Abstract
Background
Intention-to-treat analyses of POINCARE-2 trial led to inconclusive results regarding the effect of a conservative fluid balance strategy on mortality in critically ill patients. The present as-treated analysis aimed to assess the effectiveness of actual exposure to POINCARE-2 strategy on 60-day mortality in critically ill patients.
Methods
POINCARE‑2 was a stepped wedge randomized controlled trial. Eligible patients were ≥ 18 years old, under mechanical ventilation and had an expected length of stay in ICU > 24 h. POINCARE-2 strategy consisted of daily weighing over 14 days, and subsequent restriction of fluid intake, administration of diuretics, and/or ultrafiltration. We computed a score of exposure to the strategy based on deviations from the strategy algorithm. We considered patients with a score ≥ 75 as exposed to the strategy. We used logistic regression adjusted for confounders (ALR) or for an instrumental variable (IVLR). We handled missing data using multiple imputations.
Results
A total of 1361 patients were included. Overall, 24.8% of patients in the control group and 69.4% of patients in the strategy group had a score of exposure ≥ 75. Exposure to the POINCARE-2 strategy was not associated with 60-day all-cause mortality (ALR: OR 1.2, 95% CI 0.85–1.55; IVLR: OR 1.0, 95% CI 0.76–1.33).
Conclusion
Actual exposure to POINCARE-2 conservative strategy was not associated with reduced mortality in critically ill patients.
Trial registration POINCARE-2 trial is registered at ClinicalTrials.gov (NCT02765009). Registered 29 April 2016.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-023-04701-5.
Keywords: Critical care, Water–electrolyte balance, Clinical trial, Complex intervention, Instrumental variable
Background
Among critically ill patients in intensive care units (ICU), early and aggressive fluid resuscitation is indicated to tackle hemodynamic instability [1, 2]. However, after this early critical phase, excessive fluid intake can be deleterious by increasing intravascular pressure and vascular permeability, eventually leading to tissue edema [3].
Higher mortality was reported in critically ill patients with a positive fluid balance in various clinical settings, such as septic shock, acute respiratory distress syndrome (ARDS), cancers, and post-operative settings [4–7]. A meta-analysis of 11 randomized controlled trials included 2051 adults and children and showed that exposure to a strategy of conservative fluid management was associated with an increased number of mechanical ventilator-free days (MVFDs) and a reduced ICU length of stay, as compared to a liberal strategy or to standard of care. However, its effect on mortality remained uncertain [8].
POINCARE-2 stepped-wedge randomized controlled trial aimed to assess the effectiveness of a conservative strategy on 60-day all-cause mortality in a broad population of critically ill patients [9]. Intention-to-treat (ITT) analysis of POINCARE-2 trial led to inconclusive results, with a 60-day mortality of 30.5% (95% confidence interval [CI] 26.2–34.8) in the intervention group vs. 33.9% (95% CI 29.6–38.2) in the control group (p = 0.26) [10]. The strategy under scrutiny consisted of daily weighing over 14 days after admission to the ICU, and subsequent restriction of fluid intake, administration of diuretics, and/or ultrafiltration in case of renal replacement therapy (RRT) [9]. Accordingly, patients from the control group were likely to receive some of the strategy components as part of standard of care, despite the stepped-wedge design of the trial, which would result in so-called (but unavoidable) contamination and bias the results of ITT analyses towards the null [11]. Consequently, relying on ITT analyses only might lead to discredit a strategy that might otherwise prove effective if actual exposure to this strategy was taken into account.
The present as-treated analysis aimed to assess the effectiveness of actual exposure to POINCARE-2 strategy on 60-day mortality in critically ill patients.
Methods
Design and setting
The POids INtensive CARE 2 (POINCARE-2) trial was a stepped wedge cluster randomized controlled trial implemented in 12 French ICUs [9]. This trial aimed to assess the effectiveness of a fluid-balance control strategy on 60-day all-cause mortality in critically ill patients. As recommended [12], the main analysis was conducted on an intention-to-treat basis. However, to assess the actual magnitude of the strategy effect, as-treated analyses were also planned in the trial protocol as secondary analyses [9].
Critically ill patients who were admitted to one of the 12 recruiting ICUs were allocated either to the control group (and received standard of care during the control period) or to the strategy group (and received the POINCARE-2 strategy during the intervention period). They were followed up for 1 year.
Additional file 1: Fig. S1 describes the POINCARE-2 strategy. Briefly, this strategy relied on daily weighing from Day2 to Day14 after admission and subsequent daily decision to restrict salt and water for all infusion volumes (i.e., IV treatments and infusions allowing venous permeability), to administer diuretics and/or albumin, and/or to use ultrafiltration, in case of excessive weight gain.
Population and sampling
Eligible patients were ≥ 18 years old, under mechanical ventilation (through endotracheal intubation), admitted to one of the 12 participating ICUs between 48 and 72 h prior to inclusion, and with an expected length of stay after inclusion of > 24 h.
Main exclusion criteria were clinical condition or unavailability of bedside scale impeding weight assessment, multiple trauma, history of ICU stay > 24 h immediately preceding the index ICU admission, pregnancy, expected withdrawal of life-sustaining therapy < 7 days after admission, patient refusal to personal data collection (and/or use), history of ICU stay in one of the 12 recruiting ICUs during the study period, and patients under guardianship.
Assessment of exposure to the strategy
To assess exposure of patients to the strategy under scrutiny, we developed a dedicated score. This score was based on the amount of deviation from the algorithm on which the strategy relied (Additional file 1: Fig. S1), during the time over which it was supposed to be delivered (i.e., from Day2 to Day14 or the end of ICU stay which ever came first), for each patient, regardless of their group (strategy or control).
Additional file 1: Fig. S2 presents the scoring method. For each day of hospitalization in the recruiting ICU between Day2 and Day14 after admission, we incremented a counter of deviation from the algorithm at each step of the strategy, resulting in a crude score of deviation. To minimize the impact of missing data, we only considered the core components of the strategy (i.e., daily weighing, subsequent prescription of water and salt restriction, and administration of diuretics or ultrafiltration). We then normalized the crude score by dividing it by the length of ICU stay (or by 14 when length of stay was > 14 days) and transformed it to make it vary from 0 (i.e., POINCARE-2 strategy not administered at all) to 100 (i.e., POINCARE-2 strategy optimally administered as planned in the algorithm). We finally weighed it by the percentage of patient weighing performed between Day2 and Day14. To assess the validity of this score, we submitted 10 randomly selected patient files to 11 intensive care specialists. We stratified the random sampling on quintiles of score of exposure to the strategy, and randomly selected two patients per quintile. Each expert ranked the 10 patients according to his/her assessment of the compliance with the strategy, from 1 (best compliance with the strategy) to 10 (poorer compliance with the strategy). Experts were blinded to patient group, recruiting ICU and hospital, and to the score of exposure to the strategy. We found an agreement between experts for a computed score above 74.4. Accordingly, we further considered patients as actually exposed to the strategy if their score was ≥ 75, and as unexposed otherwise.
