Abstract
The ability of caseins to fulfill the amino acid requirements of Lactococcus lactis for growth was studied as a function of the type of cell envelope proteinase (PI versus PIII type). Two genetically engineered strains of L. lactis that differed only in the type of proteinase were grown in chemically defined media containing αs1-, β-, and κ-caseins (alone or in combination) as the sources of amino acids. Casein utilization resulted in limitation of the growth rate, and the extent of this limitation depended on the type of casein and proteinase. Adding different mixtures of essential amino acids to the growth medium made it possible to identify the nature of the limitation. This procedure also made it possible to identify the amino acid deficiency which was growth rate limiting for L. lactis in milk (S. Helinck, J. Richard, and V. Juillard, Appl. Environ. Microbiol. 63:2124–2130, 1997) as a function of the type of proteinase. Our results were compared with results from previous in vitro experiments in which casein degradation by purified proteinases was examined. The results were in agreement only in the case of the PI-type proteinase. Therefore, our results bring into question the validity of the in vitro approach to identification of casein-derived peptides released by a PIII-type proteinase.
Lactococci have numerous nutritional requirements for growth; in particular, nitrogen sources are required (13, 23, 33), because these organisms have a limited capacity to synthesize amino acids (3). Therefore, growth of Lactococcus lactis depends on the amino acids available in the culture medium. In milk, the concentrations of several essential amino acids, especially Ile, Leu, and Met, are very low (less than 1 mg/liter) (17, 26). On the other hand, only a small fraction of the peptides that are present in milk are utilized during growth (15, 17). In addition, the utilizable peptides are a poor source of Leu and Met (15). Consequently, caseins are the main source of amino acids and are responsible for about 90% of the growth of L. lactis in milk (17, 26). Casein utilization by L. lactis is mediated by a complex proteolytic system, which consists of a cell envelope proteinase, the oligopeptide transport system, and several intracellular peptidases (16, 19, 28). The proteinase is involved in the first step of casein degradation. Only some of the oligopeptides released by the proteinase are taken up by the oligopeptide transport system and subsequently cleaved into amino acids by intracellular peptidases (20, 21).
Two different types of proteinase (PI and PIII types) have been identified in lactococci on the basis of their specificity for caseins (30, 31, 37). PI-type proteinase cleaves β-casein preferentially, κ-casein to a lesser extent, and αs1-casein insignificantly. In contrast, PIII-type proteinase cleaves β-, κ-, and αs1-caseins equally well. The in vitro activity of purified proteinases on caseins has been studied extensively over the last few years (19). Most, if not all, of the peptides released by degradation of β-casein by purified PI-type proteinase have been identified (18). Electrophoretic (37) and reverse-phase high-performance liquid chromatography (HPLC) (31) studies have shown that the two types of proteinase cleave β-casein at significantly different cleavage sites.
Surprisingly, very little is known about the consequences of these differences on the growth of L. lactis in milk or in casein-containing media. A previous study showed that optimal growth of L. lactis is related to β- and κ-casein degradation (6). On the other hand, Kunji and coworkers (21) reported that poor growth of L. lactis occurs in a culture medium containing β-casein as the sole source of amino acids. However, these results were obtained with PI-type-proteinase-producing strains. No clear information concerning the ability of PIII-type-proteinase-producing strains to use the different caseins as sources of amino acids is available.
Two recent studies showed that the type of proteinase may influence the growth of L. lactis since (i) increasing the proteolytic activity of L. lactis cultures in milk by adding a purified lactococcal proteinase resulted in different effects, depending on the type of proteinase (11), and (ii) the associative growth of L. lactis in milk was influenced mainly by the type of proteinase produced by cocultured strains (7). The aim of the present study was to analyze the utilization of each type of casein (alone or in combination) as a source of essential amino acids for growth of L. lactis strains with different types of proteinase.
MATERIALS AND METHODS
Strains and culture conditions.
Construction of the proteinase-negative (Prt−), lactose-negative (Lac−), plasmid-free strain L. lactis MG1363 and construction of the Prt+ Lac+ strains L. lactis MG611-1 (PI-type proteinase) and SH5-1 (PIII-type proteinase) have been described elsewhere (2, 8, 11, 25). The two Prt+ strains were derived from L. lactis MG1363; they differed only in the type of proteinase that they produced. Therefore, the three strains had identical nitrogen requirements; Gln, His, Met, Leu, Ile, and Val were essential amino acids. It was also determined that the three strains had identical peptidolytic and transport abilities, as previously described (1, 7). The strains were stored at −80°C in M17 broth (35) containing glycerol (10%, vol/vol) and 5 μg of erythromycin per ml (for MG611-1) or 5 μg of chloramphenicol per ml (for SH5-1).
