Abstract
Aims
The present study sought to determine the rate and prognostic implications of post-procedural physiologically significant residual ischemia according to Murray law-based quantitative flow ratio (μQFR) after left main (LM) bifurcation percutaneous coronary intervention (PCI).
Methods and results
Consecutive patients undergoing LM bifurcation stenting at a large tertiary care center between January 2014 and December 2016 with available post-PCI μQFR were included. Physiologically significant residual ischemia was defined by post-PCI μQFR values ≤0.80 in the left anterior descending (LAD) or left circumflex artery (LCX). The primary outcome was 3-year cardiovascular death. The major secondary outcome was 3-year bifurcation-oriented composite endpoint (BOCE). Among 1170 included patients with analyzable post-PCI μQFR, 155 (13.2%) had residual ischemia in either LAD or LCX. Patients with vs. those without residual ischemia had a higher risk of 3-year cardiovascular mortality [5.4% vs. 1.3%; adjusted hazard ratio (HR) 3.20, 95% confidence interval (CI): 1.16–8.80]. The 3-year risk of BOCE was significantly higher in the residual ischemia group (17.8% vs. 5.8%; adjusted HR 2.79, 95% CI: 1.68–4.64), driven by higher incidence of the composite of cardiovascular death and target bifurcation-related myocardial infarction (14.0% vs. 3.3%; adjusted HR 4.06, 95% CI: 2.22–7.42). A significant, inverse association was observed between continuous post-PCI μQFR and the risk of clinical outcomes (per 0.1 μQFR decrease, HR of cardiovascular death 1.27, 95% CI: 1.00–1.62; HR of BOCE 1.29, 95% CI: 1.14–1.47).
Conclusion
After angiographically successful LM bifurcation PCI, residual ischemia assessed by μQFR was identified in 13.2% of patients and was associated with higher risk of 3-year cardiovascular death, indicating the superior prognostic value of post-PCI physiological assessment.
Keywords: Cardiovascular death, Left main coronary artery disease, Quantitative flow ratio, Percutaneous coronary intervention, Residual ischemia
Structured Graphical Abstract
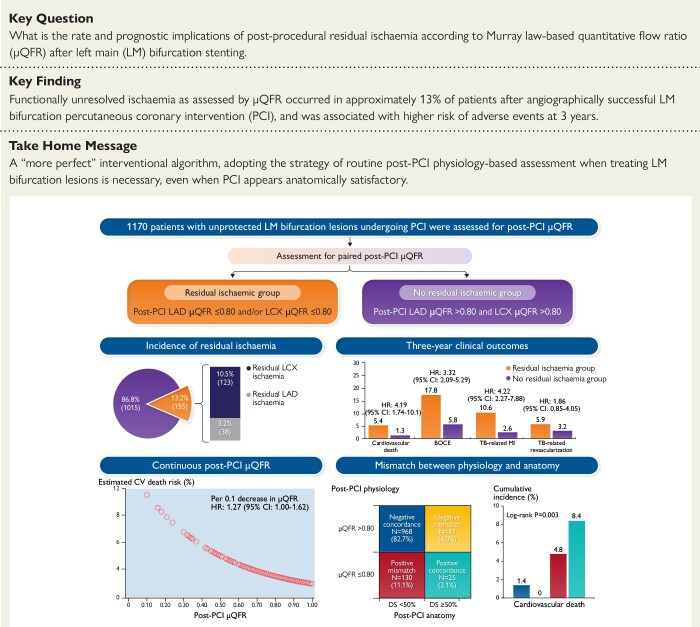
Structured Graphical Abstract.
Physiologically significant residual ischemia after LM bifurcation stenting. Among 1,170 included patients undergoing angiographically successful LM bifurcation PCI with analyzable post-PCI μQFR, 155 (13.2%) had residual ischemia in either LAD or LCX which was associated with a significantly higher risk of 3-year adverse cardiac events. The post-PCI μQFR demonstrated a continuous and inverse relationship between its numeric value and clinical events. Mismatch between post-PCI physiological and anatomical assessment for significant stenoses was identified in over 15% of patients, in whom post-PCI μQFR-based physiological assessment showed superior prognostic value for 3-year clinical outcomes.
BOCE, bifurcation-oriented composite endpoint; CABG, coronary artery bypass grafting; CI, confidence interval; CV, cardiovascular; DS, diameter stenosis; HR, hazard ratio; LAD, left anterior descending artery; LCX, left circumflex artery; LM, left main; PCI, percutaneous coronary intervention; TB, target bifurcation; μQFR, Murray law-based quantitative flow ratio.
See the editorial comment for this article ‘Left main PCI: beware the circumflex!’, by N.P. Johnson and J.-M. Ahn, https://doi.org/10.1093/eurheartj/ehad434.
Introduction
Left main (LM) coronary artery disease (CAD) has long been recognized as a crucial anatomical subset of coronary atherosclerosis associated with high mortality, for which coronary artery bypass grafting (CABG) has been established as standard of care for many years.1 Latest guidelines have stated that the less invasive percutaneous coronary intervention (PCI) with drug-eluting stents could be considered an alternative to CABG among patients with low-to-intermediate anatomical complexity,2,3 as the efficacy and safety of LM PCI greatly advanced over the ensuing decades with improved imaging technology, better PCI equipment, enhanced antithrombotic therapy, and increased operator experience.1,4 Notably, an individual patient data meta-analysis of four large randomized controlled trials demonstrated no significant difference between PCI and CABG with respect to rates of 5-year mortality among patients with LM CAD.5
Left main coronary artery disease frequently involves distal LM bifurcation, and PCI for LM bifurcation disease is usually associated with more complex procedures and inferior outcomes compared with isolated ostial or shaft treatment.1,4 In this regard, it is important to adequately evaluate the hemodynamic significance of bifurcation lesions and apply physiological concepts during bifurcation PCI for improvement in hemodynamic results and clinical outcomes.6 Post-PCI physiology assessment is one of the effective metrics to quantify residual ischemia.7 Abnormal post-PCI physiology, as measured by wire-based pressures [i.e. fractional flow reserve (FFR)], was identified in side branch [left circumflex coronary artery (LCX)] (occurring in nearly 16.9% of patients) and associated with poor prognosis after LM crossover stenting.8 However, wire-based physiology is underused among patients with bifurcation lesions due to need of dedicated pressure wire, hyperemic agents, prolonged procedural time, and difficulties to access side branch through stent struts.9,10
Artificial intelligence (AI)-powered Murray bifurcation fractal law-based quantitative flow ratio (μQFR) is a new-generation angiography-based computational coronary physiology index proven to be more appropriate for analysis of bifurcation lesions, with good diagnostic accuracy in identifying hemodynamically significant coronary stenoses compared with FFR as the reference standard.11–15 Currently, there is a lack of evidence regarding how frequently residual ischemia is present and whether this criterion associates with long-term prognosis (especially mortality) among patients with LM bifurcation undergoing PCI. Therefore, the aim of this study was (i) to analyze the incidence and predictors of residual ischemia detected by post-PCI μQFR and (ii) to determine the prognostic impact of residual ischemia on 3-year clinical outcomes in patients undergoing LM bifurcation PCI from the Fuwai LM PCI cohort.16,17
Methods
Study design and patient population
All consecutive patients undergoing unprotected LM CAD PCI at a large tertiary hospital (Fuwai Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China) between January 2014 and December 2016 were prospectively enrolled and assessed for inclusion in this study. The design and enrollment characteristics of the prospective, observational, single-center Fuwai LM CAD PCI cohort have been published previously.16,17 Eligible study participants had silent ischemia, stable angina, unstable angina, or recent myocardial infarction (MI) and underwent PCI of an unprotected lesion involving the LM bifurcation with diameter stenosis (DS) ≥50% by visual estimation. All eligible patients with LM CAD in the present study were considered for μQFR measurement. Patients with ST-elevation or non-ST elevation MI within 72 h of the index procedure and non-analyzability for post-PCI μQFR were excluded. The present study was approved by the Fuwai Hospital Institutional Review Committee and performed in accordance with the Declaration of Helsinki. All patients provided written informed consent for use of their clinical data in clinical research.
