Abstract
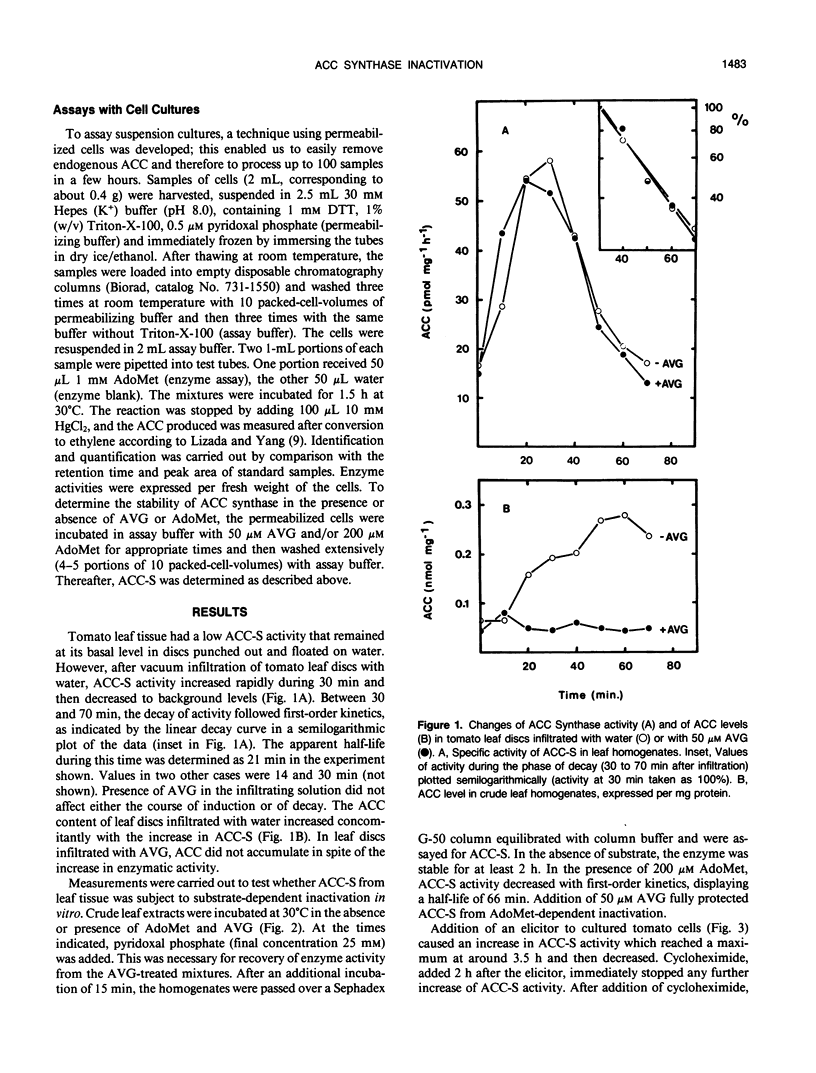
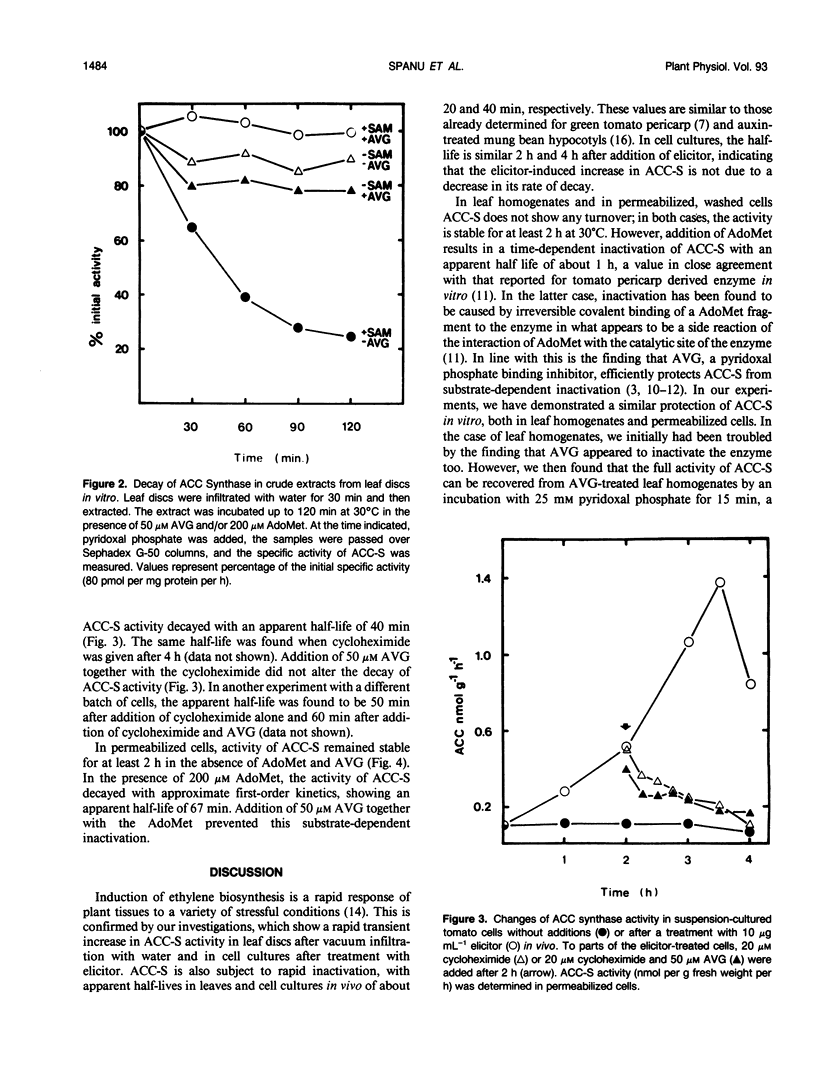
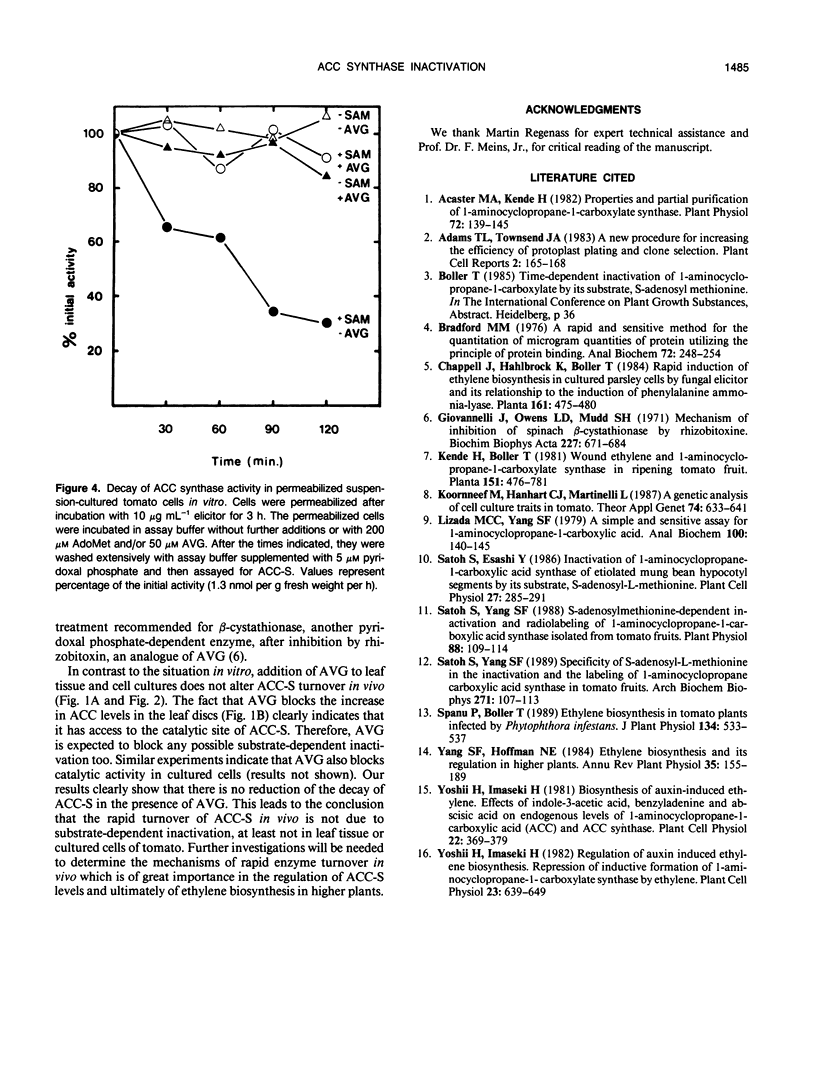
The activity of 1-aminocyclopropane carboxylate (ACC) synthase increased rapidly in tomato (Lycopersicon esculentum Mill.) leaf discs after vacuum infiltration, reached a maximum after about 30 minutes, and subsequently decayed with an apparent half-life of about 20 minutes. Aminoethoxyvinylglycine, a known inhibitor of ACC synthase, did not alter the apparent turnover of ACC synthase in vivo although it efficiently blocked inactivation of the enzyme by its substrate S-adenosylmethionine in vitro. Similar results were obtained, using a novel assay with permeabilized cells, for ACC synthase in tomato cell cultures treated with a fungal elicitor. The results indicate that inactivation of ACC synthase in vivo differs from substrate-dependent inactivation in vitro.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acaster M. A., Kende H. Properties and Partial Purification of 1-Aminocyclopropane-1-carboxylate Synthase. Plant Physiol. 