Cells were grown at 30°C in reconstituted skim milk (10% [wt/wt] Nilac Low Heat milk powder; Netherlands Dairy Research Institute, Ede, The Netherlands) or in chemically defined medium (CDM) (29). When required (Lac− strain), milk was supplemented with glucose (10 mg/ml). Pure caseins were added alone or in combination to the CDM as sources of amino acids at a final concentration of 2.4 g/liter. β-Casein and κ-casein were obtained from Sigma Chemical Co. (St. Louis, Mo.). The results obtained with these commercial caseins were confirmed by using β- and κ-caseins purified in our laboratory as previously described (9). αs1-Casein was purified from skim milk by isoelectric precipitation, anion-exchange chromatography, and hydrophobic interaction chromatography as previously described (24). When added as a mixture, the αs1-, β-, and κ-caseins were added at a ratio corresponding to the ratio in milk (i.e., 6:5:2). Culture media were inoculated with approximately 7 × 106 CFU of a preculture of the test strain in the exponential stage of growth in M17 broth per ml. Cells were washed twice in sterile 50 mM KH2PO4-K2HPO4 (pH 6.8) prior to inoculation.
Bacterial enumeration and statistical analysis.
The chains of lactococci were first disrupted for 30 s with a mechanical blender (Ultra-Turrax model T25; Janke and Kunkel, Staufen, Germany). Cell populations were then estimated by plating appropriate dilutions of each culture on M17 agar with a spiral plater (Spiral System, Cincinnati, Ohio). The accuracy and precision of this plating method have been described previously (10). All growth experiments were repeated three times, unless otherwise stated. Growth rates (μ) were calculated from the slopes (log10 CFU per milliliter per hour) by using the following formula: μ = slope/log10 2 (27). Confidence limits (P = 0.95) of the mean growth rates were calculated as described by Snedecor and Cochran (34), as follows: (t × SD)/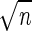 , where t is obtained from the t distribution table (t0.95 = 4.303 in the case of three repetitions), SD is the standard deviation of the mean growth rate, and n is the number of repetitions.
, where t is obtained from the t distribution table (t0.95 = 4.303 in the case of three repetitions), SD is the standard deviation of the mean growth rate, and n is the number of repetitions.
Proteinase isolation.
The Prt+ L. lactis strains were grown in milk to the end of the exponential growth phase, removed from the culture medium by centrifugation (10,000 × g for 10 min at 4°C), and washed three times in sterile 50 mM Tris-HCl (pH 8) containing 30 mM CaCl2. The proteinase was released from the cells by incubation for 30 min at 30°C in Ca2+-free buffer (22) and was purified by ion-exchange chromatography as previously described (18). The proteolytic activity of the proteinase fractions was determined by using the chromogenic peptide methoxy-succinyl-l-arginyl-l-prolyl-l-tyrosine-p-nitroanilide (Chromogenix, Möldaln, Sweden) or fluorescein isothiocyanate-labeled casein (Sigma) as the substrate (4, 36).
Milk peptide analysis.
Cells were removed by centrifugation (10,000 × g for 10 min at 4°C), and proteins were precipitated with 1% (vol/vol) trifluoroacetic acid (TFA). After the proteins were removed by centrifugation (10,000 × g for 10 min at 4°C), the supernatant was filtered through a 0.45-μm-pore-size filter (Millipore Corp., Bedford, Mass.). The 1% TFA-soluble peptides were separated at 40°C by HPLC on a reverse-phase C18 column (Nucleosil; 4.6 by 250 mm; Shandon HPLC, Cheshire, United Kingdom). Solvents A and B were 0.11% (vol/vol) TFA in MilliQ-treated water (Millipore) and 0.1% (vol/vol) TFA–60% (vol/vol) acetonitrile in MilliQ-treated water, respectively. A 40-min linear 0 to 60% solvent B gradient with a flow rate of 1 ml/min was used. The eluted peptides were detected by on-line absorbance at 214 nm and fluorescence after postcolumn derivatization of the eluted peptides with o-phthalaldehyde, as previously described (12). UV detection was monitored prior to peptide derivatization. For detection of fluorescence the excitation and emission wavelengths were 340 and 425 nm, respectively.
RESULTS
Growth of L. lactis in milk.
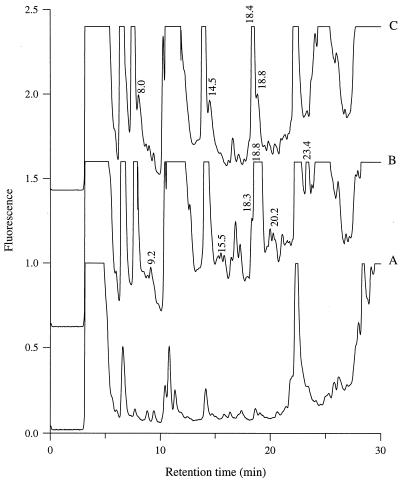
As reported previously for several lactococci (7, 11, 17), the genetically engineered strains L. lactis MG611-1 (PI-type proteinase) and L. lactis SH5-1 (PIII-type proteinase) displayed biphasic exponential growth in milk, with the change to the lower growth rate corresponding to the utilization of caseins as an amino acid source (17). The concentrations of 1% TFA-soluble peptides in the milk before and after growth of the two strains were determined by reverse-phase HPLC. The peptide content of the milk changed drastically during growth and depended on the type of proteinase (Fig. 1). For instance, the peak eluting at 14.5 min was detected at the end of growth of L. lactis SH5-1, whereas no corresponding peptide(s) was present in the milk cultured with L. lactis MG611-1. Moreover, there were quantitative differences in the relative proportions of closely eluting peptides (for instance, peptides eluting at retention times of 18.4 and 18.8 min). As expected, these differences were detected only during the second phase of growth, as caseins were utilized as the source of amino acids during this phase (17).
