Abstract
Introduction:
Per- and poly-fluoroalkyl substances (PFASs) are a large, complex group of synthetic chemicals humans can be exposed to from occupational or environmental sources. In this systematic review and meta-analysis, we examined the association between PFAS exposure, particularly Perfluorooctanoic Acid (PFOA), and Perfluorooctane Sulfonic Acid (PFOS), and risk of kidney, liver, and testicular cancer.
Methods:
We systematically searched PubMed to identify cohort and case-control studies reported after the Monograph of the International Agency for Research on Cancer and the Toxicological Profile of the Agency for Toxic Substances and Disease Registry. We assessed the quality of the studies by using a modified version of the Newcastle-Ottawa Scale (NOS). Forest relative risk (RR) plots were constructed for liver, kidney, and testicular cancer. We conducted stratified analyses by geographic region, study design, quality score, outcome, years of publication, exposure source, and PFAS type. A random-effects model was used to address heterogeneity between studies.
Results:
Fifteen studies, including ten cohort studies, three case-control studies nested in a cohort, and two case-control studies were included after removing duplicate and irrelevant reports. We found an association between overall PFAS exposure and the risk of kidney cancers (RR=1.18, 95% CI =1.05-1.32; I =52.8%, 11 studies). Also, we showed an association between high-level exposure to PFAS and kidney cancer (RR=1.74, 95% CI =1.23-2.47; p=0.005) and testicular cancer (RR=2.22, 95% CI =1.12-4.39; p=0.057). There was no association with liver cancer. We found no heterogeneity by geographical region, PFAS type, study design, outcome, quality score, year of publication, or exposure source. Only two studies reported results among women.
Conclusions:
We detected an association between overall PFAS exposure and kidney cancer and high doses of PFAS with testicular cancer. However, bias and confounding cannot be excluded, precluding a conclusion in terms of causality.
Keywords: Kidney, Occupational Factors, Liver, Testis, Malignant, Water, Perfluorooctanoic Acid, PFAS, Perfluorooctane Sulfonic Acid
Supplementary Table 1b.
PRISMA Abstract Checklist.
| Section and Topic | Item # | Checklist item | Reported (Yes/No) |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a systematic review. | Yes |
| BACKGROUND | |||
| Objectives | 2 | Provide an explicit statement of the main objective(s) or question(s) the review addresses. | Yes |
| METHODS | |||
| Eligibility criteria | 3 | Specify the inclusion and exclusion criteria for the review. | No |
| Information sources | 4 | Specify the information sources (e.g. databases, registers) used to identify studies and the date when each was last searched. | Yes |
| Risk of bias | 5 | Specify the methods used to assess risk of bias in the included studies. | Yes |
| Synthesis of results | 6 | Specify the methods used to present and synthesise results. | Yes |
| RESULTS | |||
| Included studies | 7 | Give the total number of included studies and participants and summarise relevant characteristics of studies. | Yes |
| Synthesis of results | 8 | Present results for main outcomes, preferably indicating the number of included studies and participants for each. If meta-analysis was done, report the summary estimate and confidence/credible interval. If comparing groups, indicate the direction of the effect (i.e. which group is favoured). | Yes |
| DISCUSSION | |||
| Limitations of evidence | 9 | Provide a brief summary of the limitations of the evidence included in the review (e.g. study risk of bias, inconsistency and imprecision). | No |
| Interpretation | 10 | Provide a general interpretation of the results and important implications. | Yes |
| OTHER | |||
| Funding | 11 | Specify the primary source of funding for the review. | No |
| Registration | 12 | Provide the register name and registration number. | No |
Abbrevations:
- Agency for Toxic Substances and Disease Registry; ATSDR
- International Agency for Research on Cancer; IARC
- inflammatory bowel disease; IBD
- Hepatocellolar carcinoma; HCC
- Nitrogen dioxide; NO2
- Odds ratio; OR
- Risk ratio, rate ratio;R R
- Standardized mortality ratio;SMR
- Standardized incidence ratio; SIR
- Perfluorooctanoic Acid; PFOA
- Per- and poly-fluoroalkylsubstances; PFAS
- Perfluorooctane sulfonic acid; PFOS
1. Introduction
Per- and poly-fluoroalkyl substances (PFASs) are a large, complex group of synthetic chemicals that are thermally and chemically stable in the environment [1]. Since the 1940s, these agents have been used in various industries, such as aerospace, automotive, construction, and electronics. Also, they are used to produce stain- and water-resistant fire-fighting foams, cleaning products, and paints [2].
PFAS may be released into water, air, and soil. Hence humans can be exposed to these substances through occupational or environmental sources [3, 4]. Chemically, there are several types of PFAS. However, the most common types are perfluorooctanoic acid (PFOA), perfluorooctane sulfonic acid (PFOS), and perfluorohexanesulfonic acid (PFHxS) [1].
Previous studies reported that exposure to some PFAS types may be associated with health effects [5, 6]. Cancer incidence is one of the most pressing concerns concerning PFAS exposure [7]. The International Agency for Research on Cancer (IARC) 2017 classified PFOA as a possible human carcinogen based on limited epidemiologic evidence for kidney and testicular cancer [8]. In addition, previous epidemiological and animal studies reported some association between these substances and other cancer varieties, such as liver cancer [9, 10]. Worldwide, 431,288, 905,677, and 74,458 people can be diagnosed yearly with kidney, liver, and testicular cancer [11].
To better clarify the possible effects of PFAS on cancer incidence and mortality, we conducted a systematic review and meta-analysis to examine the association between occupational and environmental exposure to PFA, emphasizing PFOS and PFOA, and the risk of kidney, liver, and testicular cancer.
2. Methods
2.1. Data Sources, Search Strategy, Selection Criteria, and Quality Assessment
First, we searched the reference lists of the IARC Monograph on PFOA [8] and the Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profile for perfluoroalkyls [12]. And then, searches were undertaken on July 8, 2023, for peer-reviewed publications in PubMed with no limit according to year of publication and language to identify more recent studies. We included studies on incidence or mortality from kidney, liver, and testicular cancers and exposure to PFAS, including PFOA and PFOS.
The search strategy was designed using MeSH terms (“PFOA”[Text Word] OR “PFOS”[Text Word] OR “PFAS”[Text Word]) AND (“kidney”[Text Word] OR “liver”[Text Word] OR “testicular”[Text Word] OR “testis”[Text Word] OR “Hepatocellular”[Text Word]) AND (“cancer”[Text Word]). We included cohort, case-control, and ecological studies of occupational and environmental exposure to PFAS, including studies based on serum levels of PFAS. Studies involving animals were excluded.