1983 May;72(1):139–145. doi: 10.1104/pp.72.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Giovanelli J., Owens L. D., Mudd S. H. Mechanism of inhibition of spinach beta-cystathionase by rhizobitoxine. Biochim Biophys Acta. 1971 Mar 10;227(3):671–684. doi: 10.1016/0005-2744(71)90016-7. [DOI] [PubMed] [Google Scholar]
- Lizada M. C., Yang S. F. A simple and sensitive assay for 1-aminocyclopropane-1-carboxylic acid. Anal Biochem. 1979 Nov 15;100(1):140–145. doi: 10.1016/0003-2697(79)90123-4. [DOI] [PubMed] [Google Scholar]
- Satoh S., Yang S. F. S-adenosylmethionine-dependent inactivation and radiolabeling of 1-aminocyclopropane-1-carboxylate synthase isolated from tomato fruits. Plant Physiol. 1988 Sep;88(1):109–114. doi: 10.1104/pp.88.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh S., Yang S. F. Specificity of S-adenosyl-L-methionine in the inactivation and the labeling of 1-aminocyclopropane-1-carboxylate synthase isolated from tomato fruits. Arch Biochem Biophys. 1989 May 15;271(1):107–112. doi: 10.1016/0003-9861(89)90260-9. [DOI] [PubMed] [Google Scholar]