FIG. 1.
(A) Peptide chromatogram for uninoculated milk. (B and C) Peptide chromatograms for milk cultures of L. lactis MG611-1 (PI-type proteinase) (B) and SH5-1 (PIII-type proteinase) (C) grown to the stationary phase.
Despite marked differences in the peptide contents of the milk following growth of L. lactis MG611-1 and SH5-1, no difference in the growth kinetics of the two strains was observed, as previously reported (7, 11). In particular, there was no significant difference (P < 0.01) between the growth rates of the two strains during the second exponential phase (0.75 ± 0.06 and 0.74 ± 0.07 h−1, respectively).
Hydrolysis of milk with purified proteinase.
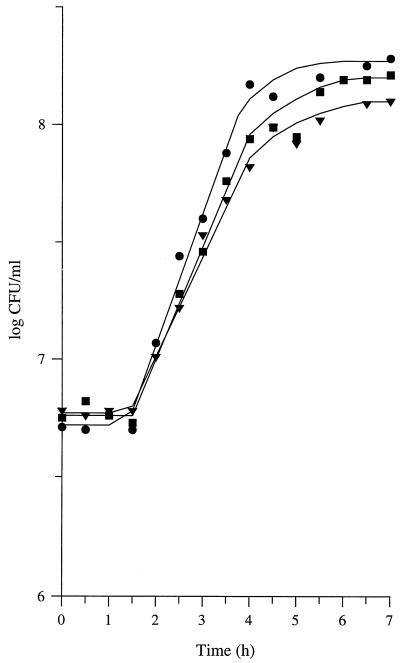
To analyze further the growth of L. lactis as a function of the type of proteinase, a complementary approach was used. Milk was incubated for 2 h with either PI- or PIII-type purified proteinase prior to inoculation with the Prt− strain L. lactis MG1363. Predigestion of milk caseins with purified proteinase stimulated the growth of L. lactis MG1363 (Fig. 2). However, the extent of the stimulation depended on the type of proteinase; the increases in the growth rate were 17% ± 6% and 37% ± 10% with the PI- and PIII-type proteinases, respectively (means of three repetitions ± confidence limits; P = 0.95). Similarly, there were differences in the extent of the increases in the maximal populations of L. lactis MG1363.
FIG. 2.
Growth of L. lactis MG1363 (proteinase negative) in control milk (▾) and in milk previously incubated for 2 h with PI-type purified proteinase (▪) or PIII-type purified proteinase (•). PI- and PIII-type proteinases were isolated from L. lactis MG611-1 and SH5-1, respectively, and were added to the milk at the same activity (4% of a 0.4% solution of fluorescein isothiocyanate-labeled casein was hydrolyzed within 1 h).
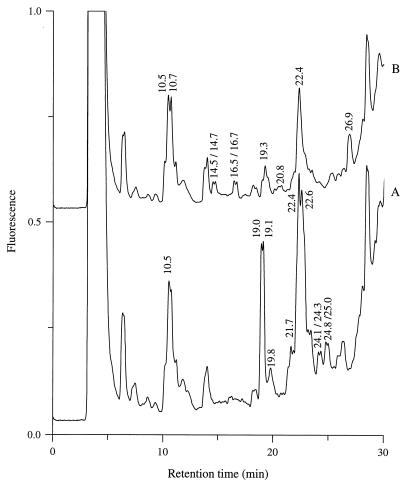
As expected, the peptide content of the milk after incubation with purified proteinase depended on the type of enzyme added (Fig. 3). Interestingly, the six HPLC peaks produced only by the PIII-type proteinase (at retention times of 14.5, 14.7, 16.5, 16.7, 19.3, and 26.9 min) were not detected at the end of growth of the Prt− strain. In contrast, only three of the peaks which were specifically produced by the PI-type proteinase (at retention times of 19.1, 24.1, and 24.8 min) disappeared during growth of the proteinase-negative strain (data not shown).
FIG. 3.
Peptide chromatograms for milk following 2 h of incubation with PI-type (A) or PIII-type (B) purified proteinase. PI- and PIII-type proteinases were isolated from L. lactis MG611-1 and SH5-1, respectively.
Utilization of caseins as the sole source of amino acids.
The results described above suggest that the amino acids obtained from caseins may be influenced by the type of proteinase. To confirm this, L. lactis MG611-1 (PI-type proteinase) and SH5-1 (PIII-type proteinase) were cultured in CDM containing αs1-, β-, or κ-casein or CDM containing combinations of caseins as the sole sources of amino acids.
