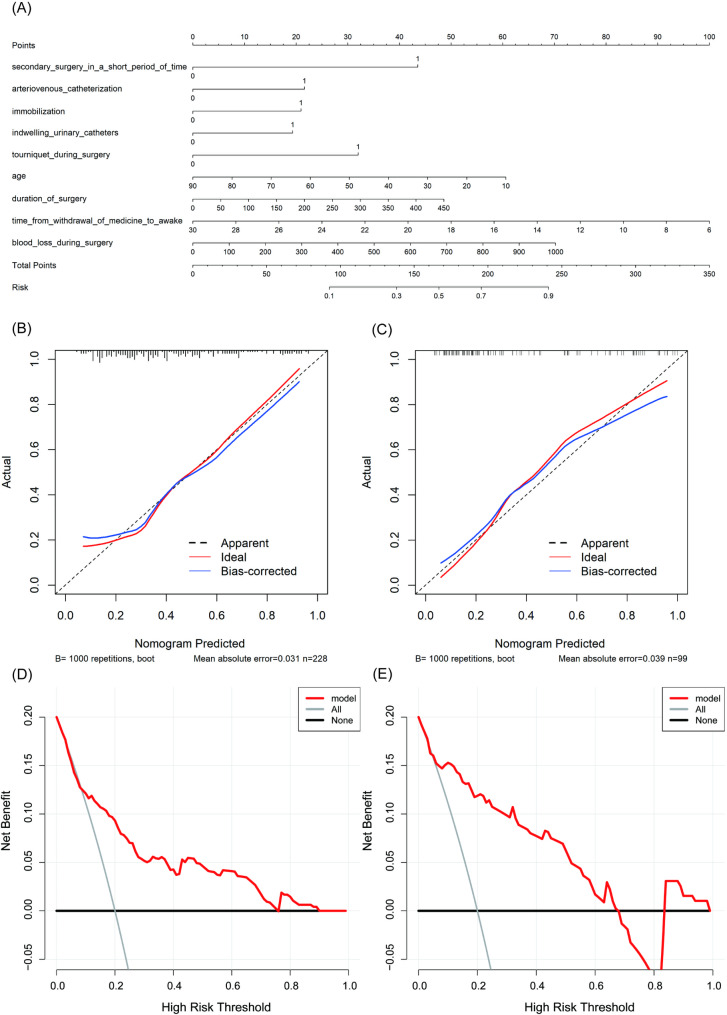
To verify the efficacy of the random forest model, we constructed a multivariate logistic regression model to predict moderate to severe acute postoperative pain, the model is visualized in Fig. 4A. Based on the multivariate analysis, three characteristics, namely shorter time from withdrawal of medicine to awake [OR 1.19, 95% CI (1.08, 1.31)], immobilization [OR 2.36, 95% CI (1.15, 4.85)], and indwelling urinary catheters [OR 2.39, 95% CI (1.09, 5.27)] were identified as independent risk factors. As shown in Fig. 4(B and C), the calibration plots showed favorable consistency between the prediction of the logistic model and actual observations in both the training and testing sets. Furthermore, As shown in Fig. 4 (D and E), the DCA plots showed that the logistic model was clinically useful and had good predictive ability in the training set.
Fig. 4.
