Abstract
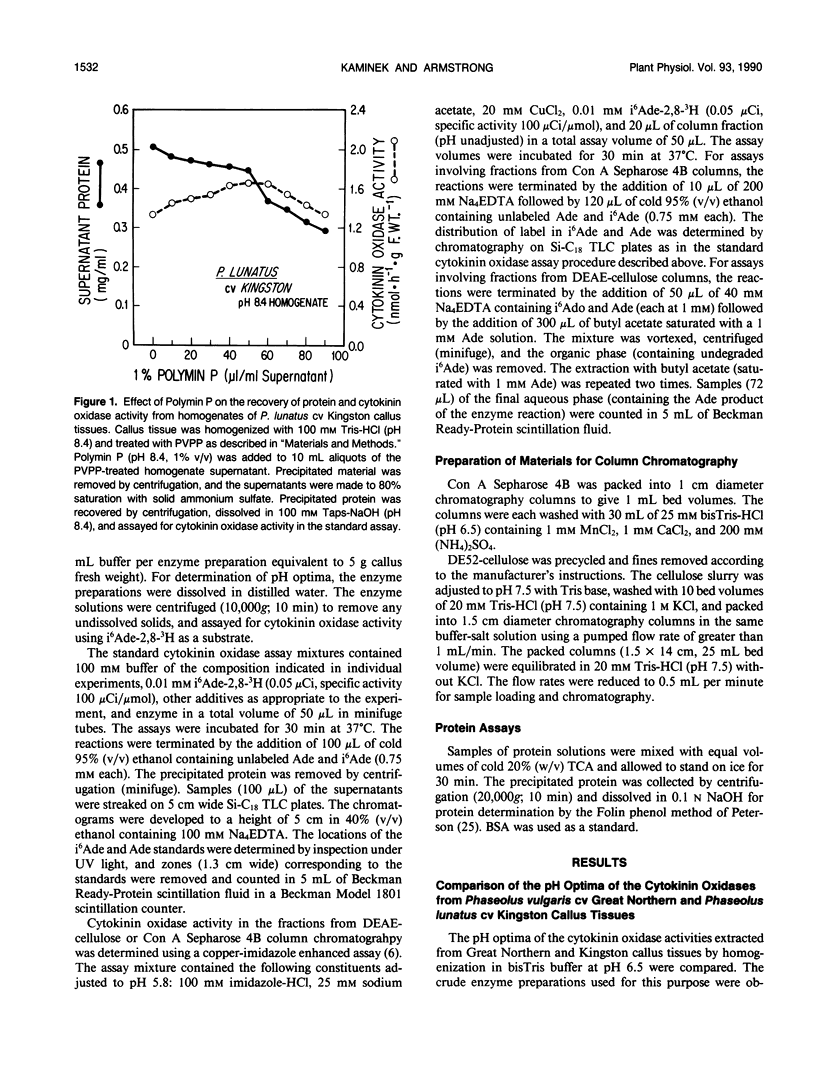
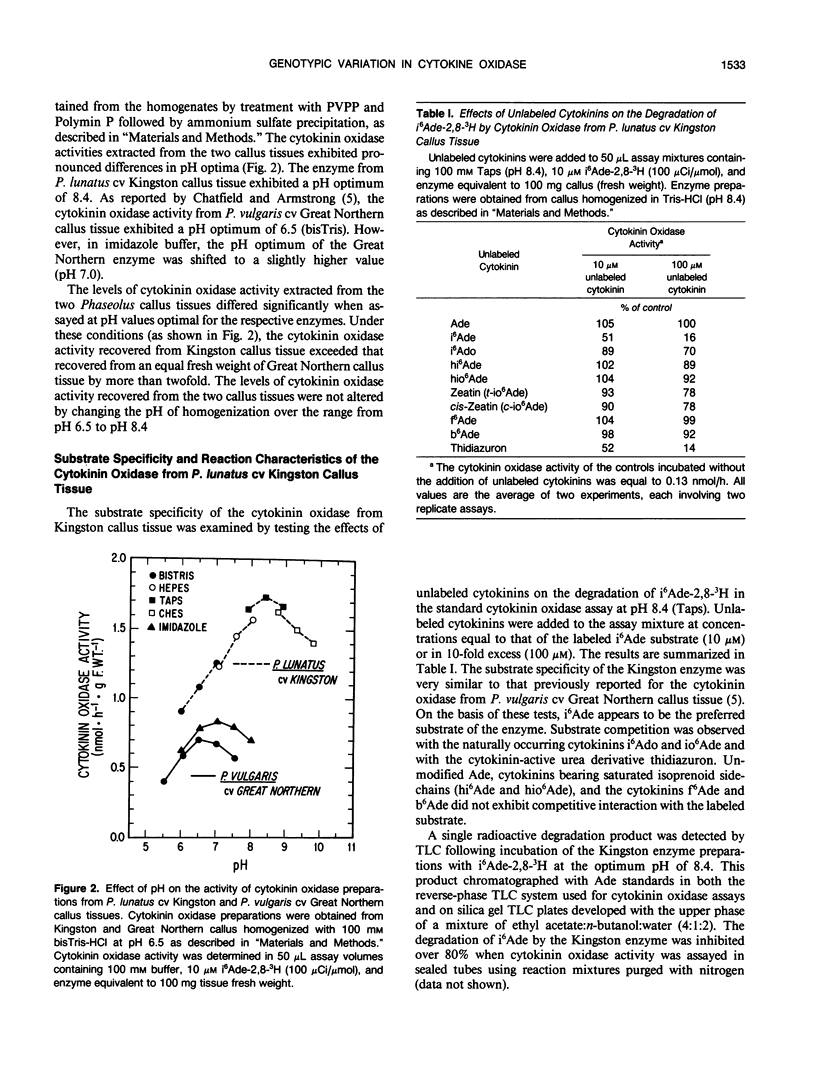
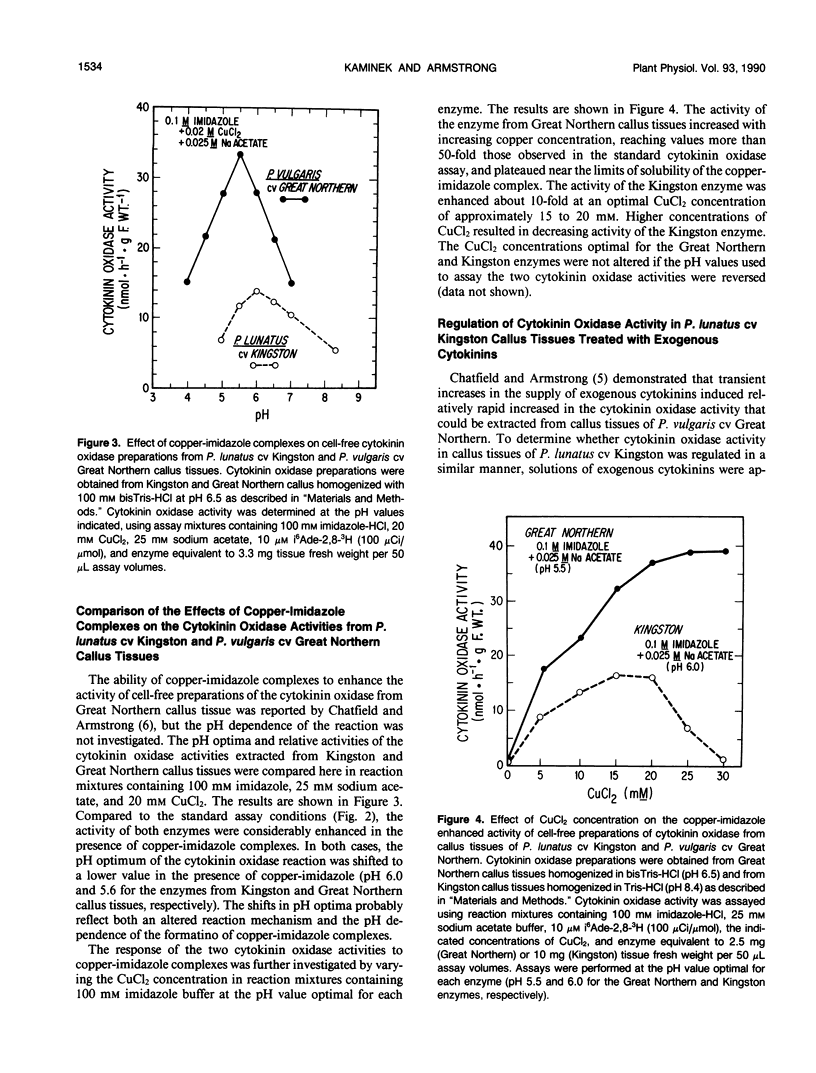
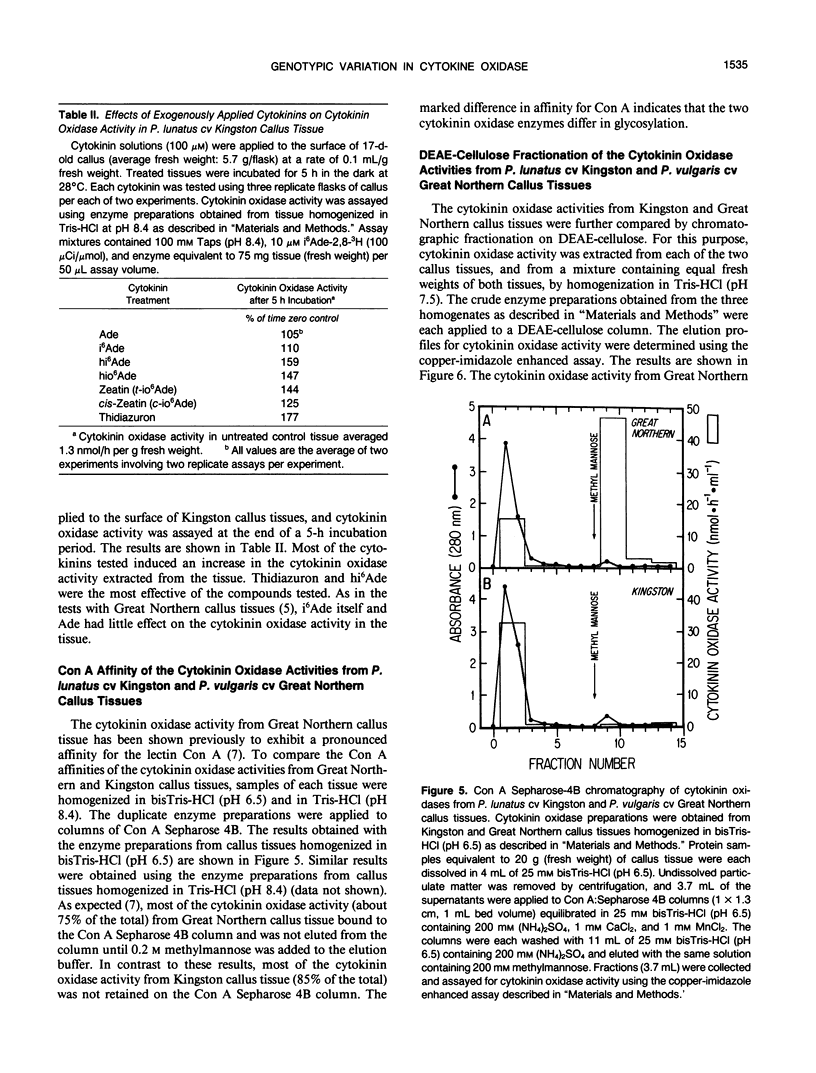
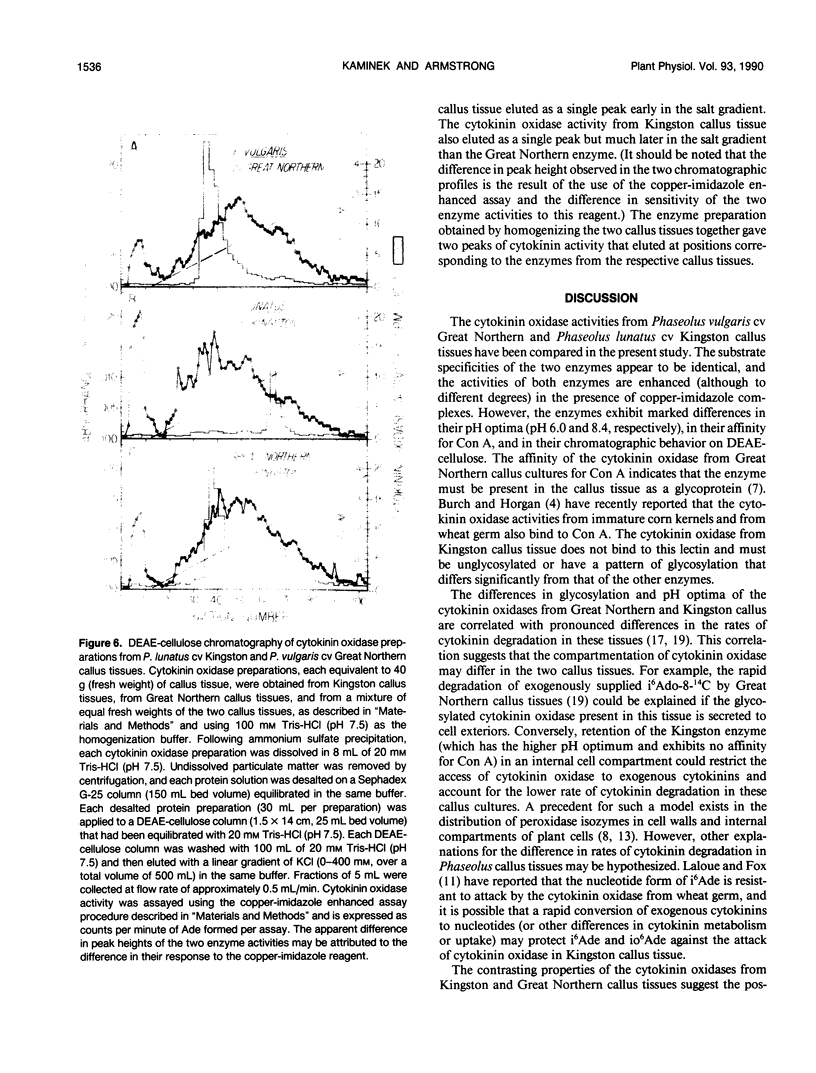
Genotypic variation in cytokinin oxidase has been detected in enzyme preparations from Phaseolus vulgaris L. cv Great Northern and Phaseolus lunatus L. cv Kingston callus cultures. Although cytokinin oxidase preparations from Great Northern and Kingston callus tissues appear to have very similar substrate specificities, the cytokinin oxidase activities from the two callus tissues were found to differ in a number of other properties. The cytokinin oxidase from P. vulgaris cv Great Northern callus tissue exhibited a pH optimum of 6.5 (bisTris) and had a strong affinity for the lectin concanavalin A. The cytokinin oxidase from P. lunatus cv Kingston callus tissue exhibited a pH optimum of 8.4 (Taps) and did not bind to concanavalin A. The two enzymes also differed in position of elution when chromatographed on