Abstract
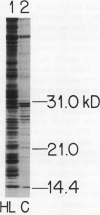
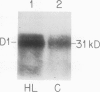
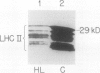
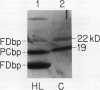
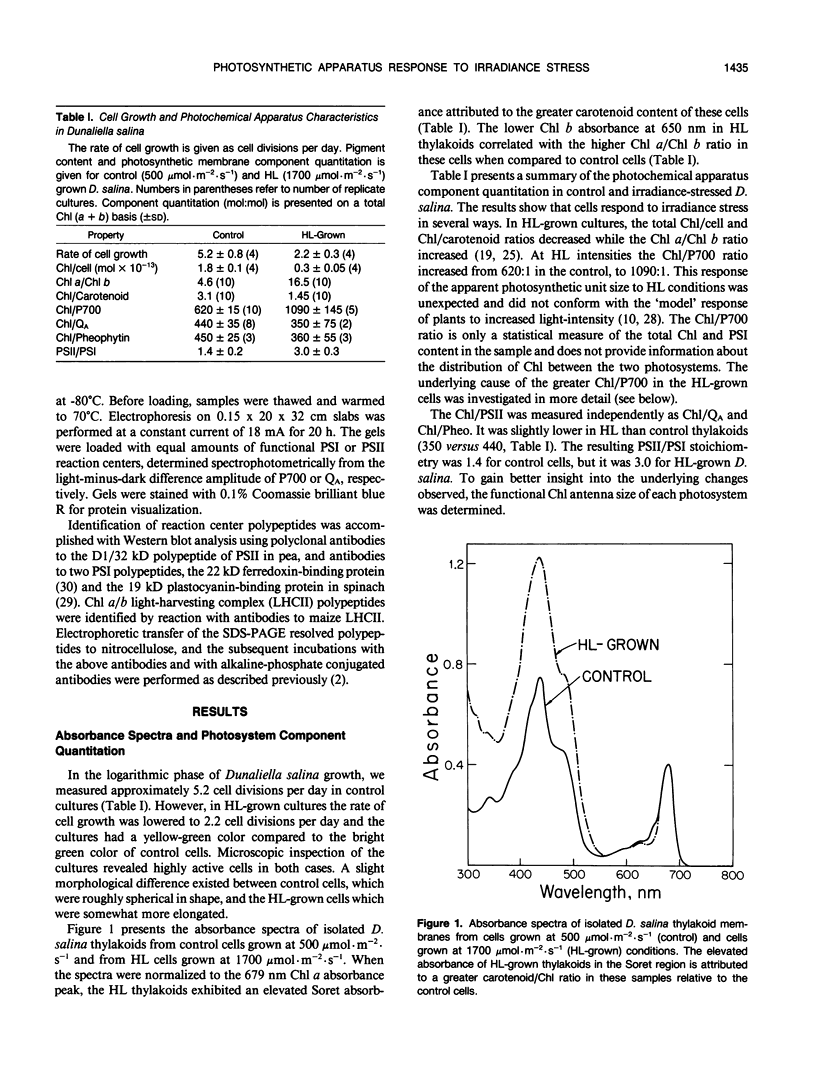
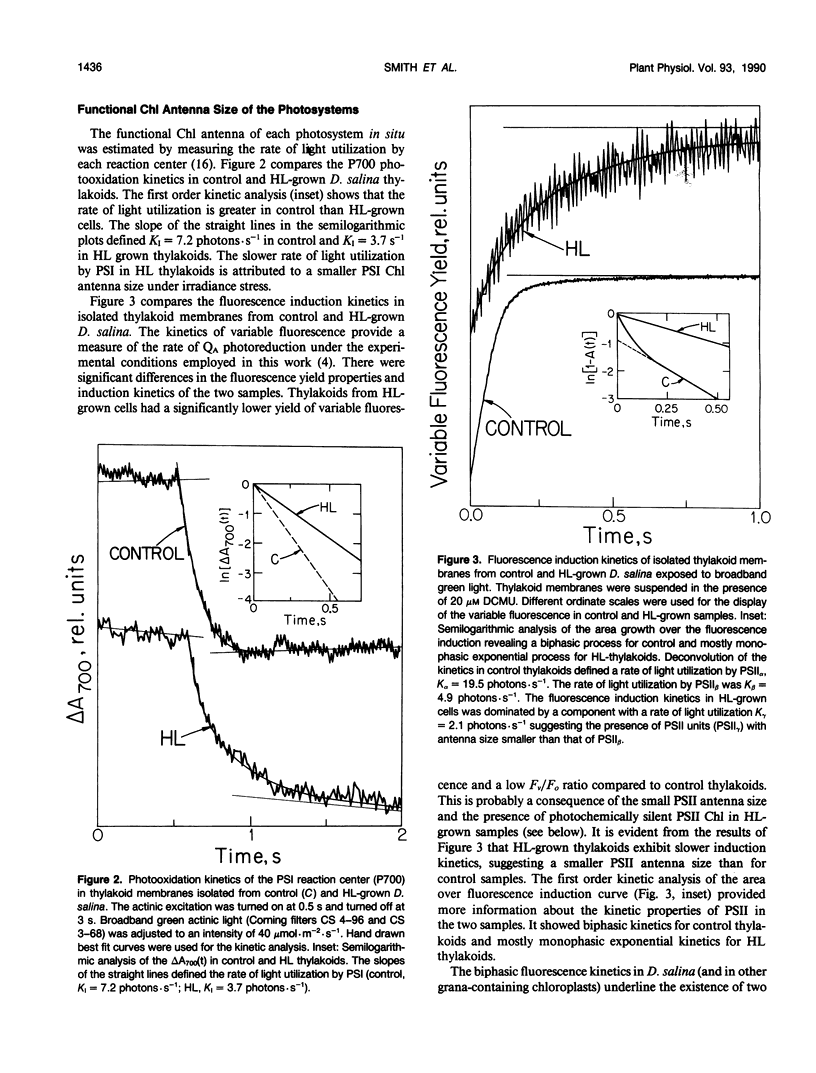
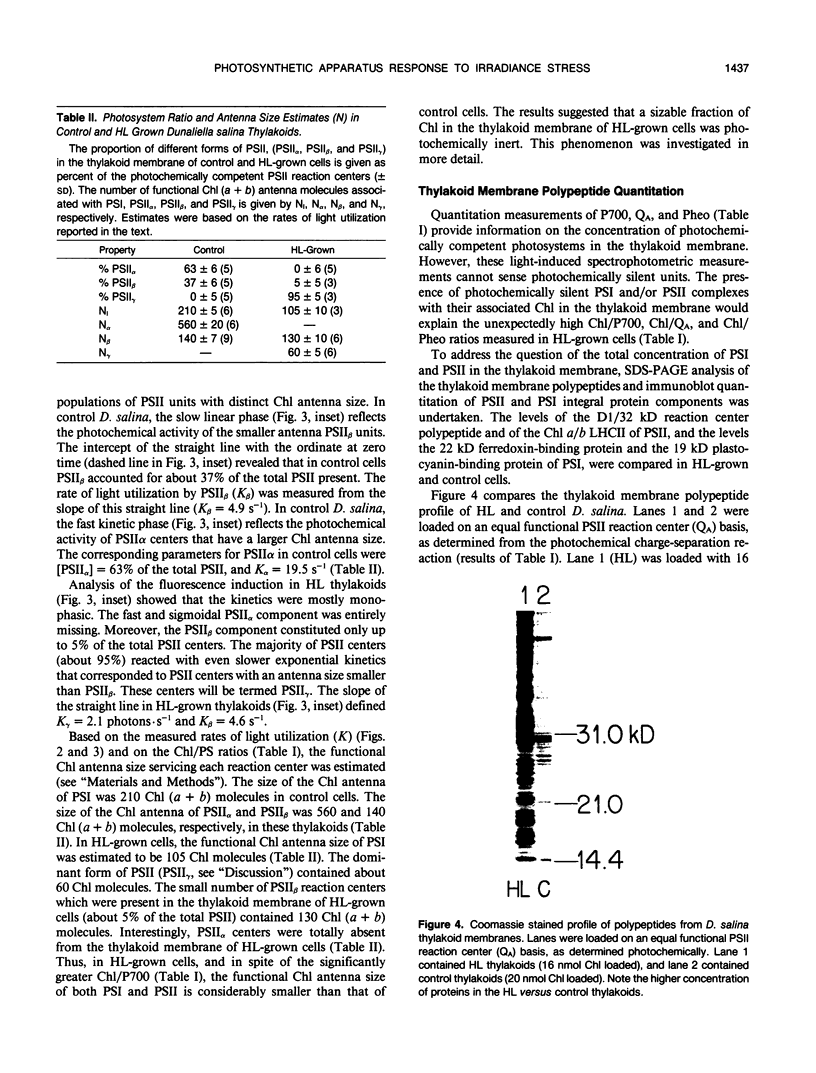
The response of the photosynthetic apparatus in the green alga Dunaliella salina, to irradiance stress was investigated. Cells were grown under physiological conditions at 500 millimoles per square meter per second (control) and under irradiance-stress conditions at 1700 millimoles per square meter per second incident intensity (high light, HL). In control cells, the light-harvesting antenna of photosystem I (PSI) contained 210 chlorophyll a/b molecules. It was reduced to 105 chlorophyll a/b in HL-grown cells. In control cells, the dominant form of photosystem II (PSII) was PSIIα(about 63% of the total PSII) containing >250 chlorophyll a/b molecules. The smaller antenna size PSIIβ centers (about 37% of PSII) contained 135 ± 10 chlorophyll a/b molecules. In sharp contrast, the dominant form of PSII in HL-grown cells accounted for about 95% of all PSII centers and had an antenna size of only about 60 chlorophyll a molecules. This newly identified PSII unit is termed PSIIγ. The HL-grown cells showed a substantially elevated PSII/PSI stoichiometry ratio in their thylakoid membranes (PSII/PSI = 3.0/1.0) compared to that of control cells (PSII/PSI = 1.4/1.0). The steady state irradiance stress created a chronic photoinhibition condition in which D. salina thylakoids accumulate an excess of photochemically inactive PSII units. These PSII units contain both the reaction center proteins and the core chlorophyll-protein antenna complex but cannot perform a photochemical charge separation. The results are discussed in terms of regulatory mechanism(s) in the plant cell whose function is to alleviate the adverse effect of irradiance stress.