Visualization and performance evaluation of the predictive model based on multivariate logistic regression. (A) The nomogram. (B) The calibration curve in the training set. (C) The calibration curve in the testing set. (D) The decision curve in the training set. (E) The decision curve in the testing set
Evaluation of predictor performance
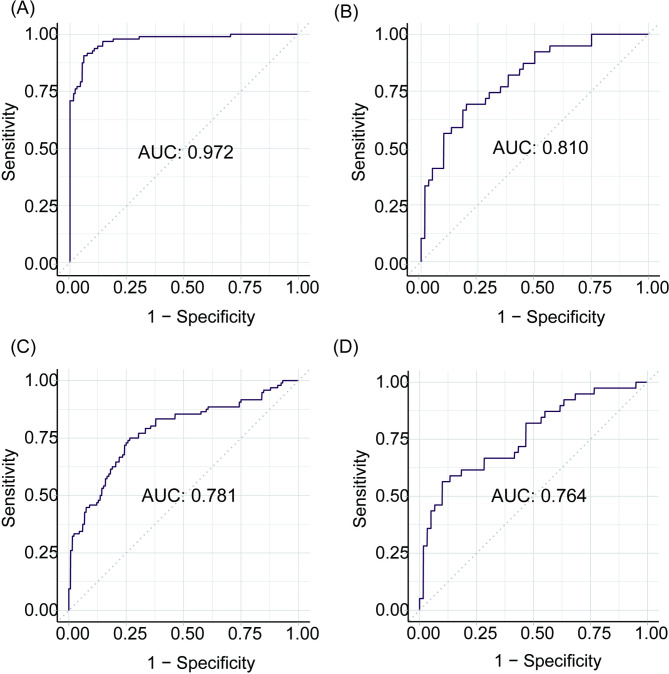
The ROC curves of prediction models constructed by random forest and traditional logistic regression in the training and testing sets are shown in Fig. 5. The AUC of the random forest algorithm-based prediction model in the training and testing sets were 0.972 and 0.810, respectively, which confirmed the good discrimination performance of the prediction model. Additionally, the AUC of the risk prediction model constructed by multivariate logistic regression in the training and testing sets were 0.781 and 0.764, respectively.
Fig. 5.
The ROC curves of the models in this study. (A) The ROC curve of the prediction model constructed by random forest in the training set. (B) The ROC curve of the prediction model constructed by random forest in the testing set. (C) The ROC curve of the prediction model constructed by multivariate logistic regression in the training set. (D) The ROC curve of the prediction model constructed by multivariate logistic regression in the testing set
The accuracy and the error rate were applied in testing the reliability of prediction models in our study. The accuracy values of the random forest algorithm-based prediction model and multivariate logistic regression-based prediction model in the training set were 0.882 and 0.724, respectively. The accuracy values of the random forest algorithm-based prediction model and logistic regression-based prediction model in the testing set were 0.747 and 0.687, respectively.
Discussion
Despite extraordinary advances in anesthesia and analgesia, a significant proportion of patients still suffer from moderate to severe pain after surgery, yet treatments and interventions for these patients are lacking [26, 27]. According to previous reports, the incidence of moderate to severe postoperative pain can be up to 66% in the United States [1]. In particular, orthopedic patients have a higher incidence of postoperative pain. In a study of 10,008 patients in Canada who underwent surgery, the incidence of acute postoperative pain was highest in orthopedic patients [28]. In this study, 41.3% of orthopedic surgery patients under general anesthesia experienced moderate to severe postoperative pain.
Early identification of patients who underwent orthopedic surgery under general anesthesia with a high risk of moderate to severe acute postoperative pain is helpful for early intervention and improving analgesic effect. There are currently no models for predicting postoperative pain in the patient of orthopedic surgery under general anesthesia, while generic postoperative pain prediction models do not accurately predict the degree of acute pain after orthopedic surgery. In addition, risk factors reported in some studies varied widely [8, 10, 13–15, 29, 30] (Table 3). In this study, we constructed a reliable risk prediction model with high discriminatory ability, which is helpful in building personalized treatment plans for patients with an increased risk of acute postoperative pain.
Table 3.
