Abstract
Background
Glioblastoma multiforme, commonly known as GBM or glioblastoma is a grade IV astrocytoma. Brain tumors are difficult to treat and lead to poor prognosis and survival in patients. Gliomas are categorized into four different grades among which GBM is the worst grade primary brain tumor with a survival of less than a year. The genomic heterogeneity of the brain tumor results in different profiles for patients diagnosed with glioblastoma. Precision medicine focuses on this specific tumor type and suggests specialized treatment for better prognosis and overall survival (OS)
Purpose
With the recent advancements in Genome-Wide Studies (GWS) and various characterizations of brain tumors based on genetic, transcriptomic, proteomic, epigenetic, and metabolomics, this review discusses the advancements and opportunities of precision medicine therapeutics, drugs, and diagnosis methods based on the different profiles of glioblastoma.
Methods
This review has exhaustively surveyed several pieces of works from various literature databases.
Conclusion
It is evident that most primary brain tumors including glioblastoma require specific and precision therapeutics for better prognosis and OS. In present and future, molecular understanding and discovering specific therapies are essential for treatment in the field of neurooncology.
Keywords: Blood brain barrier, Brain tumor, Cancer, Precision medicine, Glioblastoma
1. Introduction
Glioblastoma multiforme (GBM) is the most common primary brain tumor occurring in almost 80% of the primary malignant central nervous system (CNS) tumors. Glioblastoma occurs in about 3 in 100,000 people every year. The prevalence of the tumor is more in Caucasians compared to Asians and Africans. Furthermore, the tumor prevalence is 1.5 times more in men compared to women [1]. The overall survival (OS) of patients diagnosed with glioblastoma is less than 1 year to 14 months approximately [2].
Glioblastoma patients experience pressure in their head region due to the tumor growth and face symptoms like nausea, headache, drowsiness, personality changes and seizures. Cell cycle dysregulation is associated with enhanced glioblastoma cell proliferation [3]. As visualized through MRI (magnetic resonance imaging), most commonly the tumor occurs in the supratentorial (cerebrum) location of the brain including the frontal, temporal, and parietal lobes [4]. Glioblastoma mostly occurs in the frontal lobe, and seldom the tumor progresses in both the occipital and temporal lobes, known as the butterfly glioma [5]. Studies suggest that the emergence of glioblastoma or brain tumors is associated with exposure to ionizing radiation. Few theories suggest the cause of the tumor as a consequence of long-term mobile phone use. However, present evidences still require further confirmation and reliable data [6]. It has a high invasive ability and malignant form but it does not tend to metastasize to other parts of the human anatomy, apart from the brain and spinal cord [7]. Glioblastoma is a grade IV astrocytoma caused by the glial cells (astrocytes) in the brain and CNS. Astrocytes are one of the four glial cells that has major functions in the brain and spinal cord including their function in axon guidance, synaptic support, and further acting as a blood brain barrier (BBB). These cells are star-shaped brain cells, and thus obtaining their name astrocytes.
The astrocytes are crucial for the formation and maintenance of the BBB. The BBB, being a highly selective membrane, has a significant role in the therapeutics of glioblastoma as the complex epithelial-like tight junctions within the brain endothelium does not permit certain solutes to pass through [3]. The BBB is also regarded as a blockade as many anti-cancer drugs administered (oral and intravenous) gets restricted and is not readily penetrable. The ability of the drugs to penetrate the BBB are dependent on pharmacokinetic properties and its formulation. Low molecular size, weight and increased lipophilicity of the drugs are characteristics enabling easy permeability.
One certain incapable drug is Imatinib [8], imatinib mesylate (a tyrosine kinase inhibitor) for targeting cancer cells, which is regarded as the “Magical bullet” for the treatment of chronic myeloid lymphoma (CML) [9]. Imatinib, also known as “Gleevac” has been recorded as one of the first cancer therapies showing potential with a targeted action in CML in early 1990s. Unfortunately, Imatinib has low efficiency in targeting brain tumor cells as it penetrates the BBB poorly [10]. The formulation of Imatinib along with methamphetamine has shown positive signs of BBB penetration for treatment of both CML and glioblastoma. Hence, an understanding of different combined formulations specific for BBB penetration and glioblastoma therapies must be explored and applied for personalized treatment. The current standard of treatment for glioblastoma is non-specific and does not categorize glioblastoma patients based on the genetic profile of the tumor. Developments in the field of GWS and a better understanding of the categorized profiles paves way for specific and personalized medicine for glioblastoma therapy. Personalized combined therapies designing for different varieties of brain tumor profiles are under research and await a breakthrough.
2. Glioblastoma: A grade IV astrocytoma
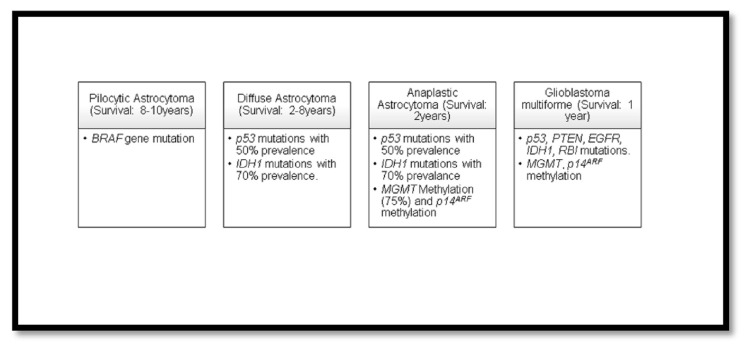
Gliomas are categorized into four different grades by the World Health Organization (WHO) on account of their malignancy and survival rates in patients. Glioblastoma (Grade IV) is the most severe one compared to the rest with OS of subjects <1 year. Pilocytic astrocytoma is categorized as grade I with survival period of 8–10 years. Diffuse astrocytoma is categorized as grade II with a survival of 7–8 years after diagnosis. Anaplastic astrocytoma is categorized as grade III with OS of 2–3 years [11]. The different grades of gliomas categorized by the WHO display different mutations. The following data [Fig. 1] provides information on different mutations occurring in the various grades of gliomas.
Fig. 1.
WHO classification of four grades of gliomas and occurrence of mutations (provide reference for this).
