Abstract
Objectives
The optimal puncture technique for neuraxial anaesthesia in different populations is unclear. We sought to obtain data from randomised controlled trials comparing the impact of ultrasound-guided technology and traditional positioning technology on the success rate of neuraxial anaesthesia.
Design
Systematic review and network meta-analysis using study populations, interventions, intervention comparisons, outcome measures and study types.
Data sources
PubMed, Embase, Cochrane Library and Web of science were searched until 31 September 2022.
Eligibility criteria
We included randomised controlled trials comparing three types of neuraxial anaesthesia: ultrasound-assisted, ultrasound real-time guidance and conventional positioning to describe which neuraxial anaesthesia modality is best for patients and to recommend the appropriate one for different populations.
Data extraction and synthesis
Five independent reviewers retrieved, screened and edited included studies using standardised methods. Assess risk of bias using the Cochrane Collaboration and Evidence Project tools. Network meta-analysis was performed using STATA V.15 statistical software.
Results
Twenty-two studies containing three different interventions were included. The SUCRA values of first-pass success rates for the three neuraxial anaesthesia methods were real-time guidance (82.8%), ultrasound-assisted (67.1%) and traditional positioning (0.1%). Both ultrasound techniques improved first-pass success rates compared with traditional localization, but there was no significant difference between the two. Subgroup analysis showed that the use of real-time ultrasound guidance for neuraxial anaesthesia in pregnant and patients with obesity improved first-pass success rates. Ultrasound-assisted technology can improve first-attempt success rates in older patients with abnormal lumbar spine anatomy.
Conclusion
Compared with conventional positioning, ultrasound guidance technology can improve the first-pass success rate of neuraxial anaesthesia, but there is no significant difference between ultrasound-assisted and real-time guidance technology. The results of subgroup analysis tell us that the most suitable neuraxial anaesthesia method is different for different groups of people.
PROSPERO registration number
PROSPERO number: CRD42022376041.
Keywords: Ultrasonography, Adult anaesthesia, Adult surgery
STRENGTHS AND LIMITATIONS OF THIS STUDY.
To the best of our knowledge, this is the first study to compare the puncture success rates of three neuraxial anaesthesia methods using a frequentist approach.
This protocol was created strictly based on the published Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines, and its research results have certain reference value for clinical anesthesiologists.
Due to the technical difficulty of real-time ultrasound guidance and the lack of evidence from clinically relevant studies, this may be one of the main limitations of this meta-analysis.
Introduction
As a commonly used method of anaesthesia, neuraxial anaesthesia has traditionally been performed by manually palpating body markers to determine the puncture site. In recent years, ultrasound technology has been increasingly used in neuraxial anaesthesia.1 There are currently two types of ultrasound technologies used for neuraxial anaesthesia: ultrasound-assisted technology and ultrasound real-time guidance technology. Preoperative ultrasound scanning helps identify puncture points and estimate puncture depth, while ultrasound real-time guidance technology (puncture under ultrasound visualisation) allows for more accurate observation of the needle’s location and trajectory. Some existing studies have compared ultrasound-assisted technology with traditional localization methods, and some have compared ultrasound real-time guidance technology with traditional localisation methods. However, few studies have compared these two ultrasound techniques. Chen’s study pointed out that ultrasound-assisted neuraxial anaesthesia has a higher first-pass success rate and higher patient satisfaction than real-time guidance technology in hip surgery in elderly patients,2 while Parli pointed out that in the proposed operation in patients with obesity undergoing lower limb surgery, the use of real-time ultrasound guidance for neuraxial anaesthesia shortens the operation time and has a higher first-pass success rate.3 There is controversy as to which of these three methods of neuraxial anaesthesia is the most effective. Therefore, we reviewed articles comparing traditional positioning, ultrasound-assisted, and real-time guidance techniques used in neuraxial anaesthesia. A systematic review of three methods of neuraxial anaesthesia was conducted through network meta-analysis (NMA).
Materials and methods
We followed the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)4 and registered the meta-analysis and systematic review in the PROSPERO database and PROSPERO network on 28 November 2022 (registration number: CRD42022376041). The current NMA is based on the protocol recommended by the Cochrane Collaboration5 and follows the PRISMA guidelines.6
Search strategy
We searched the PubMed, EMbase, Web of Science and Cochrane Library databases for all relevant articles up to 31 September 2022. Keywords: “ultrasound real-time guidance”, “ultrasound-assisted”, “landmark palpation”, “traditional positioning”, “epidural anesthesia”, “spinal anesthesia” and “combined spinal and epidural anesthesia”. Searches were conducted using a combination of subject headings and free words. The complete search strategy can be found in the online supplemental file 1.
bmjopen-2022-071253supp001.pdf (1.2MB, pdf)
Inclusion and exclusion criteria
We included randomised controlled trials (RCTs) comparing two or three methods of neuraxial anaesthesia. The information is as follows: study population: neuraxial anaesthesia, including epidural anaesthesia, spinal anaesthesia, and combined spinal and epidural anaesthesia; intervention: traditional positioning, ultrasound-assisted positioning, and ultrasound real-time guidance; intervention comparison: a neuraxial anaesthesia method; outcome measures: the primary outcome was first-pass success rate (defined as the needle successfully achieving epidural puncture in one attempt without reorientation); the secondary outcome was first-attempt success rate (defined as the needle reaching the epidural space in one insertion attempt and allows for needle reorientation), recognition time (the time from operator contact with the patient’s skin to marking the puncture site on the skin and the time from placing the probe on the skin to marking the puncture site), and puncture time (from skin contact with needle to cerebrospinal fluid time interval between outflows); study design: RCT.
