Abstract
Background:
Clinical decision support systems and telemedicine for remote monitoring can together support surgical patients’ intraoperative decision-making and care management. However, there has been limited investigation on patient perspectives about advanced health information technology use in intraoperative settings, particularly within an intraoperative telemedicine setting (eOR).
Purpose:
Our study objectives were: (1) to identify participant-rated items contributing to patient attitudes, beliefs, and level of comfort with eOR monitoring; (2) to highlight barriers and facilitators to eOR use; and (3) to develop guidelines for eOR implementation that improve patient buy-in.
Methods:
We surveyed 324 individuals representing surgical patients across the United States using Amazon Mechanical Turk, an online platform supporting internet-based work. The structured survey questions examined the level of agreement and comfort with eOR for remote patient monitoring. We calculated descriptive statistics for demographic variables and performed a Wilcoxon matched-pairs signed-rank test to assess whether participants were more comfortable with familiar clinicians from local hospitals or health systems monitoring their health and safety status during surgery than clinicians from hospitals or health systems in other regions or countries. We also analyzed open-ended survey responses using a thematic approach informed by an eight-dimensional socio-technical model.
Results:
Participants’ average age was 34.07 (SD = 10.11). Most were white (80.9%), male (57.1%), and had a high school degree or more (88.3%). Participants reported a higher level of comfort with clinicians they knew monitoring their health and safety than clinicians they did not know, even within the same healthcare system (z = −4.012, p<.001). They reported significantly higher comfort levels with clinicians within the same hospital or health system in the United States than those in a different country (z = −10.230, p<.001). Facilitators and barriers to eOR remote monitoring were prevalent across four socio-technical dimensions: 1) organizational policies, procedures, environment, and culture; 2) people; 3) workflow and communication; and 4) hardware and software. Facilitators to eOR use included perceptions of improved patient safety through a safeguard system and perceptions of streamlined care. Barriers included fears of incorrect eOR patient assessments, decision-making conflicts between care teams, and technological malfunctions.
Conclusions:
Participants expressed significant support for intraoperative telemedicine use and greater comfort with local telemedicine systems instead of long-distance telemedicine systems. Reservations centered on organizational policies, procedures, environment, culture; people; workflow and communication; and hardware and software. To improve the acceptability of remote monitoring by an OR telemedicine team and address these concerns, we highlight evidence-based guidelines applicable to telemedicine use within the context of OR workflow. Guidelines include backup plans for technical challenges, rigid care, and privacy standards, and patient education to increase understanding of telemedicine’s potential to improve patient care.
Keywords: Telemedicine, Surgery, Remote Monitoring, Operating Room
1. Introduction
Each year, approximately 7 million patients out of 300 million global surgeries (50 million in the United States [1, 2]) experience postoperative complications, resulting in over 1 million deaths annually [3]. Up to half of these complications are preventable, and many are attributed to ineffective intraoperative decision-making and care management in the operating room (OR) [3, 4]. Commonly reported barriers to intraoperative decision-making include information overload [5], inefficient communication leading to limited shared understanding between disciplines and teams [6–11], and cognitive constraints (e.g., human bias) [12, 13].
Clinical decision support systems (CDSS) [14] can monitor patient status, streamline documentation, guide evidence-based treatments [15, 16], flag critical events and patient risk factors [3, 4], and enhance situational awareness [17] to aid decision-making and care planning and reduce opportunities for decision-making errors and preventable complications [18]. However, their use is often limited by several socio-technical issues, including unclear alerts and changing guidelines to CDSS use [16, 19].
To ameliorate these CDSS limitations, researchers have evaluated the use of telemedicine for the OR (hereafter called electronic OR, eOR) across various inpatient surgical services [4, 20]. Currently, within the eOR, eOR teams (comprised of attending anesthesiologists, residents, certified/student registered nurse anesthetists) assess real-time surgical procedures through remote monitoring equipment feeding data into an eOR-based clinical decision support system. After determining patient data and clinical status, eOR team members identify red flags, contingencies, or patient complications and offer evidence-based risk mitigation strategies to the OR team through virtual consultations and feedback over an electronic message or phone call [21]. Once the OR receives the message, they determine how best to move forward with the procedure and maintain safety under time constraints and high-pressured conditions [22, 23]. Initial clinician-centered evaluations [20, 24, 25] identified barriers to eOR use, including poorly timed alerts, emotional responses, and care redundancy, and enablers including workload reduction and case-relevant alerts from eOR.
Studies conducted on telemedicine use internationally [26–28] indicate that patient-perceived and clinician-perceived factors affected its implementation. Patients in more rural areas with less education on telemedicine can resist its use [28]. Many patients are concerned about privacy [26]. Facilitators to successful implementation include collaboration between healthcare institutions and institutions of higher education or the government to provide resources, education, and training for telemedicine use [28]. In addition, many clinicians perceive telemedicine to enhance access to healthcare and offer better patient care quality [27]. While these international studies examined patient and clinician perspectives on telemedicine, studies within the US have primarily focused on clinician perspectives.
Patient-centered high-quality care should incorporate patients’ values, needs, and goals [29]. Thus obtaining patient perspectives on intraoperative care is essential to consider before large-scale implementation of the eOR. Leaders need to understand patient perspectives about using intraoperative telemedicine as part of their OR care to enhance care quality and improve intraoperative patient-centered outcomes. Towards this end, we conducted a survey study to examine the perspectives of past surgical patients on the eOR. Our study objectives were: (1) to identify perceived factors contributing to their attitudes, beliefs, and level of comfort with eOR monitoring; (2) to highlight barriers and facilitators to eOR use; and (3) to develop guidelines for eOR implementation that improve patient buy-in.
2. Methods
2.1. Study Setting and Participants
The survey was administered in October 2018 via Amazon Mechanical Turk (MTurk), an online platform supporting internet-based work [30]. Eligibility included English-speaking adults (age 18+) with internet access who underwent surgery within a year before participation. Potential participants were provided an online information sheet to read before agreeing to the survey. Washington University School of Medicine’s institutional review board deemed the study exempt (HRPO 201809038).
2.2. Conceptual Framework: 8-dimensional Socio-technical Model
We use the 8-dimensional socio-technical model to examine participant-perceived factors impacting patient acceptability towards eOR across qualitative responses [31]. The model includes eight interrelated dimensions: 1) hardware and software (e.g., all technology within the OR and eOR); 2) clinical content (e.g., clinical programs used within the OR and eOR); 3) human-computer interface (e.g., clinical program user interfaces); 4) people (e.g., OR patients and OR/eOR clinicians); 5) workflow and communication (e.g., intraoperative workflow); 6) internal organizational (e.g., hospital) policies, procedures, and cultures; 7) external rules, regulations, and pressures; and 8) system measurement and monitoring (e.g., telemedicine system) (Figure 1).
Figure 1.