Procedures and quantitative coronary angiography
Percutaneous coronary intervention was performed following standards of practices, as described in the Supplementary material online, Appendix. Data were entered in a dedicated database by independent research personnel. Coronary angiograms were collected and analyzed at the angiographic core laboratory in a blinded fashion (Interventional Cardiovascular Imaging Core Laboratory, National Center for Cardiovascular Diseases, Beijing, China). Quantitative coronary angiography (QCA) characteristics, including reference vessel diameter (RVD), minimal lumen diameter, percentage DS, and lesion length, were analyzed in all treated LM bifurcation lesions using well-validated software (QAngio software version 7.3, Medis Medical Imaging Systems, Leiden, the Netherlands). Anatomically significant residual disease was diagnosed in patients with DS ≥50% in LAD or LCX after LM bifurcation stenting.
Murray law-based quantitative flow ratio and residual ischemia
The μQFR is a new-generation angiography-based computational coronary physiology index that has been validated as having good diagnostic accuracy in identifying physiologically significant coronary stenosis while achieving several improvements in intellectualization and simplicity:11,12 (i) frame counting (for contrast flow velocity) and delineation of lumen contours on the major epicardial coronary arteries and their side branches were performed automatically by AI using a convolutional neural network based on the U-Net architecture, (ii) the RVD was reconstructed more accurately by considering the step-down phenomenon across bifurcations based on the Murray bifurcation fractal law, (iii) supporting single angiographic view computation (2D-μQFR) which proved to have comparable good diagnostic performance as 3D-μQFR by two view computation, (iv) analysis time was significantly faster (about 1 min), and (v) reducing training time while maintaining good reproducibility due to the high-level automation powered by AI.
The detailed methodology for μQFR computation has been reported previously11,12 and is described in the Supplementary material online, Appendix. For the present analysis, offline 2D-μQFR assessments were performed separately in main vessel [left anterior descending (LAD) artery] and side branch (LCX) based on different single angiographic views among all patients with LM bifurcation disease. The μQFR was analyzed from the healthy segment of proximal LM (start point) to the healthy segment distal to the farthest lesion (endpoint) in LAD or LCX. The offline pre-PCI and post-PCI μQFR analyses were performed by well-trained technicians in the angiographic core laboratory (Interventional Cardiovascular Imaging Core Laboratory, National Center for Cardiovascular Diseases, Beijing, China) blinded for any clinical data using AngioPlus Core 2.0 (Pulse Medical Imaging Technology, Shanghai, China) based on pre- and post-PCI coronary angiograms using standard methods, respectively. Representative cases of μQFR computation are presented in Supplementary material online, Figure S1.
The cut-off value of μQFR for physiological significance has been established as 0.80. Patient-level residual ischemia (namely, residual ischemia group) was diagnosed in patients with post-PCI μQFR values ≤0.80 in either LAD or LCX after LM bifurcation stenting. Residual ischemia group could be further stratified into three subgroups: (i) residual ischemia in LCX alone (post-PCI LCX μQFR ≤0.80 and post-PCI LAD μQFR >0.80), (ii) residual ischemia in LAD alone (post-PCI LCX μQFR >0.80 and post-PCI LAD μQFR ≤0.80), and (iii) residual ischemia in both LAD and LCX (post-PCI LCX μQFR ≤0.80 and post-PCI LAD μQFR ≤0.80). In addition, two modalities of patient-level μQFR metrics were calculated: (i) post-PCI minimal μQFR, defined as the lower post-PCI μQFR values of LAD and LCX, and (ii) post-PCI LM-global μQFR, defined as the sum of post-PCI μQFR values measured in LAD and LCX.
Clinical outcomes
Clinical follow-up was obtained by outpatient visits, telephone contact, or reviewing clinical reports at 1, 6, and 12 months and yearly thereafter. All clinical outcomes were adjudicated using original source documents by clinical events committee whose members were blinded to baseline clinical characteristics, angiographic details, and μQFR values. The primary outcome of the present analysis was the 3-year rate of cardiovascular death. The major secondary outcome was 3-year bifurcation-oriented composite endpoint (BOCE), defined as the composite of cardiovascular death, target bifurcation-related MI, or target bifurcation revascularization based on the Bifurcation Academic Research Consortium consensus document.18 Other secondary endpoints included other composite endpoints (i.e. a “hard” outcome composite of cardiovascular death and target bifurcation-related MI, patient-oriented composite endpoint (POCE)], individual components, stroke, and stent thrombosis. The details of endpoint definitions are reported in Supplementary material online, Appendix.
Statistical analysis
Continuous variables are expressed as mean ± SD or median [interquartile range (IQR)] and were compared using Student’s t-test or Mann–Whitney U test as appropriate. Categorical variables are presented as counts (%) and were compared using chi-square test or Fisher’s exact test as appropriate. Multivariable logistic regression analysis was performed to identify the independent predictors of post-PCI residual ischemia. Two multivariable models were constructed: (i) model 1 incorporated 15 baseline variables; (ii) model 2 included 9 procedural variables. The detailed methodology of multivariable logistic regression is described in the Supplementary material online, Appendix. The cumulative incidence of clinical events was presented as Kaplan–Meier estimates. Cox proportional hazards modeling was used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs). Multivariable Cox regression analysis with incorporation of covariates was performed to adjust possible confounders. The candidate variables for multivariable adjustment were identified using historical confounder definition based on clinical knowledge and previous literature reports, including clinical [age, sex, body mass index, diabetes, chronic kidney disease (CKD), family history of CAD, previous MI, clinical presentation, and left ventricular ejection fraction (LVEF)], angiographic (multi-vessel disease and SNYTAX score), and procedural (total stent length in LM, type of drug-eluting stent, post-PCI DS in LCX, and post-PCI DS in LM-LAD) covariates. Compared with the predictive model including traditional clinical (age, LVEF, acute coronary syndrome) and angiographic (residual SYNTAX score) risk factors, the incremental prognostic values of residual ischemia (by μQFR) beyond these risk factors were evaluated by assessing the Harrell’s C-index, category-free net reclassification index (NRI), and integrated discrimination improvement (IDI). Unless otherwise specified, a two-sided P < 0.05 was considered to indicate statistical significance. All statistical analyses were performed using SAS software, version 9.4 (SAS Institute, Cary, NC).