Two reviewers (MSS and PB) independently reviewed the list of titles and abstracts, and the final selection was based on the full text of potentially relevant articles. If multiple reports were based on the same database, we included only the most informative article, typically based on the most recent update. The meta-analysis was performed according to the STROBE statement [13] and reported according to the PRISMA statement (Supplementary Table 1) [14].
Supplementary Table 1a.
PRISMA Checklist.
| Section and Topic | Item # | Checklist item | Location where item is reported |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a systematic review. | P1 |
| ABSTRACT | |||
| Abstract | 2 | See the PRISMA 2020 for Abstracts checklist. | P24 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of existing knowledge. | P4 |
| Objectives | 4 | Provide an explicit statement of the objective(s) or question(s) the review addresses. | P4 |
| METHODS | |||
| Eligibility criteria | 5 | Specify the inclusion and exclusion criteria for the review and how studies were grouped for the syntheses. | P5 |
| Information sources | 6 | Specify all databases, registers, websites, organisations, reference lists and other sources searched or consulted to identify studies. Specify the date when each source was last searched or consulted. | P5 |
| Search strategy | 7 | Present the full search strategies for all databases, registers and websites, including any filters and limits used. | P5 |
| Selection process | 8 | Specify the methods used to decide whether a study met the inclusion criteria of the review, including how many reviewers screened each record and each report retrieved, whether they worked independently, and if applicable, details of automation tools used in the process. | P5 |
| Data collection process | 9 | Specify the methods used to collect data from reports, including how many reviewers collected data from each report, whether they worked independently, any processes for obtaining or confirming data from study investigators, and if applicable, details of automation tools used in the process. | P5 |
| Data items | 10a | List and define all outcomes for which data were sought. Specify whether all results that were compatible with each outcome domain in each study were sought (e.g. for all measures, time points, analyses), and if not, the methods used to decide which results to collect. | P5 |
| 10b | List and define all other variables for which data were sought (e.g. participant and intervention characteristics, funding sources). Describe any assumptions made about any missing or unclear information. | P5 | |
| Study risk of bias assessment | 11 | Specify the methods used to assess risk of bias in the included studies, including details of the tool(s) used, how many reviewers assessed each study and whether they worked independently, and if applicable, details of automation tools used in the process. | P5 |
| Effect measures | 12 | Specify for each outcome the effect measure(s) (e.g. risk ratio, mean difference) used in the synthesis or presentation of results. | P5 |
| Synthesis methods | 13a | Describe the processes used to decide which studies were eligible for each synthesis (e.g. tabulating the study intervention characteristics and comparing against the planned groups for each synthesis (item #5)). | P6 |
| 13b | Describe any methods required to prepare the data for presentation or synthesis, such as handling of missing summary statistics, or data conversions. | P6 | |
| 13c | Describe any methods used to tabulate or visually display results of individual studies and syntheses. | P6 | |
| 13d | Describe any methods used to synthesize results and provide a rationale for the choice(s). If meta-analysis was performed, describe the model(s), method(s) to identify the presence and extent of statistical heterogeneity, and software package(s) used. | P6 | |
| 13e | Describe any methods used to explore possible causes of heterogeneity among study results (e.g. subgroup analysis, meta-regression). | P6 | |
| 13f | Describe any sensitivity analyses conducted to assess robustness of the synthesized results. | P6 | |
| Reporting bias assessment | 14 | Describe any methods used to assess risk of bias due to missing results in a synthesis (arising from reporting biases). | P6 |
| Certainty assessment | 15 | Describe any methods used to assess certainty (or confidence) in the body of evidence for an outcome. | P6 |
| RESULTS | |||
| Study selection | 16a | Describe the results of the search and selection process, from the number of records identified in the search to the number of studies included in the review, ideally using a flow diagram. | P7,17 |
| 16b | Cite studies that might appear to meet the inclusion criteria, but which were excluded, and explain why they were excluded. | P17 | |
| Study characteristics | 17 | Cite each included study and present its characteristics. | P7 |
| Risk of bias in studies | 18 | Present assessments of risk of bias for each included study. | P7 |
| Results of individual studies | 19 | For all outcomes, present, for each study: (a) summary statistics for each group (where appropriate) and (b) an effect estimate and its precision (e.g. confidence/credible interval), ideally using structured tables or plots. | P7 |
| Results of syntheses | 20a | For each synthesis, briefly summarise the characteristics and risk of bias among contributing studies. | P7 |
| 20b | Present results of all statistical syntheses conducted. If meta-analysis was done, present for each the summary estimate and its precision (e.g. confidence/credible interval) and measures of statistical heterogeneity. If comparing groups, describe the direction of the effect. | P7 | |
| 20c | Present results of all investigations of possible causes of heterogeneity among study results. | P7 | |
| 20d | Present results of all sensitivity analyses conducted to assess the robustness of the synthesized results. | P7 | |
| Reporting biases | 21 | Present assessments of risk of bias due to missing results (arising from reporting biases) for each synthesis assessed. | P7 |
| Certainty of evidence | 22 | Present assessments of certainty (or confidence) in the body of evidence for each outcome assessed. | P7 |
| DISCUSSION | |||
| Discussion | 23a | Provide a general interpretation of the results in the context of other evidence. | P8 |
| 23b | Discuss any limitations of the evidence included in the review. | P9 | |
| 23c | Discuss any limitations of the review processes used. | P9 | |
| 23d | Discuss implications of the results for practice, policy, and future research. | P9 | |
| OTHER INFORMATION | |||
| Registration and protocol | 24a | Provide registration information for the review, including register name and registration number, or state that the review was not registered. | NA |
| 24b | Indicate where the review protocol can be accessed, or state that a protocol was not prepared. | NA | |
| 24c | Describe and explain any amendments to information provided at registration or in the protocol. | NA | |
| Support | 25 | Describe sources of financial or non-financial support for the review, and the role of the funders or sponsors in the review. | P1 |
| Competing interests | 26 | Declare any competing interests of review authors. | P1 |
| Availability of data, code and other materials | 27 | Report which of the following are publicly available and where they can be found: template data collection forms; data extracted from included studies; data used for all analyses; analytic code; any other materials used in the review. | NA |
The data extraction file contained demographic characteristics of the original studies, including author name, year of publication, country, type of study (case-control, cohort, ecologic), patient characteristics (sex, ethnicity), type of cancer, type of PFAS, exposure source (occupational or environmental), duration and level of exposure. Finally, we extracted the effect size measures, including relative risks (RRs), odds ratio (OR), risk ratio, rate ratio, standardized mortality ratio (SMR), or standardized incidence ratio (SIR), and their 95% Confidence Intervals (CI). If only results for subgroups were reported, we combined them using a fixed effect meta-analysis. If RR or CI were not reported, we calculated them from the row data if possible.