The ability of individual caseins to support growth of L. lactis did not depend on the type of proteinase, except for β-casein (Table 1). No growth was observed in the presence of αs1-casein alone, indicating that at least one essential amino acid was not present in the casein-derived peptides that the strains were able to translocate. In contrast, all of the essential amino acids required by the strains were present in the peptides released from κ-casein, regardless of the type of proteinase. However, the growth rates were significantly lower than those in the presence of a mixture of 19 free amino acids, suggesting that they were limited by the rate at which amino acids were supplied.
TABLE 1.
Growth of L. lactis MG611-1 (PI-type proteinase) and L. lactis SH5-1 (PIII-type proteinase) in CDM containing caseins as the sole sources of amino acids
| Source of amino acidsa | Growth rate (h−1)b
|
|
|---|---|---|
| L. lactis MG611-1 | L. lactis SH5-1 | |
| αs1-Casein | NGc | NG |
| β-Casein | NG | 0.09 ± 0.03 |
| κ-Casein | 0.77 ± 0.10 | 0.59 ± 0.08 |
| αs1-Casein + β-casein | 0.30 ± 0.09 | 0.12 ± 0.04 |
| αs1-Casein + κ-casein | 0.33 ± 0.03 | 0.40 ± 0.05 |
| β-Casein + κ-casein | 0.91 ± 0.13 | 0.50 ± 0.10 |
| αs1-Casein + β-casein + κ-casein | 0.62 ± 0.04 | 0.53 ± 0.06 |
| 19 free amino acids | 1.39 ± 0.06 | 1.35 ± 0.07 |
Caseins were added to a final concentration of 2.4 g/liter. When a mixture was used, the ratio of αs1-, β-, and κ-casein was the ratio in milk (i.e., 6:5:2). The amino acid concentration was the amino acid concentration established by Poolman and Konings (29).
Mean of three determinations ± confidence limits at P = 0.95.
NG, no growth.
Both L. lactis MG611-1 and SH5-1 grew to some extent in the presence of mixtures of caseins, but the growth rates were lower than the growth rates in the presence of free amino acids. Complementation between caseins as sources of amino acids was observed only with the PI-type proteinase; the growth rate of L. lactis MG611-1 in the presence of a mixture of αs1- and β-caseins or a mixture of β- and κ-caseins was higher than the growth rates obtained with individual caseins. In contrast, the growth rate of L. lactis in the presence of both αs1- and κ-caseins was significantly lower (P < 0.05) than the growth rate in the presence of κ-casein alone, regardless of the type of proteinase. This suggests that the αs1-casein-derived peptides had an inhibitory effect on the growth rate, which was partially overcome by adding β-casein to the mixture.
Nature of growth rate limitation.
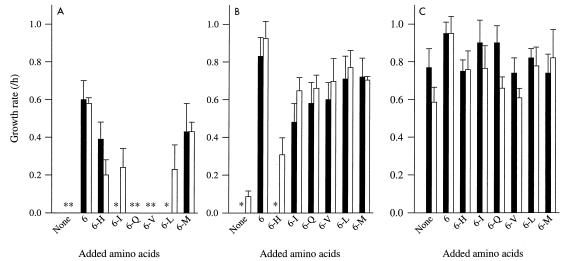
Experiments in which different combinations of five of six essential amino acids were added to CDM containing individual caseins made it possible to identify the amino acid deficiency which was responsible for the lack of growth of L. lactis when individual caseins were used as the sole sources of amino acids. For instance, the inability of β-casein to support growth of L. lactis MG611-1 was due to a lack of His-containing peptides (Fig. 4). αs1-Casein was the poorest source of essential amino acids for L. lactis, regardless of the type of proteinase. Several essential amino acids were not provided. Surprisingly, the growth rate in the presence of αs1-casein supplemented with the six essential amino acids was significantly (P < 0.05) lower than the growth rate in the presence of β- or κ-casein supplemented with the same mixture of amino acids, whatever strain was used. In addition, the growth rate in the presence of αs1-casein and the six essential amino acids was also lower than the growth rate observed during growth in the presence of only the essential amino acids (0.93 ± 0.07 h−1 for both strains). These results are consistent with the previously suggested hypothesis that the αs1-casein-derived peptides inhibit the growth rate in some way.
FIG. 4.
Growth of L. lactis in CDM containing αs1-casein (A), β-casein (B), or κ-casein (C) and supplemented with different mixtures of amino acids as nitrogen sources. Solid bars, L. lactis MG611-1 (PI-type proteinase); open bars, L. lactis SH5-1 (PIII-type proteinase). 6, mixture of His (H), Ile (I), Gln (Q), Val (V), Leu (L), and Met (M); 6-X, mixture lacking amino acid X. The amino acid concentrations were the amino acid concentrations in CDM (27), and the casein concentration was 2.4 g/liter. Error bars indicate confidence limits at P = 0.95. Asterisks indicate that no growth occurred.