Results
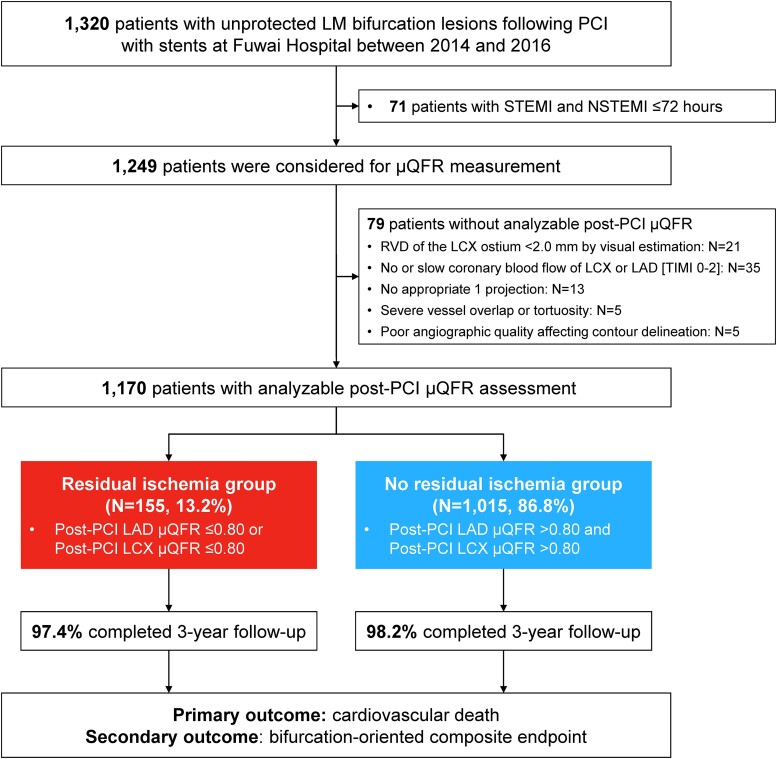
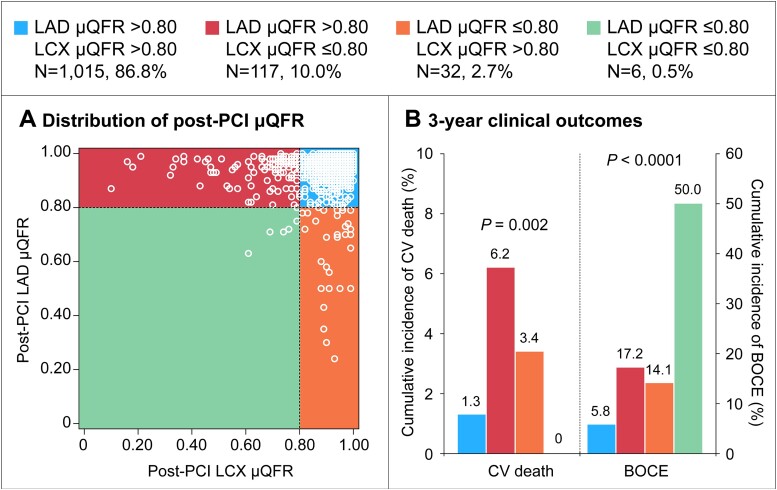
Between 1 January 2014, and 31 December 2016, a total of 1320 patients with unprotected LM bifurcation lesions following PCI with stents were prospectively enrolled. For the present analysis, 150 patients were excluded including 71 patients with ST-elevation or non-ST elevation MI ≤72 h and 79 patients without analyzable post-PCI μQFR. Therefore, 1170 patients with available post-PCI μQFR assessment were included in the present study (Figure 1). Of these, 155 (13.2%) presented with residual ischemia in either LAD or LCX, including 117 (10.0%) LCX ischemia alone, 32 (2.7%) LAD ischemia alone, and 6 (0.5%) both LAD and LCX ischemia (Figure 2).
Figure 1.
Study flowchart. Among 1170 patients with analyzable post-percutaneous coronary intervention Murray law-based quantitative flow ratio assessments, 155 (13.2%) with abnormal post-percutaneous coronary intervention Murray law-based quantitative flow ratio in either left anterior descending or left circumflex artery, in spite of “successful” percutaneous coronary intervention by angiographic criteria, were attributed to residual ischemia group. LAD, left anterior descending; LCX, left circumflex artery; LM, left main; RVD, reference vessel diameter; STEMI, ST-segment elevation myocardial infarction; NSTEMI, non-ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; μQFR, Murray law-based quantitative flow ratio.
Figure 2.
Distribution of post-percutaneous coronary intervention Murray law-based quantitative flow ratio and 3-year outcomes. (A) Among 1170 patients, 1015 (86.8%) patients had no residual ischemia, 117 (10.0%) had left circumflex artery ischemia alone, 32 (2.7%) had left anterior descending ischemia alone, and 6 (0.5%) had both left anterior descending and left circumflex artery ischemia; (B) cumulative incidence of cardiovascular death and bifurcation-oriented composite endpoint according to left circumflex artery or left anterior descending ischemia after percutaneous coronary intervention. CV, cardiovascular; BOCE, bifurcation-oriented composite endpoint; other abbreviations as in Figure 1.
Patient with vs. without residual ischemia
In brief, the mean age was 60.8 ± 10.2 years, 30.9% of patients had diabetes, and 50.3% presented with acute coronary syndrome. Baseline clinical, angiographic, procedural, and QCA characteristics of patients with vs. without residual ischemia are shown in Tables 1 and 2, and Supplementary material online, Table S1. Compared with those without residual ischemia, patients with residual ischemia had more comorbidities and lower LVEF. Regarding angiographic and procedural characteristics, the residual ischemia group was associated with more extensive CAD and a lower number of two-stent technique and final kissing balloon inflation (FKBI). In addition, clinical characteristics of the four subgroups stratified by LAD or LCX residual ischemia are presented in Supplementary material online, Tables S2 and S3.
Table 1.