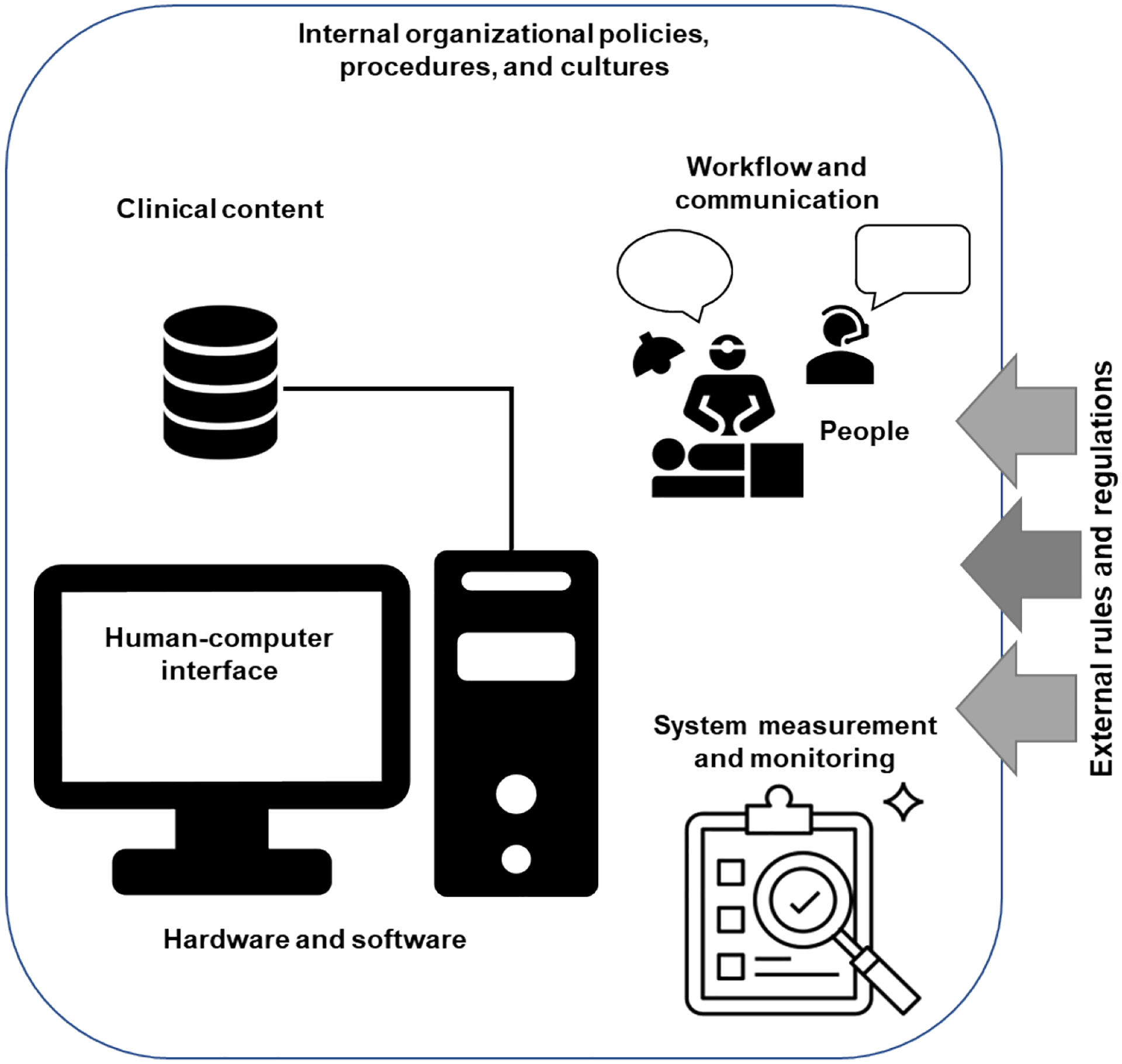
8-dimensional socio-technical model [31].
These dimensions address the socio-technical barriers and facilitators to designing, developing, implementing, and evaluating health information technology within complex healthcare systems. Without adequate support across all eight interdependent and interrelated dimensions, challenges arise in developing, implementing, and using the examined system (i.e., eOR). The socio-technical model has been used to inform institutional policies, improve clinician training, and develop and implement health information technology tools and clinical decision support interventions [32–34].
2.3. Data Collection
We developed a 40-item survey with close- and open-ended items to assess perceptions, comfort, and concerns with telemedicine remote monitoring scenarios within the local hospital, from a different hospital or health system within the US, and from a hospital or health system in another country (see Appendix for the survey with the scenarios). Our survey had four open-ended questions related to initial reactions to possible eOR use, eOR pros, eOR cons, and any other concerns. The survey development was informed by prior research [35–37] and surveys [38, 39] and was internally tested with researchers, clinicians, and patients/members of the general public (N=15) before being launched. Responses were not mandatory for all questions. An attention check question identified those who might not read each question before responding. Participants were categorized according to age (6 categories) and US Census regions (5 categories). Participants were compensated per MTurk guidelines at a rate consistent with minimum wage and the expected duration of the survey ($1.00 deposited into users’ Amazon account) [30].
2.4. Data Analysis
2.4.1. Quantitative analysis
Descriptive statistics were calculated for demographic variables and questions about their level of agreement and comfort with different scenarios. A Wilcoxon matched-pairs signed-rank test was conducted to assess whether participants were more comfortable with clinicians in a local hospital or health system monitoring the OR than clinicians from other hospitals or countries. The Wilcoxon test was selected as a non-parametric test to compare proportions for ordinal data.
2.4.2. Qualitative analysis
Thematic analyses of free-text responses were conducted using a hybrid inductive-deductive approach [40]. Responses were reviewed multiple times by two researchers (JA, AM) to familiarize themselves with the content. Relevant quotations and topics were labeled. Data was openly coded line-by-line to characterize the data’s underlying semantics, such as barriers and facilitators to intraoperative telemedicine use (i.e., inductive coding). Next, a priori codes informed by the socio-technical model [31] were applied to each transcript to categorize relevant data on eOR characteristics and patient-telemedicine relationships into one of the eight dimensions (i.e., deductive coding). Areas of similarity and overlap between codes were identified to synthesize unifying or repeated sub-themes under each relevant socio-technical dimension – for example, attention issues were categorized as a barrier to eOR use under the “People” socio-technical dimension (Table 1). Themes were finalized within and across transcripts after multiple rounds of data review and sub-theme refinement to arrive at 100% consensus between researchers.
Table 1.
Example of inductive and deductive thematic analysis.
| Clinician | Interview Transcript Text | Open Code | Sub-Themes | Socio-technical Dimension |
|---|---|---|---|---|
| P12 | ”I would be very happy to have this sort of scenario as part of my surgical care. While a single anesthesiologist and possibly nurse anesthetist would likely be enough to be vigilant about my vital signs during surgery, I would feel more comfortable with additional sets of trained eyes looking out for me. This scenario parallels telemetry units in hospitals where each patient has a nurse assigned to them, but their hearts are also being monitored by a telemetry tech.” | Safety Additional monitoring Clinician vigilance Redundancy in patient care |
Efficient workflow, education of clinicians, and stronger teamwork with checks and balances | Workflow and Communication |
| P6 | “I love this idea. It would make surgery feel much safer that an extra set of eyes are watching.” | Safety Additional monitoring |
||
| P110 | “Sometimes you need to see the patient versus just looking at vitals through a computer.” | Bedside context Telemedicine limits |
Lack of physical patient context/stressors in the eOR leading to misunderstanding or incorrect assessment |
3. Results
324 out of 360 individual responses were analyzed. Twenty-nine were removed due to incomplete response (≤ 27% completed), six were removed because the participant responded incorrectly to the attention check, and one was removed for taking 29 seconds to answer “2” for every question.
Table 2 presents demographic information for the 324 included participants. The mean age was 34.07 (SD = 10.11). Most were white (80.9%), male (57.1%), and had a high school degree or more (88.3%). Participants resided across US census regions in rural, urban, and suburban areas. Most were employed (89.4%) with a range of household incomes.
Table 2.