Eligible studies were critically appraised by two independent reviewers (MSS and PB) using a modified version of the Newcastle-Ottawa Scale (NOS) for case-control (6 items) and cohort studies (6 items) [15] (Supplementary Table 2). Studies that scored <8 corresponded to low quality, and those that scored >=8 were considered high quality.
Supplementary Table 2.
Modified Newcastle - Ottawa Quality Assessment Scale
| Case Control Studies |
| 1. Selection of controls |
| a. From study base (2) |
| b. Not from study base (1) |
| c. Other, incl. ecological, no description (0) |
| 2. Adjustment of confounders |
| a. Adjustment for most important potential confounders (2) |
| b. Adjustment for some potential confounders (1) |
| c. Adjustment for no confounders except age, gender, race/ethnicity, calendar period (0) |
| 3. Ascertainment of exposure |
| a. Objective record (eg employment records, biomarkers) (2) |
| b. Structured interview blind to case/control status, GIS (1) |
| c. Interview not blinded to case/control status, self-report, proxy (e.g., residence) (0.5) |
| d. No description (0) |
| 4. Response rate |
| a. Both groups over 90% (2) |
| b. One or both groups between 60- 90% (1) |
| c. One group under 60%, no description (0) |
| 5. Latency |
| a. Adequate latency between exposure and outcome (>15 yrs) (2) |
| b. Limited latency between exposure and outcome (5-15 yrs) (1) |
| c. Inadequate latency between exposure and outcome (<5 yrs), no description (0) |
| 6. Outcome |
| a. Cancer registration (2) |
| b. Death certificates, hospital records (1) |
| c. Self report (0.5) |
| d. No description (0) |
| Cohort Studies |
| 1. Selection of unexposed cohort |
| a. Derived from the same population as the exposed (2) |
| b. Derived from a different source (1) |
| c. Other, no description (0) |
| 2. Adjustment of confounders |
| a. Adjustment for most important potential confounders (2) |
| b. Adjustment for some potential confounders (1) |
| c. Adjustment for no confounders except age, gender, race/ethnicity, calendar period (0) |
| 3. Ascertainment of exposure |
| a. Objective record (e.g., employment records, biomarkers) (2) |
| b. Structured interview blind to outcome status, GIS (1) |
| c. Interview not blinded outcome status, self-report, proxy (e.g., residence) (0.5) |
| d. No description (0) |
| 4. Follow-up rate |
| a. Follow-up of both groups over 90% (2) |
| b. Follow-up of one or both groups between 60- 90% (1) |
| c. Follow-up of one group under 60%, no description (0) |
| 5. Latency |
| a. Adequate latency between exposure and outcome (>15 yrs) (2) |
| b. Limited latency between exposure and outcome (5-15 yrs) (1) |
| c. Inadequate latency between exposure and outcome (<5 yrs), no description (0) |
| 6. Outcome |
| a. Cancer registration (2) |
| b. Death certificates, hospital records (1) |
| c. Self report (0.5) |
| d. No description (0) |
2.2. Statistical Analysis
All analyses were completed using the STATA version 14.0 (Stata, College Station, TX, USA). We examined the exposure to PFAS and incidence and mortality from kidney, liver, and testicular cancer based on the RR and each study's corresponding 95% CIs. Heterogeneity (het.) among studies was evaluated by the Q test, based on the variation across studies rather than within studies, and the I2 statistic (the percentage of variance in a meta-analysis that is attributable to study heterogeneity) [16]. Random-effect models were used to account for heterogeneity in the design characteristics of the cohorts and case controls included in the meta-analysis [17].
First, we performed a meta-analysis, including non-overlapping studies for each cancer type separately. Then we conducted stratified analyses by geographic region (Europe, North America, others including Asia and Oceania), study design (cohort, nested case-control, case-control), level of exposure assessment (individual, ecologic), quality score (low, high quality), outcome (incidence, mortality), year of publication (<2014, >=2014), exposure source (occupational, environmental), and PFAS type (PFAS, PFOA, PFOS). In addition, we conducted a meta-regression of the RR on the quality scores.
We also abstracted dose-response results, including analyses by duration or level of exposure. We categorized results into low, medium, or high exposure. We conducted a meta-analysis of results in each category and a meta-regression of the linear trend using weights 1,2 and 4 for the exposure categories. Finally, we examined publication bias by creating a funnel plot and applying a regression asymmetry test [18].
3. Results
Based on our search of the literature and selection procedure, we included 15 independent studies in the review and meta-analysis [19-33]. The flow diagram for literature search and study selection is shown in Figure 1. The review comprised 10 cohort studies [19-21, 23, 25-28, 30, 33], three case-control studies nested in a cohort [22, 29, 32], and two case-control studies [24, 31]. All studies had individual-level assessments of PFAS exposure, except for two studies in which the assessment was ecologic-level [24, 27]. Details on these studies are provided in Supplementary Table 3.
Figure 1.
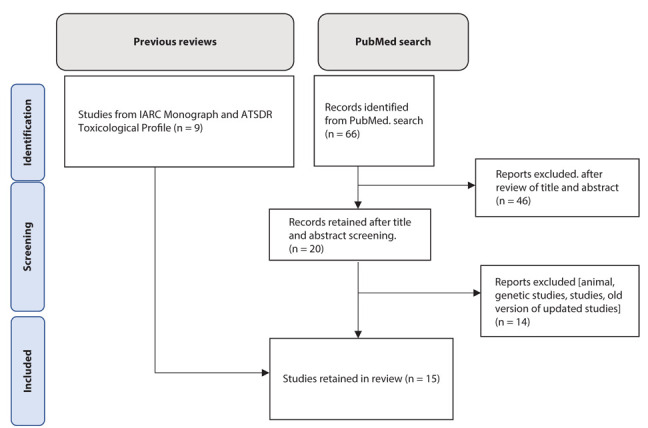
Selection of studies for inclusion in the review and meta-analysis.
Supplementary Table 3.