The risk factors of moderate to severe acute postoperative pain in previous studies
| Study (year) | Country | Number of patients | Type of surgery | Risk factors / protective factors |
|---|---|---|---|---|
|
Vasilopoulos et al. (2021) |
USA | 360 | mixed surgery | younger age, female gender, higher anxiety, and more pain behaviors. |
|
Sun et al. (2020) |
China | 1164 | thoracic surgery | younger age, high BMI, preoperative pain, smoking history, and number of chest tubes. |
|
Abrecht et al. (2019) |
USA | 126 | orthopaedic surgery | temporal summation of pain, high BMI, number of previous knee surgeries, and female gender. |
| Zaslansky et al. (2018) | International | 14,334 | orthopaedic surgery | female gender, younger age, high BMI, chronic pain, and opioid use before surgery. |
| Hartwig et al. (2017) | International | 192 |
gastric surgery |
younger age and preoperative pain. |
| Borges et al. (2016) | Brazil | 1062 | cesarean section |
preoperative anxiety, intrathecal morphine with fentanyl#. |
|
Liu et al. (2012) |
USA | 897 | orthopedic surgery | female gender, younger age, high BMI, preoperative pain, preoperative use of opioids, general anesthesia, preoperative use of anti-convulsants and anti-depressants, and prior surgery at the surgical site. |
# represents the protective factors
Most characteristics of orthopedic surgery patients between group P and group N were significantly different, so it is possible to use them to predict the clinical outcomes. Several risk prediction models were constructed to predict acute postoperative pain by typically performing univariate regression followed by multivariate logistic regression, resulting in reduced prediction accuracy. As an ensemble learning algorithm for classification, random forest is performed by constructing numerous decision trees at training time and outputting the class that is the mode of the classification of the individual trees. Compared with multivariate logistic regression, the random forest algorithm has higher accuracy in classification or prediction tasks and does not require strict assumptions about raw data [31, 32]. We applied the mlr3 package in R to establish and validate a random forest-based prediction model, which has a high ability to handle a multitude of input variables and evaluate the missing data to maintain the prediction accuracy [33].
In this study, the results of ROC analysis showed that the random forest algorithm-based prediction model had higher predictive accuracy than the logistic regression-based model in both the training and the testing sets. To our knowledge, this study is the first attempt to use random forests to predict acute postoperative pain severity in patients undergoing orthopedic surgery under general anesthesia. Our findings demonstrate the potential of random forest algorithms in predicting acute postoperative pain.
In this study, the results demonstrated that the duration of surgery, and blood loss during surgery were significantly associated with acute postoperative pain, which may be related to surgical complexity or surgical trauma size. Abrecht et al. [29] used temporal summation of pain (TSP) to predict postoperative pain accurately. Duration of surgery and blood loss during surgery may be reflections of TSP [34]. Some studies suggest that acute postoperative pain is mainly related to patients rather than surgical factors [13]. In contrast, our study found that postoperative pain was associated with surgical and anesthesia factors. In addition, the use of tourniquets during surgery, indwelling urinary catheters, and arteriovenous catheterization reflects pain from multiple causes other than surgical procedures [35, 36], all of these factors have the potential to predict postoperative pain severity. The above findings remind anesthesiologists that for surgeries that involve large tissue damage and a long operation time, they should pay attention to the dose of analgesics during and after the operation to ensure adequate analgesia. In addition, attention should be paid to the side effects of using tourniquets, indwelling urinary catheters, and drainage tubes.
In previous studies, it has been reported that preoperative pain can increase the incidence of acute postoperative pain [37]. In this study, two factors namely immobilization before surgery and secondary surgery in a short period of time caught the attention. These factors are related to the preoperative pain experience, immobilization is generally used in patients with fractures, and secondary surgery in a short period of time may indicate recently experienced pain. The ability of these two factors to predict postoperative pain has not been reported and can be further investigated in the future. These risk factors alert anesthesiologists to potential pain factors before surgery.
Time from withdrawal of medicine to awake defines the period of time from the cessation of the general anesthetic infusion to the time when the patient becomes conscious. This characteristic was extracted from the patient’s electronic anesthesia records based on our clinical experience. As far as we know, it has not been used in other studies so far. In this study, the characteristic was found to be an important risk factor or predictor of moderate to severe acute postoperative pain. In general, insufficient intraoperative analgesia leads to earlier awakening [38], so we speculate that this characteristic may reflect the adequacy of intraoperative analgesia and may be a potential predictor of acute postoperative pain. This important finding also reminds anesthesiologists to pay attention to adequate intraoperative analgesia.
