Abstract
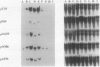
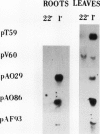
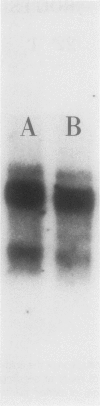
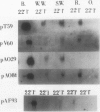
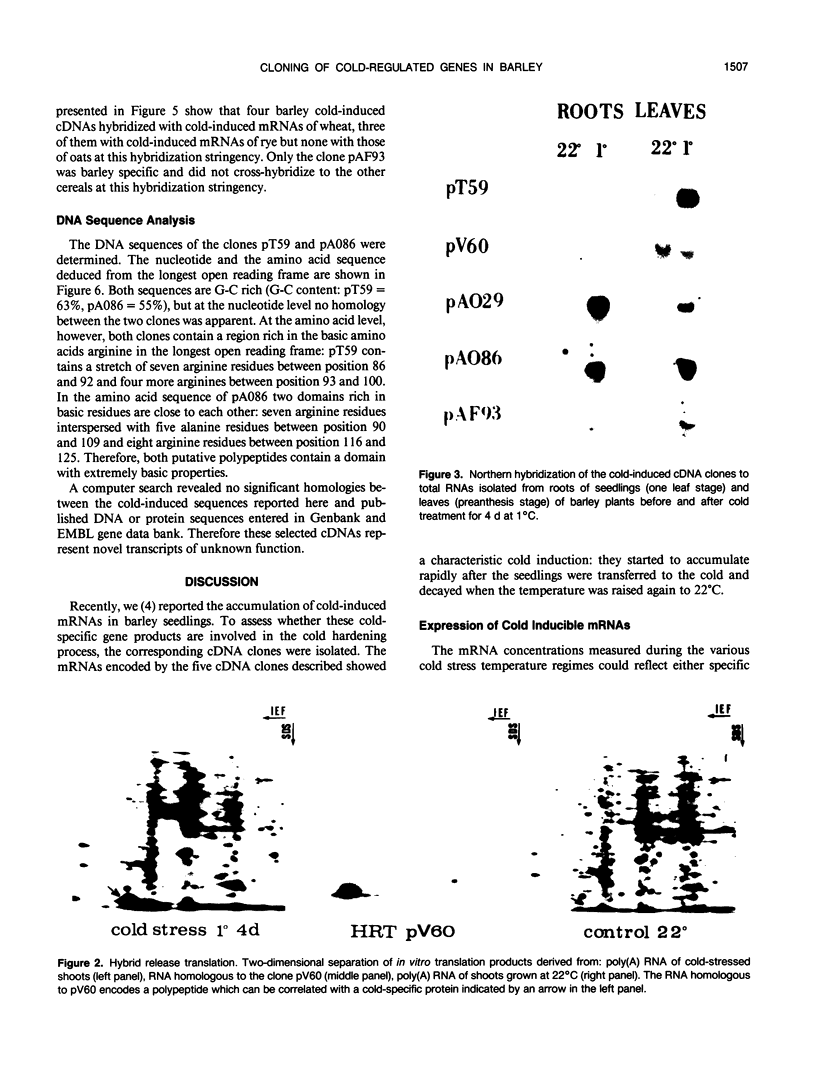
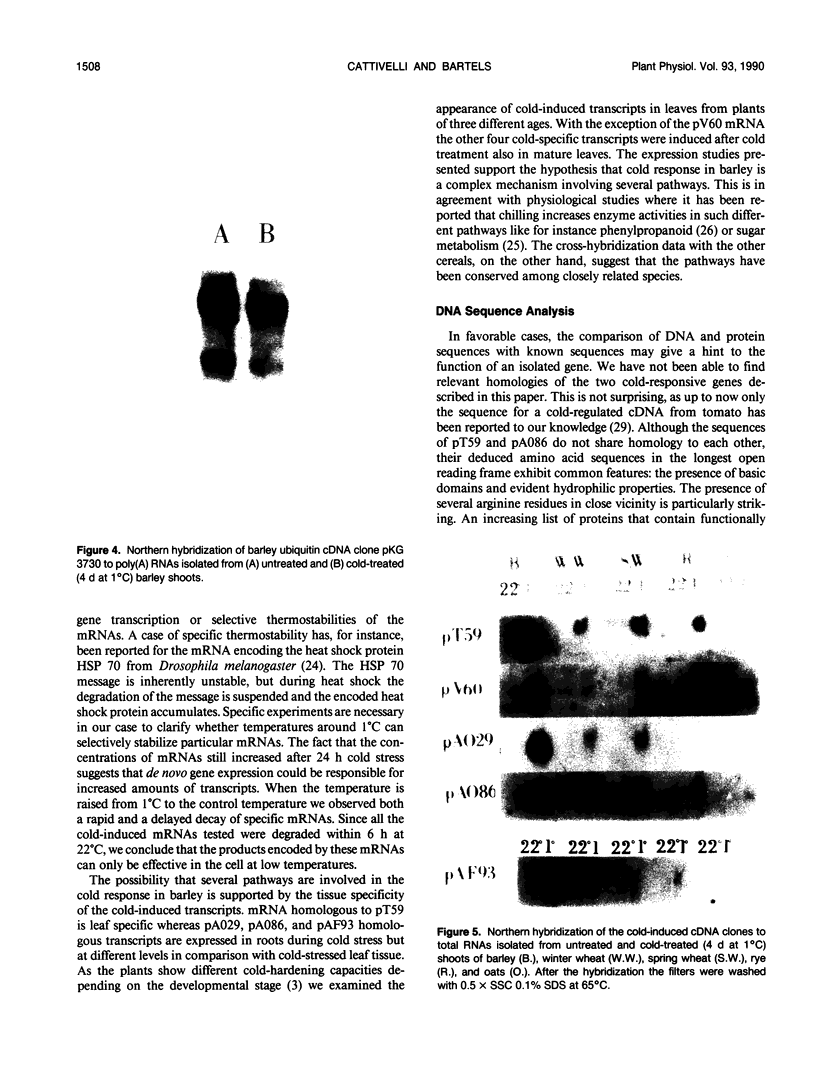
Five different cDNA clones have been isolated which are homologous to cold-regulated mRNAs in barley (Hordeum vulgare L.). The analyses of their hybridizations indicate that the transcripts accumulate to different levels during cold-treatment. Hybridization experiments using RNAs isolated from different plant tissues indicate that several cold-regulated genes are expressed in a tissue-specific manner. The expression studies suggest that in barley several different genes are involved in the cold hardening process depending on developmental stages and tissues involved. Homology has been found between the isolated cDNAs and cold-induced transcripts of related cereals. DNA sequence analysis of the clones pT59 and pA086 reveals that the proteins deduced from the longest open reading frame contain arginine rich basic domains.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartels D., Thompson R. D. The characterization of cDNA clones coding for wheat storage proteins. Nucleic Acids Res. 1983 May 25;11(10):2961–2977. doi: 10.1093/nar/11.10.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Gausing K., Barkardottir R. Structure and expression of ubiquitin genes in higher plants. Eur J Biochem. 1986 Jul 1;158(1):57–62. doi: 10.1111/j.1432-1033.1986.tb09720.x. [DOI] [PubMed] [Google Scholar]
- Gerlach W. L., Bedbrook J. R. Cloning and characterization of ribosomal RNA genes from wheat and barley. Nucleic Acids Res. 1979 Dec 11;7(7):1869–1885. doi: 10.1093/nar/7.7.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour S. J., Hajela R. K., Thomashow M. F. Cold Acclimation in Arabidopsis thaliana. Plant Physiol. 1988 Jul;87(3):745–750. doi: 10.1104/pp.87.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn M., Walbot V. Effects of cold-treatment on protein synthesis and mRNA levels in rice leaves. Plant Physiol. 1989 Nov;91(3):930–938. doi: 10.1104/pp.91.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazinski D., Grzadzielska E., Das A. Sequence-specific recognition of RNA hairpins by bacteriophage antiterminators requires a conserved arginine-rich motif. Cell. 1989 Oct 6;59(1):207–218. doi: 10.1016/0092-8674(89)90882-9. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Meza-Basso L., Alberdi M., Raynal M., Ferrero-Cadinanos M. L., Delseny M. Changes in Protein Synthesis in Rapeseed (Brassica napus) Seedlings during a Low Temperature Treatment. Plant Physiol. 1986 Nov;82(3):733–738. doi: 10.1104/pp.82.3.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra S. S., Poole R. J., Dhindsa R. S. Changes in Protein Patterns and Translatable Messenger RNA Populations during Cold Acclimation of Alfalfa. Plant Physiol. 1987 Aug;84(4):1172–1176. doi: 10.1104/pp.84.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra S. S., Wolfraim L., Poole R. J., Dhindsa R. S. Molecular cloning and relationship to freezing tolerance of cold-acclimation-specific genes of alfalfa. Plant Physiol. 1989 Jan;89(1):375–380. doi: 10.1104/pp.89.1.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Taubenberger A., Hagmann J., Noegel A., Gerisch G. Ubiquitin gene expression in Dictyostelium is induced by heat and cold shock, cadmium, and inhibitors of protein synthesis. J Cell Sci. 1988 May;90(Pt 1):51–58. doi: 10.1242/jcs.90.1.51. [DOI] [PubMed] [Google Scholar]
- Perras M., Sarhan F. Synthesis of Freezing Tolerance Proteins in Leaves, Crown, and Roots during Cold Acclimation of Wheat. Plant Physiol. 1989 Feb;89(2):577–585. doi: 10.1104/pp.89.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen R., Lindquist S. The Drosophila hsp70 message is rapidly degraded at normal temperatures and stabilized by heat shock. Gene. 1988 Dec 10;72(1-2):161–168. doi: 10.1016/0378-1119(88)90138-2. [DOI] [PubMed] [Google Scholar]
- Pressey R., Shaw R. Effect of temperature on invertase, invertase inhibitor, and sugars in potato tubers. Plant Physiol. 1966 Dec;41(10):1657–1661. doi: 10.1104/pp.41.10.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Schaffer M. A., Fischer R. L. Analysis of mRNAs that Accumulate in Response to Low Temperature Identifies a Thiol Protease Gene in Tomato. Plant Physiol. 1988 Jun;87(2):431–436. doi: 10.1104/pp.87.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veidt I., Lot H., Leiser M., Scheidecker D., Guilley H., Richards K., Jonard G. Nucleotide sequence of beet western yellows virus RNA. Nucleic Acids Res. 1988 Nov 11;16(21):9917–9932. doi: 10.1093/nar/16.21.9917. [DOI] [PMC free article] [PubMed] [Google Scholar]