Demographic information of participants (n = 324).
| Variable | N (%) |
|---|---|
| Age mean (SD) | 10.11 (34.07) |
| Census Region | |
| West | 86/323 (26.5) |
| Midwest | 38/323 (11.7) |
| Northeast | 58/323 (17.9) |
| South | 141/323 (43.5) |
| Missing | 1 (0.3) |
| Area Type | |
| Rural | 103/323 (31.9) |
| Urban | 132/323 (40.9) |
| Suburban | 88/323 (27.2) |
| Missing | 1 (0.3) |
| Latino or Hispanic | |
| Yes | 69/323 (21.4) |
| No | 254/323 (78.6) |
| Missing | 1 (0.3) |
| Race | |
| Black or African American | 35/322 (10.9) |
| American Indian or Alaska Native | 5/322 (1.6) |
| Asian | 11/322 (3.4) |
| Native Hawaiian or Pacific Islander | 9/322 (2.8) |
| White or Caucasian | 262/322 (81.4) |
| Education Level | |
| Less than high school | 1/323 (0.3) |
| Some high school | 4/323 (1.2) |
| High school diploma/GED | 32/323 (9.9) |
| Some college or technical training | 57/323 (17.7) |
| College degree | 161/323 (49.9) |
| Graduate or professional degree | 68/323 (21.1) |
| Missing | 1 (0.3) |
| Gender | |
| Female | 138/324 (42.6) |
| Male | 185/324 (57.1) |
| Employment Status | |
| Employed | 288/322 (89.4) |
| Other | 34/322 (10.6) |
| Missing | 2 (0.6) |
| Annual Income | |
| Less than $40K | 113/315 (35.9) |
| $40K - $79,999 | 138/315 (43.8) |
| Greater than $80K | 64/315 (20.3) |
| Missing | 9 (2.8) |
3.1. Participants’ comfort with remote monitoring by eOR
Table 3 shows participants’ comfort level with the use of the eOR and highlights responses around specific eOR-based scenarios.
Table 3.
Participants’ ratings on telemedicine support for OR, eOR (N=324).
| Self-reported ratings (1 = extremely comfortable, 5 = extremely uncomfortable) | Median (IQR) |
|---|---|
| Level of comfort with the use of the eOR | 2 (1–2) |
| Level of comfort with familiar doctors and nurses running the eOR during surgery | 2 (1–2) |
| Level of comfort with unfamiliar doctors and nurses within the same hospital running the eOR during surgery? | 2 (1–3) |
| Level of comfort with unfamiliar doctors and nurses within a different hospital or health system in the US running the eOR during surgery? | 2 (2–3) |
| Level of comfort with unfamiliar doctors and nurses within a hospital or health system in another country running the eOR during surgery? | 3 (2–4) |
| Self-reported ratings (1 = extremely likely, 5 = extremely unlikely) | Median (IQR) |
| Likelihood for using remote eOR tracking during surgery | 2 (1–2) |
| Likelihood that the use of the eOR would improve surgery outcomes | 2 (1–2) |
| Likelihood that the use of the eOR would worsen surgery outcomes | 4 (2–5) |
| Likelihood that the use of the eOR would improve patient care quality | 2 (1–2) |
| Likelihood that the use of the eOR would worsen patient care quality | 4 (2–5) |
| Likelihood that the use of the eOR would improve patient safety | 2 (1–2) |
| Likelihood that the use of the eOR would worsen patient safety | 4 (2–5) |
| Self-reported ratings (1 = not at all concerned, 4 = extremely concerned) | Median (IQR) |
| Level of concern with eOR using your private health information | 2 (1–3) |
| Level of concern that eOR will be a distraction to care teams in the operating room during surgery | 2 (1–3) |
| Level of concern that care teams in the operating room would rely too much on eOR during surgery | 2 (2–3) |
| Self-reported ratings (1 = strong understanding, 5 = no understanding) | Median (IQR) |
| Level of understanding about eOR use and functions | 2 (1–2) |
Results from the Wilcoxon matched-pairs signed-rank test showed participants reporting greater comfort with familiar eOR clinicians than unfamiliar clinicians within the same healthcare system (z = −4.012, p<.001). Furthermore, they reported significantly higher comfort with clinicians in the same hospital or a health system in the US than those in a different country (z = −10.230, p<.001).
3.2. Perceptions of eOR barriers and facilitators characterized by the socio-technical model
Two hundred fifty-three participants provided free-text responses to the four open-ended questions focused on their initial reactions to a hypothetical eOR use (78.1%), 250 participants reported on pros to eOR use (77.2%), 212 participants responded with cons to eOR use (65.4%), and 41 participants highlighted other concerns (12.7%). Across rating and free-text responses, participants’ perceptions of eOR use were categorized by four of eight socio-technical dimensions: organizational policies, procedures, and cultures; hardware and software; workflow and communication; and people (Figure 2). We present each dimension’s key themes, supported by quotes (Table 4).
Figure 2.
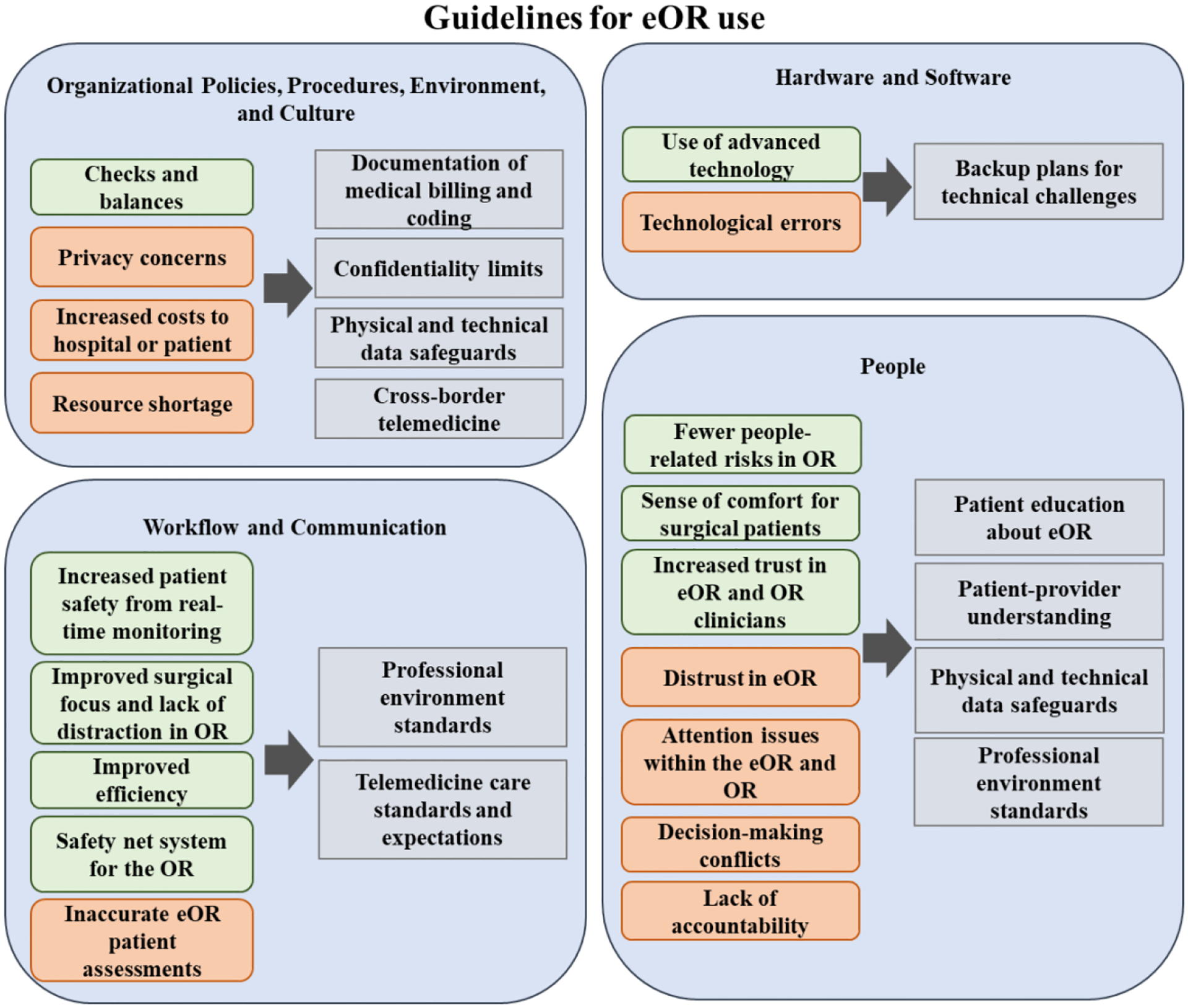
Potential guidelines to enhance patient adoption and use of telemedicine for OR (eOR), adapted from [45]). Blue outlines indicate dimensions of the socio-technical model, grey boxes indicate guidelines, green boxes indicate facilitators to eOR use, and orange boxes indicate barriers to eOR use.