DEAE-cellulose. Both cytokinin oxidase activities exhibited enhanced activity and lower pH optima in the presence of copper-imidazole complexes, but the optimum copper-imidazole ratio and the magnitude of enhancement differed for the two activities. In both callus tissues, transient increases in the supply of exogenous cytokinins induced increases in cytokinin oxidase activity. The differences in pH optima and in glycosylation (as evidenced by the observed difference in lectin affinity) of the cytokinin oxidases from Great Northern and Kingston callus tissues suggest that the compartmentation of cytokinin oxidase may differ in the two callus tissues. The possibility that enzyme compartmentation and isozyme variation in cytokinin oxidase may play a role in the regulation of cytokinin degradation in plant tissues is discussed in relation to known differences in the rates of cytokinin degradation in Great Northern and Kingston callus tissues.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong D. J., Firtel R. A. Cytokinin oxidase activity in the cellular slime mold, Dictyostelium discoideum. Dev Biol. 1989 Dec;136(2):491–499. doi: 10.1016/0012-1606(89)90274-1. [DOI] [PubMed] [Google Scholar]
- Brownlee B. G., Hall R. H., Whitty C. D. 3-Methyl-2-butenal: an enzymatic degradation product of the cytokinin, N-6-(delta-2 isopentenyl)adenine. Can J Biochem. 1975 Jan;53(1):37–41. doi: 10.1139/o75-006. [DOI] [PubMed] [Google Scholar]
- Chatfield J. M., Armstrong D. J. Cytokinin Oxidase from Phaseolus vulgaris Callus Cultures : Affinity for Concanavalin. Plant Physiol. 1988 Oct;88(2):245–247. doi: 10.1104/pp.88.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatfield J. M., Armstrong D. J. Cytokinin Oxidase from Phaseolus vulgaris Callus Tissues : Enhanced in Vitro Activity of the Enzyme in the Presence of Copper-Imidazole Complexes. Plant Physiol. 1987 Jul;84(3):726–731. doi: 10.1104/pp.84.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatfield J. M., Armstrong D. J. Regulation of Cytokinin Oxidase Activity in Callus Tissues of Phaseolus vulgaris L. cv Great Northern. Plant Physiol. 