Clinical and angiographic characteristics in patients with and without residual ischemia
| Total (N = 1170) | Residual ischemia group (n = 155) | No residual ischemia group (n = 1015) | |
|---|---|---|---|
| Age, years | 60.8 ± 10.2 | 62.1 ± 10.4 | 60.6 ± 10.2 |
| Sex | |||
| Men | 930 (79.5) | 120 (77.4) | 810 (79.8) |
| Women | 240 (20.5) | 35 (22.6) | 205 (20.2) |
| Body mass index, kg/m2 | 25.7 ± 3.0 | 25.2 ± 3.1 | 25.8 ± 3.0 |
| Hypertension | 722 (61.7) | 101 (65.2) | 621 (61.2) |
| Diabetes mellitus | 362 (30.9) | 61 (39.4) | 301 (29.7) |
| Hypercholesterolemia | 812 (69.4) | 119 (76.8) | 693 (68.3) |
| Current smoking | 317 (27.1) | 38 (24.5) | 279 (27.5) |
| Family history of CAD | 233 (19.9) | 28 (18.1) | 205 (20.2) |
| Previous myocardial infarction | 277 (23.7) | 49 (31.6) | 228 (22.5) |
| Previous PCI | 284 (24.3) | 41 (26.5) | 243 (23.9) |
| Previous stroke | 157 (13.4) | 20 (12.9) | 137 (13.5) |
| Peripheral artery disease | 106 (9.1) | 19 (12.3) | 87 (8.6) |
| Chronic kidney disease | 200 (17.1) | 26 (16.8) | 174 (17.1) |
| Clinical presentation | |||
| Silent ischemia | 118 (10.1) | 20 (12.9) | 98 (9.7) |
| Stable angina | 464 (39.7) | 59 (38.1) | 405 (39.9) |
| Unstable angina | 541 (46.2) | 68 (43.9) | 473 (46.6) |
| NSTEMI | 25 (2.1) | 4 (2.6) | 21 (2.1) |
| STEMI | 22 (1.9) | 4 (2.6) | 18 (1.8) |
| Left ventricular ejection fraction, % | 63.1 ± 7.2 | 61.2 ± 8.6 | 63.4 ± 7.0 |
| <45 | 38 (3.2) | 11 (7.1) | 27 (2.7) |
| Number of non-LM diseased vessels | |||
| 0 | 157 (13.4) | 13 (8.4) | 144 (14.2) |
| 1 | 349 (29.8) | 48 (31.0) | 301 (29.7) |
| 2 | 512 (43.8) | 57 (35.8) | 455 (44.8) |
| 3 | 152 (13.0) | 37 (23.9) | 115 (11.3) |
| LM lesion type | |||
| De novo | 1138 (97.3) | 148 (95.5) | 990 (97.5) |
| Restenosis | 32 (2.7) | 7 (4.5) | 25 (2.5) |
| Medina | |||
| 1,1,1 | 163 (13.9) | 18 (11.6) | 145 (14.3) |
| 1,0,1 | 43 (3.7) | 7 (4.5) | 36 (3.5) |
| 1,1,0 | 583 (49.8) | 91 (58.7) | 492 (48.5) |
| 1,0,0 | 264 (22.6) | 26 (16.8) | 238 (23.4) |
| 0,1,1 | 117 (10.0) | 13 (8.4) | 104 (10.2) |
| True LM bifurcation | 323 (27.6) | 38 (24.5) | 285 (28.1) |
| DEFINITION criteria | |||
| Complex LM bifurcation | 113 (40.4) | 10 (32.3) | 103 (41.4) |
| Simple LM bifurcation | 167 (59.6) | 21 (67.7) | 146 (58.6) |
| LM moderate-to-severe calcification | 217 (18.5) | 36 (23.2) | 181 (17.8) |
| LM thrombus-containing lesions | 4 (0.3) | 0 (0) | 4 (0.4) |
| LM total occlusion | 30 (2.6) | 4 (2.6) | 26 (2.6) |
| Anatomic SYNTAX score | 22.9 ± 6.9 | 25.4 ± 7.4 | 22.5 ± 6.7 |
| Low (≤22) | 613 (52.4) | 58 (37.4) | 555 (54.7) |
| Intermediate (23–32) | 454 (38.8) | 74 (47.7) | 380 (37.4) |
| High (≥33) | 103 (8.8) | 23 (14.8) | 80 (7.9) |
| Pre-PCI physiology assessment | |||
| Pre-PCI LAD μQFR | 0.64 ± 0.24 | 0.62 ± 0.22 | 0.65 ± 0.24 |
| Pre-PCI LCX μQFR | 0.75 ± 0.21 | 0.65 ± 0.24 | 0.77 ± 0.21 |
Data are mean (SD), n (%), median (IQR), or n/N (%).
CAD, coronary artery disease; LAD, left anterior descending; LCX, left circumflex artery; LM, left main; NSTEMI, non-ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction; SYNTAX, SYNergy between percutaneous coronary intervention with TAXus and cardiac surgery; μQFR, Murray law-based quantitative flow ratio.
Table 2.
Procedural characteristics in patients with and without residual ischemia
| Total (N = 1170) | Residual ischemia group (n = 155) | No residual ischemia group (n = 1015) | |
|---|---|---|---|
| Trans-radial intervention | 981 (83.8) | 135 (87.1) | 846 (83.3) |
| IVUS guidance | 597 (51.0) | 69 (44.5) | 528 (52.0) |
| Stent type | |||
| First-generation DES | 101 (8.6) | 91 (9.0) | 10 (6.5) |
| Second-generation DES | 1069 (91.4) | 924 (91.0) | 145 (93.5) |
| Total number of stents per LM lesion | 1.79 ± 0.76 | 1.75 ± 0.74 | 1.79 ± 0.76 |
| Total stent length per LM lesion, mm | 35.5 ± 19.6 | 34.4 ± 18.9 | 35.7 ± 19.7 |
| Mean LM stent diameter, mm | 3.41 ± 0.45 | 3.33 ± 0.41 | 3.42 ± 0.45 |
| Treatment strategy | |||
| Provisional one-stent technique | 871 (74.4) | 137 (88.4) | 734 (72.3) |
| Two-stent technique | 299 (25.6) | 18 (11.6) | 281 (27.7) |
| Culotte | 55 (18.4) | 1 (5.6) | 54 (19.2) |
| Crush | 192 (64.2) | 12 (66.7) | 180 (64.1) |
| T-stent | 36 (12.0) | 3 (16.7) | 33 (11.7) |
| V or simultaneous kissing stent | 16 (5.4) | 2 (11.1) | 14 (5.0) |
| POT performed | 601 (51.4) | 89 (57.4) | 512 (50.4) |
| LM bifurcation with FKBI | 581 (49.7) | 55 (35.5) | 526 (51.8) |
| Provisional one-stent technique | 315 (36.2) | 38 (27.7) | 277 (37.7) |
| Two-stent technique | 266 (89.0) | 17 (94.4) | 249 (88.6) |
| Post-dilation performed | 1055 (90.2) | 139 (89.7) | 916 (90.2) |
| IABP utilization | 43 (3.7) | 5 (3.2) | 38 (3.7) |
| Angiographic successa | 1115 (95.3) | 146 (94.2) | 969 (95.5) |
| Residual SYNTAX score | 4.2 ± 5.1 | 7.1 ± 6.5 | 3.8 ± 4.7 |
| Post-PCI physiology assessment | |||
| Post-PCI LAD μQFR | 0.95 ± 0.07 | 0.87 ± 0.15 | 0.96 ± 0.04 |
| Post-PCI LCX μQFR | 0.91 ± 0.10 | 0.72 ± 0.17 | 0.93 ± 0.05 |
Data are mean (SD), n (%), median (IQR), or n/N (%).
DES, drug-eluting stent; IVUS, intravenous ultrasound; POT, proximal optimization technique; FKBI, final kissing balloon inflation; IABP, intra-aortic balloon pulsation; other abbreviations as in Table 1.