Table 4.
Barriers and facilitators to patient acceptance of the eOR.
| Organizational Policies, Procedures, Environment, and Culture: internal structures and policies guiding clinical workflow | ||
|---|---|---|
| Barriers | Privacy concerns |
As long as there were security measures in place with regard to my privacy, I don’t see an issue. I think I’d prefer monitoring being conducted by professionals here in this country so that I would know they would be following a standard of care required by law. I would like to know what happens to the data after the surgery. – P133
There is no privacy among [clinicians] in attendance [of my surgery]. – P134 My privacy is being invaded (through eOR use). -P137 [There is a] potential loss of some privacy (during surgery). – P193 |
| Increased costs to hospitals or patients (e.g., resource wastage, insurance coverage, payment increases) |
I just do not understand the theory of what advantage [eOR use during surgery] would provide. It just seems to add cost with no improvement in outcomes. It seems wasteful with more people involved than the job requires. – P2
I’m concerned that even though you said [eOR use] won’t affect the costs of surgery, it really will - because someone will have to pay for the associated computer monitoring costs…I would wonder if my health insurance cost will go up again because of this. – P58 There are several doctors and nurses are involved in a single surgery… maybe [telemedicine use] increases the cost. – P244 Even if [remote monitoring] doesn’t cost me anything, it costs somebody something. Maybe there would also be false alarms [from the eOR] that could interfere with the surgery. – P277 |
|
| Resource shortage |
The additional tracking [from the eOR] may create a shortage of staff, causing other surgeries to be pushed back or delayed. – P242
[Staffing the eOR] might take doctors away from other patients. – P290 |
|
| Facilitators | Checks and balances (i.e., between eOR and OR teams) |
I feel transparency (between team members to hold each other accountable). – P111
Better checks and balances for the anesthesia teams (to speak up if there are concerns). – P37 |
| Hardware and Software: technology within the OR and eOR required to run the applications (i.e., computer, monitor, data display devices, data entry devices) | ||
| Barriers | Technological errors leading to communication and information transfer failures or false alarms |
The computer [in the eOR] could malfunction. – P110
Well, I suppose something could happen to the connection, and they’d lose monitoring capability. – P77 Something could get in the way of communication [between eOR and OR clinicians] at a bad time. – P80 [eOR clinicians might have] slower or worse form of communication with the surgical staff. – P113 Maybe there would be false alarms that could interfere with the surgery. – P277 False alarms [from the eOR clinicians could] lead to over-druggings. – P144 [Use of remote monitoring] is a good idea, but what if something goes wrong in the communication. Are there any monitors in the surgery room for the surgeon to look at also, or is it just in a separate room? – P315 |
| Facilitators | Use of advanced technology (i.e., telemedicine) |
Technology can be good [for remote] monitoring – P25
Advanced technology (is) very useful (for surgical) patients and doctors – P218 This scenario [using remote monitoring during surgery] looks very realistic, and I am in favor of the new technology used in medicine since it makes surgeries and any medical procedure much safer. – P228 |
| Workflow and Communication: relationships between health information technology, individual clinicians, healthcare teams, and patients to ensure smooth, cohesive patient care | ||
| Barriers | Incorrect eOR patient assessments |
Potentially [the eOR] not understanding some specifics behind my vitals or levels due to a change in the circumstances at the location of the surgery and perhaps making a wrong emergency call. – P89
Other signs of distress may not show on the computer and may only be seen in person. If there is an emergency, [eOR clinicians] have to come all the way from another room to intervene. – P276 |
| Facilitators | Increased patient safety from real-time monitoring |
[Scenarios involving remote monitoring of surgeries] seem quite normal, as this is an everyday practice in healthcare… It is quite a good idea. – P1
I would hope that the staff monitoring me from a different location would be in addition to the monitoring that would be going on in the operating room. – P9 eOR monitoring reduces complications, [resulting in] less blood loss and fewer infections. – P28 Extra people monitoring [patient surgeries] would reduce complications. – P113 The pros [to eOR use] would be the extra people monitoring your health would increase the chance that any complication would be spotted immediately. – P170 The doctors and nurses [in the eOR] keep a check on the medicine [OR clinicians] give me; that way, they could avoid an overdose, and since other doctors and nurses see me from another room, they will inform themselves and help with that. – P164 [Double checking through the eOR] seems like a great idea. – P21 An extra eye [through remote monitoring] can’t be bad at all, given all the mishaps in surgery rooms. – P26 |
| Improved surgical focus and lack of distraction in OR |
(Remote monitoring allows surgical teams in the OR) a better ability to focus on (bedside) data. – P95
(With regards to eOR use, I would like that there is) someone monitoring me who is solely focused on [monitoring my condition] because they are removed from the physical location and the commotion. – P90 [With eOR use, the OR team has] increased awareness of their surrounding environment [and can notice] things… such as noises or movements. – P103 |
|
| Improved efficiency |
I think [remote monitoring is a] more efficient process. To be able to monitor all screens at once, away from the actual surgery, would be easier to do and more efficient. – P257
[Remote monitoring of surgery] sounds efficient. – P230 |
|
| Safeguard for the OR |
My first reaction is [having an eOR to monitor surgeries] is a great situation to have. Someone watching right there, but also a “fallback” in a sense. – P11
I feel very good in this very reliable scenario [where the eOR monitors my hypothetical surgery]. – P15 [Remote monitoring] would make [surgery] safer. – P15 It’s nice to have double monitoring [between the OR and eOR]. – P29 Redundant systems are generally good. Perhaps if one set of equipment fails in the OR, [the eOR] would be a wonderful backup system. – P91 |
|
| People: humans involved in all aspects of the design, development, implementation, and use of health information technology | ||
| Barriers | Attention issues within the eOR and OR |
[eOR use could lead to] extra distractions/too many spoons in the soup – P273
[Remote monitoring] could interrupt the surgery and cause complications because of this. – P184 The biggest con [to eOR use] is that being apart and aside from the actual surgery makes people less attentive to what is actually going on. I would worry that whoever is monitoring the computer is only doing so intermittently, perhaps getting distracted by other things. – P201 What if [the eOR clinicians] get bored or are not attentive to the computer screen at all times? – P261 |
| Decision-making conflicts |
Having too many people on a [surgical] team could lead to disagreements about treatment options (between the eOR and OR clinicians). – P265
What I mean is that there are sometimes too many [clinical leaders], and they may not always agree. The people in the room may feel the patient is doing well, whereas the people monitoring from another room see it differently, and this could cause some conflict that delays action. – P271 It could end up being a too many cooks situation causing unwarranted tinkering with medications. – P42’ A con would be that there are too many people, so not all of them are paying close attention. – P177 Too many people monitoring can cause confusion. – P319 |
|
| Lack of accountability |
[eOR use] could create a sense of diminished responsibility if something goes wrong. The level of attention of the [remotely] monitoring doctors and nurses would be difficult to monitor and could mislead the operating nurses and doctors. – P125
I guess the doctors in the room might feel a little less responsible for what is happening and drop their guard a bit. – P168 Shared responsibility [between eOR and OR teams] can reduce the obligation that someone has to pay full attention [to]. – P11 There is no hierarchy of responsibility mentioned, so who is most responsible if something goes wrong - the doctor in the room or the doctor on the screen in another room? – P58 If something [in the surgery] went wrong, could someone be there to bring me back to life? – P181 [Remote care might be] less personable, I guess – P256 |
|
| Distrust in eOR |
[I am a] little distrustful of eOR clinicians. – P41
I just don’t trust doctors. You can’t fully trust doctors and nurses – P324 |
|
| Facilitators | Fewer people-related risks in OR (less infection, fewer distractions, commotion) |
I think that the fact that [the eOR clinicians] are outside the room is no issue. Things get monitored remotely all the time. – P8
I like the idea of making the room less crowded and providing a separate location where experts can monitor vitals without distraction. – P13 [eOR use] seems like a good way to monitor my condition without crowding the operating room. – P53 |
| Sense of comfort for surgical patients |
I would feel more comfortable with additional sets of trained eyes looking out for me. This scenario [where an eOR team monitors my surgery remotely] parallels telemetry units in hospitals where each patient has a nurse assigned to them, but their heart are also being monitored by a telemetry tech. – P12
[Having an additional team monitoring me] feels more assuring than my normal view of surgery. – P25 I love this idea [of remote monitoring during surgery]. It would make surgery feel much safer that an extra set of eyes are watching. – P6 |
|
| Trust in clinicians |
Doctors and nurses [are]…. Careful [during surgery]. –P33 Honestly, I’m of the mindset that I just want to get better, and so long as [those operating the eOR are] trained professionals, I trust them. – P302 I really don’t see any cons to [eOR use], especially since it would not increase the cost of care. I don’t see any privacy issues with [eOR use] either, as long as I can trust the [remote] medical team. – P78 I trust that professionals in the field (of surgery) understand and implement best practices. – P95 |
|
3.2.1. Organizational policies, procedures, environment, and culture
Participants mentioned privacy concerns, feeling self-conscious about the number of clinicians monitoring their surgeries, and worrying about health data falling into “the wrong hands.” Approximately 33% of responding participants were slightly concerned about the privacy of their health information, though 41% were unconcerned.