Selected characteristics of the studies included in the review and meta-analysis.
| Ref. # | 1st author and year | Study design | Gender* | Cancer type | Country | Type of PFAS | Exposure source | Potential confounders included in the analysis (other than sex, age, calendar period) | Quality score |
|---|---|---|---|---|---|---|---|---|---|
| 19 | Gilliland FD, 1993 | Cohort | Male | Testis | USA | PFOA | Occupational | 8 | |
| 20 | Alexander BH, 2003 | Cohort | Both | Liver | USA | PFOS | Occupational | 6 | |
| 21 | Leonard RC, 2008 | Cohort | Both | Liver, kidney, testis | USA | PFOA | Occupational | 6.5 | |
| 22 | Eriksen KT, 2009 | Nested case-control | Both | Liver | Denmark | PFOA, PFOS | Environmental | Tobacco smoking, education, alcohol drinking, occupation | 11 |
| 23 | Steenland K, 2012 | Cohort | Both | Liver, kidney, testis | USA | PFOA | Occupational | 7 | |
| 24 | Vieira VM, 2013 | Case-control ecologic | Both | Liver | USA | PFOA | Environmental | 7 | |
| 25 | Barry V, 2013 | Cohort | Both | Kidney | USA | PFOA | Environmental | 8.5 | |
| 26 | Raleigh KK, 2014 | Cohort | Both | Liver, kidney, testis | USA | PFOA | Occupational | 10 | |
| 27 | Mastrantonio M, 2018 | Cohort ecologic | Both | Liver, kidney, testis | Italy | PFAS | Environmental | 6.5 | |
| 28 | Girardi P, 2019 | Cohort | Male | Liver | Italy | PFOA | Occupational | 8 | |
| 29 | Shearer JJ, 2021 | Nested case control | Both | Kidney | USA | PFOA, PFOS | Environmental | BMI, tobacco smoking, hypertension | 12 |
| 30 | Li H, 2022 | Cohort | Both | Liver, kidney, testis | Sweden | PFAS | Environmental | 7.5 | |
| 31 | Cao L, 2022 | Case control | Both | Liver | China | PFOA, PFOS | Environmental | Education, BMI, income, tobacco smoking, medical history | 6 |
| 32 | Goodrich JA, 2022 | Nested case control | Both | Liver | USA | PFOA, PFOS | Environmental | 11 | |
| 33 | Law HD, 2023 | Cohort | Both | Liver, kidney, testis | Australia | PFAS | Environmental | Adjusted for age, sex and calendar period | 6.5 |
* Male: >75% male; both: neither gender >75%
BMI, body mass index
The studies reported 11 risk estimates for kidney cancer [21, 23, 25-27, 29, 30, 33], 16 for liver cancer [20-24, 26, 27, 28, 30-33], and 8 for testicular cancer [19, 21, 23, 26, 27, 30, 33]. The summary RR of kidney cancer for ever-PFAS exposure was 1.18 (95% CI=1.05-1.32; I2=52.8%, p-het=0.02; Figure 2a). There was no association for liver (RR=1.03, 95% CI =0.91-1.16; I2=47.9%, p-het=0.02; Figure 2b) or testicular cancer (RR=1.12, 95% CI =0.94-1.34; I2 = 0.0%, p-het=0.52; Figure 2c).
Figure 2.
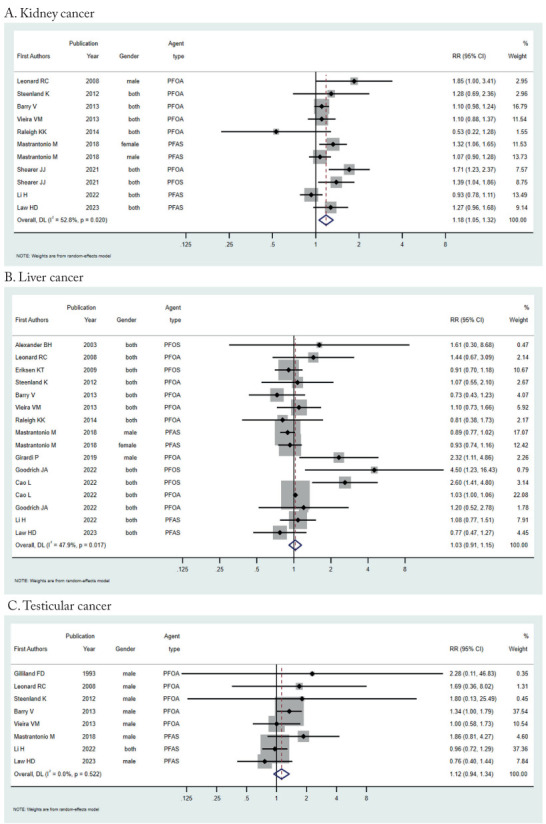
Forest plot (random-effects model) of results on the association between PFAS exposure and kidney, liver, and testicular cancer.
The results of stratified meta-analyses are reported in Table 1. No differences by type of PFAS were detected for any of the cancers under review. Stratification by geographical region, study design, outcome, quality score, year of publication, quality score, and exposure source did not reveal heterogeneity for any of the three cancers under study. Stratification analysis by study design showed heterogeneity for kidney cancer (p=0.04) but not for liver and testicular cancer. The results of the meta-regression did not suggest a relationship between RR and quality score for kidney cancer (p=0.31), liver cancer (p=0.61), or testicular cancer (p=0.59). Only two studies reported results for women.
Table 1.
Results of the metanalyses stratified by region, outcome, study design, year of publication, gender, and quality score.
| Characteristic | N risk estimates | RR, 95% CI | p heterogeneity |
|---|---|---|---|
| Kidney cancer | |||
| Region | |||
| North America | 7 | 1.25(1.04-1.50) | 0.49 |
| Europe | 3 | 1.08(0.90-1.31) | |
| Others | 1 | 1.27(0.96-1.68) | |
| Study design | |||
| Cohort | 8 | 1.12(0.99-1.26) | 0.04 |
| Nested case control | 2 | 1.52(1.22-1.89) | |
| Case control | 1 | 1.10( 0.88-1.37) | |
| Quality score | |||
| Low quality (<8) | 7 | 1.14(1.00-1.30) | 0.60 |
| High quality (>=8) | 4 | 1.24(0.93-1.65) | |
| Outcome | |||
| Incidence | 9 | 1.16(1.04-1.29) | 0.98 |
| Mortality | 3 | 1.15(0.60-2.20) | |
| Year of publication | |||
| <2014 | 4 | 1.12(1.01-1.24) | 0.54 |
| >=2014 | 7 | 1.19(1.00-1.42) | |
| Exposure | |||
| Occupational | 3 | 1.15(0.60-2.20) | 0.96 |
| Environmental | 8 | 1.17(1.05-1.31) | |
| PFAS type | |||
| PFOA | 6 | 1.23(0.99-1.51) | 0.41 |
| PFOS | 1 | 1.39(1.04-1.86) | |
| PFAS | 4 | 1.12(0.95-1.31) | |
| Dose category | |||
| Low | 7 | 0.98(0.83-1.17) | 0.03* |
| Medium | 8 | 1.38 (1.09-1.74) | |
| High | 7 | 1.74 (1.23-2.47) | |
| Liver cancer | |||
| Region | |||
| North America | 8 | 1.08(0.83-1.42) | 0.63 |
| Europe | 5 | 0.97(0.83-1.13) | |
| Others | 3 | 1.20(0.72-2.01) | |
| Study design | |||
| Cohort | 10 | 0.94(0.83-1.08) | 0.25 |
| Nested case control | 3 | 1.37(0.65-2.87) | |
| Case control | 3 | 1.31(0.85-2.00) | |
| Quality score | |||
| Low quality (<8) | 10 | 1.02(0.90-1.14) | 0.55 |