Angiographic success defined as (i) residual stenosis less than 30% for main vessel treated with stents and less than 50% for side branch treated with balloon angioplasty by visual estimation, with thrombolysis in myocardial infarction flow Grade 3 in both main vessel and side branch for LM bifurcation patients treated with one-stent technique, or (ii) residual stenosis less than 30% by visual estimation with thrombolysis in myocardial infarction flow Grade 3 in both main vessel and side branch for LM bifurcation patients treated with two-stent technique.
After PCI, both post-PCI μQFR in the LCX and LAD showed significant increase from pre-PCI values (see Supplementary material online, Figure S2). Patients with residual ischemia in either LAD or LCX had lower levels of post-PCI LCX μQFR (0.72 ± 0.17 vs. 0.93 ± 0.05) and post-PCI LAD μQFR (0.87 ± 0.15 vs. 0.96 ± 0.04) compared with those without residual ischemia in both LAD and LCX.
By multivariable logistic regression analysis, LVEF, true LM bifurcation, pre-PCI LCX RVD, anatomic SS, pre-PCI LCX μQFR, treatment strategy, post-PCI LCX %DS, and residual SYNTAX score were found to be independent predictors of post-PCI residual ischemia of LM bifurcation lesions (Table 3).
Table 3.
Univariable and multivariable predictors of residual ischemia
| Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Model 1: baseline variables | ||||
| Age | 1.01 (1.00–1.03) | 0.09 | 1.01 (0.99–1.03) | 0.36 |
| Male | 0.87 (0.58–1.30) | 0.49 | 0.98 (0.63–1.53) | 0.94 |
| Diabetes | 1.54 (1.09–2.18) | 0.02 | 1.44 (1.00–2.08) | 0.051 |
| Previous myocardial infarction | 1.60 (1.10–2.31) | 0.01 | 1.31 (0.87–1.98) | 0.20 |
| LVEF, per 10% decrease | 1.43 (1.15–1.78) | 0.001 | 1.30 (1.02–1.67) | 0.03 |
| Multi-vessel disease | 1.20 (0.85–1.70) | 0.29 | 1.08 (0.75–1.57) | 0.67 |
| True LM bifurcation | 1.20 (0.81–1.78) | 0.36 | 1.79 (1.15–2.80) | 0.01 |
| LM moderate-to-severe calcification | 1.39 (0.93–2.09) | 0.11 | 1.34 (0.87–2.07) | 0.19 |
| Pre-PCI LM-LAD RVD (per 0.1 decrease) | 1.02 (0.99–1.05) | 0.24 | 0.98 (0.95–1.02) | 0.28 |
| Pre-PCI LM-LAD %DS (per 10% increase) | 1.08 (0.98–1.19) | 0.14 | 0.98 (0.88–1.09) | 0.68 |
| Pre-PCI LCX RVD (per 0.1 decrease) | 1.05 (1.02–1.08) | 0.001 | 1.05 (1.02–1.09) | 0.04 |
| Pre-PCI LCX %DS (per 10% increase) | 0.98 (0.91–1.06) | 0.68 | 0.94 (0.86–1.02) | 0.13 |
| Anatomic SYNTAX score (per 1 increase) | 1.06 (1.03–1.08) | <0.0001 | 1.06 (1.03–1.08) | <0.0001 |
| Pre-PCI LAD μQFR (per 0.1 decrease) | 1.77 (0.88–3.56) | 0.11 | 0.96 (0.88–1.04) | 0.29 |
| Pre-PCI LCX μQFR (per 0.1 decrease) | 1.23 (1.15–1.32) | <0.0001 | 1.28 (1.18–1.39) | <0.0001 |
| Model 2: procedural variables | ||||
| IVUS guidance | 0.74 (0.53–1.04) | 0.08 | 0.95 (0.66–1.37) | 0.79 |
| Total number of stents per LM lesion | 0.92 (0.74–1.16) | 0.49 | 0.99 (0.78–1.26) | 0.94 |
| Provisional one-stent crossover technique | 2.91 (1.75–4.85) | <0.0001 | 2.03 (1.12–3.70) | 0.02 |
| POT performed | 1.32 (0.94–1.86) | 0.11 | 0.97 (0.66–1.42) | 0.87 |
| Final kissing balloon inflation | 0.51 (0.36–0.73) | 0.0002 | 0.69 (0.46–1.03) | 0.07 |
| Post-dilation performed | 0.94 (0.54–1.64) | 0.82 | 1.22 (0.66–2.26) | 0.53 |
| Post-PCI LM-LAD %DS (per 10% increase) | 1.06 (0.72–1.55) | 0.77 | 1.04 (0.69–1.55) | 0.86 |
| Post-PCI LCX %DS (per 10% increase) | 1.26 (1.14–1.38) | <0.0001 | 1.18 (1.07–1.30) | 0.001 |
| Residual SYNTAX score (per 1 increase) | 1.11 (1.08–1.14) | <0.0001 | 1.10 (1.07–1.14) | <0.0001 |
Three-year clinical outcomes
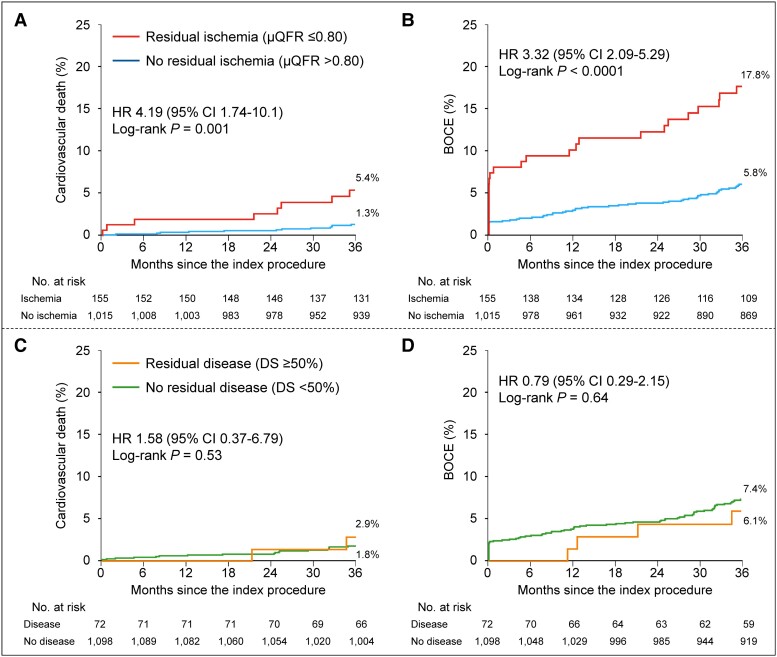
Complete 3-year follow-up data were available in 1148 of 1170 patients (98.1%), with median follow-up of 1095 days (Q1: 1095 days, Q3: 1125 days). The Kaplan–Meier estimates for primary 3-year cardiovascular death outcome were 5.4% and 1.3% of patients with and without residual ischemia, respectively (adjusted HR 3.20, 95% CI: 1.16–8.80, P = 0.02) (Figure 3, Table 4). The 3-year rate of the major secondary composite BOCE outcome was significantly higher in residual ischemia group (17.8% vs. 5.8%; adjusted HR 2.79, 95% CI: 1.68–4.64). The respective 3-year BOCE rates excluding periprocedural MI were 12.8% vs. 4.6% (adjusted HR 2.34; 95% CI: 1.30–4.21) (see Supplementary material online, Figure S3). The between-group differences in BOCE were mainly driven by higher rates of cardiovascular death and target bifurcation-related MI (10.6% vs. 2.6%; adjusted HR 3.45, 95% CI: 1.73–6.88) in the residual ischemia group. Both periprocedural (adjusted HR 3.79, 95% CI: 1.42–10.1) and non-procedural MI (adjusted HR 2.86, 95% CI: 1.14–7.15) were significantly increased in the residual ischemia group. Patients with residual ischemia showed a significantly higher risk of the “hard” endpoint (14.0% vs. 3.3%; adjusted HR 4.06, 95% CI: 2.22–7.42) (Table 4). Similar results were observed when performing two sensitivity analyses in patients presenting with de novo LM bifurcation lesions or without post-PCI angiographically