Abstract
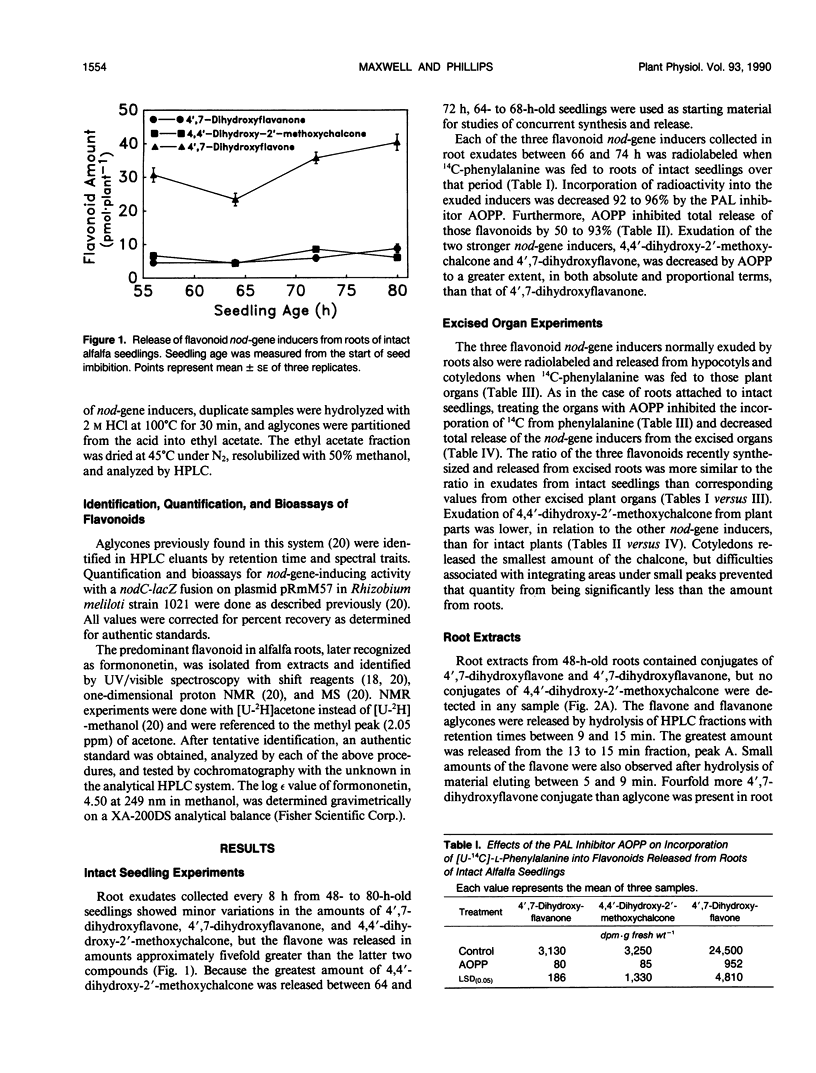
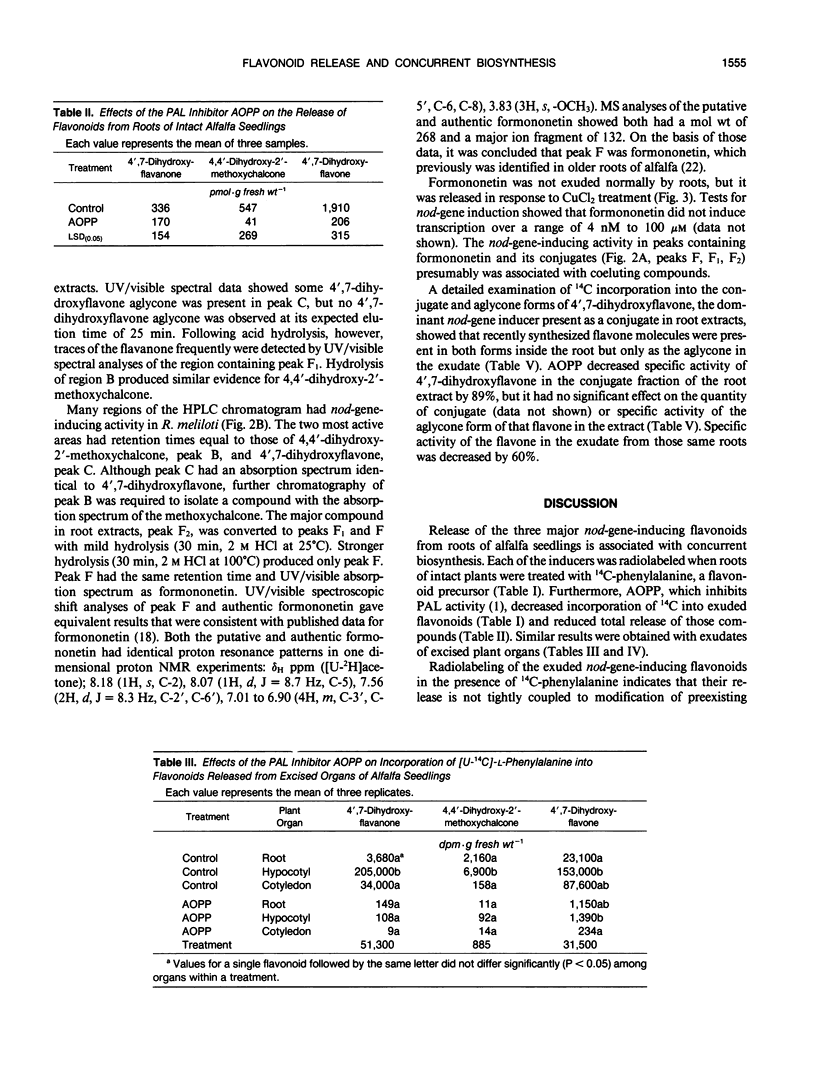
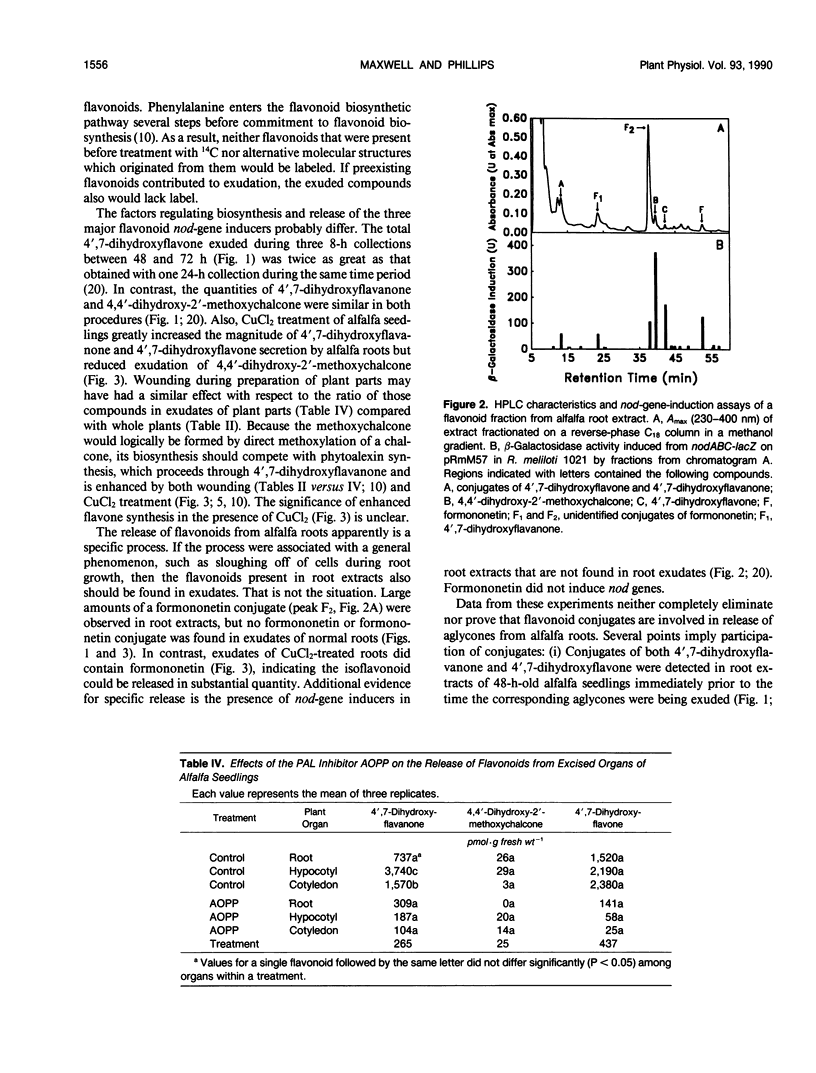
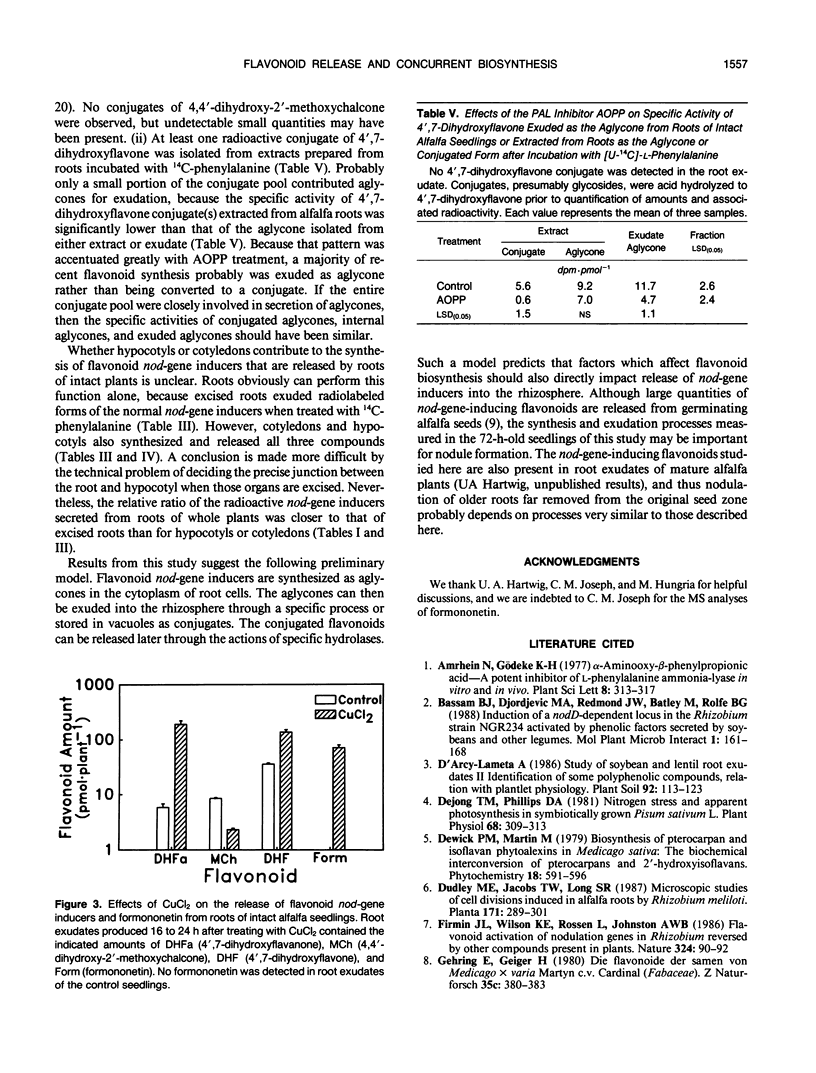
Flavonoid signals from alfalfa (Medicago sativa L.) induce transcription of nodulation (nod) genes in Rhizobium meliloti. Alfalfa roots release three major nod-gene inducers: 4′,7-dihydroxyflavanone, 4′,7-dihydroxyflavone, and 4,4′-dihydroxy-2′-methoxychalcone. The objective of the present study was to define temporal relationships between synthesis and exudation for those flavonoids. Requirements for concurrent flavonoid biosynthesis were assessed by treating roots of intact alfalfa seedlings with [U-14C]-l-phenylalanine in the presence or absence of the phenylalanine ammonia-lyase inhibitor l-2-aminoxy-3-phenylpropionic acid (AOPP). In the absence of AOPP, each of the three flavonoids in exudates contained 14C. In the presence of AOPP, 14C labeling and release of all the exuded nod-gene inducers were reduced significantly. AOPP inhibited labeling and release of the strongest nod-gene inducer, methoxychalcone, by more than 90%. Experiments with excised cotyledons, hypocotyls, and roots incubated in solution showed that the flavonoids could be synthesized in and released from each organ. However, the ratio of the three flavonoids in exudates from intact plants was most similar to the ratio recently synthesized