Outcomes
The main outcome was vital status at Day 60 (alive vs deceased). Secondary outcomes included mechanical ventilator-free days (MVFDs) and vasopressor-free days (VFDs), defined as the cumulative number of days alive with no mechanical ventilation (or no prescription of vasopressor, respectively) between Day0 and Day28; renal replacement therapy-free days (RRTFDs), defined as the cumulative number of days alive with no renal replacement therapy between Day0 and Day60; occurrence of at least one unexpected harmful event (arterial hypotension, i.e. arterial systolic pressure < 90 mmHg, between Day2 and Day14; hypernatremia, i.e. serum sodium level > 155 mmol/L, between Day2 and Day14; hypokalemia, i.e. serum potassium level < 2.8 mmol/L, between Day2 and Day14; or acute ischemic events, i.e. myocardial infarction and/or patent mesenteric ischemia, between Day3 and discharge); and renal damage, defined by a worsening in the RIFLE criteria between Day3 and Day14, as compared to the higher RIFLE criteria during the first two days of hospitalization [13].
Statistical analysis
Descriptive analyses
We first described the distribution of the score of exposure to the strategy in our sample, overall and by group. We then described patients’ characteristics at admission, overall, and stratified on (1) the quartile of score of exposure to the strategy, and (2) both the ICU and the group, using mean (standard deviation [SD]) or median (interquartile range [IQR]) for continuous variables, and counts (percentages) for categorical variables.
Then, we described survival time, vital status at Day 60, and total number (%) of events for each secondary outcome, overall and stratified on (1) the quartile of the score of exposure to the strategy, and (2) both the ICU and the group.
Main outcome
To assess the effect of actual exposure to the POINCARE-2 strategy on vital status at Day60, two different analyses were carried out: (1) a “naive” logistic regression model adjusted for potential confounders (model 1); and (2) a logistic regression model with the addition of an instrumental variable using the two-stage residual inclusion (2SRI) method [14] (model 2). Instrumental variable are useful to take into account both measured and unmeasured confounders [15]. To account for the stepped-wedge design of the trial, we adjusted the baseline model (model 0) for ICU and period.
To identify potential confounders in the association between exposure to the strategy and outcomes, we compared patients’ characteristics at admission according to the exposure to the strategy, using logistic regression models adjusted for both ICU and group. Patients’ characteristics that were associated with both the outcome of interest and the score of exposure to POINCARE-2 strategy with a p value of < 0.2 were further defined as potential confounders, and entered as dependent variables in adjusted analyses.
Hypotheses of log-linearity and absence of multiple colinearity were verified before implementation of the regression models, using the Hosmer–Lemeshow test [16] and calculation of (Generalized) Variance Inflation Factors, respectively [17].
In order to meet the assumptions of relevance, independence, and exclusion restriction, we chose the group (strategy vs. control) as an instrumental variable in model 2. We verified the relevance condition using a logistic regression explaining exposure to the strategy and the corresponding Wald test using F-value that was considered satisfactory above 10. We used a robust ridge regression estimation to account for the strong multiple collinearity between ICU, period, and group, inherent to the stepped-wedge design. We handled standard errors using bootstrap iterations.
Finally, we used the Durbin-Wu-Hausman [18] test to assess the relevance of the instrumental variable method, as compared with the naive logistic regression model.
We handled missing data using multiple imputation (MI), with the number of imputation datasets defined according to the rule of the percentage of missing observations [19]. We used the MI Boot method to combine bootstrap and multiple imputations [20].
Subgroup analyses
We added an interaction term to the naive logistic regression model (model 1) to test for a possible subgroup effect of the POINCARE-2 strategy according to the main cause of admission (septic shock, Acute Respiratory Distress Syndrome (ADRS), Central Nervous System (CNS) injury, or other).
Sensitivity analyses
We conducted sensitivity analyses for the main outcome in the subsample of complete cases.
Secondary outcomes
We used zero-inflated negative binomial (or zero-inflated Poisson) mixed models to assess the effect of exposure to the POINCARE-2 strategy on the cumulative number of MVFDs and VFDs. Logistic regression models adjusted for potential confounders were computed to examine the effect of the strategy on the occurrence of at least one unexpected harmful event or renal damage. We conducted all secondary analyses on complete cases only. We excluded from the RRTFDs analysis patients who were already at the worst stage of the RIFLE classification (i.e., “end stage renal disease”) at Day2.
We used SAS 9.4 (SAS Institute, Inc) and R v4.0.3, with a level of significance set at 0.05.
Ethics
Because the POINCARE-2 strategy focused on health care organization, written informed consent was waived in accordance with French law (Bill number 2012–300 on March 5, 2012 about research involving humans). Comité de Protection des Personnes Est III, Grand-Est, North-East France, has reviewed and approved POINCARE-2 trial (ID-RCB: 2015-A00662-47). The trial is registered at ClinicalTrials.gov (NCT 02,765,009).
Results
Patients’ characteristics at admission
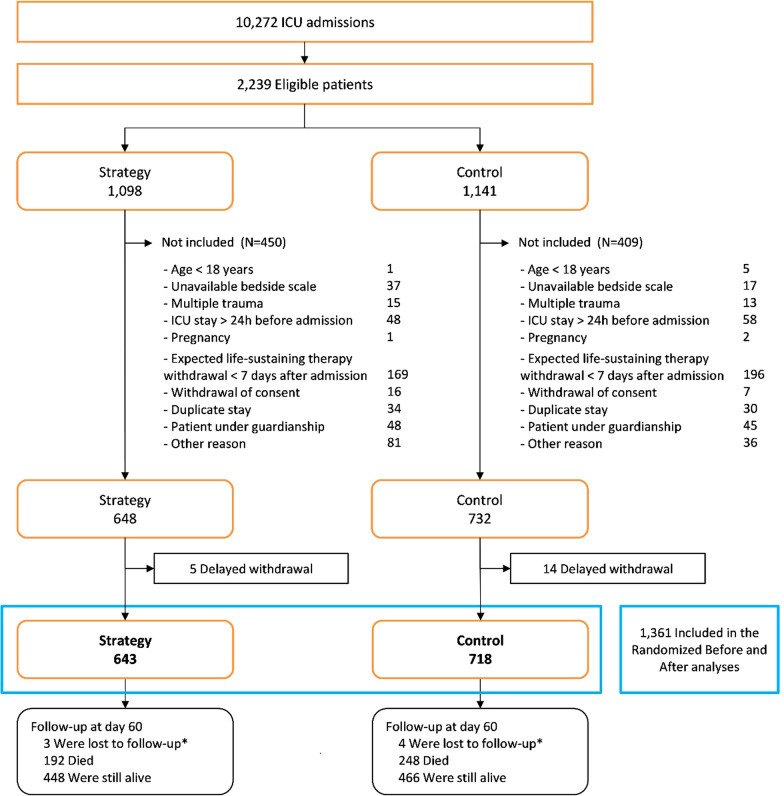
A total of 1361 patients were included in the present study, i.e. 718 in the control group and 643 in the strategy group (Fig. 1) [10]. Thirty patients were already at the worst stage of the RIFLE classification on Day2 and were further excluded for the RRTFDs analysis. We described patients’ characteristics at admission in Additional file 1: Tables S1 and S2. Mean (SD) age was 64.4 (14.6) years. Body mass index (BMI) and main cause of admission varied across ICUs, as did the presence of coexisting conditions such as heart failure or chronic kidney disease (Additional file 1: Table S2). Complete cases represented a subsample of 977 patients. Description of missing values is presented in Additional file 1: Table S3.