significant stenoses in the non-ostial (downstream) LAD or LCX, respectively (see Supplementary material online, Tables S4 and S5). The prognosis difference for the primary cardiovascular death and secondary composite BOCE outcomes between two groups were consistent across various subgroups (see Supplementary material online, Figures S4 and S5). In addition, the relative risks for 3-year adverse cardiac events with residual ischemia compared with no residual ischemia were consistent regardless stratification by LAD μQFR or LCX μQFR, and no significant interaction was observed (see Supplementary material online, Table S6). Post-PCI μQFR assessment added to the Model 1 with clinical risk factors (C-index 0.65, 95% CI: 0.52–0.78) presented with acceptable C-index (model 2: 0.70, 95% CI: 0.58–0.81). Compared with Model 1, Model 2 showed slightly improved discrimination and risk classification of 3-year cardiovascular death (category-free NRI: 0.51, P = 0.02; IDI: 0.012; P = 0.03), indicating that 51% of patients were correctly reclassified for predicted probability of cardiovascular death and the average improvement in predicted probability of cardiovascular death was 0.012. Both Model 1 and Model 2 had acceptable calibration results, as assessed by Hosmer–Lemeshow test with P-values of 0.89 and 0.75, respectively. Figure 2B and Supplementary material online, Table S7, show 3-year clinical outcomes among four groups stratified by LAD or LCX residual ischemia.
Figure 3.
Time-to-event curves for 3-year clinical outcomes stratified by physiologically significant residual ischemia or residual disease by anatomy. Kaplan–Meier time-to-first event curves showing the 3-year cumulative incidences of cardiovascular death and bifurcation-oriented composite endpoint by physiologically significant residual ischemia (A and B) and residual disease by anatomy (C and D), respectively. BOCE, bifurcation-oriented composite endpoint; CI, confidence interval; CV, cardiovascular; DS, diameter stenosis; HR, hazard ratio; PMI, periprocedural myocardial infarction; other abbreviations as in Figure 1.
Table 4.
Clinical outcomes at 3 years in patients with and without residual ischemia
| Residual ischemia group (N = 155) | No residual ischemia group (N = 1015) | Unadjusted HRa (95% CI) | P-value | Adjusted HRa,b (95% CI) | P-value | |
|---|---|---|---|---|---|---|
| CV death | 8 (5.4) | 13 (1.3) | 4.19 (1.74–10.1) | 0.001 | 3.20 (1.16–8.80) | 0.02 |
| BOCEc | 26 (17.8) | 56 (5.8) | 3.32 (2.09–5.29) | <0.0001 | 2.79 (1.68–4.64) | <0.0001 |
| Target bifurcation-related MI | 16 (10.6) | 26 (2.6) | 4.22 (2.27–7.88) | <0.0001 | 3.45 (1.73–6.88) | 0.0005 |
| Target bifurcation revascularization | 8 (5.9) | 30 (3.2) | 1.86 (0.85–4.05) | 0.12 | 1.46 (0.64–3.32) | 0.37 |
| BOCE excluding periprocedural MI | 18 (12.8) | 44 (4.6) | 2.87 (1.66–4.97) | 0.0002 | 2.34 (1.30–4.21) | 0.005 |
| CV death and target bifurcation-related MI | 21 (14.0) | 33 (3.3) | 4.42 (2.56–7.63) | <0.0001 | 4.06 (2.22–7.42) | <0.0001 |
| CV death and target bifurcation-related non-procedural MI | 12 (8.2) | 20 (2.1) | 4.13 (2.02–8.45) | 0.0001 | 3.61 (1.66–7.87) | 0.001 |
| POCEd | 37 (24.5) | 117 (11.8) | 2.29 (1.58–3.31) | <0.0001 | 1.95 (1.31–2.91) | 0.001 |
| All-cause death | 10 (6.7) | 30 (3.0) | 2.26 (1.10–4.62) | 0.03 | 1.91 (0.88–4.13) | 0.10 |
| Any MI | 17 (11.3) | 30 (3.0) | 3.90 (2.15–7.07) | <0.0001 | 3.38 (1.75–6.52) | 0.0003 |
| Periprocedural MI | 9 (5.8) | 14 (1.4) | 4.21 (1.82–9.73) | 0.001 | 3.79 (1.42–10.1) | 0.008 |
| Non-procedural MI | 8 (5.5) | 16 (1.6) | 3.43 (1.47–8.02) | 0.004 | 2.86 (1.14–7.15) | 0.02 |
| Any revascularization | 18 (12.3) | 68 (6.9) | 1.84 (1.09–3.09) | 0.02 | 1.54 (0.89–2.69) | 0.12 |
| Target vessel revascularization | 9 (6.6) | 41 (4.3) | 1.53 (0.74–3.14) | 0.25 | 1.25 (0.58–2.65) | 0.57 |
| Stroke | 2 (1.4) | 16 (1.7) | 0.86 (0.20–3.72) | 0.84 | 0.79 (0.17–3.64) | 0.76 |
| Definite or probable stent thrombosis | 4 (2.7) | 10 (1.0) | 2.70 (0.85–8.61) | 0.09 | 1.58 (0.43–5.86) | 0.49 |
Event rates are n (Kaplan–Meier estimated %). The primary endpoint was the 3-year rate of cardiovascular death.
BOCE, bifurcation-oriented composite endpoint; CV, cardiovascular; MI, myocardial infarction; POCE, patient-oriented composite endpoint; other abbreviations as in Tables 1 and 2.
No residual ischemia group as reference.
The included covariates in the multivariable-adjusted model were age, sex, body mass index, diabetes, CKD, family history of CAD, previous MI, clinical presentation, LVEF, multi-vessel disease, SYNTAX score, total stent length in LM, type of DES, post-PCI diameter stenosis in LCX, post-PCI diameter stenosis in LM-LAD.
Bifurcation-oriented composite endpoint (BOCE), defined as the composite of cardiovascular death, target bifurcation-related MI, or target bifurcation revascularization.
Patient-oriented composite endpoint (POCE), defined as the composite of all-cause death, any MI, or any revascularization.