Currently, many previous studies reported that some demographic characteristics were associated with moderate to severe acute postoperative pain in patients undergoing orthopedic surgery, such as sex, age, and BMI [8, 15, 29]. In this study, after the univariate screening, age was entered into the multivariate logistic regression model and random forest model, younger age was identified as an independent risk factor. However, studies have found that factors such as age are associated with only statistically significant but not clinically significant associations with postoperative pain [39]. In this study, after the univariate screening, sex and BMI were not entered into the models. Therefore, female and high BMI were not included as independent risk factors in this study, which differed from the results of some other studies [8, 15, 29]. We suspect that female and high BMI were widely recognized as risk factors for postoperative pain, timely perioperative interventions, such as multimodal analgesia, were introduced. Therefore, the difference in sex and BMI between group P and group N was not significant.
For the risk factors identified in this study, orthopedic surgeons, anesthesiologists, and nurses need to focus on these factors in their daily work and effectively intervene to reduce acute postoperative pain. The real value of this model is that it can comprehensively evaluate the impact of many variables on outcomes and overcome the limitations of single risk factors. In real-world practice, outcome prediction can be achieved by entering the specific values of each variable included in the model, thereby helping doctors take timely intervention measures for high-risk patients. In the future, the prediction model can be packaged into applications with the help of computer science and other related knowledge, making clinical applications more convenient.
Some limitations of this study are worth mentioning. First, our study was retrospective. In our study, we included as many variables as possible, however, there were still a few characteristics that were not included, such as smoking and drinking habits. Therefore, some valuable factors may not be included. Further studies are needed to investigate whether adding these variables could improve the accuracy of the prediction model. Second, the datasets in our study were collected from a single center and were not large enough. Further studies with large multi-center samples are needed. Last, as a real-world clinical study, the postoperative management of these patients employed different methods for pain management, which varied depending on the patient’s condition, likely contributing somewhat to the variability in pain scores between individuals. Therefore, the risk factors screened out in this study need to be verified by rigorous RCT studies in the future.
Conclusions
This study addresses the high incidence of acute moderate to severe postoperative pain in orthopedic surgery patients under general anesthesia. We successfully developed a robust predictive model, utilizing the random forest algorithm, which demonstrated strong discriminatory power. The model holds the potential to aid healthcare professionals in early intervention and personalized pain management strategies for orthopedic surgery patients. In addition, this study identified some risk factors that have not been reported in the past and deserve attention in future clinical work.
Acknowledgements
We thank the staff of the Anesthesiology Department of Shanxi Bethune Hospital for their help in clinical data collection.
List of abbreviations
- ASA
the American Society of Anesthesiologists
- AUC
area under the receiver operating characteristic curve
- BMI
body mass index
- CI
confdence interval
- DCA
decision curve analysis
- EMR
electronic medical record
- Lasso
the least absolute shrinkage and selection operator
- OR
odds ratio
- PCIA
patient-controlled intravenous analgesia
- RCT
randomized controlled trial
- ROC
receiver operating characteristic
- SD
standard deviations
- TRIPOD
the transparent reporting of a multivariable prediction model for individual prognosis or diagnosis
- TSP
temporal summation of pain
- VAS
visual analogue scale
- WHO
World Health Organization
Authors’ contributions
Guarantor of integrity of the entire study: QY and PH; Study design: GS and GL; Data analysis: GS, QG, and QW; Manuscript preparation: GS; Manuscript editing: LW; Manuscript review: QY and SZ. All authors read and approved the final manuscript.
Funding
This study was supported by the Department of Education of Shanxi Province [2021Y364], and the Key R&D Projects of Shanxi Province [202102130501003] & [201903D311011].
Data Availability
The datasets used and analyzed during this current study are available from the corresponding author on reasonable request.
Declarations
Ethical approval
The study was approved and monitored by the Ethics Committee of Shanxi Bethune Hospital (Third Hospital of Shanxi Medical University).