Glioblastoma is further classified into two types, primary and secondary glioblastoma. The primary glioblastoma is more malignant and has a poor prognosis compared to the secondary. Majority of the primary glioblastoma mutations include those of EGFR (epidermal growth factor receptor) amplifications (40%–60%), p53 (tumor protein P53) mutations (30%), PTEN (phosphatase and TENsin homolog) mutations (25%), and the loss of entire chromosome 10 (70%). Most cases of primary glioblastoma tumor involve MGMT (O (6)-methylguanine-DNA methyltransferase) methylation at a very low occurrence of 36%. The secondary glioblastoma has mutations in EGFR (10% amplification), p53 (65%), IDH1 (isocitrate dehydrogenase) (70% mutations), and MGMT (methylation 75%). The significance of methylation of the mutated gene plays a major role in the disease prognosis, recurrence, and therapy [12]. The low-grade gliomas such as the grade I (Pilocytic astrocytoma) and II (Diffuse astrocytoma) transform into the secondary glioblastoma at later stages, while the ones which form directly (de novo) are known as the primary glioblastoma. Secondary glioblastoma also appears in patients of younger age of 40 when compared to primary glioblastoma, occurring in elderly at an age of 60 [13,14].
2.1. Genetic heterogeneity in glioblastoma
Many novel and developing cancer treatments involve the identification of a single genomic alteration and targeting the same for therapy. The same principle may be impractical and unrealistic in glioblastoma. The reason is due to the multiple alterations in its genomic, transcriptomic, and epigenetic profile in glioblastoma. This indicates that in the case of glioblastoma, it undergoes more than a single genetic or cellular event and hence, requiring multiple combinations of therapies to target individual events.
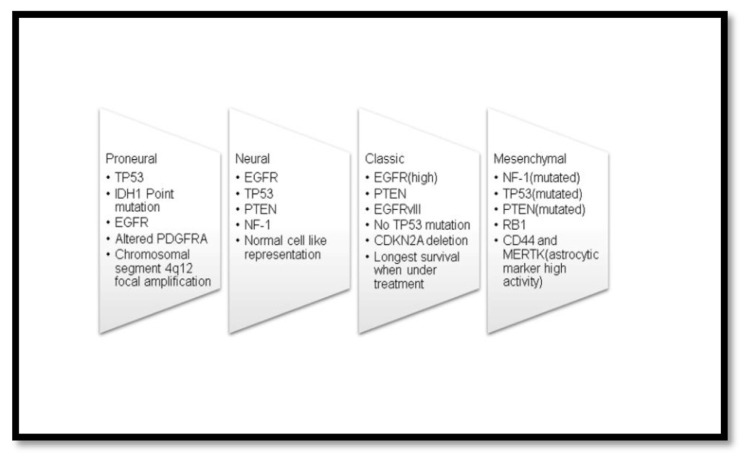
Glioblastoma shows both inter-tumoral and intra-tumoral heterogeneity [13]. Inter-tumoral heterogeneity is the various mutations that occur in different glioblastoma patients. The genetic profile of the glioblastoma tumor differs among patients, thus demanding specific/personalized treatments. Inter-tumoral heterogeneity is further classified into four subtypes classic, neural, pro-neural, and mesenchymal subtypes [14]. The classification of the subtypes is based upon the molecular alterations. The prognosis of patients in mesenchymal subtype is the worst amongst the four subtypes.
Generation of genomic profiles of glioblastoma has been possible with the progression of The Cancer Genome Atlas (TCGA) [15]. Molecular characterization of different subtypes of glioblastoma along with its genetic alterations has major significance in novel therapies [1]. Development of mouse models with molecular alterations in the aforementioned genes [Fig. 2], has been in progress by the employment of genetic deletion systems like the recombinase enzyme Cre-loxP system® [16]. These types of approaches for the development of mouse models are in research to enhance the preclinical trials and track down therapies using specialized anti-cancer agents for glioblastoma. Robust mouse models for each subtype are still under development.
Fig. 2.
Four subtypes of inter tumor heterogeneity and occurrence of genetic alterations amongst the four subtypes.
EGFR (epidermal growth factor receptor) activating mutations exist dominantly in the classic subtypes. The mesenchymal subtype, which has the worst prognosis, is often associated with NF-1 and TP53 mutations [17,18]. PDGFR (platelet-derived growth factor receptor) mutation often exists dominantly in the pro-neural subtypes. PDGFR activation and related VEGF (vascular endothelial growth factor) show neovascularization and tumor growth in glioblastoma. Pre-clinical trials using anti-angiogenic agents targeting PDGFR deactivation are under research. Clinical trials demonstrated that the drug Imatinib (Gleevac), was effective against the PDGFR with 6 months progression free survival and median OS of 48.9 weeks [19–21]. Novel drugs are thus desired as Imatinib does not effectively pass through the BBB. Furthermore, several non-coding constraint mutations display regulatory potential by multiple mutations overlapping transcription factor binding sites, and reducing the DNA binding capacity. Hence, these act as additional candidate glioblastoma genes for tumor regulation in glioblastoma [22].
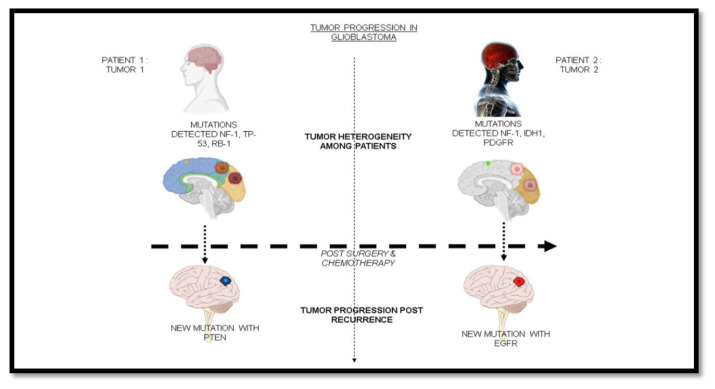
Apart from the inter-tumor heterogeneity among patients diagnosed with glioblastoma, glioblastoma tumors display an intra-tumor heterogeneity, i.e. varied mutations existing within the tumor. Hence, targeting a single mutation does not lead to therapy in all cases [Fig. 3]. Multiple combinations of drugs are required for existing intra-tumor heterogeneity. The tumor often arises with mutations leading to signal transduction pathway activation of those downstream of tyrosine kinase receptors, such as EFGR and PDGFR [23]. Evidence shows that the cells carry mutations in PDGFR, EFGR, and Receptor Tyrosine Kinase (RTK), all existing together in glioblastoma [24].
Fig. 3.
Tumor progression amongst profiles in GBM along with heterogeneity of mutations at different stages of the tumor growth.