Association of continuous post-percutaneous coronary intervention Murray law-based quantitative flow ratio with clinical outcomes
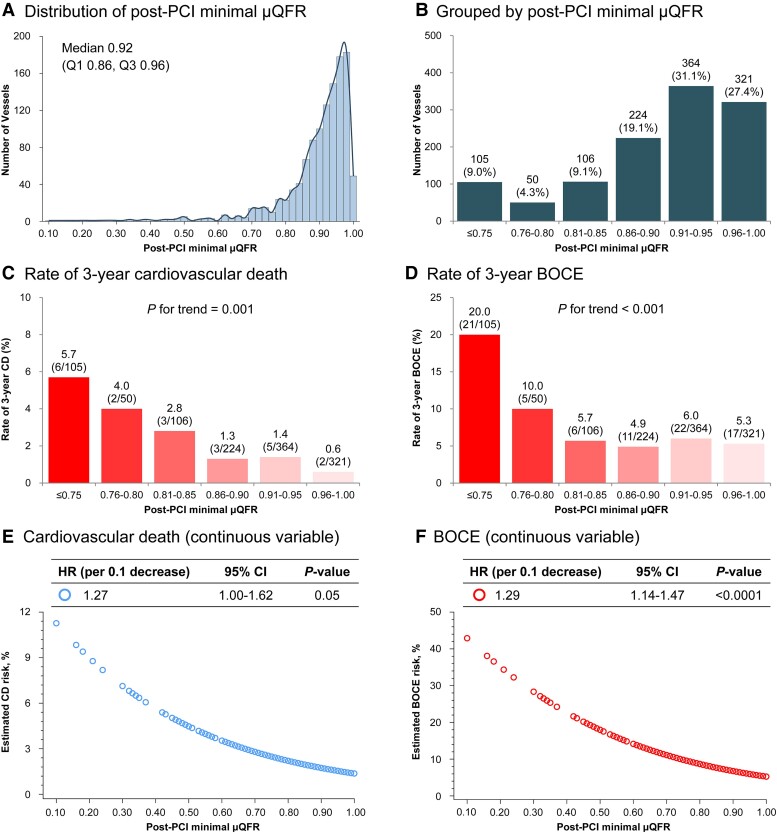
The distributions of continuous post-PCI LAD μQFR, LCX μQFR, minimal μQFR, and LM-global μQFR are shown in Figure 4 and Supplementary material online, Figures S7–S9; the median (IQR) were 0.97 (0.94–0.99), 0.94 (0.88–0.97), 0.92 (0.86–0.96), and 1.89 (1.81–1.94), respectively. There were increasing trends in 3-year rates of cardiovascular death and BOCE with decreasing post-PCI μQFR values. Clinical outcomes were inversely and continuously associated with post-PCI μQFR. Per 0.1 decrease in post-PCI μQFR value, the risk of cardiovascular death increased for LAD μQFR (HR 1.21, 95% CI: 0.79–1.86), LCX μQFR (HR 1.32, 95% CI: 1.04–1.68), minimal μQFR (HR 1.27, 95% CI: 1.00–1.62), and LM-global μQFR (HR 1.28, 95% CI: 1.04–1.58), with similar results observed for BOCE.
Figure 4.
Distribution and clinical outcomes by patient-level post-percutaneous coronary intervention minimal Murray law-based quantitative flow ratioa. aDefined as the lower post-percutaneous coronary intervention Murray law-based quantitative flow ratio value in left anterior descending and left circumflex artery. (A and B) Distribution of post-percutaneous coronary intervention minimal Murray law-based quantitative flow ratio; (C and D) Rates of 3-year adverse events in each 0.05 post-percutaneous coronary intervention minimal Murray law-based quantitative flow ratio unit; (E and F) Estimated risks of 3-year adverse events according to continuous post-percutaneous coronary intervention minimal Murray law-based quantitative flow ratio. Abbreviations as in Figure 1.
Physiology vs. anatomy
Continuous post-PCI μQFR ranged from 0.10 to 1.00 (0.89 ± 0.11) and DS from 0.1% to 79.1% (23.0 ± 14.6%). There was a weak correlation between post-PCI μQFR and %DS (r = −0.19, 95% CI: −0.25 to −0.14, P < 0.0001). The rate of adverse cardiac events was significantly higher in patients with physiologically significant residual ischemia (μQFR ≤0.80), whereas the rates of cardiovascular death (2.9% vs. 1.8%, P = 0.53) and BOCE (6.1% vs. 7.4%, P = 0.64) were comparable in the groups with and without anatomically significant residual disease (DS ≥50%) (Figure 3). The post-PCI μQFR and angiographic %DS values for physiological or anatomical significance were mismatched in 177 patients (15.1%), including 130 (11.1%) presented with abnormal physiology (positive mismatch: μQFR ≤0.80 and DS <50%) and 47 (4.0%) with abnormal anatomy (negative mismatch: μQFR >0.80 and DS ≥50%), in whom the rate of adverse cardiac events were higher in abnormal physiology than abnormal anatomy (4.8% vs. 0 for cardiovascular death; 18.0% vs. 0 for BOCE) (see Supplementary material online, Figure S10). The area under the curve (AUC) of post-PCI μQFR and %DS value for predicting 3-year cardiovascular death was 0.68 (95% CI: 0.56–0.79) and 0.57 (95% CI: 0.43–0.71), respectively (see Supplementary material online, Figure S11).
Discussion
The major findings from the present study are as follows (Structured Graphical Abstract): (i) functionally unresolved ischemia as assessed by μQFR occurred in approximately 13% of patients; (ii) LVEF, true LM bifurcation, pre-PCI LCX RVD, anatomic SS, pre-PCI LCX μQFR, treatment strategy, post-PCI LCX %DS, and residual SYNTAX score were found to be independent predictors of post-PCI residual ischemia; (iii) patients with residual ischemia had significantly higher risk of 3-year cardiovascular death and BOCE, compared with no residual ischemia; (iv) integration of functionally significant residual ischemia assessed by post-PCI μQFR into the model with clinical factors showed significantly increased discrimination and reclassification ability for cardiovascular death; (v) continuous, inverse associations were observed between post-PCI μQFR and the risk of adverse cardiac events; and (vi) mismatch between post-PCI physiological and anatomical assessment for significant stenoses were identified over 15% of patients, in whom post-PCI μQFR-based physiological assessment showed superior prognostic value for 3-year clinical outcomes. Taken together, AI-powered μQFR was a feasible and safe clinical strategy that identified residual ischemia in a large proportion of patients undergoing angiographically successful LM bifurcation PCI. Post-PCI physiological assessment by μQFR could help operators understand the residual ischemia of LM bifurcation disease, delineate branch involvement and clinical relevance, and save the patient unnecessary additional intervention after LM bifurcation PCI.