Exclusion criteria were as follows: review articles, case reports, case series, letters to the editor, reviews, conference proceedings, laboratory science studies and any other irrelevant studies, as well as studies that did not report the results of interest.
Study selection
Two authors, Yinzhou Zhang and Junying Wei, respectively, searched the database according to the above search strategy. The type of RCT or clinical trial was selected through filters in online databases. The retrieved documents were saved and deduplicated through document management software (NoteExpress). The titles and abstracts of the selected literature were read one by one, and if the title and abstract met the criteria, the full text was evaluated to see if the results of interest were reported. Yinzhou Zhang, Junying Wei and Jieling Huang also discussed whether each study should be included or excluded to reach consensus. Disagreements regarding inclusion or exclusion were resolved in discussions with Yuhui Li and Wuhua Ma.
Date extraction
All relevant data from the included studies were independently extracted and entered into standardised forms by Yinzhou Zhang and Junying Wei, and then cross-checked. The standardised form included the following items: title, author name, publication date, patient type, surgery type, body mass index, age, anaesthesia method, sample size, first pass success rate, first attempt success rate, identification time, procedure time, intervention method and the best way to intervene. Age and body mass index data were extracted as mean±SD and median (IQR). When data from included studies were presented in the form of IQRs, we followed appropriate methods for transformation7–9 and finally used mean±SD for statistical analysis.
Study quality
Jieling Huang and Wuhua Ma conducted independent assessments using the risk of bias tool in Review Manager (V.5.3). Quality was assessed using the following possible sources of bias: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessments, incomplete outcome data and selective reporting. The methods of each study were rated as ‘high’, ‘low’ or ‘unclear’, reflecting the risk of bias.5
Statistical analysis
Multiple treatment comparison is a meta-analytic summary method that includes direct and indirect comparisons of treatments. We used STATA V.15 software to download the network package for statistical analysis. The effect value of dichotomous variables used RR values, and the effect value of continuous variables used SMD. When the p value was >0.05, the inconsistency model was used to test consistency, and the node splitting method was used for local inconsistency analysis. Perform a ring inconsistency test on the network diagram that forms a closed loop. If the 95% CI does not include 0, the heterogeneity is large, and sensitivity and subgroup analysis are required.
A network diagram was formed connecting all included studies to indicate the type of neuraxial anaesthesia, the number of patients in the different studies, and the number of pairwise comparisons. Nodes show different neuraxial anaesthesia methods, and lines show direct comparisons between neuraxial anaesthesia methods. Cumulative probability plots for each neuraxial anaesthesia method and pairwise comparisons for each intervention were plotted. We used cumulative ranking area under the curve (SUCRA) values to present the effect of neuraxial anaesthesia methods on first-pass success rate and first-attempt success rate. SUCRA is a relative ranking metric with a statistical range from 0% to 100% that indicates the likelihood that the therapy will be rated the best.10 Higher SUCRA values are considered better outcomes for individual interventions.
Patient and public involvement
No patients participated in the study
Results
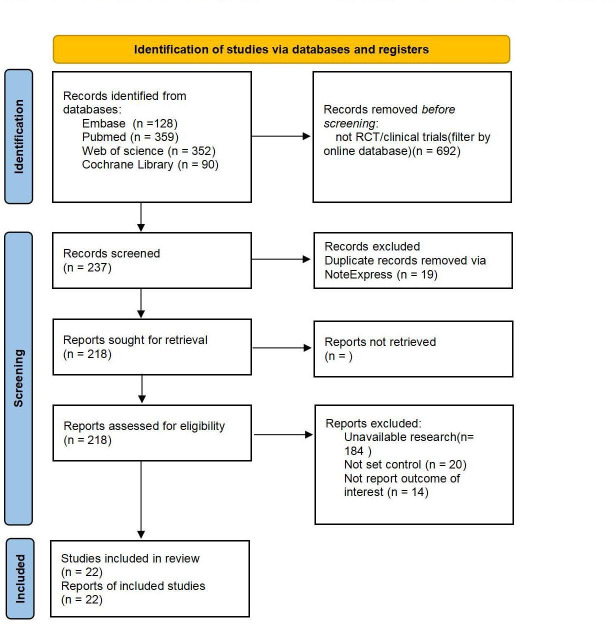
PubMed and Embase databases were searched, and 128 and 359 studies were initially assessed. In addition, we searched the Web of science and Cochrane Library databases and retrieved 352 and 90 studies, respectively, yielding a total of 929 publications. Online database filters were used to screen for RCTs or clinical trials and 692 studies were excluded. After removing duplicates using literature management software, 218 studies remained. Titles, abstracts and full texts of the remaining studies were reviewed in detail; 184 studies were not available, 20 studies were excluded due to lack of controls, and 14 studies did not report the outcome of interest (figure 1).
Figure 1.
Flow diagram. RCTs, randomised controlled trials.
Research characteristics
Tables 1 and 2 summarise the characteristics of the 22 studies. All experiments were two or three arms. Among them, 13 studies compared ultrasound-assisted localisation with conventional localisation,11–23 5 studies compared ultrasound-assisted localisation with conventional localisation24–28 and 3 studies compared ultrasound-assisted localisation with real-time guidance in the spinal anaesthesia.2 3 19 One study compared the use of three methods in spinal anaesthesia.20 Table 1 lists the first author and publication year of the literature, as well as basic information such as patient type, surgical method, patient age and body mass index. Table 2 lists the anaesthesia method, study sample size, intervention measures and main outcome indicators (first time passing success rates) and better intervention outcomes. In all included studies, the probes used for ultrasound were portable low-frequency convex array probes, excluding special puncture probes.