“I know many people value their privacy a lot and would want as few people witnessing their surgery as possible (P205).”
Others worried that eOR implementation would be “a waste of resources (P122),” increasing patient or hospital costs. A few felt that even if patient bills did not increase, resource wastage would “cost somebody extra money (P2)” or increase insurance payments. Other logistical concerns involved resource shortages, with telemedicine barring staff and resources from other hospital tasks. Some worried that “[the eOR] might take doctors away from other patients (P291).” Others agreed that “additional tracking may create a shortage of staff, causing other surgeries to be… delayed (P242).”
Positive perceptions surrounding improvements to organizational safety culture emphasized a system of checks and balances with the eOR providing an unbiased risk assessment and alerting OR clinicians of feedback and concerns. Participants felt comforted by this transparency between team members.
“[The eOR] could provide more input and feedback for [clinicians] and improve their expertise. The [eOR] might be more unbiased and unafraid to speak up if they see something wrong (P125).”
3.2.2. Hardware and software
Despite some apprehension, participants expressed optimism overall about use of advanced technology with the eOR intervention. Participants believed that if “there is no extra cost that would not be covered by insurance,” the eOR would be “helpful (P90)” and provide “additional caretakers at no additional cost (P242).” Some also felt that “technology has advanced so much that this doesn’t seem risky, but an added bonus to [patient] welfare (P62).”
Common concerns involved technological errors leading to communication and information transfer failures or false alarms. As one participant stated, “Doctors may not listen to calls, and sometimes you need to see the patient versus just looking at… a computer (P110).” A few worried that “[system] malfunctions (P56)” or “technical problems (P107)” could prevent accurate communication between eOR and OR clinicians. About 42.7% of responding participants were slightly concerned that the OR team would rely too much on eOR reports during high-risk cases, indicating distrust in technology.
“Being too heavily relied on technology… can be a risky thing to do in case… the machines aren’t working optimally (P267).”
Some speculated that data could overwhelm eOR clinicians (P66), resulting in inaccurate or inefficient interpretations. A few others worried that “there could be a misinterpretation of [data and eOR feedback] (P62),” leading to inaccurate and harmful decision-making.
“The technology [might] mess up and [the clinicians might] do something based on faulty data (P179).”
3.2.3. Workflow and communication
Many felt the eOR would increase patient safety from real-time monitoring, improving clinician workflow and team communication, and reducing patient risks and complications.
“Early abnormality detection, data storage easily accessible and monitored by healthcare professionals (P274).”
In addition to patient surveillance tasks, one participant also suggested that the eOR could provide a teaching tool to clinicians (P316).
Participants also felt that by assigning monitoring tasks to eOR clinicians, clinicians could improve their surgical focus without distractions in the OR. As one participant explained, “[Bedside doctors] do not have to devote mental effort to tracking certain quantities when performing more complex tasks (P258).”
Furthermore, eOR clinicians could improve efficiency by monitoring multiple patients at a time, “allowing clinicians to carefully examine patient status without being in the [OR] (P175).” This safeguard for the OR offered more reliable patient care.
However, some participants felt that miscommunication or team disconnect could disrupt workflow, as “the work could be outsourced and lead to communication issues between [eOR and OR teams] (P165).” Primarily, a lack of bedside context could potentially lead to inaccurate eOR assessments and information transfer.
“Not being in the room, they may miss physical vital signs. (P114).”
3.2.4. People
Participants speculated that distributing clinicians into the eOR would reduce people-related risks in the OR, such as distractions and infection risks, improving patient safety and reflecting across survey ratings (extremely or somewhat likely = 81.7%).
“No one would be disturbing the doctor, the room would be less crowded, less chance of… something to go wrong (P64).”
Within the eOR, clinicians could focus on the case, smoothly integrating telemedicine care into the existing workflow. As one participant stated, within the eOR, “there is no distraction, just… focus on the computer screen (P114).” In reducing cognitive workload for the OR team, both the eOR and OR would run at maximum efficiency, resulting in quicker risk and concern detection. Many felt that an eOR intervention could reduce patient blood loss and infections (P28). This perception aligned with survey ratings, as most felt confident that intraoperative remote monitoring would improve surgery outcomes (extremely likely or likely = 78.7%).
Participants predicted that the eOR intervention would enhance patients’ overall surgical experience by increasing their sense of comfort and security with care from multiple experienced clinicians. One participant explained, “It makes me feel safer and more comfortable, like the healthcare professionals… are really paying attention to my body and are doing everything in their power to make sure nothing goes wrong (P3).”
Most indicated they trusted their clinicians to take care of them and therefore trusted the intervention. One participant confidently stated, “I trust that professionals in the field understand and implement best practices (P95).” Many agreed, reporting similarly high comfort with doctors and nurses they knew keeping track of their health and safety outside of the OR (extremely comfortable or comfortable = 83.2%) and with unfamiliar doctors and nurses who were part of the same hospital (extremely comfortable or comfortable = 74.6%). Over half of the participants (extremely comfortable or comfortable = 60.3%) felt comfortable with unfamiliar doctors and nurses in different hospitals. 43.3% felt extremely comfortable or comfortable with unfamiliar doctors and nurses who were part of a hospital system in another country (extremely uncomfortable or uncomfortable = 35.9%).
Of those who felt uncomfortable with eOR use, many stated that they did not fully trust their care teams and eOR technology. These participants worried that remote monitoring could worsen surgery outcomes, although more than half of respondents strongly or somewhat disagreed (56.1%). This response was similar to whether clinicians tracking their wellbeing would worsen the quality of care (strongly or somewhat disagree = 57.3%) or worsen safety (strongly or somewhat disagree = 57.6%).