| High quality (>=8) | 6 | 1.16(0.77-1.74) | |
| Outcome | |||
| Incidence | 13 | 1.03(0.90-1.18) | 0.23 |
| Mortality | 5 | 1.31(0.90-1.90) | |
| Year of publication | |||
| <2014 | 6 | 0.96(0.80-1.16) | 0.43 |
| >=2014 | 10 | 1.06(0.91-1.24) | |
| Exposure | |||
| Occupational | 5 | 1.31(0.90-1.90) | 0.18 |
| Environmental | 11 | 1.00(0.88-1.13) | |
| PFAS type | |||
| PFOA | 8 | 1.05(0.93-1.18) | 0.07 |
| PFOS | 4 | 1.86(0.81- 4.25) | |
| PFAS | 4 | 0.91(0.82-1.02) | |
| Dose category | |||
| Low | 8 | 1.12 (0.85-1.48) | 0.37* |
| Medium | 4 | 1.22 (0.66-2.25) | |
| High | 9 | 1.01 (0.68-1.50) | |
| Testicular cancer | |||
| Region | |||
| North America | 5 | 1.28(0.99-1.64) | 0.33 |
| Europe | 2 | 1.19(0.65-2.17) | |
| Others | 1 | 0.76(0.40-1.44) | |
| Study design | |||
| Cohort | 7 | 1.14(0.94-1.37) | 0.67 |
| Nested case control | 0 | - | |
| Case control | 1 | 1.00(0.58-1.73) | |
| Quality score | |||
| Low quality (<8) | 6 | 1.00(0.79-1.26) | 0.11 |
| High quality (>=8) | 2 | 1.35(1.01-1.80) | |
| Outcome | |||
| Incidence | 5 | 1.10(0.88-1.39) | 0.44 |
| Mortality | 3 | 1.80(0.53-6.14) | |
| Year of publication | |||
| <2014 | 5 | 1.28(0.99-1.64) | 0.30 |
| >=2014 | 3 | 1.01(0.69-1.46) | |
| Exposure | |||
| Occupational | 3 | 1.80(0.53-6.14) | 0.44 |
| Environmental | 5 | 1.10(0.88-1.39) | |
| PFAS type | |||
| PFOA | 5 | 1.28(0.99-1.64) | 0.30 |
| PFOS | 0 | - | |
| PFAS | 3 | 1.01(0.70-1.46) | |
| Dose category | |||
| Low | 2 | 0.86(0.59-1.24) | 0.02* |
| Medium | 2 | 1.01(0.33-3.12) | |
| High | 3 | 2.22(1.12-4.39) | |
*denotes the p-value of test for linear trend.
An analysis of stratification by low, medium, and high PFAS exposure showed an association between increased exposure and kidney (RR=1.74, 95% CI=1.23-2.47; p-trend=0.03) and testicular cancer (RR=2.22, 95% CI=1.12-4.39; p-trend=0.02), while the results for liver cancer did not reveal any trend (p= 0.37) (Supplementary Table 4).
Supplementary Table 4.
Results on dose-response relationship.
| First Author, year | Exposure level | Dose detail | RR (95% CI) | PFAS type | Cancer type |
|---|---|---|---|---|---|
| Alexander BH (2003) | High | N/A | 2.00 (0.05-11.10) | PFOS | Liver |
| Low | N/A | 3.94 (0.1-21.88) | |||
| Girardi P (2019) | High | > 16,956 ng /mL-years | 3.07 (1.15-8.18) | PFOA | Liver |
| Medium | 4034–16,956 ng/mL-years | 2.76 (0.69-11.00) | |||
| Low | ≤4,034 ng/mL-years | 1.02 (0.12-7.21) | |||
| Shearer JJ (2021) | High | >7.3-27.2 µg /-l | 2.63 (1.33-5.20) | PFOA | Kidney |
| Medium | >5.5-7.3 µg /-l | 1.24 (0.64-2.41) | |||
| Low | ≥4-5.5 µg /l | 1.47 (0.77-2.80) | |||
| High | >49.9-154.2 µg /-l | 2.51 (1.28-4.92) | PFOS | ||
| Medium | >38.4-49.9 µg /-l | 0.92 (0.45-1.88) | |||
| Low | >26.3-38.4 µg /-l | 1.67 (0.84-3.30) | |||
| Li H (2022) | High | N/A | 1.07 (0.75-1.54) | PFAS | Kidney |
| Low | N/A | 0.88 (0.72-1.09) | |||
| High | N/A | 0.98 (0.45-1.86) | Liver | ||
| Low | N/A | 1.12 (0.72-1.66) | |||
| High | N/A | 1.28 (0.70-2.15) | Testis | ||
| Low | N/A | 0.85 (0.57-1.21) | |||
| Raleigh KK (2014) | High | >7.9×10 − 4 µg/m3 years. | 0.73 (0.21-2.48) | PFOA | Kidney |
| Medium | 2.9×10-5 - 1.5×10-4 µg /m3 years | 1.07 (0.36-3.17) | |||
| Medium | 1.5×10-4 - 7.9×10-4 µg /m3 years. | 0.98 (0.33-2.92) | |||
| Low | <2.9×10-5 µg /m3 years | 1.07 (0.36-3.16) | |||
| High | >1.5×10-4 µg /m3 years | 0.67 (0.14-3.27) | Liver | ||
| Low | <1.5×10-4 µg /m3 years | 2.09 (0.69-6.31) | |||
| Eriksen KT (2009) | Low | N/A | 0.62 (0.29-1.33) | PFOS | Liver |
| Medium | N/A | 0.72 (0.33-1.56) | |||
| High | N/A | 0.59 (0.27-1.27) | |||
| High | N/A | 0.60 (0.26-1.37) | PFOA | ||
| Low | N/A | 1.00 (0.44-2.23) | |||
| Steenland K (2012) | High | ≥2,700 ppm-years | 2.66 (1.15-5.24) | PFOA | Kidney |
| Medium | 904–<1,520 ppm-years | 1.37 (0.28-3.99) | |||
| Low | 0–<904 ppm-years | 1.07 (0.02-3.62) | |||
| High | ≥2,700 ppm-years | 0.32 (0.01-1.76) | Liver | ||
| Medium | 1,520–<2,700 ppm-years | 2.01 (0.65-4.68) | |||
| Low | 0–<904 ppm-years | 2.39 (0.65-6.13) | |||
| Vieira VM (2013) | High | 110–655 µg/L | 2.8 (0.8-9.2) | PFOA | Testis |
| Medium1 | 12.9–30.7 µg/L | 0.6( 0.2-2.2) | |||
| Medium2 | 30.8–109 µg/L | 0.3 (0-2.7) | |||
| Low | 3.7–12.8 µg/L | 0.2 (0-1.6) | |||
| High | 30.8–109 µg/L | 1.0 (0.3-3.1) | Liver | ||
| Medium1 | 12.9–30.7 µg/L | 0.9 (0.3-2.5) | |||
| Low | 3.7–12.8 µg/L | 1.1 (0.4-1.5) | |||
| High | 110–655 µg/L | 2.0 (1.0-3.9) | Kidney | ||
| Medium1 | 12.9–30.7 µg/L | 1.2 (0.7-2.0) | |||
| Medium2 | 30.8–109 µg/L | 2.0 (1.3-3.2) | |||
| Low | 3.7–12.8 µg/L | 0.8( 0.4-1.5) | |||
| Barry V (2013) | High | N/A | 1.58 (0.88-2.84) | PFOA | Kidney |
| Medium | N/A | 1.48 (0.84-2.60) | |||
| Low | N/A | 1.23 (0.70-2.17) | |||
| High | N/A | 3.17 (0.75-1.45) | Testis | ||
| Medium | N/A | 1.91 (0.47-7.75) | |||
| Low | N/A | 1.04 (0.26-4.22) |
No publication bias was detected for kidney cancer (p=0.31), liver cancer (p=0.51), or testicular cancer (p=0.53); the funnel plots are shown in Figure 3.