Fig. 1.
Flowchart of critically ill patients recruited and followed up in the POINCARE-2 trial
Exposure to the POINCARE-2 strategy
Mean (SD) score of exposure to the strategy was 80.5 (19.3) in the strategy group vs. 55.2 (24.6) in the control group (overall, 67.1 (25.6)). Overall, 446 (69.4%) patients in the strategy group vs. 178 patients (24.8%) in the control group, had a score of exposure to the strategy ≥ 75 and were further considered as exposed to the strategy. Accordingly, despite higher scores of exposure, implementation of the strategy was not optimal in the strategy group, and a significant proportion of patients from the control group received at least some part of the strategy.
Implementation of the various components of the POINCARE-2 strategy are described in Table 1. The average daily fluid intake from Day2 to Day14 did not vary with exposure to the strategy (p = 0.249). Total dose of diuretics from Day0 to Day14 differed between groups, with the highest dose in the [75;100] class of exposure to the strategy.
Table 1.
Characteristics of patients’ stays and implementation of the POINCARE-2 strategy
| Score of exposure to the strategy | Total N = 1361 |
Tests | |||||
|---|---|---|---|---|---|---|---|
| [0;25[ | [25;50[ | [50;75[ | [75;100] | ||||
| N = 74 | N = 288 | N = 375 | N = 624 | ||||
| Duration of stays in icu (days) | Kruskal–Wallis: 0.020 | ||||||
| N | 73 | 284 | 369 | 610 | 1336 | ||
| Mean (std) | 14.1 (10.84) | 15.0 (10.48) | 13.5 (9.83) | 15.9 (11.76) | 15.0 (10.98) | ||
| Min–median–max | 2–11.0–58 | 4–12.0–58 | 3–11.0–56 | 2–12.0–60 | 2–11.0–60 | ||
| 2 | 1 (1.4%) | 0 (0.0%) | 0 (0.0%) | 1 (0.2%) | 2 (0.1%) | ||
| 3 | 7 (9.5%) | 0 (0.0%) | 6 (1.6%) | 7 (1.1%) | 20 (1.5%) | ||
| 4 | 5 (6.8%) | 22 (7.6%) | 23 (6.1%) | 15 (2.4%) | 65 (4.8%) | ||
| 5 | 0 (0.0%) | 18 (6.3%) | 19 (5.1%) | 50 (8.0%) | 87 (6.4%) | ||
| 6 | 7 (9.5%) | 20 (6.9%) | 22 (5.9%) | 42 (6.7%) | 91 (6.7%) | ||
| 7 | 3 (4.1%) | 10 (3.5%) | 42 (11.2%) | 35 (5.6%) | 90 (6.6%) | ||
| 8 | 5 (6.8%) | 13 (4.5%) | 30 (8.0%) | 32 (5.1%) | 80 (5.9%) | ||
| 9 | 2 (2.7%) | 16 (5.6%) | 19 (5.1%) | 44 (7.1%) | 81 (6.0%) | ||
| 10 | 5 (6.8%) | 24 (8.3%) | 23 (6.1%) | 34 (5.4%) | 86 (6.3%) | ||
| 11 | 2 (2.7%) | 18 (6.3%) | 22 (5.9%) | 37 (5.9%) | 79 (5.8%) | ||
| 12 | 3 (4.1%) | 10 (3.5%) | 16 (4.3%) | 23 (3.7%) | 52 (3.8%) | ||
| 13 | 3 (4.1%) | 10 (3.5%) | 22 (5.9%) | 28 (4.5%) | 63 (4.6%) | ||
| 14 or more | 31 (41.9%) | 127 (44.1%) | 131 (34.9%) | 276 (44.2%) | 565 (41.5%) | ||
| Extra-renal purification during d0-14 | 8 (10.8%) | 37 (12.8%) | 64 (17.1%) | 180 (28.8%) | 289 (21.2%) | Chi-2: < 0.001 | |
| Total ultrafiltration from day 0 to day 14 (ml) | |||||||
| N | 5 | 29 | 54 | 155 | 243 | ||
| Mean (std) | 941.8 (549.09) | 5900.4 (7994.25) | 7621.5 (7286.60) | 12098.5 (10496.76) | 10134.4 (9844.67) | ||
| Min–median–max | 250–1000.0–1740 | 120–4200.0–39976 | 28–4866.0–30033 | 50–9824.0–56181 | 28–7156.0–56181 | ||
| Average daily fluid intake from day 0 to day 14 (ml) | F-test: 0.249 | ||||||
| N | 74 | 288 | 375 | 624 | 1361 | ||
| Mean (std) | 2297.7 (723.21) | 2469.1 (871.77) | 2478.4 (858.66) | 2404.2 (860.61) | 2432.6 (855.99) | ||
| Min–median–max | 254–2189.9–4487 | 377–2345.1–7233 | 239–2420.3–5411 | 469–2292.4–5913 | 239–2328.3–7233 | ||
| Total dose of albumin 20% from day 0 to day 14 (ml) | Kruskal–Wallis: 0.016 | ||||||
| N | 12 | 48 | 51 | 91 | 202 | ||
| Mean (std) | 583.3 (404.15) | 471.9 (372.59) | 672.5 (643.14) | 818.7 (803.73) | 685.4 (673.15) | ||
| Min–median–max | 100–450.0–1500 | 100–300.0–1500 | 100–400.0–3700 | 100–600.0–5100 | 100–500.0–5100 | ||
| Total dose of diuretics from day 0 to day 14 (mg) | Kruskal–Wallis: < 0.001 | ||||||
| N | 45 | 160 | 263 | 485 | 953 | ||
| Mean (std) | 408.4 (1208.74) | 326.0 (515.01) | 665.6 (1605.31) | 770.7 (1414.45) | 649.9 (1366.05) | ||
| Min–median–max | 10–120.0–8140 | 20–140.0–3500 | 10–210.0–12636 | 20–300.0–13432 | 10–220.0–13432 | ||
| Contraindications | 65 (87.8%) | 252 (87.5%) | 349 (93.1%) | 590 (94.6%) | 1256 (92.3%) | Chi-2: 0.001 | |
| At least one fluid vascular loading (over d2-d14) | 44 (59.5%) | 171 (59.4%) | 250 (66.7%) | 392 (62.8%) | 857 (63.0%) | Chi-2: 0.242 | |
| Arterial hypotension (at least one episode over d2-d14) | 45 (60.8%) | 204 (70.8%) | 287 (76.5%) | 488 (78.2%) | 1024 (75.2%) | Chi-2: 0.002 | |
| At least one episode of hypernatremia > 155 mmol/l (over d2-d14) | Fisher's Exact: 0.024 | ||||||
| Missing | 1 | 0 | 2 | 0 | 3 | ||
| No | 67 (90.5%) | 284 (98.6%) | 362 (96.5%) | 600 (96.2%) | 1313 (96.5%) | ||
| Yes | 6 (8.1%) | 4 (1.4%) | 11 (2.9%) | 24 (3.8%) | 45 (3.3%) | ||
| At least one episode of hypokalemia < 2.8 mmol/l (over d2-d14) | Chi-2: 0.715 | ||||||
| Missing | 1 | 0 | 1 | 0 | 2 | ||
| No | 67 (90.5%) | 264 (91.7%) | 351 (93.6%) | 576 (92.3%) | 1258 (92.4%) | ||
| Yes | 6 (8.1%) | 24 (8.3%) | 23 (6.1%) | 48 (7.7%) | 101 (7.4%) | ||
| At least one episode of non-normal rifle (over d2-d14) | 51 (68.9%) | 173 (60.1%) | 266 (70.9%) | 503 (80.6%) | 993 (73.0%) | Chi-2: < 0.001 | |
| Percentage of weighing done from day 2 to day 14 | Kruskal–Wallis: < 0.001 | ||||||
| N | 74 | 288 | 375 | 624 | 1361 | ||
| Mean (std) | 16.4 (16.16) | 47.3 (17.16) | 70.0 (13.90) | 92.9 (8.35) | 72.8 (25.69) | ||
| Min–median–max | 0–16.0–92 | 25–44.4–100 | 50–66.7–100 | 75–100.0–100 | 0–77.8–100 | ||
Patients exposed to POINCARE-2 strategy were more likely to be older (p < 0.001), to have a higher McCabe score at admission (p = 0.016), and to have a cancer (p = 0.031) or a chronic kidney disease under RRT (p = 0.008) than patients less exposed to the strategy (Additional file 1: Table S1).