The absence of 1p/19q co-deletion in IDH1 mutations exhibited greater significance in the survival rates of patients [25]. General mutations involved with glioblastoma are MGMT, IDH1, TP53, RB1, RTK, RAS, EGFR, cyclin D1/3, MDM2, PTEN, CDK4, PDGFRA, PIK3CA, NF1, PIK3R1, LZTR1, BRAF, FGFR1, FGFR2, FGFR3, ATRX, TERT, NOTCH1, and FUBP1 [26]. The intra-tumor heterogeneity is currently vague in understanding and hence requires further research for the development of successful models for pre-clinical trials.
3. Diagnosis and imaging the brain tumor
Diagnosis of the brain tumor is primarily important for the identification and consideration of various drugs for the tumor. MRI is one of the standards for the diagnosis of glioblastoma. Properties, such as, the diffused rate of water, cerebral blood volume, blood flow rate, and transit time indicate the presence of tumor in the brain. The tracer 2-[18F] fluoro-2-deoxy-d-glucose (FDG) used in PET scan is ineffective in characterizing gliomas due to the presence of high levels of glucose in the brain tissues compared to the brain cells. An alternative to the positron emission tomography (PET) scan, the FET O-(2-[18F] fluoroethyl)-l-tyrosine has better resolution for brain imaging as the presence of massive levels of glucose does not permit PET scans effectively in brain tumors [27]. For detection of the Inter-tumor heterogeneity, MRI combined with NMR (nuclear magnetic resonance) spectroscopy indicated a positive approach in detecting of mesenchymal subtype of glioblastoma [28]. Magnetic resonance spectroscopy imaging (MRSI) is another commonly used technique that monitors the lactate, choline, creatine, N-acetyl aspartate and lipids in determining the tumor. The cellular levels measured by the MRSI indicate the tumor activity and it is a developing approach for the identification of various clones present in the tumor.
Fluorescent marker 5-aminolevulinic acid is a novel method in its phase 3 trials showing better surgical resection efficiency. The technique is a way of employing the marker 5-aminolevulinic which is taken up by the tumor cells, and fluorescence is observed under filtered light. The fluorescent dye is administered orally 2–5 h before the procedure showing maximal fluorescence at 6–8 h [29]. However, the fluorescent dye uptake does not occur in the normal brain tissue, blood vessels, and olfactory traces. The method is approved by the Food and Drug Administration (FDA) in the year 2017 [1] and it has shown promising potentials in improving the progression free survival (PFS). Fluorescence guided resection has greater scope in increasing the EOR (extent of resection) and survival in glioblastoma patients [30].
4. Conventional therapeutics for glioblastoma
The existing therapeutics for the treatment of glioblastoma are mainly dependent on surgical resection of the brain tumor. The surgery is subsequently followed by radiation therapy and chemotherapy. The surgical resection does not completely remove the tumor as the brain tumor often tends to infiltrate nearby adjacent normal brain cells’ parenchyma and it is unreliable to remove without affecting the normal cells of the brain [31]. Complete surgical resection in any case of brain tumor is not technically possible. Hence, the recurrence of the tumor from the remaining cells is inevitable. The EOR is important for the suppression of tumors [32].
However, EOR is observed to be restricted to a certain limit. For several decades, resection and radiation therapies were practiced in glioblastoma. Furthermore, there was no FDA-approved drug for treatment of glioblastoma until the year 2005 [33].
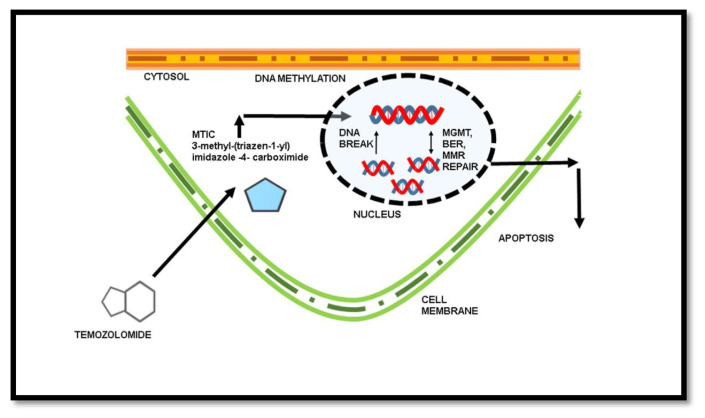
The drug temozolomide (TMZ) obtained Food and Drug Administration (FDA) approval for chemotherapy in glioblastoma in the year 2005. The implication of standard resection, radiotherapy and chemotherapy using TMZ has been in practice until recent advancements and drug approvals. The TMZ is a DNA alkylating agent and works in a way that causes the methylation of DNA in tumor cells [Fig. 4]. The methylation of DNA further promotes the cells to undergo apoptosis in the absence of MMR (mismatch repair), BER (base excision repair), and MGMT activity [34].
Fig. 4.
Mechanism of TMZ in the treatment of cancers by DNA methylation.
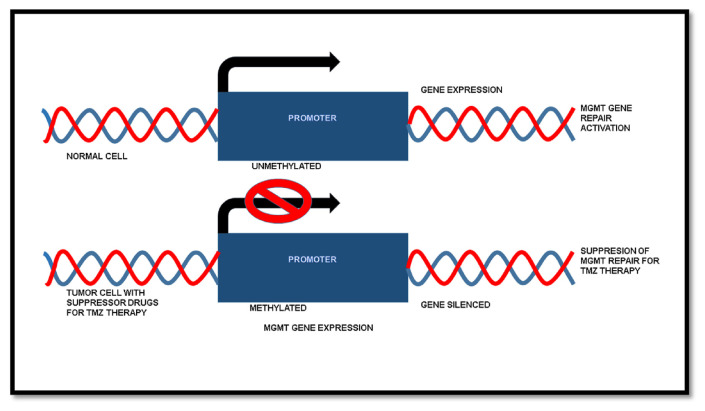
TMZ would have been a breakthrough in the treatment of glioblastoma, nonetheless, the MGMT (O-6-methylguanine DNA methyltransferase) gene in tumors is responsible for the repair of DNA, and hence nullifies the effect of TMZ [34]. In normal cells, the MGMT expression is regulated as a DNA repair mechanism, whereas, in tumor cells, the gene is silenced in certain profiles. The MGMT gene is mostly obtained as unmethylated in primary glioblastoma brain tumors, while in secondary glioblastoma brain tumors, the MGMT occurs as a methylated one. Therefore, the treatment with TMZ is found to be very effective in brain tumors with methylated MGMT promoter, i.e. in the secondary glioblastoma, as it plays a critical factor for the efficacy of TMZ [35]. MGMT promoter methylation acts as a biomarker that has a major significance in determining the prognosis of patients under treatment [36]. However, research to identify adjuvants with TMZ that causes the methylation of MGMT promoter is still in progress [Fig. 5]. The role of MGMT gene expression in the treatment of glioblastoma has a major significance. Screening of drugs for silencing the MGMT promoter might provide better treatment and prognosis in TMZ therapy [37]. Advancements in specific drug discoveries for targeting MGMT promoters, occurring in glioblastoma, can lead to better prognosis and OS in several cases with MGMT unmethylated tumor cells, i.e. in primary glioblastoma patients.