Figure 3.
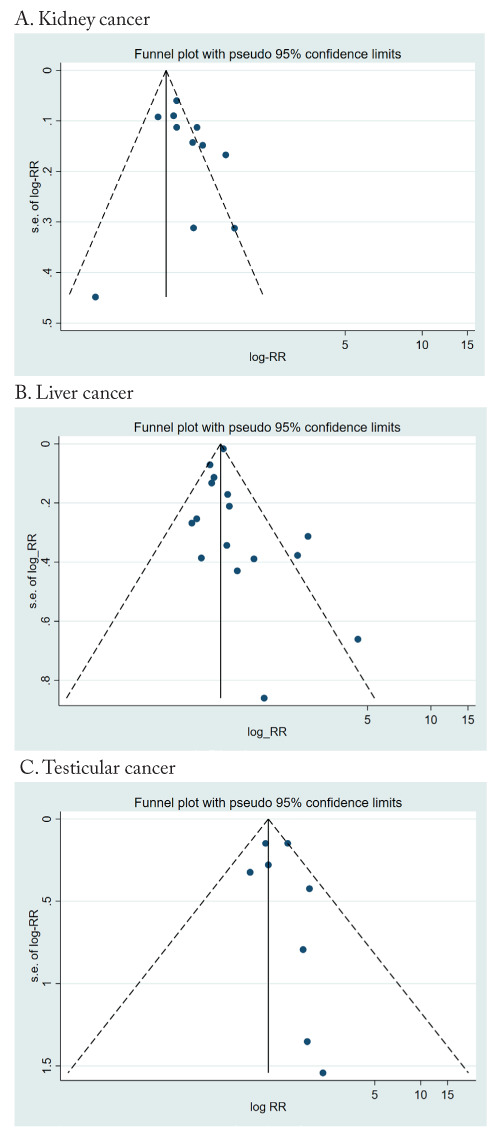
Funnel plot of results on the association between PFAS exposure and kidney, liver, and testicular cancer.
4. Discussion
Our systematic review and meta-analysis presented an association between overall PFAS exposure and the risk of kidney cancer. Also, we found a dose-response relationship for kidney and testicular cancer. Conversely, we did not find an association with liver cancer.
The human body is exposed to PFAS through several sources and pathways, including ingestion through water, packaging materials, and food items; inhalation through air, and dermal absorption through various consumer products (e.g., waxes, leather, outdoor textiles, cosmetics, and impregnation spray) [9].
PFAS have a long half-life in the environment and inside the human body. It has been reported that the half-life of PFOA ranges from 2 to 3 years, whereas that of PFOS and other PFAS is longer, up to 4 to 7 years. This factor is associated with the amount of PFAS stored and the possible effects in different organs [34, 35]. The long half-life of these agents may explain that, despite a decrease in exposure over time in most populations, we did not find a difference in our analysis according to the year of publication [36].
When entering the body, this group of agents can affect it by various mechanisms [37]. PFAS are nephrotoxic through oxidative stress and epigenetic mechanisms linked to tubular reabsorption, leading to high concentrations in renal parenchyma [38, 39]. Also, the liver is an important storage organ for PFAS, which can lead to lipid metabolism alteration and non-alcoholic fatty liver disease, and ultimately to the subsequent development of cancer [40-42]. In addition, PFAS influences immunological processes and hormonal balance, resulting in possible reproductive effects on this group of organs in men and women [43-48].
Several confounding risk factors can affect the results of kidney, liver, and testicular cancer studies. Regarding liver cancer, major risk factors include chronic alcohol consumption, hepatitis B and C virus infection, tobacco smoking, and increased body mass. Concerning kidney cancer, it is critical to consider tobacco smoking, body size, hypertension, and other chronic kidney diseases [49]. In addition, perinatal exposures (such as maternal smoking) and medical conditions (cryptorchidism) are important risk factors for testicular cancer. There is also limited evidence that occupational exposures during firefighting and aircraft maintenance and environmental exposures, like that to organochlorine pesticides, may cause testicular cancer [50].
The results of the stratification analysis did not reveal any statistically significant heterogeneity. However, there was a suggestion of a stronger association between occupation exposure and testicular and liver cancer than environmental exposure. Workers are probably exposed to higher concentrations and more prolonged periods than the community exposed to environmental sources. Additionally, many factors affect the concentration of PFAS in water, soil, and food, such as seasonal rain levels, the use of private wells or tap public water, and geographical locations. However, regarding kidney cancer, this difference was not apparent. Results based on PFOS exposure suggested a stronger association with kidney and liver cancer risk compared to results based on PFOA or other unspecified PFAS. However, the difference was not statistically significant (no results were available on PFOS exposure and testicular cancer). Given the multiplicity of comparisons in the stratified analysis, these results should be interpreted cautiously. The analysis by geographic region revealed that few studies were conducted outside Europe and North America but did not show any consistent pattern.
There is some evidence that exposure to high doses of PFAS increases the risk of cancers like liver, testis, bladder, prostate, and breast. [7, 51] Despite the use of heterogeneous categories of exposure, which might have resulted in non-differential misclassification, our meta-regression suggested that high doses of PFAS are associated and seem to show a dose-response trend with kidney and testicular cancer but not liver cancer.