Some discussed attention issues within the eOR and OR, such as multitasking, as “the doctors [could be] disconnected/distracted from the task at hand if they had to keep referring to a screen (P202).” Some also speculated that the eOR would be distracting if eOR clinicians sent messages or called the OR during a surgical case; 43% of responding participants reported that they would be slightly concerned about eOR distractions affecting patient care.
Another common concern was that too many members within a patient care team could lead to decision-making conflicts through coordinating issues. One participant explained that “many doctors watching the situation… could be distracting at a certain point and could jeopardize the operation (P301).” P301 elaborated that confusion and distractions could “especially happen if the doctors [argue about] how things are going.” In the event that one team inaccurately predicted patient risks or that “somebody could have an outlying opinion, meaning they think something is wrong when nothing is, in fact, wrong (P268),” some expressed concern about decision-making and confusion about leadership and coordination during eOR/OR conversations, asking, “Who has the final authority to make decisions… if several avenues of treatment arose (P170)?”
Other participants agreed that using multiple care teams would lead to a sense of shared responsibility for errors and a subsequent lack of accountability. This “less personable” patient care (P256) could lead to disjointed teamwork.
“There will be a lot of different people involved in communication, making coordination potentially more complicated if there are no clear rules (P258).”
4. Discussion
This exploratory study highlights recent surgical patients’ attitudes and beliefs towards intraoperative telemedicine use to support care delivery and management. Participants generally supported the use of remote monitoring technology from eOR clinicians to improve team coordination, ensure shared understanding, foster situational awareness, and serve as a backup care team and safeguard to support clinical decisions, all leading to improvements in care quality and safety outcomes.
Participants reported significantly more comfort with locally managed eOR teams retrieving their data, assessing clinical status, and providing feedback and suggestions to their OR team than distant eOR services, which potentially indicates a decrease in comfort as their familiarity with eOR staff decreased. Similarly, they reported comfort with eOR teams managed within the US healthcare system compared to internationally hosted eOR services. Cross-border telemedicine is often seen as a low-cost solution that does not take local clinicians away from their responsibilities [41]; for example, a telemedicine center in the Caribbean monitored pediatric inpatient units of a Canadian hospital and provided feedback and decision support to their bedside care teams [42]. Benefits of such international telemedicine use included expedition of patient case review processes, development of informal clinical education forums between centers, and sustainable allocation of resources to provide specialized patient care. Nevertheless, several other factors, such as cost of eOR implementation, training, staff and resources, healthcare reimbursement structure, etc., play into which scenario is feasible and cost-effective, as highlighted by our participants.
Irrespective of the scenario of eOR use, participants identified four dimensions in the 8-dimensional socio-technical model to be critical for its acceptance: organizational policies, procedures, environment, and culture; workflow and communication; people; and hardware and software. Key facilitators to eOR use include checks and balances between eOR and OR teams, increased patient safety with real-time risk monitoring and patient surveillance, improved focus and lack of distraction within the OR, increased trust in clinicians due to a safeguard system, use of advanced technology, and enhanced patient comfort. Significant barriers to eOR use included increased hospital costs or patient costs, privacy concerns, incorrect eOR clinician assessments, technology malfunctions, eOR clinician attention issues, decreased accountability for errors, and eOR-OR decision-making conflicts. Similar barriers have been reported in studies on CDSS use in surgical outpatient clinics (e.g., privacy [43]) and studies evaluating telemonitoring (e.g., data interpretation difficulties and overtreatment [44]).
To establish successful intraoperative telemedicine use, institutions need intraoperative telemedicine guidelines, similar to those developed for primary and urgent care by the American Telemedicine Association (ATA) [45] that can account for strategies for addressing these barriers and facilitators to eOR adoption and use by patients. Informed by findings from this study, we present preliminary guidelines that can address the four socio-technical dimensions to enhance patient acceptance and comfort levels towards telemedicine and its embedded technologies (e.g., CDSS, EHR) and their integration within the routine intraoperative workflow (Figure 2).
Guidelines for organizational policies, procedures, environment, and culture:
To foster a system of checks and balances between the eOR and OR teams, professional environment standards can be established through rigid protocols to emphasize methods of ensuring best practices, quality care, and decision-making with a fresh perspective [45]. Furthermore, to mitigate privacy concerns, patient education on confidentiality limits and the introduction of physical and technical data safeguards can minimize the risk of data compromise. As patients undergo surgery from a multidisciplinary team of care providers, some may be out-of-network, resulting in surprise medical bills [46, 47]. As such, patients should also be reassured that eOR use will not waste resources or increase costs to them through accurate and complete documentation of medical billing and coding. Lastly, although some patients expressed more comfort with local telemedicine use, resource shortages within local institutions can be prevented through cross-border telemedicine, which provides many previously mentioned benefits [42].
Guidelines for hardware and software:
To make the most of telemedicine’s advanced technology and sustain the infrastructure, we recommend building a backup system for the eOR in the event of any technical errors or malfunctions. If technology fails or user error prevents adequate access to patient data or online communication, other means of accessing necessary information via mobile phone or tablet would be useful [48].
Guidelines for workflow and communication:
To enhance patient safety from real-time remote monitoring of the OR and improve efficiency across the intraoperative workflow, rigid and evidence-based telemedicine care standards and expectations recommended by the ATA can be applied [45]. These telemedicine care standards would encourage clear communication between eOR and OR clinicians to avoid misunderstandings and prevent inaccurate eOR patient assessments while serving as a safeguard for the OR. Furthermore, professional environment standards must be established through rigid OR protocols to improve OR team attention and limit distraction in the OR [45].
Guidelines for people:
To instill a sense of comfort for surgical patients in the eOR, we suggest patient education about eOR functions and their role, along with the implementation of physical and technical data safeguards. Elucidating the eOR process, benefits, and resources could improve patient understanding and buy-in [49]. Additionally, patient trust can be promoted through discussion using simple, standardized common language to ensure patient-provider shared knowledge [45]. Professional environment standards must continue to be upheld to further combat distrust, avoid distractions within the eOR and OR, OR-eOR decision-making conflicts, and related lack of accountability. Our findings on patient-perceived socio-technical barriers and facilitators related to the use of eOR in the US are similar to the ones reported in studies on telemedicine use internationally (i.e., outpatient settings, robotic-assisted surgery) [50] (i.e., in outpatient settings) [51]. Hence, the proposed guidelines can be tailored to address the unique contextual needs and related socio-technical factors for widespread telemedicine adoption internationally (e.g., emphasizing telemedicine infrastructure and financial planning in regions with little government healthcare support).
Our findings should be interpreted within the context of several study limitations. First, the survey was developed for this study based on the literature and existing surveys because validated instruments were not available for these topics. We tested the survey for clarity before administering the survey to participants, but future studies could develop validated measures to assess eOR perceptions. Second, although Amazon MTurk provides an online service for users to complete research studies in exchange for payment [52] and is often more representative than other convenience samples [53, 54], respondents were younger, more likely to be female, more educated (and possibly more technically savvy), less wealthy (household income of < $150,000 yearly), and less likely to be Hispanics or Black [55]. Lastly, we compensated participants $1.00 for participation, a rate higher than many MTurk studies and consistent with minimum wage [56]. However, the low incentive could lead to hasty responses. We included an attention check question and excluded those who did not respond correctly to it to check for this possibility. In other studies, MTurk participants consistently produced the same standard decision-making biases as those from different samples [57].
Conclusion
eOR acceptability was influenced by barriers and facilitators across four socio-technical dimensions regarding successful eOR implementation: organizational policies, procedures, environment, and culture; people; workflow and communication; and hardware and software. Our study provides insights to inform intraoperative telemedicine guidelines for surgical settings that can address barriers and facilitators to eOR acceptability identified by previous surgical patients. These insights can inform eOR design and implementation strategies and, increasing acceptability, adoption and use, and effectiveness for future large-scale implementation.