and released from excised roots. A portion of recently synthesized flavonoid aglycones was found conjugated, presumably as glycosides, in root extracts and may have been involved in the release process. Data from root extracts showed that formononetin, an isoflavonoid which does not induce nod genes, was present in conjugated and aglycone forms but was not released by normal intact roots. In contrast, roots stressed with CuCl2 did release the aglycone formononetin. Thus, the release process responsible for exudation of nod-gene inducers appears to be specific rather than a general phenomenon such as a sloughing off of cells during root growth. The synthesis and specific concurrent release of flavonoid nod-gene inducers in this study is consistent with the physiological requirement for nodule formation of the 3-day-old seedlings used.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassam B. J., Djordjevic M. A., Redmond J. W., Batley M., Rolfe B. G. Identification of a nodD-dependent locus in the Rhizobium strain NGR234 activated by phenolic factors secreted by soybeans and other legumes. Mol Plant Microbe Interact. 1988 Apr;1(4):161–168. doi: 10.1094/mpmi-1-161. [DOI] [PubMed] [Google Scholar]
- Dejong T. M., Phillips D. A. Nitrogen Stress and Apparent Photosynthesis in Symbiotically Grown Pisum sativum L. Plant Physiol. 1981 Aug;68(2):309–313. doi: 10.1104/pp.68.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig U. A., Maxwell C. A., Joseph C. M., Phillips D. A. Chrysoeriol and Luteolin Released from Alfalfa Seeds Induce nod Genes in Rhizobium meliloti. Plant Physiol. 1990 Jan;92(1):116–122. doi: 10.1104/pp.92.1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hósel W., Barz W. Beta-Glucosidases from Cicer arietinum L. Purification and Properties of isoflavone-7-O-glucoside-specific beta-glucosidases. Eur J Biochem. 1975 Sep 15;57(2):607–616. doi: 10.1111/j.1432-1033.1975.tb02336.x. [DOI] [PubMed] [Google Scholar]
- Kosslak R. M., Bookland R., Barkei J., Paaren H. E., Appelbaum E. R. Induction of Bradyrhizobium japonicum common nod genes by isoflavones isolated from Glycine max. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7428–7432. doi: 10.1073/pnas.84.21.7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köster J., Strack D., Barz W. High Performance Liquid Chromatographic Separation of Isoflavones and Structural Elucidation of Isoflavone 7-O-glucoside 6''-malonates from Cicer arietinum. Planta Med. 1983 Jul;48(7):131–135. doi: 10.1055/s-2007-969907. [DOI] [PubMed] [Google Scholar]
- Matern U., Heller W., Himmelspach K. Conformational changes of apigenin 7-O-(6-O-malonylglucoside), a vacuolar pigment from parsley, with solvent composition and proton concentration. Eur J Biochem. 1983 Jun 15;133(2):439–448. doi: 10.1111/j.1432-1033.1983.tb07483.x. [DOI] [PubMed] [Google Scholar]