Table 1.
| The author | Time of publication | Type of patient | Type of surgery | Age (*‡†) | BMI (*‡†) |
| Karthikeyan | 2018 | Adult | Knee and hip surgery | 65.3±9.7† 68.2±10.3* |
30.1±6.4† 30.6±4.7* |
| Sangeeta Dhanger | 2018 | Maternal | Caesarean section | 23.06±3.01† 24.03±3.43* |
27.2±3.8† 27.2±4.2* |
| Cristian Arzola | 2015 | Maternal | Childbirth | 32.3±5.8† 32.7±4.7* |
29±5.1† 29.3±6* |
| Y. C. Lim | 2014 | Adult | Lower limb surgery | 61.1±13.3† 63.7±12.6* |
25.4±5.6† 25.0±5.9 |
| Chin | 2018 | Maternal | Caesarean section | NM | 30.2§(27.0–36.5)† 30.5§(26.9–34.2)* |
| Bingdong Tao | 2021 | Maternal | Caesarean section | 32.3±5.2† 30.6±3.8* |
28.3±3.0† 28.3±2.2* |
| Mohd Anas Khan | 2022 | Orthopaedic patient | Lower limb surgery | 54.5±12.8† 57.7±13.2* |
29.3±4.6† 27.7±3.8* |
| Mengzhu Li | 2019 | Patients with obesity | Caesarean section | 29.5±3.9† 30.1±4.5* |
NM |
| Sun-Kyung Park | 2019 | Old age patient | Lower limb surgery | 71.1±7.2† 71.2±6.1* |
25.8±3.1† 25.8±3.1* |
| Mohamed Mohamed Tawfik | 2017 | Maternal | Caesarean section | 27.7±4† 26.7±3.8* |
29.2±3† 29.2±2.9* |
| Sun-Kyung Park | 2020 | Anatomic abnormality of lumbar spine | Lower limb surgery | 70.5±8.8† 66.5±13.2* |
26.1±3.2† 25.9±2.9* |
| Bo Qu | 2020 | Old age patient | Hip surgery | 83.3±6.7† 82.3±7.1* |
21.6±3.6† 20.6±3.0* |
| Xiu Ni | 2021 | Patients with obesity | Caesarean section | 31.8±4.8† 31.4±4.2* |
33.5±2.1† 33.0±2.1* |
| Bertam | 2017 | Adult | Lower limb surgery | NM | NM |
| Tanya Mital | 2021 | Children | Chest and abdominal surgery | 2.4±1.3‡ 3.0±1.7* |
NM |
| Jatuporn Pakpirom | 2020 | Adult | Chest and abdominal surgery | 60.0§(51.0–67.0)‡ 58.5§(53.75–70.25)* | 23.4±4.0‡ 22.8±3.5* |
| Jindi Jiang | 2021 | Overweight mothers | Childbirth | 29.2±3.1‡ 28.4±3.4* |
35.6±2.0‡ 35.2±2.4* |
| Hesham | 2017 | Anatomic abnormality of lumbar spine | Knee and hip surgery | 69±10‡ 70±10* |
34±11‡ 33±8* |
| Luying Chen | 2021 | Old age patient | Hip surgery | 82.7±6.6‡ 84.5±6.2† |
21.9±3.1‡ 21.3±3.4† |
| Yasser Mohamed | 2020 | Maternal | Childbirth | 25.4±5.1‡ 26.8±5.65† |
37.9±4.3‡ 38.1±4.2† |
| Parli Raghavan Ravi | 2021 | Patients with obesity | Lower limb surgery | 58.5§(50.3, 65.8)‡ 59.5§(52.3, 65.8)† | 34.9§(33.1, 36.35)‡ 34.9§(33.1, 36.40)† |
| Deepak Bhardwaj | 2022 | Adult | Lower limb surgery | 39.66±13.27* 42.88±12.72† 43.6±15.24‡ |
22.8±2.8* 22.4±3.4† 23.9±3.0‡ |
*Landmark group.
†Ultrasound assisted group.
‡Real time group.
§Median (IQR).
CSE, combined spinal and epidural anesthesia; E, epidural anesthesia; NM, no mention; S, spinal anesthesia.
Table 2.