Supplementary Material
Summary table.
What was already known regarding this topic:
Clinical decision support systems (CDSS) support intraoperative decision-making through streamlined data interpretation to produce patient alerts, documentation and care recommendations.
Telemedicine is increasingly being adopted within the operating room to provide virtual consultations and real-time feedback to surgical teams.
Clinicians have found intraoperative CDSS and telemedicine to support resilience in patient safety through reduced workload and a safeguard system for the surgical team.
Clinicians have identified alert timing, emotional responses, and care redundancy as barriers to the use of intraoperative CDSS and telemedicine.
What this study added to our knowledge:
Patients envision intraoperative CDSS and telemedicine (eOR) to enhance surgical team attention and increase patient safety through real-time monitoring and transparency between care teams.
Patient perceptions of organizational policies, procedures, environment, and culture; workflow and communication; people; and hardware and software are critical dimensions affecting patient acceptability and adoption of the eOR.
Patients are more comfortable with intraoperative remote monitoring by familiar or local clinicians than by unfamiliar or international clinicians.
Highlights.
Patients perceive intraoperative telemedicine (eOR) to enhance patient safety through real-time remote monitoring.
Patient perceptions of organizational policies, procedures, environment, and culture; workflow and communication; people; and hardware and software are critical dimensions affecting patient acceptability and adoption of the eOR.
Patients are more comfortable with intraoperative remote monitoring by familiar or local clinicians than by unfamiliar or international clinicians.
Acknowledgments
We would like to thank participants for their involvement in the study.
Funding
Work included in this manuscript was partly supported by the National Institute of Nursing Research [R01 NR017916] and the Agency for Healthcare Research and Quality [R21 HS24581]. The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
Declarations of interest: none.
References
- 1.National Quality Forum, NQF-Endorsed Measures for Surgical Procedures. 2015.
- 2.Nepogodiev D, Martin J, Biccard B, Makupe A, Bhangu A, et al. , Global burden of postoperative death. Lancet, 2019. 393(401): p. 33139–8. [DOI] [PubMed] [Google Scholar]
- 3.Weiser TG and Gawande A, Excess surgical mortality: strategies for improving quality of care. 2015. [PubMed]
- 4.King CR, Abraham J, Kannampallil TG, Fritz BA, Abdallah AB, et al. , Protocol for the effectiveness of an anesthesiology control tower system in improving perioperative quality metrics and clinical outcomes: The TECTONICS randomized, pragmatic trial. F1000Research, 2019. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Melby L and Toussaint P. Supporting operating nurses’ collaborative work: preventing information overload and tailoring information access. in 2009 22nd IEEE International Symposium on Computer-Based Medical Systems. 2009. IEEE. [Google Scholar]
- 6.Brilli RJ, McClead RE Jr, Crandall WV, Stoverock L, Berry JC, et al. , A comprehensive patient safety program can significantly reduce preventable harm, associated costs, and hospital mortality. The Journal of Pediatrics, 2013. 163(6): p. 1638–1645. [DOI] [PubMed] [Google Scholar]
- 7.Davenport DL, Ferraris VA, Hosokawa P, Henderson WG, Khuri SF, et al. , Multivariable predictors of postoperative cardiac adverse events after general and vascular surgery: results from the patient safety in surgery study. Journal of the American College of Surgeons, 2007. 204(6): p. 1199–1210. [DOI] [PubMed] [Google Scholar]
- 8.de Vries EN, Prins HA, Crolla RM, den Outer AJ, van Andel G, et al. , Effect of a comprehensive surgical safety system on patient outcomes. New England Journal of Medicine, 2010. 363(20): p. 1928–1937. [DOI] [PubMed] [Google Scholar]
- 9.Jameson JK, Transcending intractable conflict in health care: an exploratory study of communication and conflict management among anesthesia providers. Journal of Health Communication, 2003. 8(6): p. 563–581. [DOI] [PubMed] [Google Scholar]
- 10.Kane M and Smith A, An American tale–professional conflicts in anaesthesia in the United States: implications for the United Kingdom. Anaesthesia, 2004. 59(8): p. 793–802. [DOI] [PubMed] [Google Scholar]
- 11.Pronovost PJ and Freischlag JA, Improving teamwork to reduce surgical mortality. Journal of the American Medical Association, 2010. 304(15): p. 1721–1722. [DOI] [PubMed] [Google Scholar]
- 12.Stiegler MP and Ruskin KJ, Decision-making and safety in anesthesiology. Current Opinion in Anesthesiology, 2012. 25(6): p. 724–729. [DOI] [PubMed] [Google Scholar]
- 13.Stiegler MP and Tung A, Cognitive processes in anesthesiology decision making. Anesthesiology, 2014. 120(1): p. 204–217. [DOI] [PubMed] [Google Scholar]
- 14.Chau A and Ehrenfeld JM, Using real time clinical decision support to improve performance on perioperative quality and process measures. Anesthesiology Clinics, 2011. 29(1): p. 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durieux P, Nizard R, Ravaud P, Mounier N, and Lepage E, A clinical decision support system for prevention of venous thromboembolism: effect on physician behavior. Journal of the American Medical Association, 2000. 283(21): p. 2816–2821. [DOI] [PubMed] [Google Scholar]
- 16.Karlen W, Dumont GA, Petersen C, Gow J, Lim J, et al. Human-centered phone oximeter interface design for the operating room. in Proceedings of the International Conference on Health Informatics. 2011. [Google Scholar]
- 17.Levine WC, Meyer M, Brzezinski P, Robbins J, and Sandberg WS. Computer automated total perioperative situational awareness and safety systems. in International Congress Series. 2005. Elsevier. [Google Scholar]
- 18.Sim I, Gorman P, Greenes RA, Haynes RB, Kaplan B, et al. , Clinical decision support systems for the practice of evidence-based medicine. Journal of the American Medical Informatics Association, 2001. 8(6): p. 527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okumura LM, Veroneze I, Bugardt CI, and Fragoso MF, Effects of a computerized provider order entry and a clinical decision support system to improve cefazolin use in surgical prophylaxis: a cost saving analysis. Pharmacy Practice (Granada), 2016. 14(3): p. 0–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gregory S, Murray-Torres TM, Fritz BA, Abdallah AB, Helsten DL, et al. , Study protocol for the Anesthesiology Control Tower—Feedback Alerts to Supplement Treatments (ACTFAST-3) trial: a pilot randomized controlled trial in intraoperative telemedicine. F1000Res, 2018. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abraham J, Meng A, Sona C, Wildes T, Avidan M, et al. , An observational study of postoperative handoff standardization failures. International Journal of Medical Informatics, 2021. 151: p. 104458. [DOI] [PubMed] [Google Scholar]
- 22.Carter J Telemedicine in the operating room: the ‘new normal’ for MedTech. 2020; Available from: www.medicaldesignandoutsourcing.com/telemedicine-in-the-operating-room-the-new-normal-for-medtech/.
- 23.Wicklund E Opening up the OR: using telemedicine to enhance collaboration. 2018; Available from: mhealthintelligence.com/news/opening-up-the-or-using-telemedicine-to-enhance-collaboration.