1986 Feb;80(2):493–499. doi: 10.1104/pp.80.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laloue M., Fox J. E. Cytokinin oxidase from wheat: partial purification and general properties. Plant Physiol. 1989 Jul;90(3):899–906. doi: 10.1104/pp.90.3.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok M. C., Mok D. W., Armstrong D. J. Differential cytokinin structure-activity relationships in phaseolus. Plant Physiol. 1978 Jan;61(1):72–75. doi: 10.1104/pp.61.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok M. C., Mok D. W., Dixon S. C., Armstrong D. J., Shaw G. Cytokinin structure-activity relationships and the metabolism of N-(delta-isopentenyl)adenosine-8-C in phaseolus callus tissues. Plant Physiol. 1982 Jul;70(1):173–178. doi: 10.1104/pp.70.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paces V., Kamínek M. Effect of ribosylzeatin isomers on the enzymatic degradation of N6-(delta2-isopentenyl) adenosine. Nucleic Acids Res. 1976 Sep;3(9):2309–2314. doi: 10.1093/nar/3.9.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paces V., Werstiuk E., Hall R. H. Conversion of N-(Delta-Isopentenyl)adenosine to Adenosine by Enzyme Activity in Tobacco Tissue. Plant Physiol. 1971 Dec;48(6):775–778. doi: 10.1104/pp.48.6.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. Determination of total protein. Methods Enzymol. 1983;91:95–119. doi: 10.1016/s0076-6879(83)91014-5. [DOI] [PubMed] [Google Scholar]
- Terrine C., Laloue M. Kinetics of N-(Delta-Isopentenyl)Adenosine Degradation in Tobacco Cells: EVIDENCE OF A REGULATORY MECHANISM UNDER THE CONTROL OF CYTOKININS. Plant Physiol. 1980 Jun;65(6):1090–1095. doi: 10.1104/pp.65.6.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. C., Katterman F. R. Cytokinin activity induced by thidiazuron. Plant Physiol. 1986 Jun;81(2):681–683. doi: 10.1104/pp.81.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitty C. D., Hall R. H. A cytokinin oxidase in Zea mays. Can J Biochem. 1974 Sep;52(9):789–799. doi: 10.1139/o74-112. [DOI] [PubMed] [Google Scholar]