Mortality and safety during follow-up
Vital status at Day 60 and safety outcomes are described in Table 2. During an overall median (min–max) follow-up of 11 (2–60) days, a total of 440 patients (32.3%) died, i.e. 39.2% (28.8%, 30.1%, and 34.5%) of patients with a score of exposure to POINCARE-2 strategy comprised between [0–25[([25–50[, [50–75[, and [75–100], respectively). Seven patients were lost to follow-up.
Table 2.
Occurrence of primary and secondary outcomes
| Score of exposure to the strategy | Total N = 1361 |
|||||
|---|---|---|---|---|---|---|
| [0;25[ | [25;50[ | [50;75[ | [75;100] | |||
| N = 74 | N = 288 | N = 375 | N = 624 | |||
| Primary outcome | ||||||
| Vital status at day 60 | ||||||
| Lost of follow-up | 0 | 1 | 4 | 2 | 7 | |
| Alive | 45 (60.8%) | 204 (70.8%) | 258 (68.8%) | 407 (65.2%) | 914 (67.2%) | |
| Deceased | 29 (39.2%) | 83 (28.8%) | 113 (30.1%) | 215 (34.5%) | 440 (32.3%) | |
| Survival time (days)—truncated at Day 60 | ||||||
| N | 74 | 288 | 375 | 624 | 1361 | |
| Mean (std) | 43.5 (22.28) | 48.3 (19.85) | 47.0 (20.82) | 46.2 (20.36) | 46.7 (20.50) | |
| Min–median–max | 3–60.0–60 | 4–60.0–60 | 3–60.0–60 | 2–60.0–60 | 2–60.0–60 | |
| Secondary outcomes | ||||||
| Effectiveness | ||||||
| Number of days without mechanical ventilation during Day0 – Day28 | ||||||
| N | 74 | 288 | 375 | 624 | 1361 | |
| Mean (std) | 8.4 (8.90) | 10.2 (8.26) | 9.9 (8.61) | 9.4 (8.54) | 9.7 (8.52) | |
| Min–median–max | 0–5.0–26 | 0–10.0–26 | 0–9.0–28 | 0–8.0–27 | 0–9.0–28 | |
| Number of days without vasopressor during Day0 – Day28 | ||||||
| N | 74 | 288 | 375 | 624 | 1361 | |
| Mean (std) | 15.0 (10.07) | 17.0 (9.59) | 14.8 (9.48) | 15.1 (9.46) | 15.4 (9.55) | |
| Min–median–max | 0–14.5–28 | 0–20.0–28 | 0–14.0–28 | 0–16.0–28 | 0–16.0–28 | |
| Number of days without RRT (#) during Day0 – Day60 | ||||||
| N | 74 | 288 | 375 | 624 | 1361 | |
| Mean (std) | 27.3 (20.50) | 30.0 (19.90) | 26.7 (19.57) | 27.2 (20.31) | 27.7 (20.05) | |
| Min–median–max | 0–21.5–60 | 0–27.0–60 | 0–21.0–60 | 0–21.5–60 | 0–22.0–60 | |
| Vital status at Day 28 | ||||||
| Missing | 0 | 0 | 1 | 2 | 3 | |
| Alive | 51 (68.9%) | 225 (78.1%) | 279 (74.4%) | 458 (73.4%) | 1013 (74.4%) | |
| Deceased | 23 (31.1%) | 63 (21.9%) | 95 (25.3%) | 164 (26.3%) | 345 (25.3%) | |
| In-hospital mortality at Day 60 | ||||||
| Missing | 1 | 0 | 0 | 3 | 4 | |
| No | 46 (62.2%) | 209 (72.6%) | 266 (70.9%) | 414 (66.3%) | 935 (68.7%) | |
| Yes | 27 (36.5%) | 79 (27.4%) | 109 (29.1%) | 207 (33.2%) | 422 (31.0%) | |
| Safety outcomes | 47 (63.5%) | 207 (71.9%) | 293 (78.1%) | 495 (79.3%) | 1042 (76.6%) | |
| Arterial hypotension (at least one episode over D2-D14) | 45 (60.8%) | 204 (70.8%) | 287 (76.5%) | 488 (78.2%) | 1024 (75.2%) | |
| At least one episode of hypernatremia > 155 mmol/L (over D2-D14) | ||||||
| Missing | 1 | 0 | 2 | 0 | 3 | |
| No | 67 (90.5%) | 284 (98.6%) | 362 (96.5%) | 600 (96.2%) | 1313 (96.5%) | |
| Yes | 6 (8.1%) | 4 (1.4%) | 11 (2.9%) | 24 (3.8%) | 45 (3.3%) | |
| At least one episode of hypokalemia < 2.8 mmol/L (over D2-D14) | ||||||
| Missing | 1 | 0 | 1 | 0 | 2 | |
| No | 67 (90.5%) | 264 (91.7%) | 351 (93.6%) | 576 (92.3%) | 1258 (92.4%) | |
| Yes | 6 (8.1%) | 24 (8.3%) | 23 (6.1%) | 48 (7.7%) | 101 (7.4%) | |
| Mesenteric ischemia or myocardial infarction | 0 (0.0%) | 0 (0.0%) | 7 (1.9%) | 7 (1.1%) | 14 (1.0%) | |
| Mesenteric ischemia | 0 (0.0%) | 0 (0.0%) | 2 (0.5%) | 3 (0.5%) | 5 | (0.4%) |
| Myocardial infarction | 0 (0.0%) | 0 (0.0%) | 5 (1.3%) | 4 (0.6%) | 9 (0.7%) | |
| Renal damage over D3-D14 | ||||||
| N/A | 0 | 2 | 6 | 22 | 30 | |
| No RIFLE after D2 | 4 | 0 | 1 | 3 | 8 | |
| No D1-D2 RIFLE | 0 | 1 | 1 | 4 | 6 | |
| No | 58 (78.4%) | 230 (79.9%) | 298 (79.5%) | 467 (74.8%) | 1053 (77.4%) | |
| Yes | 12 (16.2%) | 55 (19.1%) | 69 (18.4%) | 128 (20.5%) | 264 (19.4%) | |
(#) RRT: Renal-replacement therapy
Forty-five (3.3%) patients presented at least one episode of hypernatremia > 155 mmol/L, and 14 (1.0%) had an episode of mesenteric or myocardial infarction during follow-up.