To our knowledge, this is the first systematic review and meta-analysis of studies dealing with the possible association between environmental and occupational PFAS exposure and liver, kidney, and testicular cancers. A severe limitation of our review is the relatively small number of available studies, particularly those addressing exposure to specific PFAS other than PFOA, those reporting results among female workers, and those conducted in countries outside North America and Europe, especially locations with a high prevalence of these cancers including East Asia and sub-Saharan Africa [11]. Thus, stratified analyses have limitations. The lack of adjustment for potential confounders is a severe drawback of many available studies.
In conclusion, we identified an association between overall PFAS exposure and kidney cancer and between high-dose exposure and kidney and testicular cancer. Residual confounding and other sources of bias prevent concluding the causal nature of these associations. Additional studies are needed to elucidate the carcinogenic risk from PFAS exposure fully.
Acknowledgments:
The authors thank Germana Giupponi for assisting in identifying articles included in the review.
Conflicts of Interest:
PB acted as an expert in litigation involving PFAS exposure unrelated to the present work. MSS declares no conflict of interest.
References
- Ying L, Fletcher T, Mucs D, et al. Half-lives of PFOS, PFHxS and PFOA after end of exposure to contaminated drinking water. Occup Environ Med. 2018;75:46–51. doi: 10.1136/oemed-2017-104651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaines LGT. Historical and current usage of per- and polyfluoroalkyl substances (PFAS): A literature review. Am J Ind Med. 2023;66:353–378. doi: 10.1002/ajim.23362. [DOI] [PubMed] [Google Scholar]
- Exner M, Färber H. Perfluorinated surfactants in surface and drinking waters. Environ Sci Pollut Res. 2006;13:299–307. doi: 10.1065/espr2006.07.326. [DOI] [PubMed] [Google Scholar]
- Panieri E, Baralic K, Djukic-Cosic D, Buha Djordjevic A, Saso L. PFAS Molecules: A Major Concern for the Human Health and the Environment. Toxics. 2022;10:44. doi: 10.3390/toxics10020044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Environment Agency. Emerging chemical risks in Europe – PFAS. Copenhagen, EEA, 2019. 2019 https://www.eea.europa.eu/themes/human/chemicals/emerging-chemical-risks-in-europe. [Google Scholar]
- Fenton SE, Ducatman A, Boobis A, et al. Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environ Toxicol Chem. 2021;40:606–630. doi: 10.1002/etc.4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenland K, Winquist A. PFAS and cancer, a scoping review of the epidemiologic evidence. Environ Res. 2021;194:110690. doi: 10.1016/j.envres.2020.110690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Agency for Research on Cancer. Some Chemicals Used as Solvents and in Polymer Manufacture. Vol. 110. Lyon, IARC: 2017. PFOA. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; pp. 37–110. [PubMed] [Google Scholar]
- Ding N, Harlow SD, Randolph JF Jr, Loch-Caruso R, Park SK. Perfluoroalkyl and polyfluoroalkyl substances (PFAS) and their effects on the ovary. Hum Reprod Update. 2020;26:724–752. doi: 10.1093/humupd/dmaa018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang ET, Adami HO, Boffetta P, Wedner HJ, Mandel JS. A critical review of perfluorooctanoate and perfluorooctanesulfonate exposure and immunological health conditions in humans. Crit Rev Toxicol. 2016;46:279–331. doi: 10.3109/10408444.2015.1122573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry. Toxicological Profile for Perfluoroalkyls. Atlanta, GA, ATSDR: 2021. [PubMed] [Google Scholar]
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE)statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–9. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altmann DG. The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann Intern Med. 2009;151:264–69. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- Stan A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder CE. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliland FD, Mandel JS. Mortality among employees of a perfluorooctanoic acid production plant. J Occup Med. 1993;35:950–4. doi: 10.1097/00043764-199309000-00020. [DOI] [PubMed] [Google Scholar]
- Alexander BH, Olsen GW, Burris JM, Mandel JH, Mandel JS. Mortality of employees of a perfluorooctanesulphonyl fluoride manufacturing facility. Occup Environ Med. 2003;60:722–9. doi: 10.1136/oem.60.10.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard RC, Kreckmann KH, Sakr CJ, Symons JM. Retrospective cohort mortality study of workers in a polymer production plant including a reference population of regional workers. Ann Epidemiol. 2008;18:15–22. doi: 10.1016/j.annepidem.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Eriksen KT, S⊘rensen M, McLaughlin JK, et al. Perfluorooctanoate and perfluorooctanesulfonate plasma levels and risk of cancer in the general Danish population. J Natl Cancer Inst. 2009;101:605–9. doi: 10.1093/jnci/djp041. [DOI] [PubMed] [Google Scholar]
- Steenland K, Woskie S. Cohort mortality study of workers exposed to perfluorooctanoic acid. Am J Epidemiol. 2012;176:909–17. doi: 10.1093/aje/kws171. [DOI] [PubMed] [Google Scholar]
- Vieira VM, Hoffman K, Shin HM, Weinberg JM, Webster TF, Fletcher T. Perfluorooctanoic acid exposure and cancer outcomes in a contaminated community: a geographic analysis. Environ Health Perspect. 2013;121:318–23. doi: 10.1289/ehp.1205829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry V, Winquist A, Steenland K. Perfluorooctanoic acid (PFOA) exposures and incident cancers among adults living near a chemical plant. Environ Health Perspect. 2013;121:1313–8. doi: 10.1289/ehp.1306615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raleigh KK, Alexander BH, Olsen GW, et al. Mortality and cancer incidence in ammonium perfluorooctanoate production workers. Occup Environ Med. 2014;71:500–6. doi: 10.1136/oemed-2014-102109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrantonio M, Bai E, Uccelli R, Cordiano V, Screpanti A, Crosignani P. Drinking water contamination from perfluoroalkyl substances (PFAS): an ecological mortality study in the Veneto Region, Italy. Eur J Public Health. 2018;28:180–185. doi: 10.1093/eurpub/ckx066. [DOI] [PubMed] [Google Scholar]
- Girardi P, Merler E. A mortality study on male subjects exposed to polyfluoroalkyl acids with high internal dose of perfluorooctanoic acid. Environ Res. 2019;179:108743. doi: 10.1016/j.envres.2019.108743. [DOI] [PubMed] [Google Scholar]
- Shearer JJ, Callahan CL, Calafat AM, et al. Serum Concentrations of Per- and Polyfluoroalkyl Substances and Risk of Renal Cell Carcinoma. J Natl Cancer Inst. 2021;113:580–587. doi: 10.1093/jnci/djaa143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Hammarstrand S, Midberg B, et al. Cancer incidence in a Swedish cohort with high exposure to perfluoroalkyl substances in drinking water. Environ Res. 2022;204:112217. doi: 10.1016/j.envres.2021.112217. [DOI] [PubMed] [Google Scholar]