Fig. 5.
Silencing of MGMT promoter and gene expression as a Glioblastoma multiforme therapy for suppressing MGMT repair.
5. Advancements in precision medicine
The inquisitiveness in the area of research in precision medicine has bloomed over recent years with the advancement in precision medicine and breakthroughs in leukemia with drugs like “Gleevac” (Imatinib) acting as a signal transduction inhibitor [38]. The intense research has further led to the discovery of drugs and therapies, particularly for glioblastoma. Meanwhile, several drugs have been FDA approved and are under trials showing diverse results in patients with glioblastoma as shown in [Table 1]. The combination of CCNU (lomustine) along with temozolomide (TMZ) has shown effective and reliable results in patients with methylated MGMT promoter. All patients considered for the study had a Karnofsky performance score of >70 i.e., all patients were able to perform normal activity with few signs of symptoms and difficulties. The study observed a significant difference in median OS of patients and concluded the combined therapy to be better compared to the standard TMZ therapy. The research has further provided consistent results suggesting the use of combination therapy or personalized therapy, viz., both CCNU/temozolomide for newly diagnosed glioblastoma patients with methylated MGMT promoter being superior to the use of single therapy [39].
Table 1.
FDA-approved drugs for treatment of glioblastoma - a high grade glioma (HGG).
| Sl No. | Drug/Therapy employed | FDA approval and year | Mechanism | Approved for | Application Route | OS | PFS @6months duration |
|---|---|---|---|---|---|---|---|
| 1. | TMZ (Temozolomide) | FDA approval in 2005 | Nonspecific alkylating agent that causes mismatch repair in DNA by methylation at the O6 position of guanine | All high grades of glioma | Oral | 14.616.1 months | 53.90% [1] |
| 2. | Lomustine (CCNU) | FDA approval in 1976 | Nonspecific alkylating agent that causes crosslinking of DNA and RNA in dividing cells triggering cell death | Recurrent HGG (High Grade Glioma) | Oral | 11.5 months | No supporting data yet [42] |
| 3. | Carmustine (BCNU) | FDA approval in 1977 | Nonspecific alkylating agent that causes crosslinking of DNA and RNA in dividing cells; also binds to and modifies glutathione reductase | Recurrent HGG | IV | 11.75 months | No supporting data yet [42] |
| 4. | Carmustine wafer implants (BCNU wafers) | FDA approval in 2003 | Nonspecific alkylating agent that causes crosslinking of DNA and RNA in dividing cells; also binds to and modifies glutathione reductase | Recurrent HGG | Implanted | 13.9 months | No supporting data yet [42] |
| 5. | Bevacizumab (BVZ) | FDA approval in 2009 | Targeted therapeutic antibody that binds and inhibits VEGF protein in tumor cells | Recurrent HGG | IV | 9.3 months | 36% [1] |
| 6. | TTF (Tumor treating Fields) | FDA approval in 2015 | Low-intensity (1–3 V/cm), intermediate frequency (200 kHz) alternating electric fields that disrupt mitosis in tumor cells | Recurrent and new HGG | On scalp | 20.520.9 months | 56% [43] |
The use of monoclonal antibodies and tumor immunology-based therapies have also come to light. The brain cell’s exposure to immune cells such as the dendritic cells has shown tumor suppression [11,40]. Use of peptide vaccines for the EGFR mutation, a mutant tyrosine kinase, called as ACTIVATE, is in Phase II trials showing potential possibilities in clinical trials. Clinical trials showed positive results with increased survival to 26 months for glioblastoma patients [41].
For recurrent/post progression resection glioblastoma, it has been demonstrated that the monoclonal antibody Bevacizumab against VEGF displayed effective results with PFS for >6 months. Bevacizumab, approved in 2009, have also shown prospects in therapy against recurrent glioblastoma in combinations like CCNU/Bevacizumab [1].
BCNU Wafers (polycarboxyphenoxypropane/sebacic acid anhydride) containing nitrosourea carmustine also displays an effective delivery system through an implantable controlled – release approach. It is a technique by which the drug, approved by the FDA in 2003, is released to the site of the tumors through diffusion [42]. This system of implantable release of drugs has been shown to be effective for increasing PFS in glioblastoma.
Catheter-based convection-enhanced delivery (CED) is a system that employs the use of positive pressure infusion into the brain parenchyma with lower toxicity and effective delivery to a larger area of the tumor [44]. Real time tracking of the procedure can be done using intraoperative MRI. Drugs like Irinotecan and Imatinib can be effectively infused using the catheter-based CED. This type of CED delivery systems demonstrate effective advantages in targeting the tumor without promoting toxicity, and also enabling the ability to bypass the BBB [45]. Selective targeting of tumor cells enables both preclinical and clinical trials to be conducted with accuracy. Drug delivery systems developed over the years have proven to be reliable and efficient in cancer treatments.
Tyrosine kinase inhibitors (TKI) have also been used in trials for the inhibition of glioblastoma in most cases. Many TKIs showed negative outcomes and impacts in clinical trials in patients. The inability of TKIs to penetrate the BBB has also been a chief indication of its lower application in treating glioblastoma [46]. Few of the notable second generation TKI’s include neratinib, dacomitinib, afatinib, while the first-generation gefitinib, and the peptide vaccine rindopepimut (also known as CDX-110) have shown promising results so far. Use of multiple combinations of immunotherapies for targeting the over-expressive immunosuppression by PD-L1 (programmed cell death protein - ligand 1) and CTLA4 (cytotoxic T-lymphocyte-associated antigen 4) have effective potential in the suppression of glioblastoma [47]. Drugs having immunotherapeutic approaches also include nivolumab, durvalumab, DCVax-L, pembrolizumab, and tremelimumab, and are better known to target PD-L1 for effective immune response and therapy.