Effect of the exposure to the POINCARE-2 strategy on 60-day mortality
Results from the naive logistic regression
Results for the main outcome analyses are presented in Table 3 and Fig. 2. As compared with patients considered less exposed to the POINCARE-2 strategy, patients with a score of exposure ≥ 75 had a significantly higher mortality at Day60 (ORmodel0 1.47, 95% CI 1.12–1.93, p = 0.005). Analyses on the complete cases subsample led to similar results (ORmodel0 1.43, 95% CI 1.04–1.98, p = 0.029). Adjustment for potential confounders (i.e., age, presence or absence of heart failure or chronic kidney disease under RRT, McCabe Score at admission, SAPS II, main cause of admission, SOFA, and RRT at Day0) led to similar yet insignificant results (MI dataset: ORmodel1 1.15, 95% CI 0.85–1.55, p = 0.35; complete case dataset: ORmodel1 1.12, 95% CI 0.78–1.60, p = 0.547).
Table 3.
Effect of the exposure to the POINCARE-2 strategy on vital status at Day60
| Complete casesa | Multiple imputationb | |||||
|---|---|---|---|---|---|---|
| OR (Score > 75%) | 95% CI | p | OR (Score > 75%) | 95% CI | p | |
| Unadjustedc | 1.43 | 1.04–1.98 | 0.029 | 1.47 | 1.12–1.93 | 0.005 |
| Adjusted for measured confoundersd | 1.12 | 0.78–1.60 | 0.547 | 1.15 | 0.85–1.55 | 0.356 |
| Adjusted for measured and unmeasured confounderse | 0.97 | 0.70–1.34 | 0.843 | 1.01 | 0.76–1.33 | 0.956 |
Models:
cAdjusted only on class variables: Center and Secular time
dadjusted on Center, Secular time, Age (years), Heart failure, Chronic kidney disease under RRT, McCabe Score at admission, SAPS II (*), Main cause of admission, SOFA ( ~), RRT (#) at day 0
e2SRI instrumental variable model adjusted on Center, Secular time, Age (years), Cirrhosis, Cancer, Immunodeficiency, Heart failure, Chronic respiratory failure, Chronic kidney disease under RRT, McCabe Score at admission, SAPS II (*), Main cause of admission, BMI (IOTF classification), Weight (kg), Serum bicarbonate (mmol/L), Serum potassium (mmol/L), SOFA ( ~), Serum creatinine (mg/dL), PaO2/FiO2 (mmHg), RRT (#) at day 0
Analyses:
aObservations with missing data are excluded (n = 977)
bNumber of imputation datasets = 4 (n = 1361)
*SAPS: Simplified Acute Physiology Score
~ SOFA: Sequential Organ Failure Assessment
#RRT: Renal-replacement therapy
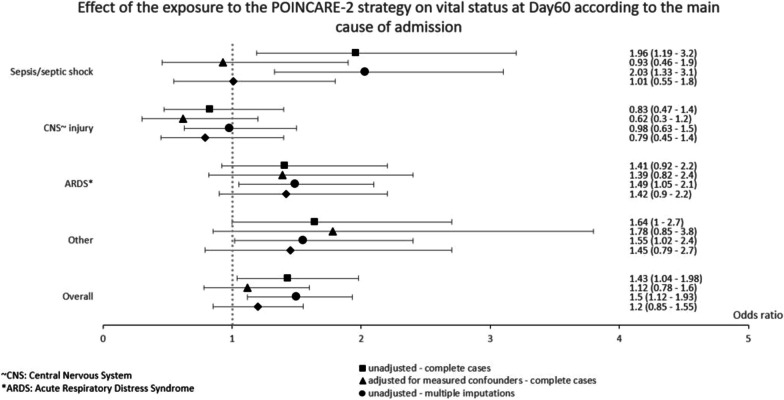
Fig. 2.
Effect of the exposure to the POINCARE-2 strategy on vital status at Day60 according to the main cause of admission
Results from instrumental variable analysis
Exposure to the POINCARE-2 strategy was not associated with 60-day mortality both in the MI dataset (ORmodel2 1.01, 95% CI 0.76–1.33, p = 0.956) and in the complete case dataset (ORmodel2 0.97, 95% CI 0.70–1.34, p = 0.843).
Results according to cause of admission: subgroup analyses
Results of the subgroup analyses are presented in Table 4. They showed that exposure to the strategy was associated with an increased 60-day mortality in case of ARDS, sepsis or septic shock, or other causes of admission. However, these results were no longer statistically significant when adjusting for potential confounders. Analyses on both MI and complete case datasets led to similar results.
Table 4.
Effect of the exposure to the POINCARE-2 strategy on vital status at Day60 according to the main cause of admission
| Complete cases dataset | Imputed dataset | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bivariate | Multivariate | Bivariate | Multivariate | |||||||||
| OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | |
| ARDS* | 1.41 | 0.92–2.2 | 0.113 | 1.39 | 0.82–2.4 | 0.226 | 1.49 | 1.05–2.1 | 0.027 | 1.42 | 0.90–2.2 | 0.128 |
| CNS ~ injury | 0.83 | 0.47–1.4 | 0.524 | 0.62 | 0.30–1.2 | 0.174 | 0.98 | 0.63–1.5 | 0.922 | 0.79 | 0.45–1.4 | 0.408 |
| Other | 1.64 | 1.00–2.7 | 0.047 | 1.78 | 0.85–3.8 | 0.126 | 1.55 | 1.02–2.4 | 0.041 | 1.45 | 0.79–2.7 | 0.230 |
| Sepsis/septic shock | 1.96 | 1.19–3.2 | 0.007 | 0.93 | 0.46–1.9 | 0.829 | 2.03 | 1.33–3.1 | 0.001 | 1.01 | 0.55–1.8 | 0.980 |
*ARDS: Acute respiratory distress syndrome
~ CNS: Central nervous system
Effect of exposure to the POINCARE-2 strategy on secondary outcomes
Results for secondary outcomes are presented in Additional file 1: Tables S4 and S5.
MVFDs
After adjustment for potential confounders, we found a negative association between exposure to the strategy and MVFDs, both on the imputed and on the complete case datasets (Additional file 1: Table S4).
VFDs
We found no significant association of the exposure to the POINCARE-2 strategy with the cumulative number of VFDs between Day0 and Day28 (Additional file 1: Table S4).
Unexpected harmful events
We found no significant association between exposure to the POINCARE-2 strategy and occurrence of at least one unexpected harmful event (i.e., arterial hypotension between Day2 and Day14, hypernatremia between Day2 and Day14, hypokalemia between Day2 and Day14, and acute ischemic events between Day3 and discharge) (Additional file 1: Table S5).
Renal damage
We found no significant association between exposure to the POINCARE-2 strategy and worsening in the RIFLE criteria between Day3 and Day14 (Additional file 1: Table S5).