- Cao L, Guo Y, Chen Y, Hong J, Wu J, Hangbiao J. Per-/polyfluoroalkyl substance concentrations in human serum and their associations with liver cancer. Chemosphere. 2022;296:134083. doi: 10.1016/j.chemosphere.2022.134083. [DOI] [PubMed] [Google Scholar]
- Goodrich JA, Walker D, Lin X, et al. Exposure to perfluoroalkyl substances and risk of hepatocellular carcinoma in a multiethnic cohort. JHEP Rep. 2022;4:100550. doi: 10.1016/j.jhepr.2022.100550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law HD, Armstrong BK, D’este C, et al. Relative rates of cancers and deaths in Australian communities with PFAS environmental contamination associated withfire-fighting foams: A cohort study using linked data. Cancer Epidemiol. 2023;82:102296. doi: 10.1016/j.canep.2022.102296. [DOI] [PubMed] [Google Scholar]
- Li Y, Xu Y, Scott K, et al. Half-lives of PFOA, PFPeS, PFHxS, PFHpS and PFOS after end of exposure to contaminated drinking water. Environ Epidemiol. 2019;3:237. doi: 10.1136/oemed-2017-104651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Fletcher T, Mucs D, et al. Half-lives of PFOS, PFHxS and PFOA after end of exposure to contaminated drinking water. Occup Environ Med. 2018;75:46–51. doi: 10.1136/oemed-2017-104651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan NM, Evans AT, Fritz MK, Peak SA, von Holst HE. Trends in the Regulation of Per- and Polyfluoroalkyl Substances (PFAS): A Scoping Review. Int J Environ Res Public Health. 2021;18:10900. doi: 10.3390/ijerph182010900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton SE, Ducatman A, Boobis A, et al. Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environ Toxicol Chem. 2021;40:606–630. doi: 10.1002/etc.4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen LL, Lin CY, Chou HC, Chang CC, Lo HY, Juan SH. Perfluorooctanesulfonate mediates renal tubular cell apoptosis through PPARgamma inactivation. PLoS One. 2016;11:e0155190. doi: 10.1371/journal.pone.0155190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanifer JW, Stapleton HM, Souma T, Wittmer A, Zhao X, Boulware LE. Perfluorinated chemicals as emerging environmental threats to kidney health: A scoping review. Clin J Am Soc Nephrol. 2018;13:1479–1492. doi: 10.2215/CJN.04670418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan HT, Zhao YG, Wei X, Hui KY, Giesy JP, Wong CK. PFOS-induced hepatic steatosis, the mechanistic actions on beta-oxidation and lipid transport. Biochim Biophys Acta. 2012;1820:1092–1101. doi: 10.1016/j.bbagen.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Hui Z, Li R, Chen L. The impact of exposure to environmental contaminant on hepatocellular lipid metabolism. Gene. 2017;622:67–71. doi: 10.1016/j.gene.2017.04.024. [DOI] [PubMed] [Google Scholar]
- Massoud O, Charlton M. Non-alcoholic fatty liver disease/non-alcoholic steatohepatitis and hepatocellular carcinoma. Clin Liver Dis. 2018;22:201–211. doi: 10.1016/j.cld.2017.08.014. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Heilmann C, Weihe P, Nielsen F, Mogensen UB, Budtz-Jorgensen E. Serum vaccine antibody concentrations in adolescents exposed to perfluorinated compounds. Environ Health Perspect. 2017;125:077018. doi: 10.1289/EHP275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coperchini F, Awwad O, Rotondi M, Santini F, Imbriani M, Chiovato L. Thyroid disruption by perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) J Endocrinol Invest. 2017;40:105–121. doi: 10.1007/s40618-016-0572-z. [DOI] [PubMed] [Google Scholar]
- Barry V, Winquist A, Steenland K. Perfluorooctanoic acid (PFOA) exposures and incident cancers among adults living near a chemical plant. Environ Health Perspect. 2013;121:1313–1318. doi: 10.1289/ehp.1306615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabovic I, Cosci I, De Toni L, et al. Perfluoro-octanoic acid impairs sperm motility through the alteration of plasma membrane. J Endocrinol Invest. 2020;43:641–652. doi: 10.1007/s40618-019-01152-0. [DOI] [PubMed] [Google Scholar]
- Cheng L, Albers P, Berney DM, et al. Testicular cancer. Nat Rev Dis Primers. 2018;4:29. doi: 10.1038/s41572-018-0029-0. [DOI] [PubMed] [Google Scholar]
- Di Nisio A, Rocca MS, Sabovic I, et al. Perfluorooctanoic acid alters progesterone activity in human endometrial cells and induces reproductive alterations in young women. Chemosphere. 2020;242:125208. doi: 10.1016/j.chemosphere.2019.125208. [DOI] [PubMed] [Google Scholar]
- Scelo G, Larose TL. Epidemiology and Risk Factors for Kidney Cancer. J Clin Oncol. 2018;36:3574–3581. doi: 10.1200/JCO.2018.79.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlynn KA, Trabert B. Adolescent and adult risk factors for testicular cancer. Nat Rev Urol. 2012;9:339–49. doi: 10.1038/nrurol.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imir OB, Kaminsky AZ, Zuo QY, et al. Per- and Polyfluoroalkyl Substance Exposure Combined with High-Fat Diet Supports Prostate Cancer Progression. Nutrients. 2021;13:3902. doi: 10.3390/nu13113902. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1b.
PRISMA Abstract Checklist.
| Section and Topic | Item # | Checklist item | Reported (Yes/No) |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a systematic review. | Yes |
| BACKGROUND | |||
| Objectives | 2 | Provide an explicit statement of the main objective(s) or question(s) the review addresses. | Yes |
| METHODS | |||
| Eligibility criteria | 3 | Specify the inclusion and exclusion criteria for the review. | No |
| Information sources | 4 | Specify the information sources (e.g. databases, registers) used to identify studies and the date when each was last searched. | Yes |
| Risk of bias | 5 | Specify the methods used to assess risk of bias in the included studies. | Yes |
| Synthesis of results | 6 | Specify the methods used to present and synthesise results. | Yes |
| RESULTS | |||
| Included studies | 7 | Give the total number of included studies and participants and summarise relevant characteristics of studies. | Yes |
| Synthesis of results | 8 | Present results for main outcomes, preferably indicating the number of included studies and participants for each. If meta-analysis was done, report the summary estimate and confidence/credible interval. If comparing groups, indicate the direction of the effect (i.e. which group is favoured). | Yes |
| DISCUSSION | |||
| Limitations of evidence | 9 | Provide a brief summary of the limitations of the evidence included in the review (e.g. study risk of bias, inconsistency and imprecision). | No |
| Interpretation | 10 | Provide a general interpretation of the results and important implications. | Yes |
| OTHER | |||
| Funding | 11 | Specify the primary source of funding for the review. | No |
| Registration | 12 | Provide the register name and registration number. | No |