| The author | Time of publication | Method of anaesthesia(E, S, CSE) | Sample size (*†,‡) | Intervention | First pass success rate (%) | Effect estimate (better) |
| Karthikeyan | 2018 | S | 59† 60* |
Landmark versus ultrasound assisted | 43† 22* |
ND |
| Sangeeta Dhanger | 2018 | S | 50† 50* |
Landmark versus ultrasound assisted | 18† 74* |
Ultrasound assisted |
| Cristian Arzola | 2015 | E | 60† 68* |
Landmark versus ultrasound assisted | 50† 60* |
ND |
| Y. C. Lim | 2014 | S | 85† 85* |
Landmark versus ultrasound assisted | 7† 15* |
ND |
| Chin | 2018 | CSE | 105† 110* |
Landmark versus ultrasound assisted | 38.2† 63.8* |
Ultrasound assisted |
| Bingdong Tao | 2021 | CSE | 64† 64* |
Landmark versus ultrasound assisted | 68.8† 93.8* |
Ultrasound assisted |
| Mohd Anas Khan | 2022 | CSE | 50† 50* |
Landmark versus ultrasound assisted | 60† 86* |
Ultrasound assisted |
| Mengzhu Li | 2019 | CSE | 40† 40* |
Landmark versus ultrasound assisted | 52.5† 87.5* |
Ultrasound assisted |
| Sun-Kyung Park | 2019 | S | 40† 40* |
Landmark versus ultrasound assisted | 17.5† 65.0* |
Ultrasound assisted |
| Mohamed Mohamed Tawfik | 2017 | CSE | 53† 55* |
Landmark versus ultrasound assisted | 60† 58.5* |
ND |
| Sun-Kyung Park | 2020 | S | 22† 22* |
Landmark versus ultrasound assisted | 9.1† 50* |
Ultrasound assisted |
| Bo Qu | 2020 | CSE | 40† 40* |
Landmark versus ultrasound assisted | 20† 70* |
Ultrasound assisted |
| Xiu Ni | 2021 | CSE | 40† 40* |
Landmark versus ultrasound assisted | 40† 72.5* |
Ultrasound assisted |
| Bertam | 2017 | S | 30‡ 30* |
Landmark versus real time | 47‡ 30* |
Real time |
| Tanya Mital | 2021 | E | 23‡ 22* |
Landmark versus real time | 82.6‡ 40.9* |
Real time |
| Jatuporn Pakpirom | 2020 | E | 48‡ 48* |
Landmark versus real time | 68.6‡ 35.4* |
Real time |
| Jindi Jiang | 2021 | E | 30‡ 30* |
Landmark versus real time | 56.7‡ 30* |
Real time |
| Hesham | 2017 | S | 14‡ 18* |
Landmark versus real time | 72.2‡ 83.3* |
ND |
| Luying Chen | 2021 | S | 57‡ 57† |
ultrasound assisted versus real time | 31.6‡ 63.2† |
Ultrasound assisted |
| Yasser Mohamed | 2020 | E | 50‡ 50† |
ultrasound assisted versus real time | 90‡ 74† |
Real time |
| Parli Raghavan Ravi | 2021 | S | 40‡ 40† |
ultrasound assisted versus real time | 40‡ 10† |
Real time |
| Deepak Bhardwaj | 2022 | S | 50* 50† 50‡ |
Landmark versus ultrasound assisted versus real time | 82* 78† 80‡ |
ND |
*Landmark group.
†Ultrasound-assisted group.
‡Real-time group.
CSE, combined spinal and epidural anesthesia; E, epidural anesthesia; ND, no difference; S, spinal anesthesia.
Risk of bias assessment
The quality indicators of the included studies are shown in figure 2. All studies used random sequence generation, 12 of which had allocation concealment. Thirteen of the studies did not specify how participants were blinded. One study had a high risk of bias in blinding the operator, which could be explained by the difficulty in achieving blinding of the procedure. Most studies had incomplete outcome data, but five of the studies had unspecified risks. None of the studies reported results selectively.
Figure 2.
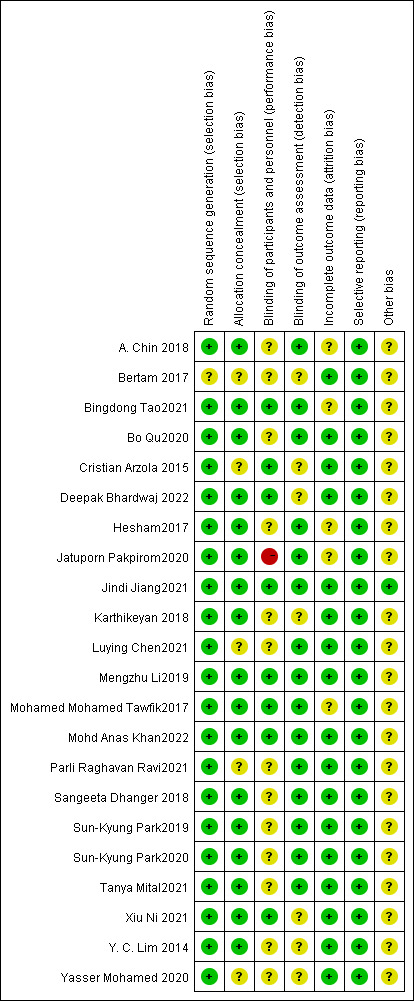
Consensus risk-of-bias assessment of the included studies. Green, low risk; yellow, unclear; red, high risk.
Synthesis of results
For all results for each outcome measure, we present network plots, forest plots for individual studies, forest plots for pairwise comparisons, and cumulative ranking curves. The results are shown in online supplemental figures 1 to 7. Results of inconsistency model detection, consistency analysis, local inconsistency analysis, ring inconsistency detection and funnel plots can be found in the online supplemental file. From model testing and funnel plots, the heterogeneity of the study was minimal.
First pass success rate
Nineteen two-arm studies and one three-arm study documented first-pass success rates and were pooled for analysis.2 3 11–17 19–27 29 30 Across all studies, traditional positioning was the most frequently cited (online supplemental figure 1A). In this study, the puncture success rate of the ultrasound-assisted group and real-time guidance group seemed to be higher than that of the traditional positioning group (online supplemental figure 1B). However, there was no significant difference between the ultrasound-assisted group and the real-time guidance group (online supplemental figure 1C). The probabilities of conventional positioning, assisted positioning and real-time guidance were analysed by plotting a cumulative ranking graph (online supplemental figure 1D). According to SUCRA data, the first puncture success rate is highest for real-time guidance (82.8%), followed by ultrasound assistance (67.1%), and finally conventional positioning (0.1%). The funnel plot is shown in online supplemental figure 1E.