- 24.Murray-Torres T, Casarella A, Bollini M, Wallace F, Avidan MS, et al. , Anesthesiology Control Tower—Feasibility Assessment to Support Translation (ACTFAST): mixed-methods study of a novel telemedicine-based support system for the operating room. JMIR Human Factors, 2019. 6(2): p. e12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murray-Torres TM, Wallace F, Bollini M, Avidan MS, and Politi MC, Anesthesiology Control Tower: Feasibility Assessment to Support Translation (ACT-FAST)—a feasibility study protocol. Pilot Feasibility Study, 2018. 4(1): p. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abd Ghani MK and Jaber MM, The Effect of Patient Privacy on Telemedicine Implementation in Developing Countries: Iraq Case Study. Research Journal of Applied Sciences, Engineering and Technology, 2015. 11(11): p. 1233–1237. [Google Scholar]
- 27.El-Mahalli AA, El-Khafif SH, and Al-Qahtani MF, Successes and challenges in the implementation and application of telemedicine in the eastern province of Saudi Arabia. Perspectives in health information management/AHIMA, American Health Information Management Association, 2012. 9(Fall). [PMC free article] [PubMed] [Google Scholar]
- 28.Kifle M, Mbarika VW, and Bradley RV, Global diffusion of the Internet X: the diffusion of telemedicine in Ethiopia: potential benefits, present challenges, and potential factors. Communications of the Association for Information Systems, 2006. 18(1): p. 30. [Google Scholar]
- 29.Panda N and Haynes AB, Prioritizing the patient perspective in oncologic surgery. Annals of surgical oncology, 2020. 27(1): p. 43–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Casler K, Bickel L, and Hackett E, Separate but equal? A comparison of participants and data gathered via Amazon’s MTurk, social media, and face-to-face behavioral testing. Computers in human behavior, 2013. 29(6): p. 2156–2160. [Google Scholar]
- 31.Sittig DF and Singh H, A new socio-technical model for studying health information technology in complex adaptive healthcare systems, in Cognitive Informatics for Biomedicine. 2015, Springer. p. 59–80. [Google Scholar]
- 32.Feldstein AC, Smith DH, Perrin N, Yang X, Simon SR, et al. , Reducing warfarin medication interactions: an interrupted time series evaluation. Archives of Internal Medicine, 2006. 166(9): p. 1009–1015. [DOI] [PubMed] [Google Scholar]
- 33.Sittig DF, Ash JS, Zhang J, Osheroff JA, and Shabot MM, Lessons from “Unexpected increased mortality after implementation of a commercially sold computerized physician order entry system”. Pediatrics, 2006. 118(2): p. 797–801. [DOI] [PubMed] [Google Scholar]
- 34.Smith DH, Perrin N, Feldstein A, Yang X, Kuang D, et al. , The impact of prescribing safety alerts for elderly persons in an electronic medical record: an interrupted time series evaluation. Archives of Internal Medicine, 2006. 166(10): p. 1098–1104. [DOI] [PubMed] [Google Scholar]
- 35.Dario C, Luisotto E, Dal Pozzo E, Mancin S, Aletras V, et al. , Assessment of patients’ perception of telemedicine services using the service user technology acceptability questionnaire. International journal of integrated care, 2016. 16(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lau M, Wong R, Bezjak A, and Levin W, Factors that Influence the Acceptability of Telemedicine as a means to Evaluate Treatment Outcome for Patients Completing Palliative Radiotherapy. Journal of Medical Imaging and Radiation Sciences, 2014. 45(2): p. 169. [Google Scholar]
- 37.Sekhon M, Cartwright M, and Francis JJ, Acceptability of healthcare interventions: an overview of reviews and development of a theoretical framework. BMC health services research, 2017. 17(1): p. 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nelson D, Kreps G, Hesse B, Croyle R, Willis G, et al. , The health information national trends survey (HINTS): development, design, and dissemination. Journal of health communication, 2004. 9(5): p. 443–460. [DOI] [PubMed] [Google Scholar]
- 39.NHS England and Department of Health and Social Care. NHS Patient Surveys. 2018; Available from: nhssurveys.org.
- 40.Fereday J and Muir-Cochrane E, Demonstrating rigor using thematic analysis: a hybrid approach of inductive and deductive coding and theme development. International Journal of Qualitative Methods, 2006. 5(1): p. 80–92. [Google Scholar]
- 41.Saliba V, Legido-Quigley H, Hallik R, Aaviksoo A, Car J, et al. , Telemedicine across borders: a systematic review of factors that hinder or support implementation. International journal of medical informatics, 2012. 81(12): p. 793–809. [DOI] [PubMed] [Google Scholar]
- 42.Gillis G, Newsham D, and Maeder A, Global telehealth 2015: integrating technology and information for better healthcare. Vol. 209. 2015: IOS Press. [PubMed] [Google Scholar]
- 43.Bator EX, Gleason JM, Lorenzo AJ, Kanaroglou N, Farhat WA, et al. , The burden of attending a pediatric surgical clinic and family preferences toward telemedicine. Journal of Pediatric Surgery, 2015. 50(10): p. 1776–1782. [DOI] [PubMed] [Google Scholar]
- 44.Ure J, Pinnock H, Hanley J, Kidd G, Smith EM, et al. , Piloting tele-monitoring in COPD: a mixed methods exploration of issues in design and implementation. Primary Care Respiratory Journal, 2012. 21(1): p. 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harting MT, Wheeler A, Ponsky T, Nwomeh B, Snyder CL, et al. , Telemedicine in pediatric surgery. Journal of Pediatric Surgery, 2019. 54(3): p. 587–594. [DOI] [PubMed] [Google Scholar]
- 46.Cooper Z, Scott Morton F, and Shekita N, Surprise! Out-of-network billing for emergency care in the United States. Journal of Political Economy, 2020. 128(9): p. 3626–3677. [Google Scholar]
- 47.Dekhne MS, Nuliyalu U, Schoenfeld AJ, Dimick JB, and Chhabra KR, “Surprise” out-of-network billing in orthopaedic surgery: charges from surprising sources. Annals of Surgery, 2020. 271(5): p. e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Henry TA 11 telehealth tweaks that help team-based care flourish. 2021.
- 49.Franco D, Montenegro T, Gonzalez GA, Hines K, Mahtabfar A, et al. , Telemedicine for the spine surgeon in the age of COVID-19: multicenter experiences of feasibility and implementation strategies. Global Spine Journal, 2020: p. 2192568220932168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Al-Samarraie H, Ghazal S, Alzahrani AI, and Moody L, Telemedicine in middle eastern countries: progress, barriers, and policy recommendations. International Journal of Medical Informatics, 2020. 141: p. 104232. [DOI] [PubMed] [Google Scholar]
- 51.Dodoo JE, Al-Samarraie H, and Alzahrani AI, Telemedicine use in sub-Saharan Africa: barriers and policy recommendations for Covid-19 and beyond. International Journal of Medical Informatics, 2021: p. 104467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berinsky AJ, Huber GA, and Lenz GS, Evaluating online labor markets for experimental research: Amazon. com’s Mechanical Turk. Political analysis, 2012. 20(3): p. 351–368. [Google Scholar]
- 53.Buhrmester M, Kwang T, and Gosling SD, Amazon’s Mechanical Turk: A new source of inexpensive, yet high-quality data? 2016. [DOI] [PubMed]
- 54.Minton E, Gurel-Atay E, Kahle L, and Ring K. Comparing data collection alternatives: Amazon Mturk, college students, and secondary data analysis. in AMA Winter Educators’ Conference Proceedings. 2013. [Google Scholar]
- 55.Moss A and Litman L, Demographics of People on Amazon Mechanical Turk, in CloudResearch. 2020, Prime Research Solutions LLC. [Google Scholar]
- 56.Cheung JH, Burns DK, Sinclair RR, and Sliter M, Amazon Mechanical Turk in organizational psychology: An evaluation and practical recommendations. Journal of Business and Psychology, 2017. 32(4): p. 347–361. [Google Scholar]
- 57.Goodman JK, Cryder CE, and Cheema A, Data collection in a flat world: The strengths and weaknesses of Mechanical Turk samples. Journal of Behavioral Decision Making, 2013. 26(3): p. 213–224. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