It has been demonstrated previously that the rate of casein hydrolysis limits the growth rate of L. lactis in milk or in casein-containing media (11). Thus, a comparison of the growth rates in CDM containing individual caseins and the growth rates in CDM supplemented with different combinations of the essential amino acids should provide information concerning the rate of production of peptides containing each of the essential amino acids. For instance, the growth rate in CDM containing β-casein and a mixture of all of the essential amino acids except Ile depends on the rate of release of Ile-containing peptides from β-casein. In this medium, the growth rate of L. lactis MG611-1 was lower than the growth rate of L. lactis SH5-1, indicating that the amount of Ile-containing peptides released by the PI-type proteinase limits the growth rate to a greater extent than the amount of Ile-containing peptides released by the PIII-type proteinase. Limitation of the growth rate in CDM containing β-casein was almost entirely overcome by adding His, Ile, and Gln, with the growth rate equivalent to 92 and 88% of the growth rates of L. lactis MG611-1 and SH5-1 in CDM containing β-casein and the six essential amino acids, respectively. Similarly, the availability of His, Val, and Met in peptides released from κ-casein limited the growth of L. lactis MG611-1 because (i) addition of a mixture lacking one of these amino acids did not increase the growth rate and (ii) addition of His, Val, and Met to the culture medium slightly increased the growth rate (0.83 ± 0.06 h−1, compared with 0.77 ± 0.10 h−1 without amino acid addition). The limitation of the growth rate of L. lactis SH5-1 in CDM containing κcasein was due to a deficiency of Val- and Gln-containing peptides; addition of these two amino acids resulted in a 30% increase in the growth rate of L. lactis SH5-1.
Quantitation of the limitation of the growth rate of L. lactis by individual caseins also made it possible to explain the previously observed complementation between caseins as the sole source of essential amino acids. For instance, hydrolysis of β-casein by L. lactis MG611-1 did not provide His-containing peptides but produced Ile-containing peptides. In contrast, hydrolysis of αs1-casein by the same strain released His-containing peptides but no Ile-containing peptides. Therefore, complementation between these two types of caseins could be expected. Moreover, the limitation of the growth rate by Ile-containing peptides released from β-casein was less than the limitation of the growth rate by His-containing peptides derived from αs1-casein (Fig. 4). Therefore, we expected that the growth rate of L. lactis MG611-1 in CDM containing both β- and αs1-caseins as the sole source of amino acids would be limited by the amount of His-containing peptides. Consequently, this growth rate should be in the same range as the growth rate in CDM containing αs1-casein and a mixture of all of the essential amino acids except His. The respective growth rates were 0.30 ± 0.09 and 0.39 ± 0.09 h−1.
In contrast, no complementation between peptides released from β- and αs1-caseins was expected with the PIII-type proteinase, although all of the amino acid deficiencies of the peptides released from αs1-casein could be overcome by peptides derived from β-casein. Both β-casein and αs1-casein released a growth-limiting amount of His-containing peptides. The growth rate in CDM containing αs1-casein and a mixture of all of the essential amino acids except His was lower than the growth rate in CDM containing β-casein and the same mixture of amino acids (Fig. 4). Consequently, the growth rate of L. lactis SH5-1 in the presence of β- and αs1-caseins as the sole source of amino acids should be in the same range as the growth rate in CDM containing only β-casein. This was observed, with growth rates of 0.12 ± 0.04 and 0.09 ± 0.03 h−1, respectively.
DISCUSSION
The use of genetically engineered strains of L. lactis that differed only in the type of proteinase produced made it possible to study the contribution of caseins to the amino acid supply. Growth experiments showed that κ-casein is the best source of amino acids for growth and that a mixture of β- and κ-caseins results in high growth rates of L. lactis strains containing either PI- or PIII-type proteinase. This is consistent with results reported previously for the PI-type-proteinase-producing strain L. lactis subsp. cremoris HP (6) and suggests that a mixture of β- and κ-caseins fulfills the amino acid requirements of any L. lactis strain.
Biochemical and physiological analyses of casein hydrolysis led to opposite conclusions. Biochemical studies on the specificity of casein hydrolysis by purified proteinases indicated that (i) β-casein is the preferred substrate for PI-type proteinases and (ii) αs1-casein is not significantly cleaved by PI-type proteinases (31, 37). From the present study, it is clear that β-casein is not the optimum source of amino acids for L. lactis. In addition, αs1-casein provides His and Met to a PI-type-proteinase-containing strain. The biochemical approach focuses mainly on the amount of substrate that is hydrolyzed; degradation of a small amount of caseins is considered insignificant, regardless of the nature of the peptides released. In contrast, the physiological approach focuses on only some of the released peptides (i.e., the peptides that can be translocated into the cell by the oligopeptide transport system). Because growth of L. lactis in milk up to the maximum yield (e.g., 2 × 109 CFU/ml) requires the synthesis of approximately 200 μg of bacterial proteins (14), hydrolysis of only 1% of the milk caseins should be sufficient to sustain maximum growth.