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Darr S. C., Somerville S. C., Arntzen C. J. Monoclonal antibodies to the light-harvesting chlorophyll a/b protein complex of photosystem II. J Cell Biol. 1986 Sep;103(3):733–740. doi: 10.1083/jcb.103.3.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiyama T., Ke B. Difference spectra and extinction coefficients of P 700 . Biochim Biophys Acta. 1972 Apr 20;267(1):160–171. doi: 10.1016/0005-2728(72)90147-8. [DOI] [PubMed] [Google Scholar]
- Klimov V. V., Klevanik A. V., Shuvalov V. A., Kransnovsky A. A. Reduction of pheophytin in the primary light reaction of photosystem II. FEBS Lett. 1977 Oct 15;82(2):183–186. doi: 10.1016/0014-5793(77)80580-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mattoo A. K., Edelman M. Intramembrane translocation and posttranslational palmitoylation of the chloroplast 32-kDa herbicide-binding protein. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1497–1501. doi: 10.1073/pnas.84.6.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pick U., Gounaris K., Barber J. Dynamics of Photosystem II and Its Light Harvesting System in Response to Light Changes in the Halotolerant Alga Dunaliella salina. Plant Physiol. 1987 Sep;85(1):194–198. doi: 10.1104/pp.85.1.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pick U., Karni L., Avron M. Determination of Ion Content and Ion Fluxes in the Halotolerant Alga Dunaliella salina. Plant Physiol. 1986 May;81(1):92–96. doi: 10.1104/pp.81.