- Maxwell C. A., Hartwig U. A., Joseph C. M., Phillips D. A. A Chalcone and Two Related Flavonoids Released from Alfalfa Roots Induce nod Genes of Rhizobium meliloti. Plant Physiol. 1989 Nov;91(3):842–847. doi: 10.1104/pp.91.3.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan J. T., Long S. R. Induction of Rhizobium meliloti nodC expression by plant exudate requires nodD. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6609–6613. doi: 10.1073/pnas.82.19.6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters N. K., Frost J. W., Long S. R. A plant flavone, luteolin, induces expression of Rhizobium meliloti nodulation genes. Science. 1986 Aug 29;233(4767):977–980. doi: 10.1126/science.3738520. [DOI] [PubMed] [Google Scholar]
- Sadowsky M. J., Olson E. R., Foster V. E., Kosslak R. M., Verma D. P. Two host-inducible genes of Rhizobium fredii and characterization of the inducing compound. J Bacteriol. 1988 Jan;170(1):171–178. doi: 10.1128/jb.170.1.171-178.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaat S. A., Schripsema J., Wijffelman C. A., van Brussel A. A., Lugtenberg B. J. Analysis of the major inducers of the Rhizobium nodA promoter from Vicia sativa root exudate and their activity with different nodD genes. Plant Mol Biol. 1989 Aug;13(2):175–188. doi: 10.1007/BF00016136. [DOI] [PubMed] [Google Scholar]