Growth of L. lactis in casein-containing media is limited by the rate of casein hydrolysis, regardless of the type of proteinase (11). Because translocated peptides are instantaneously cleaved to amino acids by intracellular peptidases (20), the amino acid deficiency responsible for the growth rate limitation depends on the ability of the strain to transport casein-derived peptides. Elucidation of the specificity of oligopeptide utilization by L. lactis MG1363 (15) makes it possible to compare our results with the results of in vitro analyses of peptides released from caseins by purified proteinases (18, 30–32). The ability of κ-casein to support growth is consistent with the observation that hydrolysis of κ-casein by purified PI- and PIII-type-proteinase resulted in the early release of four and three different oligopeptides, respectively, which contained all of the amino acids required for growth of L. lactis MG1363 (30). In the present study, significant differences in the nature of growth limitation were observed for the two types of proteinases. The peptides initially released from κ-casein by a purified PI-type proteinase are QILQWQVL, ARHPHPHLSFM, LSFM, and (to a lesser extent) KYIPIQYVL (30). Only one of these peptides, KYIPIQYVL, is expected to be translocated rapidly into cells by the oligopeptide transport system, because it is a basic peptide whose molecular weight ranges from 600 to 1,100 (15). This peptide does not provide His or Met to the cells, but it contains two Ile residues. In contrast, the three other peptides should be transported at lower rates, either because they are not basic or because their molecular weights are not in the optimum range (15). These three peptides contain few Val residues. Therefore, the growth rate of L. lactis MG611-1 in CDM containing κ-casein should be limited by the rate at which Met, Val, and His are supplied. This expectation is consistent with the data obtained from growth experiments. In contrast, the same approach suggests that the growth rate of L. lactis SH5-1 (PIII-type proteinase) in CDM containing κ-casein should be limited by the rate at which Met and Ile are supplied, because the peptides initially released by the purified proteinase are AVRSPAQILQWQVL (molecular weight, 1,609; pI 11.3), ARHPHPHLSFM (molecular weight, 1,329; pI 11.3), and TVQVTSTAV (molecular weight, 905; pI 6.1) (30). This is not consistent with our observation that growth was limited by the rate at which Val and Gln were produced. There are two hypotheses that can be used to explain this discrepancy. First, some other peptides which have not been detected and/or identified in previous studies, may be released early from κ-casein by purified proteinase. Consequently, the present estimates of amino acid supply may be incorrect. The other possible explanation is that the specificity of peptide bond cleavage with a purified PIII-type proteinase might differ significantly from the specificity of peptide bond cleavage with a native PIII-type proteinase (i.e., a proteinase bound to the cell envelope). It is worth noting that the PIII-type proteinase has been reported to cleave the 1-23 fragment of αs1-casein in a different manner when it was used as a purified enzyme or bound to the cell envelope (5). Similarly, the identities of growth-limiting amino acids obtained from growth experiments performed with L. lactis MG611-1 in CDM containing β-casein were also consistent with the identities deduced from an analysis of the peptides released from β-casein by purified PI-type proteinase (18, 31). In contrast, there was a discrepancy between the results of growth experiments performed in CDM containing αs1- or β-casein and the results of an analysis of the products of hydrolysis of αs1- and β-caseins by purified PIII-type proteinase (31, 32).
The contribution of caseins to the amino acid supply of L. lactis depends on the type of proteinase. As a result, L. lactis MG611-1 (PI-type proteinase) grows at a higher rate than L. lactis SH5-1 (PIII-type proteinase) in CDM containing a mixture of caseins. However, the two strains grow at the same rate in milk. The growth rate in milk during the second phase (i.e., the phase corresponding to casein utilization) (17) is significantly higher than the growth rate in CDM containing caseins. Therefore, the data suggest that there is complementation between peptides released from caseins and the other sources of amino acids in milk, (i.e., the free amino acids and the peptides that are initially present in milk). Moreover, the complementation should be more efficient with the PIII-type proteinase than with the PI-type proteinase. As L. lactis MG1363, MG611-1, and SH5-1 differ only in the proteinases that they produce, the causes of the growth arrest of L. lactis MG1363 (Prt− variant) are similar to the causes of the change in the growth rates of the Prt+ strains. The growth of L. lactis MG1363 in milk stops because the milk lacks sources of Met, Ile, Leu, and Val other than caseins (15). The growth of L. lactis MG611-1 (PI-type proteinase) in CDM containing caseins as the sole source of amino acids is limited by the availability of His, Met, and Val. Therefore, the second growth phase of L. lactis MG611-1 in milk should be limited by the amount of Met and Val. Addition of these two amino acids to milk resulted in a 20% (± 4%) stimulation of the growth rate during the second exponential growth phase (mean of four repetitions ± confidence limits; P = 0.95). On the other hand, κ-casein was found to be the main source of Met and Val for L. lactis MG611-1. As the growth of L. lactis in milk is limited by the rate of casein hydrolysis (11), the growth rate in the second exponential growth phase of L. lactis MG611-1 should be limited by the rate of κ-casein hydrolysis. Consequently, it should be in the same range as the growth rate in CDM containing κ-casein and a mixture of essential amino acids without Met and/or Val (i.e., 0.74 h−1). This is in full agreement with our observations; the growth rate of L. lactis MG611-1 during the second growth phase in milk was 0.75 ± 0.06 h−1. Similarly, the growth rate of L. lactis SH5-1 (PIII-type proteinase) in CDM containing caseins was limited by the rate at which Val was supplied. Therefore, the second growth phase of this strain in milk should be limited by the amount of Val. Addition of this amino acid to the milk resulted in a 32% (± 5%) stimulation of the growth rate during the second exponential growth phase (mean of four repetitions ± confidence limits; P = 0.95). As the main source of Val for L. lactis SH5-1 is β-casein, the growth rate in milk during the second phase should be in the same range as the growth rate in CDM containing β-casein and a mixture of all of the essential amino acids except Val. Again, that is what was observed.