On the other hand, enasidenib and ivosidenib, act as inhibitors of IDH (isocitrate dehydrogenase), an essential enzyme of the TCA cycle. Detailed description of the mechanism of drugs on various targets have been illustrated below [Table 2 and Fig. 6]. However, evidences from existing reports reveal several constraints that are yet to be revised and redesigned for better prognosis in patients.
Table 2.
Recent advancements in agents available for future therapies.
| Sl No. | Agent | Mechanism | Approval/Clinical/Pre-clinical stage | Administration | Grade | Results |
|---|---|---|---|---|---|---|
| 1. | Nivolumab (Opdivo) | Nivolumab is an immunoglobulin that inhibits PD-1 antibody. | Phase III | IV | Recurrent and new gliomas | Median OS 9.5 months [40] |
| 2. | Gefitinib | Targets the EGFR TKI | Phase II | Oral | Recurrent GBM | No supporting data yet [42] |
| 3. | Durvalumab | It is an immunoglobulin G1 kappa monoclonal antibody that inhibits the PD-1, PD-2 and CD80. | Phase II | IV | Recurrent and new GBM | OS 59% and PFS 50% [44] |
| 4. | DCVax-L | Use of patients’ dendritic cells for inhibiting recurrence of GBM | Phase II | Direc t | Recurrent GBM alone | >12 months with 93.5% efficiency [40] |
| 5. | Afatinib | Targets the EGFR and EGFRv III (deletion of exons 2–7) TK1 | Phase I, II | Oral | Recurrent GBM | No supporting data yet [41] |
| 6. | Tremelimumab and Durvalumab | Targets the PD-1 and CTLA-4 by inhibition | Phase II | IV | All and recurrent HGG | No supporting data yet. Clinical Data Identifier NCT02794883 [47] |
| 7. | AEE788 (7Hpyrrolo [2,3-D] pyrimidine lead scaffold) | EGFR (epidermal growth factor receptor)/VEGFR (vascular endothelial growth factor receptor) TKI | Phase I | Oral | Recurrent GBM | No supporting data/study discontinued [48] |
| 8. | Dacomitinib (a Pan-HER irreversible inhibitor) | EGFR TKI 2nd generation | Phase II | Oral | Recurrent GBM | NCT01520870 (PF-299804) [46] |
| 9. | Neratinib | EGFR TKI 2nd generation | Phase II | Oral | Recurrent GBM | NCT01953926 (HER mutation study [46] |
| 10. | Nivolumab + Bevacizumab | Inhibits PD-1 antibody and VEGF protein in tumor cells. | Phase III | IV | Recurrent GBM | 10 months OS [47] |
| 11. | Pembrolizumab | Blocks the protein PD1 | Phase II | Oral | Recurrent GBM | PFS at 6 months [47] |
| 12. | Enasidenib | Allosteric inhibiter of mutant IDH2 | Phase II | Oral | All types of gliomas | No supporting data [46] |
| 13. | Ivosidenib | Small molecular inhibitor for mutant IDH1 | Phase I | Oral | All types of gliomas | PFS 13 months [46] |
| 14. | Erlotinib | Targets EGFRvIII (deletion of exons 2–7) TKI |
Phase II | Oral | Recurrent glioma | OS at 12 months, PFS at 6 months [41] |
| 15. | Rindopepimut (EGFRvIII peptide vaccine) | Targets EGFRvIII mutation | Phase III | Oral | Newly diagnosed GBM | OS at 20.1 months. Ineffective & discontinued after Phase III [41] |
| 16. | Asunercept | Targets CD95/CD95 ligand & Blocks CD95 ligand | Phase II | IV | Recurrent GBM | PFS at 11.2–33.4 for rRT + APG101(Asune rcept) [42] |
| 17. | Depatux-M (depatuxizumab mafodotin) | EGFR antibody drug conjugate, release of anti-microtubule agent | Phase III | IV | Newly diagnosed GBM | Ineffective results, study discontinued [42] |
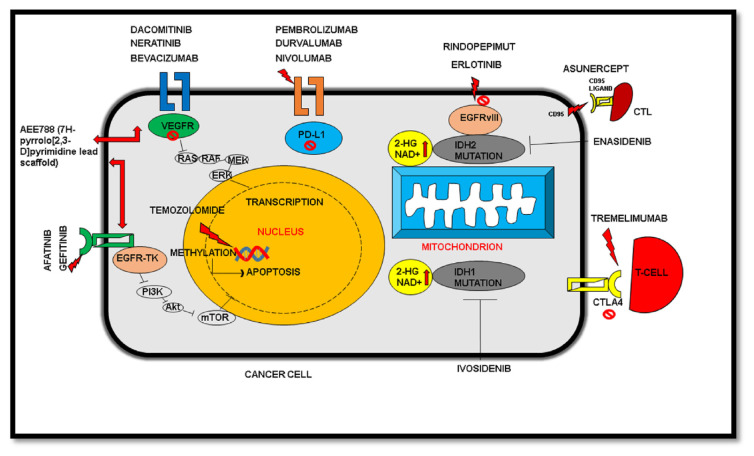
Fig. 6.
Mechanistic pathways for drugs and their targets under trial. PD-L1: Programmed cell death –ligand 1. EGFR: Epidermal growth factor receptor. VEGFR: Vascular endothelial growth factor receptor. IDH1 & IDH2: Isocitrate Dehydrogenase 1&2. TK: Tyrosine kinase. CTLA-4: Cytotoxic T Lymphocyte-associated antigen. CTL: Cytotoxic T Lymphocytes.