Discussion
Similarly to the intention-to-treat analyses [10], adjusted as-treated analyses showed no statistically significant association between exposure to the POINCARE-2 strategy and 60-day all-cause mortality in critically ill patients. The use of an instrumental variable to account for unmeasured confounders, as well as subgroup analyses according to main cause of admission, yielded similar findings. Taken together, these results make the effect of the POINCARE-2 intervention on mortality in a general population of critically ill patients unlikely.
These results are in line with previous findings. While the deleterious effect of fluid overload on mortality has been repeatedly reported in observational studies in critically ill patients [21], patients suffering from septic shock [22, 23], ARDS [24, 25], or traumatic brain injury [26], the beneficial effect of fluid balance control strategies has been more difficult to demonstrate. So far, trials failed to report any significant effect of a conservative strategy on mortality in patients with ARDS [27, 28] or septic shock [29, 30]. A recent meta-analysis led to similar conclusions with an overall RR 0.92; 95% CI 0.82–1.02 regarding the effect of conservative (vs. liberal) fluid balance control strategies on mortality [8]. However, conservative strategies have proven effective on other outcomes of interest, such as ICU length of stay and number of mechanical ventilator-free days in patients with ARDS [27, 28]. Although the effect of the POINCARE-2 strategy on such outcomes was not significant in intention-to-treat analyses [10], we found a significant decrease in the cumulative number of MVFDs in as-treated analyses, suggesting the POINCARE-2 intervention might actually be effective on this outcome in a broad population of critically-ill patients.
The highest evidence derived from randomized controlled trials (RCT) comes from intention-to-treat analyses of individual RCT [31]. However, in case of contamination [11], i.e., non-null exposure to the strategy under scrutiny in the control arm, or suboptimal exposure to the strategy in the strategy arm, results of intention-to-treat analyses are biased towards the null. As shown by our dedicated score of exposure to the strategy, we observed both contamination and suboptimal exposure to POINCARE-2 strategy in our trial. POINCARE-2 strategy relied on components, such as weighing, administration of diuretics or albumin, or RRT, that are commonly used as part of standard of care in ICU, and are indicated in many other clinical conditions than prevention of fluid overload [32–34]. It is thus very unlikely that patients from the control group of a RCT assessing the effectiveness of a fluid-balance control strategy would have no exposure to the strategy at all, even under optimal experimental conditions. In fact, only 5% of patients included in POINCARE-2 trial had a score of exposure to the strategy < 25. In the same way, as some clinical conditions contraindicate the administration of diuretics or fluid restriction, such as severe dehydration or established anuria [35], maximal exposure to the strategy could not be observed in all patients from the strategy group. In fact, in POINCARE-2 trial, although we observed a higher mean score of exposure to the strategy in the strategy group, not all patients from this group had a score of 100.
Having a closer look at the components of POINCARE-2 strategy, our results suggest that the proportion of daily weighing performed, as well as the total dose of diuretics, increased with an increasing exposure to the strategy. The daily average fluid intake, however, did not vary in the same way with the score of exposure to the strategy. This suggests that intensivists were more adherent to components of the strategy favoring fluid depletion than those favoring water and salt restriction to control fluid balance. They might also not be aware enough of how much creep fluid accounts for daily fluid intake. These findings are in line with the ones of a cross-sectional study among 524 critical care specialists showing that diuretics were the most frequently prescribed treatment to prevent fluid overload (66%). In this study, most intensivists reported using diuretics to treat fluid overload on at least 50% of days working in ICU [36].
Our study suffers from some limitations. First, although experts agreed to rank the patients with a score of exposure to the strategy > 75 as most exposed, their agreement on exposure to the strategy in patients with lower score of exposure was less consensual. Accordingly, the defined unexposed group mixed unexposed patients and somewhat exposed patients, without us being able to separate the last ones from the first ones. This might have biased our results towards the null. In addition, the highest 60-day mortality was observed both in the lowest ([0–25[) and in the highest ([75–100]) score of exposure categories, which either corroborates a potential bias on measurement of exposure to the strategy in the lowest score category or suggests that the effect of exposure to the strategy on mortality is J-shaped. This would mean that rather than discrediting conservative strategies, we might consider applying them using restrictive therapeutic targets and/or during a limited time window. Second, the participating ICUs were equipped with different weigh scales. Despite our recommendation to tare each scale before each weigh assessment, this might have led to measurement bias. Third, we did not size the trial sample to handle adjustment for multiple confounders or to use an instrumental variable without loss of power. This might have resulted in our inability to detect a significant effect of the strategy.
Our study further highlights the difficulty of demonstrating an effect of a conservative strategy in critical care practice, despite evidence of the deleterious effect of fluid overload. Given the dynamic nature of the POINCARE-2 strategy, and because of the constant need to adapt this strategy to patients’ clinical status, which varies over time, alternative statistical methods might be required in future studies. Especially, the target trial emulation approach, by simulating the various possible intervention regimens over time, could help compare the various treatment sequences and determine the most appropriate time to start fluid balance control after ICU admission [37].
Furthermore, the POINCARE-2 strategy relied on multiple components and involved multiple health professionals (i.e., nurses and assistant nurses for weighing and administration of prescribed medications, and intensivists for prescriptions). As thus, it can be considered as a complex intervention, as defined by the Medical Research Council [38]. Implementation and effects of such interventions may vary due to their interactions with the context of implementation [39]. In fact, patients’ characteristics (e.g., indications and contraindications of each of the strategy components), ICUs organizational characteristics (e.g., availability of weighing material, nursing staff size, inter professional conflicts…), and health professionals’ attitudes towards fluid balance control were likely to influence POINCARE-2 strategy implementation, and may have hindered optimal conditions to assess its effectiveness. As a consequence, only a complete process evaluation, as planned in POINCARE-2 trial protocol [9], could help untangle the interventional and contextual factors that may have influenced the actual implementation of the POINCARE-2 strategy [40].
Conclusions
As-treated analyses did not show a significant effect of the POINCARE-2 fluid balance control strategy on mortality in a broad population of critically ill patients. Further research, such as target trial emulation or a complete process evaluation, might help understand the conditions required for conservative strategies to be effective.
Supplementary Information
Additional file 1: Supplementary tables and figures.