First attempt rate
A total of 16 trials provided data on first-attempt success rates.2 3 11 12 14 18–22 24–28 30 The network node diagram is shown in online supplemental figure 2A. Forest plot results showed that the use of ultrasound was associated with first-attempt success rate (online supplemental figure 2B). However, there was no significant difference between ultrasound-assisted and real-time guidance (online supplemental figure 2C). The cumulative ranking chart shows that ultrasound-assisted first attempt success rate is the highest (75.3%), followed by real-time guidance (74.6%) and traditional positioning (0.1%) (online supplemental figure 2D). The funnel plot is shown in online supplemental figure 2E.
Identification time
The network diagrams and forest diagrams of each study are shown in online supplemental figure 3A,B. The results2 3 11 12 17–19 21–23 26 27 30 show that the traditional positioning method has the shortest positioning time (online supplemental figure 3D), but the ultrasound-assisted and real-time guided puncture positioning time does not show significant differences (online supplemental figure 3C). The funnel plot is shown in figure 3SE.
Duration of spinal anaesthesia
A total of nine studies, eight two-arm studies and one three-arm study3 11 12 14 18 19 21 28 30 were collected to compare the entire operation process from the puncture needle contacting the skin to the outflow of cerebrospinal fluid. The network diagrams and forest diagrams of each study are shown in online supplemental figure 4A,B. Comprehensive analysis showed that the ultrasound-assisted operation time was the shortest (online supplemental figure 4D), and there was no significant difference between the traditional positioning group and the real-time guidance group (online supplemental figure 4C). The funnel plot is shown in online supplemental figure 4E.
Subgroup analysis
In the first subgroup, we included nine studies in adults with obesity and pregnant women (obese or not), and analysed the results of first pass success rate3 12 13 15 16 20 23 27 29 and first attempt success rate.3 12 18 20 27 For first-pass success rate, a network plot (online supplemental figure 5A), a forest plot for a single study (online supplemental figure 5B), a forest plot for pairwise comparisons (online supplemental figure 5C), a cumulative ranking curve (online supplemental figure 5D) and a funnel plot (online supplemental figure 5E) are shown. The network diagram of the first puncture success rate (online supplemental figure 6A), the forest diagram of a single study (online supplemental figure 6B), the forest diagram of pairwise comparison (online supplemental figure 6C), the cumulative ranking curve (online supplemental figure 6D) and the funnel plot (online supplemental figure 6E) are as shown in the figure.
In a second subgroup analysis, we included patients with a mean age over 70 years and those with abnormal lumbar anatomy (previous lumbar surgery or scoliosis).2 19 21 22 28 The network diagram and forest diagram are shown in online supplemental figure 7A,B. The results of the meta-analysis showed that the first-attempt success rate seemed to be higher in the ultrasound-assisted group (online supplemental figure 7C), and the cumulative ranking chart also showed that ultrasound-assisted was the most recommended (online supplemental figure 7D). The funnel plot is shown in online supplemental figure 7E.
Discussion
In recent years, there has been increasing interest in ultrasound guidance for spinal, epidural or combined spinal-epidural anaesthesia.31–33 Research supports the use of this technique to increase puncture success rates and reduce complications.34 35 The UK National Institute for Health and Clinical Excellence has published guidance36 recommending that ultrasound can be used both as a preoperative assessment tool and as a live puncture.
Two major indicators of difficulty in neuraxial anaesthesia are the number of needle turns required for successful puncture and the time required for the entire procedure. Multiple needle sticks are an independent predictor of complications such as dural penetration, vascular injury and paresthesias.37 Ideal neuraxial anaesthesia requires a successful puncture.24 Minimising the number of attempts can help reduce the risk of complications and improve patient satisfaction.38 Previous studies have shown that ultrasound scanning before puncture can improve the success rate of puncture and reduce the number of punctures.39 The characteristic of real-time guidance technology is to observe the needle trajectory in real time during the puncture process, which improves the puncture success rate.24 40–42 This is consistent with our analysis.
But the analysis showed no significant difference in first-pass and first-attempt success rates between ultrasound-assisted and real-time guidance. However, subgroup analysis showed that real-time guidance technology was more beneficial for pregnant women and obese people. Ultrasound-assisted technology is more recommended for older patients and patients with abnormal spinal anatomy.