Statement of human rights
All procedures in this study were conducted in accordance with the Ethics Committee of Shanxi Bethune Hospital (Third Hospital of Shanxi Medical University) approved protocols.
Statement of informed consent
Informed consent for patient information to be published in this article was not obtained because of the retrospective nature of the study and the patient’s identity information has been concealed. The requirement for informed consent was waived by the Ethics Committee of Shanxi Bethune Hospital.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Peifeng He, Email: hepeifeng2006@126.com.
Qi Yu, Email: yuqi@sxmu.edu.cn.
References
- 1.Buvanendran A, Fiala J, Patel KA, Golden AD, Moric M, Kroin JS. The incidence and severity of Postoperative Pain following Inpatient Surgery. Pain Med. 2015;16:2277–83. doi: 10.1111/pme.12751. [DOI] [PubMed] [Google Scholar]
- 2.Gramke HF, de Rijke JM, van Kleef M, Raps F, Kessels AG, Peters ML, et al. The prevalence of postoperative pain in a cross-sectional group of patients after day-case Surgery in a university hospital. Clin J Pain. 2007;23:543–8. doi: 10.1097/AJP.0b013e318074c970. [DOI] [PubMed] [Google Scholar]
- 3.Gan TJ. Poorly controlled postoperative pain: prevalence, consequences, and prevention. J Pain Res. 2017;10:2287–98. doi: 10.2147/JPR.S144066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fregoso G, Wang A, Tseng K, Wang J. Transition from Acute to Chronic Pain: evaluating risk for Chronic Postsurgical Pain. Pain Physician. 2019;22:479–88. [PubMed] [Google Scholar]
- 5.Scholten W, Nygren-Krug H, Zucker HA. The World Health Organization paves the way for action to free people from the shackles of pain. Anesth Analg. 2007;105:1–4. doi: 10.1213/01.ane.0000267542.72315.34. [DOI] [PubMed] [Google Scholar]
- 6.Pitchon DN, Dayan AC, Schwenk ES, Baratta JL, Viscusi ER. Updates on Multimodal Analgesia for Orthopedic Surgery. Anesthesiol Clin. 2018;36:361–73. doi: 10.1016/j.anclin.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Wang LH, Chen FY, Su ZC, Zhang J. Effect of General Anesthesia and combined spinal-epidural anesthesia on the postoperative pains, adverse reactions and Analgesia Effect of Senile patients with hip joint replacement. Syst Med. 2018;3(05):1–3. doi: 10.19368/j.cnki.2096-1782.2018.05.001. [DOI] [Google Scholar]
- 8.Liu SS, Buvanendran A, Rathmell JP, Sawhney M, Bae JJ, Moric M, et al. Predictors for moderate to severe acute postoperative pain after total hip and knee replacement. Int Orthop. 2012;36:2261–7. doi: 10.1007/s00264-012-1623-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biau G, Scornet E. A random forest guided tour. Test. 2016;25:197–227. doi: 10.1007/s11749-016-0481-7. [DOI] [Google Scholar]
- 10.Borges NC, Pereira LV, de Moura LA, Silva TC, Pedroso CF. Predictors for Moderate to Severe Acute Postoperative Pain after Cesarean Section. Pain Res Manag. 2016; 2016: 5783817. 10.1155/2016/5783817. [DOI] [PMC free article] [PubMed]
- 11.Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ. 2015;350:g7594. doi: 10.1136/bmj.g7594. [DOI] [PubMed] [Google Scholar]
- 12.Gerbershagen HJ, Rothaug J, Kalkman CJ, Meissner W. Determination of moderate-to-severe postoperative pain on the numeric rating scale: a cut-off point analysis applying four different methods. Br J Anaesth. 2011;107:619–26. doi: 10.1093/bja/aer195. [DOI] [PubMed] [Google Scholar]