Acknowledgements
Data safety management board: Jean-François Timsit, MD PhD, Hôpital Bichat, Paris, Yves Le Tulzo, MD PhD, CHU de Rennes, Gilles Hilbert, MD PhD, CHU de Bordeaux. POINCARE-2 group: Centre hospitalier régional universitaire de Nancy, Inserm, Université de Lorraine, Centre d’investigation clinique, épidémiologie clinique, Nancy. Nelly Agrinier, Marie Buzzi, Camille Alleyrat, Marc Soudant, Jean-Marc Virion: Centre hospitalier régional universitaire de Nancy, Medical ICU, Nancy. Pierre-Edouard Bollaert, Sébastien Gibot, Jérémie Lemarie, Marc Soudant: Centre hospitalier régional universitaire de Nancy, Surgical ICU, Nancy. Ionel Alb, Claire Charpentier, Pascal Welfringer: Centre hospitalier régional de Metz-Thionville, Mercy, Metz. Rostane Gaci, Guillaume Louis: Centre hospitalier régional de Metz-Thionville, Bel Air, Thionville. Michel Bemer, Eric Delaveuve, Elsa Tahon: Centre hospitalier universitaire de Dijon, Dijon. Pascal Andreu, Marie Labruyere, Jean-Pierre Quenot, Jean-Baptiste Roudaut: Centre hospitalier de Verdun, Verdun. Bruno Maire, Laurent Ziegler: Centre hospitalier régional universitaire de Strasbourg, hôpital de Hautepierre, Strasbourg. Vincent Castelain, Francis Schneider: Centre hospitalier régional universitaire de Strasbourg, Nouvel hôpital civil, Strasbourg. Alexandra Monnier: Hôpital St Joseph, Paris. Cédric Bruel, François Philippart, Marc Tran: Hospices civils de Lyon, Hôpital Edouard Herriot, Lyon. Laurent Argaud, Martin Cour, Marie Simon, Neven Stevic: Centre hospitalier de Poissy, Poissy. Jann Hayon, Matthieu Jamme, Hervé Outin: Centre hospitalier de Prévenans, Belfort-Montbéliard. Julio Badie, Fernando Berdaguer, Hakim Slimani. We thank the patients who participated in this study and staff at the participating centres. We acknowledge support from the staff and resources of CIC-EC Nancy. We also thank POINCARE-2 clinical trial coordinators (Nadine JUGE, Andreia CARVALHO DE FREITAS, and Sophie GENDARME), the clinical trial coordinating nurse (Isabelle PETITGENET), the project manager for the sponsor (Yasmine KIDOUCH), Camille Alleyrat for statistical analyses, and clinical trial technicians (*or resident) involved in data collection (Nathalie DUMONT [DRCI CHRU Nancy], Bérengère VALLA, Catherine FERY, Hélène SENAC-DELAGE, Françoise HURSTEL, Amélie LEGRAND, Astiana BOYER*, Christelle BALDELLI, Catherine CARON, Audrey MASSARD, Solenne VILLOT, Nadia MEKAHLI, Guillaume FOUQUET, Sandrine GERSET, Nathalie DUMONT [CIC-EC CHRU Nancy], Sylvie L'HOTELLIER, Michel MASUCCIO, Sophie HATSCH, Anaïs MARTIN, Kelly TIERCELET, Roxane BAI, Laurence TRICOT, Hayat ALLAM, Vicenta FRANJA, Samir CHENAF, Sylvie DE LA SALLE, Catherine FERY, Arielle URBING, Joseph DU CHEYRON, John BECKRICH, Charlotte BOURGOUIN, Magali CLERIN).
Abbreviations
- ARDS
Acute respiratory distress syndrome
- CRA
Cluster randomized analyses
- ICU
Intensive care unit
- ITT
Intention-to-treat
- MI
Multiple imputation
- MVFD
Mechanical ventilator-free days
- OR
Odds ratio
- RBAA
Randomized before-and-after analyses
- RCT
Randomized controlled trial
- RR
Relative risk
- RRTFD
Renal replacement therapy-free day
- VFD
Vasopressor-free day
Author contributions
POINCARE-2 trial was designed jointly by NA and PEB. CHRU Nancy, Inserm, Université de Lorraine, CIC, Epidémiologie Clinique was in charge of data collection, management, analyses, and interpretation in collaboration with the authors. Randomization and data management were supervised by JMV, and statistical analyses conducted by AM, CA, and MS. AM and NA first drafted the manuscript. All authors revised the manuscript critically for important intellectual content. All authors vouch for completeness and accuracy of clinical data and analyses and for adherence to the trial protocol.
Funding
POINCARE-2 trial was supported by a grant from the French Ministry of Health, Direction générale de l’offre de soins (Programme hospitalier de recherche clinique national, PHRC-14-0224).
Availability of data and materials
Due to restrictions pertaining to French laws, the datasets generated and/or analyzed during POINCARE-2 trial are not publicly available. However, data transfer agreement remains possible and data can be made available upon reasonable request to the corresponding author.
Declarations
Ethics approval and consent to participate
The Comité de Protection des Personnes Est III, Grand-Est, North-East France, has reviewed and approved POINCARE-2 trial (ID-RCB: 2015-A00662-47).
Patients and their kin were informed about the trial protocol and their right to refuse to participate. Written informed consent was waived in accordance with the French law (Bill number 2012-300 on March 5, 2012 about research involving humans).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Marie Buzzi, Email: m.buzzi@chru-nancy.fr.
the POINCARE-2 group:
Camille Alleyrat, Jean-Marc Virion, Pierre-Edouard Bollaert, Jérémie Lemarie, Ionel Alb, Pascal Welfringer, Rostane Gaci, Michel Bemer, Eric Delaveuve, Elsa Tahon, Pascal Andreu, Marie Labruyere, Jean-Baptiste Roudaut, Bruno Maire, Laurent Ziegler, Vincent Castelain, François Philippart, Marc Tran, Martin Cour, Marie Simon, Neven Stevic, Jann Hayon, Matthieu Jamme, Fernando Berdaguer, and Hakim Slimani
References
- 1.Wang N, Jiang L, Zhu B, Wen Y, Xi XM, Beijing Acute Kidney Injury Trial (BAKIT) Workgroup Fluid balance and mortality in critically ill patients with acute kidney injury: a multicenter prospective epidemiological study. Crit Care. 2015;19:371. doi: 10.1186/s13054-015-1085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 3.Malbrain MLNG, Marik PE, Witters I, Cordemans C, Kirkpatrick AW, Roberts DJ, et al. Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: a systematic review with suggestions for clinical practice. Anaesthesiol Intensive Ther. 2014;46(5):361–380. doi: 10.5603/AIT.2014.0060. [DOI] [PubMed] [Google Scholar]
- 4.Lee J, de Louw E, Niemi M, Nelson R, Mark RG, Celi LA, et al. Association between fluid balance and survival in critically ill patients. J Intern Med. 2015;277(4):468–477. doi: 10.1111/joim.12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyd JH, Forbes J, Aki NT, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011;39(2):259–265. doi: 10.1097/CCM.0b013e3181feeb15. [DOI] [PubMed] [Google Scholar]
- 6.Roch A, Guervilly C, Papazian L. Fluid management in acute lung injury and ards. Ann Intensive Care. 2011;1(1):16. doi: 10.1186/2110-5820-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Almeida JP, Palomba H, Galas FRBG, Fukushima JT, Duarte FA, Nagaoka D, et al. Positive fluid balance is associated with reduced survival in critically ill patients with cancer. Acta Anaesthesiol Scand. 2012;56(6):712–717. doi: 10.1111/j.1399-6576.2012.02717.x. [DOI] [PubMed] [Google Scholar]
- 8.Silversides JA, Major E, Ferguson AJ, Mann EE, McAuley DF, Marshall JC, et al. Conservative fluid management or deresuscitation for patients with sepsis or acute respiratory distress syndrome following the resuscitation phase of critical illness: a systematic review and meta-analysis. Intensive Care Med. 2017;43(2):155–170. doi: 10.1007/s00134-016-4573-3. [DOI] [PubMed] [Google Scholar]