In conclusion, the contribution of bovine milk caseins to the amino acid supply for L. lactis depends on the type of cell envelope proteinase. Because the growth rate of L. lactis in milk is limited by the rate of casein hydrolysis (11), the nature of the growth rate limitation in milk depends on the type of proteinase produced.
ACKNOWLEDGMENTS
B. Flambard and S. Helinck contributed equally to this work. We thank D. Le Bars for help with αs1-casein purification.
REFERENCES
- 1.Bellengier P, Richard J, Foucaud C. Nutritional requirements of Leuconostoc mesenteroides subsp. mesenteroides and subsp. dextranicum for growth in milk. J Dairy Res. 1997;64:95–103. [Google Scholar]
- 2.Bruinenberg P G, Vos P, de Vos W. Proteinase overproduction in Lactococcus lactis strains: regulation and effect on growth and acidification in milk. Appl Environ Microbiol. 1992;58:78–84. doi: 10.1128/aem.58.1.78-84.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chopin A. Organization and regulation of genes for amino acid biosynthesis in lactic acid bacteria. FEMS Microbiol Rev. 1993;12:21–38. doi: 10.1111/j.1574-6976.1993.tb00011.x. [DOI] [PubMed] [Google Scholar]
- 4.Exterkate F A. Differences in short peptide-substrate cleavage by two cell-envelope-located serine proteinases of Lactococcus lactis subsp. cremoris are related to secondary binding specificity. Appl Microbiol Biotechnol. 1990;33:401–406. doi: 10.1007/BF00176654. [DOI] [PubMed] [Google Scholar]
- 5.Exterkate F A, Alting A C. The conversion of the αs1-casein-(1-23)-fragment by the free and bound form of the cell-envelope proteinase of Lactococcus lactis subsp. cremoris under conditions prevailing in cheese. Syst Appl Microbiol. 1993;16:1–8. [Google Scholar]
- 6.Exterkate F A, de Veer G J C M. Optimal growth of Streptococcus cremoris HP in milk is related to β- and κ-casein degradation. Appl Microbiol Biotechnol. 1987;25:471–475. [Google Scholar]
- 7.Flambard B, Richard J, Juillard V. Interaction between proteolytic strains of Lactococcus lactis influenced by different types of proteinase during growth in milk. Appl Environ Microbiol. 1997;63:2131–2135. doi: 10.1128/aem.63.6.2131-2135.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gasson M J. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J Bacteriol. 1983;154:1–9. doi: 10.1128/jb.154.1.1-9.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guillou H, Miranda G, Pelissier J-P. Analyse quantitative des caséines dans le lait de vache par chromatographie liquide rapide d’échange d’ions (FPLC) Lait. 1987;67:135–148. [Google Scholar]
- 10.Hassan A I, Deschamps N, Richard J. Précision des mesures de vitesse de croissance des streptocoques lactiques mésophiles dans le lait basées sur la méthode de dénombrement microbien par formation de colonies. Etude de référence avec Lactococcus lactis. Lait. 1989;69:433–447. [Google Scholar]
- 11.Helinck S, Richard J, Juillard V. The effects of adding lactococcal proteinase on the growth rate of Lactococcus lactis in milk depend on the type of the enzyme. Appl Environ Microbiol. 1997;63:2124–2130. doi: 10.1128/aem.63.6.2124-2130.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herraiz T, Casal V, Polo M C. Reverse-phase HPLC analysis of peptides in standard and dairy samples using one-line absorbance and post-column OPA-fluorescence detection. Z Lebensm Unters Forsch. 1994;199:265–269. doi: 10.1007/BF01193309. [DOI] [PubMed] [Google Scholar]
- 13.Jensen P R, Hammer K. Minimal requirements for exponential growth of Lactococcus lactis. Appl Environ Microbiol. 1993;59:4363–4366. doi: 10.1128/aem.59.12.4363-4366.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Juillard, V. Unpublished results.