- 13.Vasilopoulos T, Wardhan R, Rashidi P, Fillingim RB, Wallace MR, Crispen PL, et al. Patient and procedural determinants of Postoperative Pain trajectories. Anesthesiology. 2021;134:421–34. doi: 10.1097/ALN.0000000000003681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun K, Liu D, Chen J, Yu S, Bai Y, Chen C, et al. Moderate-severe postoperative pain in patients undergoing video-assisted thoracoscopic Surgery: a retrospective study. Sci Rep. 2020;10:795. doi: 10.1038/s41598-020-57620-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zaslansky R, Meissner W, Chapman CR. Pain after orthopaedic Surgery: differences in patient reported outcomes in the United States vs internationally. An observational study from the PAIN OUT dataset. Br J Anaesth. 2018;120:790–7. doi: 10.1016/j.bja.2017.11.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li GA-O, Walco JP, Mueller DA, Wanderer JP, Freundlich RE. Reliability of the ASA physical status classification system in predicting surgical morbidity: a retrospective analysis. J Med Syst. 2021;45:83. 10.1007/s10916-021-01758-z [DOI] [PMC free article] [PubMed]
- 17.Schreiber KL, Belfer I, Miaskowski C, Schumacher M, Stacey BR, Van De Ven T. AAAPT diagnostic criteria for acute pain following breast surgery. J Pain. 2020;21:294–305. 10.1016/j.jpain.2019.08.008 [DOI] [PMC free article] [PubMed]
- 18.Katz J, Seltzer Z. Transition from acute to chronic postsurgical pain: risk factors and protective factors. Expert Rev Neurother. 2009;9:723–44. 10.1586/ern.09.20 [DOI] [PubMed]
- 19.Chen YK, Boden KA, Schreiber KL. The role of regional anaesthesia and multimodal analgesia in the prevention of chronic postoperative pain: a narrative review. Anaesthesia. 2021;76(Suppl 1):8–17. 10.1111/anae.15256 [DOI] [PMC free article] [PubMed]
- 20.Weinstein EJ, Levene JL, Cohen MS, Andreae DA, Chao JY, Johnson M, et al. Local anaesthetics and regional anaesthesia versus conventional analgesia for preventing persistent postoperative pain in adults and children. Cochrane Database Syst Rev. 2018;6:Cd007105. 10.1002/14651858.CD007105 [DOI] [PMC free article] [PubMed]
- 21.Subedi A, Pokharel K, Sah BP, Chaudhary P. Association of preoperative pain catastrophizing with postoperative pain after lower limb trauma surgery. J Psychosom Res. 2021;149:110575. 10.1016/j.jpsychores.2021.110575 [DOI] [PubMed]
- 22.Carmel Neiderman NN, Frisch M, Oron Y, Handzel O, Abu Eta R, Muhanna N, et al. Preoperative anxiety levels and postoperative middle ear surgery pain levels. Otol Neurotol. 2023;44:e235–40. 10.1097/mao.0000000000003837 [DOI] [PubMed]
- 23.Dasinger EA, Graham LA, Wahl TS, Richman JS, Baker SJ, Hawn MT, et al. Preoperative opioid use and postoperative pain associated with surgical readmissions. Am J Surg. 2019;218:828–35. 10.1016/j.amjsurg.2019.02.033 [DOI] [PubMed]
- 24.de Hoogd S, Ahlers SJ, van Dongen EP, van de Garde EM, Hamilton-Ter Brake TA, Dahan A, et al. Is intraoperative remifentanil associated with acute or chronic postoperative pain after prolonged surgery? An update of the literature. Clin J Pain. 2016;32:726–35. 10.1097/ajp.0000000000000317 [DOI] [PubMed]
- 25.de Hoogd S, Ahlers S, van Dongen EPA, van de Garde EMW, Daeter EJ, Dahan A, et al. Randomized controlled trial on the influence of intraoperative remifentanil versus fentanyl on acute and chronic pain after cardiac surgery. Pain Pract. 2018;18:443–51. 10.1111/papr.12615 [DOI] [PubMed]
- 26.Apfelbaum JL, Chen C, Mehta SS, Gan TJ. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg. 2003;97:534–40. doi: 10.1213/01.ANE.0000068822.10113.9E. [DOI] [PubMed] [Google Scholar]