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powles S. B., Osmond C. B. Photoinhibition of intact attached leaves of c(3) plants illuminated in the absence of both carbon dioxide and of photorespiration. Plant Physiol. 1979 Dec;64(6):982–988. doi: 10.1104/pp.64.6.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulles M. P., Van Gorkom H. J., Verschoor G. A. Primary reactions of photosystem II at low pH. 2. Light-induced changes of absorbance and electron spin resonance in spinach chloroplasts. Biochim Biophys Acta. 1976 Jul 9;440(1):98–106. doi: 10.1016/0005-2728(76)90116-x. [DOI] [PubMed] [Google Scholar]
- Schuster G., Timberg R., Ohad I. Turnover of thylakoid photosystem II proteins during photoinhibition of Chlamydomonas reinhardtii. Eur J Biochem. 1988 Nov 1;177(2):403–410. doi: 10.1111/j.1432-1033.1988.tb14389.x. [DOI] [PubMed] [Google Scholar]
- Thielen A. P., van Gorkom H. J. Quantum efficiency and antenna size of photosystems II alpha, II beta and I in tobacco chloroplasts. Biochim Biophys Acta. 1981 Mar 12;635(1):111–120. doi: 10.1016/0005-2728(81)90012-8. [DOI] [PubMed] [Google Scholar]
- Wynn R. M., Malkin R. Interaction of plastocyanin with photosystem I: a chemical cross-linking study of the polypeptide that binds plastocyanin. Biochemistry. 1988 Aug 9;27(16):5863–5869. doi: 10.1021/bi00416a007. [DOI] [PubMed] [Google Scholar]
- Zilber A. L., Malkin R. Ferredoxin Cross-Links to a 22 kD Subunit of Photosystem I. Plant Physiol. 1988 Nov;88(3):810–814. doi: 10.1104/pp.88.3.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gorkom H. J. Identification of the reduced primary electron acceptor of photosystem II as a bound semiquinone anion. Biochim Biophys Acta. 1974 Jun 28;347(3):439–442. doi: 10.1016/0005-2728(74)90081-4. [DOI] [PubMed] [Google Scholar]