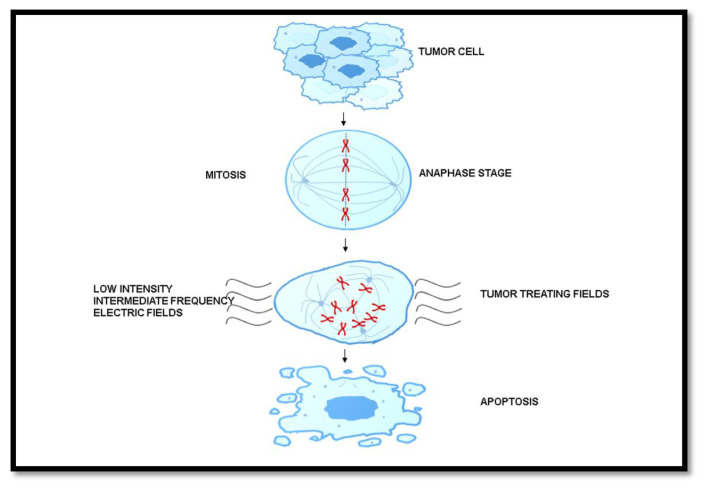
Tumor Treating Fields (TTF) is a technique of employing low-intensity intermediate frequency electric fields and applied on the patients’ shaved scalps [43]. Such types of treatments are antimitotic that target rapidly dividing cancer cells. These electric fields disrupt the microtubule stability, thereby disrupting mitotic division and causing cell deaths [Fig. 7]. Patients with newly diagnosed glioblastoma and those treated with TTF for 18 h per day together with adjuvant TMZ had a significantly improved OS from 16 months in all the TMZ group alone, and 20.9 months in the TTF plus TMZ combined groups. Such treatments resulted in the increased survivability of subjects by two-fold [49]. However, there are certain limitations to the TTF therapy, wherein the tedious treatment procedure applied on patients resulted in decline in their quality of life. Numerous patients are reported to discontinue the TTF therapy owing to its tedious process. Yet, TTF, amongst other therapies so far known, stands out from other treatments as it does not have side effects caused by chemotherapy [48]. Also, premature termination of the TTF treatment may have adverse effects on the patient and that may cause variations in survival efficiency [50].
Fig. 7.
Mechanism of Tumor Treating Fields (TTF) during glioblastoma treatment. Disruption of cell at anaphase stage by inducing low intensity intermediate frequency electric fields are shown.
6. Challenges in precision medicine
The most regarded barrier in the advancement of novel drugs and therapies has been the incapability of delivering drugs to the target tumor due to the presence of the BBB. Another major perspective on glioblastoma is the decline in the quality of life of patients as the cancer progresses. Poor lifestyle changes and therapy procedures inversely affect the well-being and survival of patients. This study has shown significant strategies to target the tumor by employing various methods. The advancement of drugs in the field of precision medicine for glioblastoma needs to be characterized among patients into specific subdivisions for identification and betterment of the treatment provided.
7. Conclusion
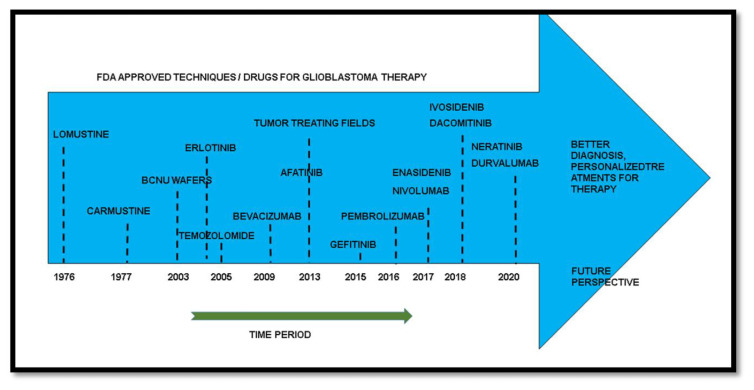
It is evident that the conventional treatments of glioblastoma need alterations and are not efficient due to the malignancy and diversity of the tumor occurring among patients. GWS has shown the potential ability to treat the disease with information and studying of various genomic profiles. Targeting glioblastoma based on the genomic profile of the patient proves to be a viable way of approaching the tumor with potential therapeutic ability. The time period on discovery of drugs over the years has been represented in the illustration [Fig. 8]. Temozolomide, being a standard therapy for glioblastoma patients along with surgery and chemotherapy has evolved over the years into different drugs targeting a variety of pathways for better prognosis in patients. These drugs in different combinations show potential for targeting the patient’s tumor based on the profile.
Fig. 8.
Food and Drug Administration (FDA) approved drugs over the period time for the treatment of glioblastoma.
The specific tumor characterization in patients using advanced diagnosis methods like FET (O-(2 [(18)F]fluoroethyl)-l-tyrosine) and MRSI (Magnetic resonance spectroscopic imaging) have showed a way for effective treatment using specific or different combination of drugs. Characterization of different models of glioblastoma based on the genomic profile is a better way to hypothesize therapeutic outcomes. Therapeutics by means of converging tumor characteristics and drug mechanisms may show potential in tumor treatment. The future perspective of personalized medicine in glioblastoma is inclined towards a novel method of treatment with precise combination of drugs. Many drugs are under trials and possess potentiality to treat patients with glioblastoma by specific and multiple therapies. The attitude of adhering to conventional methods of treatment using surgery and radioactive therapies by the scientific and medicinal community due to ethical reasons has been a minor limitation for the dawdling type progress of precision medicinal therapeutics. This study has provided significant therapeutic approaches for a better prognosis of glioblastoma. The evolution in the field of GWS and both availability and unrestricted access of medicinal advancements throughout the communities can demonstrate development in the field. Early prediction and diagnosis using genomic markers can display major significances in the treatment and lifestyle of individuals.
Advancements and findings in therapies using precision medicine approach for cancer treatments especially in the field of glioblastoma is inevitable. Expansion of the study can improve prognosis and the current situation of glioblastoma in patients. Further research and outcomes from the scientific community through precision medicine promises a breakthrough in the treatment of glioblastoma.
Acknowledgements
This work was supported by the M.Tech Biotechnology PG Teaching Program funded by the Department of Biotechnology (Government of India), All India Council for Technical Education (AICTE) for the Doctoral Fellowship to SK, and the Department of Biotechnology (MAKAUT, WB).
Funding Statement
This work was supported by the M.Tech Biotechnology PG Teaching Program funded by the Department of Biotechnology (Government of India), All India Council for Technical Education (AICTE) for the Doctoral Fellowship to SK, and the Department of Biotechnology (MAKAUT, WB).