Let us analyse the reasons for this difference. It is difficult for pregnant and obese patients to achieve the ideal puncture position during neuraxial anaesthesia, and difficulty in palpation may lead to an increase in the number of punctures, resulting in patient discomfort or puncture failure.43 During pregnancy, lumbar protrusion increases and the pelvis expands and rotates, resulting in a deeper and narrower epidural space and a narrower ‘safe zone’ between the ligamentum flavum and the dura mater.44 These individuals are generally younger, have soft lumbar ligaments, clear muscle-fat boundaries, and the anterior and posterior complexes are clearly visible under ultrasound, which can significantly reduce the number of needle adjustments and are suitable for real-time guidance technology.34 45 However, real-time puncture is difficult for elderly patients. In elderly patients, due to vertebral body and ligament hyperplasia and intervertebral space narrowing, ordinary ultrasound probes are more likely to block the puncture needle path, thus affecting the observation of the puncture needle trajectory. The advantage of ultrasound-assisted positioning is that it can shorten the anaesthesia operation time. Studies have shown that real-time guidance technology is not superior to ultrasound-assisted localisation because real-time guidance requires longer operation time, especially in elderly patients, which reduces satisfaction scores.2
Of course, we cannot ignore other factors that influence our results. The puncture paths used by the researchers were not entirely consistent. According to previous studies,46 the paramedian puncture route is better than the median position because it avoids the supraspinal and interspinous ligaments, and ligament calcification will make puncture more difficult for the operator and increase the number of attempts. The experience of the operator cannot be ignored either. Operators included in the literature were almost all anesthesiologists skilled in the use of ultrasound techniques for neuraxial anaesthesia. The anaesthetist’s qualifications are also a factor that affects the success rate of puncture, and its effect may affect the success rate of puncture, exaggerating the advantages of ultrasound-guided technology.47 In addition, real-time ultrasound guidance technology is difficult, requiring the operator to hold the probe and ensure image stability while observing the needle trajectory. This is also a challenge for anesthesiologists with many years of experience in ultrasound-assisted localization. This technical difference also affects our results. On the other hand, the choice of ultrasound probe will also affect real-time guidance of puncture. Due to the common low-frequency convex array probe, the contact surface of the probe does not completely fit the skin, and the curved shell of the probe blocks the angle of the needle during puncture. Recently, TranD45 used a new puncture probe. An epidural needle holder is provided on the side of the probe to adjust the needle angle in the plane of the probe. According to the prepositioned intervertebral space and the preset needle insertion angle, the operator only needs to pay attention to the needle insertion depth to complete the puncture. This method keeps the needle in the same plane as the probe so that the needle trajectory is always visible.
This study also has some limitations. Due to the difficulty of real-time guidance technology, there are fewer studies in this field and the sample size is smaller than assisted positioning, which will also affect our analysis. Therefore, our results cannot be extrapolated to other related studies.
Conclusion
This study demonstrates that ultrasound guidance technology has significant advantages in improving the first-pass success rate of neuraxial anaesthesia. Furthermore, subgroup analysis showed that real-time ultrasound guidance had a significant advantage in first-pass success rate. Ultrasound real-time guidance technology is more suitable for pregnant and patients with obesity, and ultrasound-assisted technology is more suitable for elderly patients with abnormal lumbar spine anatomy. Current research evidence is insufficient, mainly because study designs vary, real-time guidance techniques are difficult and there are currently few studies. Future research should focus on ultrasound real-time guidance technology and expand the application of visualisation technology in neuraxial anaesthesia.
Supplementary Material
Acknowledgments
The authors would like to thank the teachers of the Department of Anesthesiology, the First Affiliated Hospital of Guangzhou University of Chinese Medicine for their help.
Footnotes
Contributors: YZ and MP are cofirst authors of this manuscript. YZ, JH, JW, WM and YL were involved in the design and conception of the research scheme. YZ and JW will screen the title, abstract and full text. If disagreement arises on inclusion or exclusion, it will be resolved through discussion with other authors (JH, WM and/or YL). YZ and JW will extract data from the article independently, and third party reviewers (JH and/or WM) will check the integrity and correctness of the extracted data in the results evaluation.YL is the guarantor of the review. All authors drafted and revised this research protocol and approved it for publication.
Funding: This work was fully supported by the Department of Anesthesiology, the First Affiliated Hospital of Guangzhou University of Chinese Medicine.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1.Tran D, Kamani AA, Lessoway VA, et al. Rohling RN.Preinsertion Paramedian ultrasound guidance for epidural anesthesia. Anesth Analg 2009;109:661–7. 10.1213/ane.0b013e3181a94c75 [DOI] [PubMed] [Google Scholar]