- 15.Juillard V, Guillot A, Le Bars D, Gripon J-C. Specificity of milk peptide utilization by Lactococcus lactis. Appl Environ Microbiol. 1998;64:1230–1236. doi: 10.1128/aem.64.4.1230-1236.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Juillard V, Foucaud C, Desmazeaud M, Richard J. Utilisation des sources d’azote du lait par Lactococcus lactis. Lait. 1996;76:13–24. [Google Scholar]
- 17.Juillard V, Le Bars D, Kunji E R S, Konings W N, Gripon J-C, Richard J. Oligopeptides are the main source of nitrogen for Lactococcus lactis during growth in milk. Appl Environ Microbiol. 1995;61:3024–3030. doi: 10.1128/aem.61.8.3024-3030.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Juillard V, Laan H, Kunji E R S, Jeronimus-Stratingh C M, Bruins A P, Konings W N. The extracellular PI-type proteinase of Lactococcus lactis hydrolyzes β-casein into more than one hundred different oligopeptides. J Bacteriol. 1995;177:3472–3478. doi: 10.1128/jb.177.12.3472-3478.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kunji E R S, Mireau I, Hagting A, Poolman B, Konings W N. The proteolytic systems of lactic acid bacteria. Antonie Leeuwenhoek. 1996;70:187–221. doi: 10.1007/BF00395933. [DOI] [PubMed] [Google Scholar]
- 20.Kunji E R S, Mierau I, Poolman B, Kok J, Konings W N, Venema G. The fate of peptides in peptidase deficient mutants of Lactococcus lactis. Mol Microbiol. 1996;21:123–131. doi: 10.1046/j.1365-2958.1996.6231339.x. [DOI] [PubMed] [Google Scholar]
- 21.Kunji E R S, Hagting A, de Vries C J, Juillard V, Haandrikman A J, Poolman B, Konings W N. Transport of β-casein derived peptides by the oligopeptide transport system is a crucial step in the proteolytic pathway of Lactococcus lactis. J Biol Chem. 1995;270:1569–1574. doi: 10.1074/jbc.270.4.1569. [DOI] [PubMed] [Google Scholar]
- 22.Laan H, Konings W N. Mechanism of proteinase release from Lactococcus lactis subsp. cremoris Wg2. Appl Environ Microbiol. 1989;55:3101–3106. doi: 10.1128/aem.55.12.3101-3106.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Law B A, Sezgin E, Sharpe M E. Amino acid nutrition of some commercial cheese starters in relation to their growth in supplemented whey media. J Dairy Res. 1976;43:291–300. [Google Scholar]
- 24.Le Bars D, Gripon J-C. Hydrolysis of αs1-casein by bovine plasmin. Lait. 1993;73:337–344. [Google Scholar]
- 25.Leenhouts K J, Gietema J, Kok J, Venema G. Chromosomal stabilization of the proteinase genes in Lactococcus lactis. Appl Environ Microbiol. 1991;57:2568–2575. doi: 10.1128/aem.57.9.2568-2575.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mills O E, Thomas T D. Nitrogen sources for growth of lactic streptococci in milk. N Z J Dairy Sci Technol. 1981;16:43–55. [Google Scholar]
- 27.Monod J. The growth of bacterial cultures. Annu Rev Microbiol. 1949;3:371–394. [Google Scholar]
- 28.Poolman B, Kunji E R S, Hagting A, Juillard V, Konings W N. The proteolytic pathway of Lactococcus lactis. J Appl Bacteriol Symp Suppl. 1995;79:65S–75S. [PubMed] [Google Scholar]
- 29.Poolman B, Konings W N. Growth of Streptococcus lactis and Streptococcus cremoris in relation to amino acid transport. J Bacteriol. 1988;170:700–707. doi: 10.1128/jb.170.2.700-707.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reid J R, Coolbear T, Pillidge C J, Pritchard G G. Specificity of hydrolysis of bovine κ-casein by cell-envelope-associated proteinases from Lactococcus lactis strains. Appl Environ Microbiol. 1994;60:801–806. doi: 10.1128/aem.60.3.801-806.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reid J R, Ng K H, Moore C H, Coolbear T, Pritchard G G. Comparison of bovine β-casein hydrolysis by PI- and PIII-type proteinases from Lactococcus lactis subsp. cremoris. Appl Microbiol Biotechnol. 1991;36:344–351. doi: 10.1007/BF00208154. [DOI] [PubMed] [Google Scholar]
- 32.Reid J R, Moore C H, Midwinter G G, Pritchard G G. Action of a cell wall proteinase from Lactococcus lactis subsp. cremoris SK11 on bovine αs1-casein. Appl Microbiol Biotechnol. 1991;35:222–227. doi: 10.1007/BF00184690. [DOI] [PubMed] [Google Scholar]
- 33.Reiter R, Oram J D. Nutritional studies on cheese starters. I. Vitamin and amino acid requirements of single strain starters. J Dairy Res. 1962;29:63–77. [Google Scholar]
- 34.Snedecor G W, Cochran W G. Statistical methods. Ames: Iowa State University Press; 1957. [Google Scholar]
- 35.Terzaghi B E, Sandine W E. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975;29:807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Twining S S. Fluorescein isothiocyanate-labeled casein assay for proteolytic enzymes. Anal Biochem. 1984;143:30–34. doi: 10.1016/0003-2697(84)90553-0. [DOI] [PubMed] [Google Scholar]
- 37.Visser S, Exterkate F A, Slangen C J, de Veer G J C M. Comparative study of action of cell wall proteinases from various strains of Streptococcus cremoris on bovine αs1-, β-, and κ-casein. Appl Environ Microbiol. 1986;52:1162–1166. doi: 10.1128/aem.52.5.1162-1166.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]