Footnotes
Authors’ contribution
The original concept and design of the review has been done by JB and SK. SK and KI did data Compilation and article drafting. KI, SS, and SB performed image processing.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1. Bush NA, Jumper SL, Berger MS. Management of glioblastoma, present and future. World Neurosurg. 2019;131:328–38. doi: 10.1016/j.wneu.2019.07.044. [DOI] [PubMed] [Google Scholar]
- 2. Li J, Di C, Mattox AK, Wu L, Adamson DC, Robert P, et al. The future role of personalized medicine in the treatment of glioblastoma multiforme. Pharmgenomics Pers Med. 2010;3:111–27. doi: 10.2147/PGPM.S6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21(21):2683–710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- 4. Kanu OO, Mehta A, Di C, Lin N, Bortoff K, Bigner DD, et al. Glioblastoma multiforme: a review of therapeutic targets. Expert Opin Ther Targets. 2009;13(6):701–18. doi: 10.1517/14728220902942348. [DOI] [PubMed] [Google Scholar]
- 5. Zhang YY, Ruan LX, Zhang S. Rapid progression of glioblastoma multiforme: a case report. Oncol Lett. 2016;12(6):4803–6. doi: 10.3892/ol.2016.5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yang M, Guo WW, Yang CS, Tang JQ, Huang Q, Feng SX, et al. Mobile phone use and glioma risk: a systematic review and meta-analysis. PLoS One. 2017;12(5):1–13. doi: 10.1371/journal.pone.0175136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hanif F, Muzaffar K, Perveen K, Malhi SM, Simjee SU. Glioblastoma multiforme: a review of its epidemiology and pathogenesis through clinical presentation and treatment. Asian Pac J Cancer Prev APJCP. 2017;18(1):3–9. doi: 10.22034/APJCP.2017.18.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Argyriou AA, Giannopoulou E, Kalofonos HP. Angiogenesis and anti-angiogenic molecularly targeted therapies in malignant gliomas. Oncology. 2009;77(1):1–11. doi: 10.1159/000218165. [DOI] [PubMed] [Google Scholar]
- 9. Iqbal N, Iqbal N. Imatinib: a breakthrough of targeted therapy in cancer. Chemother Res Pract. 2014;2014:1–9. doi: 10.1155/2014/357027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kast RE, Focosi D. Three paths to better tyrosine kinase inhibition behind the blood-brain barrier in treating chronic myelogenous leukemia and glioblastoma with imatinib. Transl Oncol. 2010;3(1):13–5. doi: 10.1593/tlo.09280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ene C, Holland E. Personalized medicine for gliomas. Surg Neurol Int. 2015;6(2):S89–95. doi: 10.4103/2152-7806.151351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Limam S, Missaoui N, Abdessayed N, Mestiri S, Selmi B, Mokni M, et al. Prognostic significance of MGMT methylation and expression of MGMT, P53, EGFR, MDM2 and PTEN in glioblastoma multiforme. Ann Biol Clin. 2019;77(3):307–17. doi: 10.1684/abc.2019.1448. [DOI] [PubMed] [Google Scholar]
- 13. DeCordova S, Shastri A, Tsolaki AG, Yasmin H, Klein L, Singh SK, et al. Molecular heterogeneity and immunosuppressive microenvironment in glioblastoma. Front Immunol. 2020;11:1402. doi: 10.3389/fimmu.2020.01402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vartanian A, Singh SK, Agnihotri S, Jalali S, Burrell K, Aldape KD, et al. GBM’s multifaceted landscape: highlighting regional and microenvironmental heterogeneity. Neuro Oncol. 2014;16(9):1167–75. doi: 10.1093/neuonc/nou035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McLendon R, Friedman A, Bigner D, Van Meir EG, Brat DJ, Mastrogianakis GM, et al. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–8. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hicks WH, Bird CE, Traylor JI, Shi DD, El Ahmadieh TY, Richardson TE, et al. Contemporary mouse models in glioma research. Cells. 2021;10(3):712. doi: 10.3390/cells10030712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Behnan J, Finocchiaro G, Hanna G. The landscape of the mesenchymal signature in brain tumours. Brain. 2019;142(4):847–66. doi: 10.1093/brain/awz044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9(3):157–73. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 19. Wen PY, Yung WKA, Lamborn KR, Dahia PL, Wang Y, Peng B, et al. Phase I/II study of imatinib mesylate for recurrent malignant gliomas: North American Brain Tumor Consortium Study 99-08. Clin Cancer Res. 2006;12(16):4899–907. doi: 10.1158/1078-0432.CCR-06-0773. [DOI] [PubMed] [Google Scholar]
- 20. Raymond E, Brandes AA, Dittrich C, Fumoleau P, Coudert B, Clement PMJ, et al. Phase II study of imatinib in patients with recurrent gliomas of various histologies: a European organisation for research and treatment of cancer brain tumor group study. J Clin Oncol. 2008;26(28):4659–65. doi: 10.1200/JCO.2008.16.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Desjardins A, Quinn JA, Vredenburgh JJ, Sathornsumetee S, Friedman AH, Herndon JE, et al. Phase II study of imatinib mesylate and hydroxyurea for recurrent grade III malignant gliomas. J Neuro Oncol. 2007;83(1):53–60. doi: 10.1007/s11060-006-9302-2. [DOI] [PubMed] [Google Scholar]
- 22. Sakthikumar S, Sakthikumar S, Roy A, Haseeb L, Pettersson ME, Sundström E, et al. Whole-genome sequencing of glioblastoma reveals enrichment of non-coding constraint mutations in known and novel genes. Genome Biol. 2020;21(1):1–22. doi: 10.1186/s13059-020-02035-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Holland EC. Glioblastoma multiforme: the terminator. Proc Natl Acad Sci U S A. 2000;97(12):6242–4. doi: 10.1073/pnas.97.12.6242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Szerlip NJ, Pedraza A, Chakravarty D, Azim M, McGuire J, Fang Y, et al. Intratumoral heterogeneity of receptor tyrosine kinases EGFR and PDGFRA amplification in glioblastoma defines subpopulations with distinct growth factor response. Proc Natl Acad Sci U S A. 2012;109(8):3041–6. doi: 10.1073/pnas.1114033109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kawaguchi T, Sonoda Y, Shibahara I, Saito R, Kanamori M, Kumabe T, et al. Impact of gross total resection in patients with WHO grade III glioma harboring the IDH 1/2 mutation without the 1p/19q codeletion. J Neuro Oncol. 2016;129(3):505–14. doi: 10.1007/s11060-016-2201-2. [DOI] [PubMed] [Google Scholar]