- 2.Chen L, Huang J, Zhang Y, et al. Real-time ultrasound-guided versus ultrasound-assisted spinal anesthesia in elderly patients with hip fractures. Anesthesia & Analgesia 2022;134:400–9. 10.1213/ANE.0000000000005778 [DOI] [PubMed] [Google Scholar]
- 3.Ravi P, Naik S, Joshi M, et al. Real-time ultrasound-guided spinal anaesthesia vs Pre- procedural ultrasound-guided spinal anaesthesia in obese patients. Indian J Anaesth 2021;65:356. 10.4103/ija.IJA_446_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Res Synth Methods 2012;3:80–97. 10.1002/jrsm.1037 [DOI] [PubMed] [Google Scholar]
- 5.Higgins JPT, Green S, Cochrane Collaboration . Cochrane handbook for systematic reviews of interventions. Chichester, England; Hoboken, NJ: Wiley-Blackwell, 2008. [Google Scholar]
- 6.Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015;162:777–84. 10.7326/M14-2385 [DOI] [PubMed] [Google Scholar]
- 7.Shi J, Luo D, Weng H, et al. Optimally estimating the sample standard deviation from the five-number summary. Res Synth Methods 2020;11:641–54. 10.1002/jrsm.1429 [DOI] [PubMed] [Google Scholar]
- 8.Luo D, Wan X, Liu J, et al. Optimally estimating the sample mean from the sample size, median, mid-range and/or mid-Quartile range. Stat Methods Med Res 2018;27:1785–805. 10.1177/0962280216669183 [DOI] [PubMed] [Google Scholar]
- 9.Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or Interquartile range. BMC Med Res Methodol 2014;14:135. 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salanti G, Ades AE, Ioannidis JPA. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and Tutorial. J Clin Epidemiol 2011;64:163–71. 10.1016/j.jclinepi.2010.03.016 [DOI] [PubMed] [Google Scholar]
- 11.Srinivasan KK, Leo A-M, Iohom G, et al. Pre-procedure ultrasound-guided Paramedian spinal anaesthesia at L5-S1: is this better than landmark-guided midline approach? A randomised controlled trial. Indian J Anaesth 2018;62:53–60. 10.4103/ija.IJA_448_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhanger S, Vinayagam S, Vaidhyanathan B, et al. Comparison of landmark versus pre-procedural Ultrasonography-assisted midline approach for identification of subarachnoid space in elective Caesarean section: A randomised controlled trial. Indian J Anaesth 2018;62:280–4. 10.4103/ija.IJA_488_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arzola C, Mikhael R, Margarido C, et al. Spinal ultrasound versus Palpation for epidural catheter insertion in labour: A randomised controlled trial. Eur J Anaesthesiol 2015;32:499–505. 10.1097/EJA.0000000000000119 [DOI] [PubMed] [Google Scholar]
- 14.Lim YC, Choo CY, Tan KTJ. A randomised controlled trial of ultrasound-assisted spinal anaesthesia. Anaesth Intensive Care 2014;42:191–8. 10.1177/0310057X1404200205 [DOI] [PubMed] [Google Scholar]
- 15.Chin A, Crooke B, Heywood L, et al. A randomised controlled trial comparing needle movements during combined spinal-epidural anaesthesia with and without ultrasound assistance. Anaesthesia 2018;73:466–73. 10.1111/anae.14206 [DOI] [PubMed] [Google Scholar]
- 16.Tao B, Liu K, Ding M, et al. Ultrasound increases the success rate of spinal needle placement through the epidural needle during combined spinal-epidural anaesthesia: A randomised controlled study. Eur J Anaesthesiol 2021;38:251–8. 10.1097/EJA.0000000000001380 [DOI] [PubMed] [Google Scholar]
- 17.Khan M, Gupta M, Sharma S, et al. A comparative study of ultrasound assisted versus landmark technique for combined spinal-epidural anaesthesia in patients undergoing lower limb Orthopaedic surgery. Indian J Anaesth 2022;66:272. 10.4103/ija.ija_775_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li M, Ni X, Xu Z, et al. Ultrasound-assisted technology versus the conventional landmark location method in spinal anesthesia for cesarean delivery in obese Parturients: A randomized controlled trial. Anesth Analg 2019;129:155–61. 10.1213/ANE.0000000000003795 [DOI] [PubMed] [Google Scholar]
- 19.Park S-K, Yoo S, Kim WH, et al. Ultrasound-assisted vs. landmark-guided Paramedian spinal anaesthesia in the elderly: A randomised controlled trial. Eur J Anaesthesiol 2019;36:763–71. 10.1097/EJA.0000000000001029 [DOI] [PubMed] [Google Scholar]
- 20.Tawfik MM, Atallah MM, Elkharboutly WS, et al. Does Preprocedural ultrasound increase the first-pass success rate of epidural Catheterization before cesarean delivery? A randomized controlled trial. Anesth Analg 2017;124:851–6. 10.1213/ANE.0000000000001325 [DOI] [PubMed] [Google Scholar]
- 21.Park S-K, Bae J, Yoo S, et al. Ultrasound-assisted versus landmark-guided spinal anesthesia in patients with abnormal spinal anatomy: A randomized controlled trial. Anesth Analg 2020;130:787–95. 10.1213/ANE.0000000000004600 [DOI] [PubMed] [Google Scholar]
- 22.Qu B, Chen L, Zhang Y, et al. Correction to: landmark-guided versus modified ultrasound-assisted Paramedian techniques in combined spinal-epidural anesthesia for elderly patients with hip fractures: a randomized controlled trial. BMC Anesthesiol 2020;20. 10.1186/s12871-020-01183-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ni X, Li M-Z, Zhou S-Q, et al. Accuro ultrasound-based system with computer-aided image interpretation compared to traditional Palpation technique for neuraxial anesthesia placement in obese Parturients undergoing cesarean delivery: a randomized controlled trial. J Anesth 2021;35:475–82. 10.1007/s00540-021-02922-y [DOI] [PubMed] [Google Scholar]
- 24.Chong SE, Mohd Nikman A, Saedah A, et al. Real-time ultrasound-guided Paramedian spinal anaesthesia: evaluation of the efficacy and the success rate of single needle pass. Br J Anaesth 2017;118:799–801. 10.1093/bja/aex108 [DOI] [PubMed] [Google Scholar]