- 27.Baca Q, Marti F, Poblete B, Gaudilliere B, Aghaeepour N, Angst MS. Predicting Acute Pain after Surgery: a multivariate analysis. Ann Surg. 2021;273:289–98. doi: 10.1097/SLA.0000000000003400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung F, Ritchie E, Su J. Postoperative pain in ambulatory Surgery. Anesth Analg. 1997;85:808–16. doi: 10.1097/00000539-199710000-00017. [DOI] [PubMed] [Google Scholar]
- 29.Abrecht CR, Cornelius M, Wu A, Jamison RN, Janfaza D, Urman RD, et al. Prediction of Pain and Opioid utilization in the Perioperative period in patients undergoing primary knee arthroplasty: psychophysical and psychosocial factors. Pain Med. 2019;20:161–71. doi: 10.1093/pm/pny020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hartwig M, Allvin R, Backstrom R, Stenberg E. Factors Associated with increased experience of Postoperative Pain after laparoscopic gastric bypass Surgery. Obes Surg. 2017;27:1854–8. doi: 10.1007/s11695-017-2570-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhong M, Zhang H, Yu C, Jiang J, Duan X. Application of machine learning in predicting the risk of postpartum depression: a systematic review. J Affect Disord. 2022;318:364–79. doi: 10.1016/j.jad.2022.08.070. [DOI] [PubMed] [Google Scholar]
- 32.Xing F, Luo R, Liu M, Zhou Z, Xiang Z, Duan XA. New Random Forest Algorithm-based prediction model of post-operative mortality in geriatric patients with hip fractures. Front Med (Lausanne) 2022;9:829977. doi: 10.3389/fmed.2022.829977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Binder M, Pfisterer F, Lang M, Schneider L, Kotthoff L. And Bischl B. mlr3pipelines—Flexible machine learning pipelines in r. J Mach Learn Res. 2021;22:8314–20. [Google Scholar]
- 34.Staud R, Cannon RC, Mauderli AP, Robinson ME, Price DD, Vierck CJ., Jr Temporal summation of pain from mechanical stimulation of muscle tissue in normal controls and subjects with fibromyalgia syndrome. Pain. 2003;102:87–95. doi: 10.1016/s0304-3959(02)00344-5. [DOI] [PubMed] [Google Scholar]
- 35.Kumar N, Yadav C, Singh S, Kumar A, Vaithlingam A, Yadav S. Evaluation of pain in bilateral total knee replacement with and without tourniquet; a prospective randomized control trial. J Clin Orthop Trauma. 2015;6:85–8. doi: 10.1016/j.jcot.2015.01.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li S, Li P, Wang R, Li H. Different interventions for preventing postoperative catheter-related bladder discomfort: a systematic review and meta-analysis. Eur J Clin Pharmacol. 2022;78:897–906. doi: 10.1007/s00228-021-03251-5. [DOI] [PubMed] [Google Scholar]
- 37.Yang MMH, Hartley RL, Leung AA, Ronksley PE, Jette N, Casha S, et al. Preoperative predictors of poor acute postoperative pain control: a systematic review and meta-analysis. BMJ Open. 2019;9:e025091. doi: 10.1136/bmjopen-2018-025091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meijer F, Honing M, Roor T, Toet S, Calis P, Olofsen E, et al. Reduced postoperative pain using Nociception Level-guided fentanyl dosing during sevoflurane anaesthesia: a randomised controlled trial. Br J Anaesth. 2020;125:1070–8. doi: 10.1016/j.bja.2020.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kanaan SF, Melton BL, Waitman LR, Simpson MH, Sharma NK. The effect of age and gender on acute postoperative pain and function following lumbar spine surgeries. Physiother Res Int. 2021;26:e1888. doi: 10.1002/pri.1888. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during this current study are available from the corresponding author on reasonable request.