- 26. deSousa JF, Serafim RB, deFreitas LM, Fontana CR, Valente V. DNA repair genes in astrocytoma tumorigenesis, progression and therapy resistance. Genet Mol Biol. 2020;43(1):1–15. doi: 10.1590/1678-4685-GMB-2019-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Langen K, Stoffels G, Filss C, Heinzel A, Stegmayr C, Lohmann P, et al. Imaging of amino acid transport in brain tumours : positron emission tomography with O-(2-[18 F] fluoroethyl) - L -tyrosine (FET) 2017;130:124–34. doi: 10.1016/j.ymeth.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 28. Naeini KM, Pope WB, Cloughesy TF, Harris RJ, Lai A, Eskin A, et al. Identifying the mesenchymal molecular subtype of glioblastoma using quantitative resonance images. Neuro Oncol. 2013;15(5):626–34. doi: 10.1093/neuonc/not008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hadjipanayis CG, Fiske CT, Jones TF, Warkentin J, Shepherd BE, Maruri F, et al. What is the surgical benefit of utilizing 5-ALA for fluroscence-guided-surgery of malignant gliomas? Neurosurgery. 2015;143(5):951–9. [Google Scholar]
- 30. Leroy HA, Vermandel M, Lejeune JP, Mordon S, Reyns N. Fluorescence guided resection and glioblastoma in 2015: a review. Laser Surg Med. 2015;47(5):441–51. doi: 10.1002/lsm.22359. [DOI] [PubMed] [Google Scholar]
- 31. Bougnaud S, Golebiewska A, Oudin A, Keunen O, Harter PN, Mäder L, et al. Molecular crosstalk between tumour and brain parenchyma instructs histopathological features in glioblastoma. Oncotarget. 2016;7(22):31955–71. doi: 10.18632/oncotarget.7454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shonka NA, Aizenberg MR. Extent of resection in glioblastoma. J Oncol Pract. 2017;13(10):641–2. doi: 10.1200/JOP.2017.027599. [DOI] [PubMed] [Google Scholar]
- 33. Cohen MH, Johnson JR, Pazdur R. Food and drug administration drug approval summary: temozolomide plus radiation therapy for the treatment of newly diagnosed glioblastoma multiforme. Clin Cancer Res. 2005;11(19 I):6767–71. doi: 10.1158/1078-0432.CCR-05-0722. [DOI] [PubMed] [Google Scholar]
- 34. Schreck KC, Grossman SA. Role of temozolomide in the treatment of cancers involving the central nervous system. ONCOLOGY (United States) 2018;32(11):555–60. 569. [PubMed] [Google Scholar]
- 35. Weller M, Stupp R, Reifenberger G, Brandes AA, Van Den Bent MJ, Wick W, et al. MGMT promoter methylation in malignant gliomas: ready for personalized medicine? Nat Rev Neurol. 2010;6(1):39–51. doi: 10.1038/nrneurol.2009.197. [DOI] [PubMed] [Google Scholar]
- 36. Rao AM, Quddusi A, Shamim MS. The significance of mgmt methylation in glioblastoma multiforme prognosis. J Pakistan Med Assoc. 2018;68(7):1137–9. [PubMed] [Google Scholar]
- 37. Yu W, Zhang L, Wei Q, Shao A. O6-Methylguanine-DNA methyltransferase (MGMT): challenges and new opportunities in glioma chemotherapy. Front Oncol. 2020;9:1547. doi: 10.3389/fonc.2019.01547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sacha T. Imatinib in chronic myeloid leukemia : an overview. Mediterr J Hematol Infect Dis. 2014;6(1):e2014007. doi: 10.4084/MJHID.2014.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Das S, Sahgal A, Perry JR. Commentary: lomustine-temozolomide combination therapy versus standard temozolomide therapy in patients with newly diagnosed glioblastoma with methylated MGMT promoter (CeTeG/NOA-09): a randomised, open-label, phase 3 trial. Front Oncol. 2020;10:66. doi: 10.3389/fonc.2020.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Phuphanich S, Wheeler CJ, Rudnick JD, Mazer M, Wang H, Nuño MA, et al. Phase i trial of a multiepitope-pulsed dendritic cell vaccine for patients with newly diagnosed glioblastoma. Cancer Immunol Immunother. 2013;62(1):125–35. doi: 10.1007/s00262-012-1319-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Choi BD, Archer GE, Mitchell DA, Heimberger AB, McLendon RE, Bigner DD, et al. EGFRvIIItargeted vaccination therapy of malignant glioma. Brain Pathol. 2009;19(4):713–23. doi: 10.1111/j.1750-3639.2009.00318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fisher JP, Adamson DC. Current FDA-approved therapies for high-grade malignant gliomas. Biomedicines. 2021;9(3):324. doi: 10.3390/biomedicines9030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stupp R, Taillibert S, Kanner A, Read W, Steinberg DM, Lhermitte B, et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma a randomized clinical trial. JAMA, J Am Med Assoc. 2017;318(23):2306–16. doi: 10.1001/jama.2017.18718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mathew EN, Berry BC, Yang HW, Carroll RS, Johnson MD. Delivering therapeutics to glioblastoma: overcoming biological constraints. Int J Mol Sci. 2022;23(3):1711–44. doi: 10.3390/ijms23031711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shi M, Sanche L. Convection-enhanced delivery in malignant gliomas: a review of toxicity and efficacy. J Oncol. 2019;2019:9342796. doi: 10.1155/2019/9342796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kim G, Ko YT. Small molecule tyrosine kinase inhibitors in glioblastoma. Arch Pharm Res (Seoul) 2020;43(4):385–94. doi: 10.1007/s12272-020-01232-3. [DOI] [PubMed] [Google Scholar]
- 47. Preddy I, Nandoliya K, Miska J, Ahmed AU. Checkpoint: inspecting the barriers in glioblastoma immunotherapies. Semin Cancer Biol. 2022;86(Pt 3):473–48147. doi: 10.1016/j.semcancer.2022.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fields T, Review TTFA, Fabian D, Guillermo P, Eibl P, Alnahhas I, et al. Treatment of glioblastoma ( GBM ) with the addition of tumor-treating fields (TTF): a review. Cancers. 2019;11(2):17450. doi: 10.3390/cancers11020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rominiyi O, Vanderlinden A, Clenton SJ, Bridgewater C, Al-Tamimi Y, Collis SJ. Tumour treating fields therapy for glioblastoma: current advances and future directions. Br J Cancer. 2021;124(4):697–709. doi: 10.1038/s41416-020-01136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhu P, Zhu JJ. Tumor treating fields: a novel and effective therapy for glioblastoma: mechanism, efficacy, safety and future perspectives. Chin Clin Oncol. 2017;6(4):1–15. doi: 10.21037/cco.2017.06.29. [DOI] [PubMed] [Google Scholar]