- 25.Mital T, Kamal M, Kumar M, et al. Comparison of landmark and real-time ultrasound-guided epidural catheter placement in the pediatric population: a prospective randomized comparative trial. Anesth Pain Med 2021;16:368–76. 10.17085/apm.21035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pakpirom J, Thatsanapornsathit K, Kovitwanawong N, et al. Real-time ultrasound-guided versus anatomic landmark-based Thoracic epidural placement: a prospective, randomized, superiority trial. BMC Anesthesiol 2022;22:198. 10.1186/s12871-022-01730-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang YX, He M. Real-time ultrasound-guided vs. anatomical landmark-guided Paramedian epidural anesthesia in overweight Parturients undergoing analgesic labor: a randomized controlled trial. Signa Vitae 2021;17:66. [Google Scholar]
- 28.Elsharkawy H, Maheshwari A, Babazade R, et al. Real-time ultrasound-guided spinal anesthesia in patients with predicted difficult anatomy. Minerva Anestesiol 2017;83:465–73. 10.23736/S0375-9393.16.11610-4 [DOI] [PubMed] [Google Scholar]
- 29.Osman YMM. Mohamed Mohamed Osman.comparing between ultrasound-assisted epidural catheter placement using Accuro and the use of ultrasound real time with acoustic puncture-assisted device to confirm epidural space end point. Egypt J Anaesth 2020;36:140–6. 10.1080/11101849.2020.1801296 [DOI] [Google Scholar]
- 30.Bhardwaj D, Thakur L, Sharma S, et al. Comparative evaluation of three techniques for Paramedian subarachnoid block: point-of-care Preprocedural ultrasound assisted, real-time ultrasound guided and landmark based. Indian J Anaesth 2022;66:102. 10.4103/ija.ija_373_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nassar M, Abdelazim IA. Pre-puncture ultrasound guided epidural insertion before vaginal delivery. J Clin Monit Comput 2015;29:573–7. 10.1007/s10877-014-9634-y [DOI] [PubMed] [Google Scholar]
- 32.Rasoulian A, Lohser J, Najafi M, et al. Utility of Prepuncture ultrasound for localization of the Thoracic epidural space. Can J Anaesth 2011;58:815–23. 10.1007/s12630-011-9548-9 [DOI] [PubMed] [Google Scholar]
- 33.Hurdle M-F. Ultrasound-guided spinal procedures for pain: A review. Phys Med Rehabil Clin N Am 2016;27:673–86. 10.1016/j.pmr.2016.04.011 [DOI] [PubMed] [Google Scholar]
- 34.Grau T, Leipold RW, Conradi R, et al. Efficacy of ultrasound imaging in obstetric epidural anesthesia. J Clin Anesth 2002;14:169–75. 10.1016/s0952-8180(01)00378-6 [DOI] [PubMed] [Google Scholar]
- 35.Grau T, Leipold RW, Conradi R, et al. Ultrasound imaging facilitates localization of the epidural space during combined spinal and epidural anesthesia. Reg Anesth Pain Med 2001;26:64–7. 10.1053/rapm.2001.19633 [DOI] [PubMed] [Google Scholar]
- 36.Institute of Health and Clinical Excellence . Ultrasound guided regional nerve block (IPG285); 2013Jan.
- 37.Chin KJ, Ramlogan R, Arzola C, et al. The utility of ultrasound imaging in predicting ease of performance of spinal anaesthesia in an orthopedic patient population. Reg Anesth Pain Med 2013;38:34–8. 10.1097/AAP.0b013e3182734927 [DOI] [PubMed] [Google Scholar]
- 38.Vogt M, van Gerwen DJ, Lubbers W, et al. Optimal point of insertion and needle angle in neuraxial blockade using a midline approach: a study in computed tomography scans of adult patients. Reg Anesth Pain Med 2017;42:600–8. 10.1097/AAP.0000000000000653 [DOI] [PubMed] [Google Scholar]
- 39.Kim H, Won D, Chang J-E, et al. Ultrasound assessment of the anatomic landmarks for spinal anesthesia in elderly patients with hip fracture: a prospective observational study. Medicine 2019;98:e16388. 10.1097/MD.0000000000016388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karmakar MK, Li X, Ho AM-H, et al. Real-time ultrasound-guided Paramedian epidural access:evaluation of a novel in-plane technique. Br J Anaesth 2009;102:845–54. 10.1093/bja/aep079 [DOI] [PubMed] [Google Scholar]
- 41.Conroy PH, Luyet C, McCartney CJ, et al. Real-time ultrasound-guided spinal anaesthesia: a prospective observational study of a new approach. Anesthesiol Res Pract 2013;2013:525818. 10.1155/2013/525818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Y, Qian W, Ke X, et al. Real-time ultrasound-guided spinal anesthesia using a new Paramedian transverse approach. CURR MED SCI 2018;38:910–3. 10.1007/s11596-018-1961-7 [DOI] [PubMed] [Google Scholar]
- 43.Shaikh F, Brzezinski J, Alexander S, et al. Ultrasound imaging for lumbar punctures and epidural Catheterisations: systematic review and meta-analysis. BMJ 2013;346:bmj.f1720. 10.1136/bmj.f1720 [DOI] [PubMed] [Google Scholar]
- 44.Celleno D, Gollo E, Uskok M. Analgesia anestesia terapia intensiva in ostetricia. Torino: Edizioni Minerva Medica, 2006. [Google Scholar]
- 45.Tran D, Kamani AA, Al-Attas E, et al. Single-operator real-time ultrasound-guidance to aim and insert Alumbar epidural needle. Can J Anaesth 2010;57:313–21. 10.1007/s12630-009-9252-1 [DOI] [PubMed] [Google Scholar]
- 46.Park S-K, Cheun H, Kim Y-W, et al. Ultrasound-assisted spinal anesthesia: A randomized comparison between midline and Paramedian approaches. J Clin Anesth 2022;80:110823. 10.1016/j.jclinane.2022.110823 [DOI] [PubMed] [Google Scholar]
- 47.Perna P, Gioia A, Ragazzi R, et al. Can pre-procedure Neuroaxial ultrasound improve the identification of the potential epidural space when compared with anatomical landmarks? A prospective randomized study. Minerva Anestesiol 2017;83:41–9. 10.23736/S0375-9393.16.11399-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-071253supp001.pdf (1.2MB, pdf)
Data Availability Statement
No data are available.