- 9.Agrinier N, Monnier A, Argaud L, Bemer M, Virion JM, Alleyrat C, et al. Effect of fluid balance control in critically ill patients: design of the stepped wedge trial POINCARE-2. Contemp Clin Trials. 2019;83:109–116. doi: 10.1016/j.cct.2019.06.020. [DOI] [PubMed] [Google Scholar]
- 10.Bollaert PE, Monnier A, Schneider F, Argaud L, Badie J, Charpentier C, et al. Fluid balance control in critically ill patients: results from POINCARE-2 stepped wedge cluster-randomized trial. Crit Care. 2023;27(1):66. doi: 10.1186/s13054-023-04357-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torgerson DJ. Contamination in trials: is cluster randomisation the answer? BMJ. 2001;322(7282):355–357. doi: 10.1136/bmj.322.7282.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta SK. Intention-to-treat concept: a review. Perspect Clin Res. 2011;2(3):109–112. doi: 10.4103/2229-3485.83221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, the ADQI workgroup Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the second international consensus conference of the acute dialysis quality initiative (ADQI) group. Crit Care. 2004;8(4):204. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Terza JV, Basu A, Rathouz PJ. Two-stage residual inclusion estimation: addressing endogeneity in health econometric modeling. J Health Econ. 2008;27(3):531–543. doi: 10.1016/j.jhealeco.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Labrecque J, Swanson SA. Understanding the assumptions underlying instrumental variable analyses: a brief review of falsification strategies and related tools. Curr Epidemiol Rep. 2018;5(3):214–220. doi: 10.1007/s40471-018-0152-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hosmer DW, Jr, Lemeshow S, Sturdivant RX. Applied logistic regression. Wiley; 2013. p. 528. [Google Scholar]
- 17.Fox J, Monette G. Generalized collinearity diagnostics. J Am Stat Assoc. 1992;87(417):178–183. doi: 10.1080/01621459.1992.10475190. [DOI] [Google Scholar]
- 18.Davidson R, MacKinnon JG. Estimation and inference in econometrics. New York: Oxford University Press; 1993. p. 874. [Google Scholar]
- 19.Buuren S van. Stef van Buuren. 2018 [cited 2023 Apr 21]. Flexible Imputation of Missing Data. Second Edition. Available from: https://stefvanbuuren.name/publication/2018-01-01_vanbuuuren2018/
- 20.Schomaker M, Heumann C. Bootstrap inference when using multiple imputation. Stat Med. 2018;37(14):2252–2266. doi: 10.1002/sim.7654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Messmer AS, Zingg C, Müller M, Gerber JL, Schefold JC, Pfortmueller CA. Fluid overload and mortality in adult critical care patients-A systematic review and meta-analysis of observational studies. Crit Care Med. 2020;48(12):1862–1870. doi: 10.1097/CCM.0000000000004617. [DOI] [PubMed] [Google Scholar]
- 22.Abulebda K, Cvijanovich NZ, Thomas NJ, Allen GL, Anas N, Bigham MT, et al. Post-ICU admission fluid balance and pediatric septic shock outcomes: a risk-stratified analysis. Crit Care Med. 2014;42(2):397–403. doi: 10.1097/CCM.0b013e3182a64607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Acheampong A, Vincent JL. A positive fluid balance is an independent prognostic factor in patients with sepsis. Crit Care. 2015;19(1):251. doi: 10.1186/s13054-015-0970-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenberg AL, Dechert RE, Park PK, Bartlett RH, NIH NHLBI ARDS Network Review of a large clinical series: association of cumulative fluid balance on outcome in acute lung injury: a retrospective review of the ARDSnet tidal volume study cohort. J Intensive Care Med. 2009;24(1):35–46. doi: 10.1177/0885066608329850. [DOI] [PubMed] [Google Scholar]
- 25.Murai A, Ishikura H, Matsumoto N, Nakamura Y, Ohta D, Muranishi K, et al. Impact of fluid management during the three ICU days after admission in patients with ARDS. Crit Care. 2014;18(2):P25. doi: 10.1186/cc14028. [DOI] [Google Scholar]
- 26.Wiegers EJA, Lingsma HF, Huijben JA, Cooper DJ, Citerio G, Frisvold S, et al. Fluid balance and outcome in critically ill patients with traumatic brain injury (CENTER-TBI and OzENTER-TBI): a prospective, multicentre, comparative effectiveness study. Lancet Neurol. 2021;20(8):627–638. doi: 10.1016/S1474-4422(21)00162-9. [DOI] [PubMed] [Google Scholar]
- 27.National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 28.Hu W, Lin CW, Liu BW, Hu WH, Zhu Y. Extravascular lung water and pulmonary arterial wedge pressure for fluid management in patients with acute respiratory distress syndrome. Multidiscipl Resp Med. 2014;9(1):3. doi: 10.1186/2049-6958-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richard JC, Bayle F, Bourdin G, Leray V, Debord S, Delannoy B, et al. Preload dependence indices to titrate volume expansion during septic shock: a randomized controlled trial. Crit Care. 2015;19(1):5. doi: 10.1186/s13054-014-0734-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hjortrup PB, Haase N, Bundgaard H, Thomsen SL, Winding R, Pettilä V, et al. Restricting volumes of resuscitation fluid in adults with septic shock after initial management: the CLASSIC randomised, parallel-group, multicentre feasibility trial. Intens Care Med. 2016;42(11):1695–1705. doi: 10.1007/s00134-016-4500-7. [DOI] [PubMed] [Google Scholar]
- 31.Hollis S, Campbell F. What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ. 1999;319(7211):670–674. doi: 10.1136/bmj.319.7211.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mehta RL, Pascual MT, Soroko S, Chertow GM, PICARD Study Group Diuretics, mortality, and nonrecovery of renal function in acute renal failure. JAMA. 2002;288(20):2547–2553. doi: 10.1001/jama.288.20.2547. [DOI] [PubMed] [Google Scholar]
- 33.McFall A, Peake SL, Williams PJ. Weight and height documentation: Does ICU measure up? Aust Crit Care. 2019;32(4):314–318. doi: 10.1016/j.aucc.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 35.Arumugham VB, Shahin MH. Therapeutic Uses Of Diuretic Agents. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 [cited 2023 Mar 6]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK557838/ [PubMed]
- 36.Silversides JA, McAuley DF, Blackwood B, Fan E, Ferguson AJ, Marshall JC. Fluid management and deresuscitation practices: a survey of critical care physicians. J Intensive Care Soc. 2020;21(2):111–118. doi: 10.1177/1751143719846442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Labrecque JA, Swanson SA. Target trial emulation: teaching epidemiology and beyond. Eur J Epidemiol. 2017;32(6):473–475. doi: 10.1007/s10654-017-0293-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M, et al. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008;337:a1655. doi: 10.1136/bmj.a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hawe P, Shiell A, Riley T, Gold L. Methods for exploring implementation variation and local context within a cluster randomised community intervention trial. J Epidemiol Commun Health. 2004;58(9):788–793. doi: 10.1136/jech.2003.014415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: medical research Council guidance. BMJ. 2015;350:h1258. doi: 10.1136/bmj.h1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary tables and figures.
Data Availability Statement
Due to restrictions pertaining to French laws, the datasets generated and/or analyzed during POINCARE-2 trial are not publicly available. However, data transfer agreement remains possible and data can be made available upon reasonable request to the corresponding author.