Coronary physiology, especially post-PCI physiology assessment, has been validated as an efficient index to allow precise identification of the vessel accounting for residual ischemia, which could enable operators to perform accurate PCI optimization.7 In this regard, post-procedural physiology can define functional and clinical relevance of LM bifurcation lesions and is expected to further improve prognosis.6 However, wire-based physiology is underused among patients with LM bifurcation lesions due to various reasons (e.g. increased costs, prolonged operation time, and possible complications)10 as well as technical challenges in measurement of a jailed side branch after stenting the main vessel.9 Previous studies reported almost 10% of failure when physicians tried to pass pressure wires through stent struts into side branch.9 The μQFR is a novel computational method that enables accurate estimation of FFR based on analysis of a single angiographic view, adjusting both the RVD and the outgoing flow through side branches according to fractal geometry which has been validated as a more appropriate diagnostic approach for bifurcation lesions.11–13 This simple-to-use μQFR would be a reliable alternative in patients with LM bifurcation; however, no prior studies have assessed the proportion of patients undergoing LM bifurcation PCI presenting with residual ischemia detected by μQFR and its association with long-term prognosis.
In this post hoc, blinded, in silico analysis from a prospective cohort of patients with LM bifurcation disease who received PCI, we found that ∼13% of patients had residual ischemia, ∼11% in the LCX territory, and 3% in the LAD area. As recognized, careful attention was paid upon the intervention effect on the main vessel (LM–LAD) because of its greater length and larger myocardium at risk resulting in lower rate of residual LAD ischemia (similar to that in general coronary vessels), while the proportion of residual LCX ischemia was significantly higher in line with a previous study using wire-based physiology (rate of residual LCX ischemia: 16.9%).8 Notably, the relative risks for 3-year adverse cardiac events with residual ischemia compared with no residual ischemia were consistent regardless of the ischemic territory (no significant vessel interaction). A previous study showed that downstream epicardial disease does affect the functional assessment of intermediate LM CAD, but the effect is small and clinically irrelevant, unless the downstream disease is severe.19 In the present study, the relative risks for long-term adverse prognosis in residual ischemia group were consistent after excluding patients with post-PCI angiographically significant stenoses in the non-ostial (downstream) LAD or LCX. Moreover, several baseline and procedural characteristics were identified as independent predictors of residual ischemia, suggesting that active side branch treatment strategy (e.g. elective two-stent strategy) should be considered for patients with true LM bifurcation and poor pre-PCI LCX physiology even for side branches with smaller anatomical importance (e.g. smaller RVD), to achieve an acceptable post-procedural physiological result and improved clinical prognosis.
Compared with other studies mainly including patients with non-LM bifurcation lesions,20 the post-PCI physiological results in this study were slightly higher, especially in LAD. The main reasons are as follows: (i) 32% (373/1170) of patients presented with pre-PCI non-ostial lesions in LAD; in other words, 68% of patients did not actually have non-ostial lesions in LAD before PCI; (ii) the main vessel, usually the LM-LAD, is more likely to be fully treated and stented for LM bifurcation lesions owing to significant myocardial volume (>50% left ventricular myocardial mass) supplied by the LAD;21 and (iii) the post-PCI assessment based on computational physiological indices (e.g. QFR) was insensitive to small intra-stent pressure gradients, which may explain higher QFR values and lower in-stent correlation compared with paired FFR assessments.22
Based on offline QCA analysis by an independent core laboratory, nearly 6% of patients presented with anatomically significant residual disease (DS ≥50%) after LM bifurcation stenting, yet post-PCI anatomical significance was not associated with higher relative risks for 3-year adverse cardiac events. Mismatch between post-PCI physiology (μQFR) and anatomy (%DS) assessments was identified in over 15% of patients in whom abnormal physiology (μQFR ≤0.80; DS <50%) rather than abnormal anatomy (μQFR >0.80; DS ≥50%) was related to long-term adverse prognosis, suggesting that the outcomes of post-PCI LM bifurcation lesions are more likely determined by its hemodynamic significance rather than its angiographic appearance. Therefore, the observed impact of physiologically significant residual ischemia on long-term outcomes, especially mortality, strongly suggests that post-PCI physiology-based assessment should be performed when treating LM bifurcation lesions in daily practice, even when PCI appears anatomically successful.
Study limitations
First, the original study protocol was not designed to facilitate μQFR analysis, resulting in exclusion of about 6% of patients due to failure of the angiographic images to meet analysis requirements. Of note, success rates of QFR analyses are much larger with single-view μQFR compared to QFR based on two projections. Second, as this was a post hoc analysis from a single-center prospective cohort between 2014 and 2016 (more than 6 years ago), the observed associations could theoretically have been ameliorated by recent advances in device technology, PCI bifurcation technique, and adjunctive pharmacotherapy. Indeed, the application of optimization techniques [e.g. proximal optimization technique (POT) and FKBI] were relatively infrequent in this study. The extent to which the present results may have differed had a greater reliance on these techniques been used is uncertain. Although these data were obtained in a high-volume single center with extensive LM CAD PCI operator experience,16,17,23 we found that post-procedural coronary physiology assessment by μQFR potentially paved the way for further optimization of LM bifurcation PCI at large, using the generalizable angiography-based μQFR computational approach. The observed link between physiological results and long-term events emphasizes the necessity of “functional optimization of coronary intervention” before leaving catheterization laboratory. Third, some baseline characteristics between study subgroups were imbalanced. While the difference in outcomes favoring the no ischemia group persisted after multivariable adjustment for most of these variables, the impact of unmeasured confounders cannot be discounted. Fourth, this is a proof-of-concept study that retrospectively analyzed the Fuwai LM PCI patient cohort; therefore, the effect of further optimization of PCI based on poor post-PCI physiology cannot be assessed from the present study. Future large-scale randomized controlled trials are needed to investigate the efficacy of μQFR-based physiology-guided incremental optimization strategy [if post-PCI μQFR is ≤0.80 in either LAD or LCX ≤0.80—stent optimization (e.g. POT and KBI) and/or further intervention (conversion of one-stent to two-stent strategy) to achieve final post-PCI μQFR values >0.80 as possible] compared with conventional angiography-guided strategy after angiographically successful LM bifurcation PCI. This prospective study is anticipated to recruit approximate 3000 subjects to detect relative risk reduction for the comparison between two groups. Fifth, the present study could not investigate the correlation between μQFR and wire-based physiological metrics (e.g. FFR) due to the lack of wire-based assessment in original cohort. Finally, the applicability of the μQFR approach needs to be verified in daily practice given the possible measurement differences between the catheterization laboratory and the angiographic core laboratory. Thus, these striking study findings remain hypothesis generating, and the possibility of play-of-chance observations from a post hoc analysis should be ruled out by future prospective research.
Conclusions
For LM bifurcation treated with PCI, residual ischemia assessed by μQFR was identified in 13.2% of patients and was associated with a significantly higher risk of 3-year cardiovascular death. The post-PCI μQFR demonstrated a continuous and inverse relationship between its numeric value and clinical events. Mismatch between post-PCI physiological and anatomical assessment for significant stenoses was identified in over 15% of patients, in whom post-PCI μQFR-based physiological assessment showed superior prognostic value for 3-year clinical outcomes. Toward the prospect of more physiologically “precise” PCI treatment, these results support the importance of post-PCI physiologic assessment and the role of post-PCI μQFR as a procedural quality metric after drug-eluting stent implantation in LM bifurcation disease.
Supplementary data
Supplementary data is available at European Heart Journal online.
Supplementary Material
Contributor Information
Hao-Yu Wang, Cardiometabolic Medicine Center, National Clinical Research Center for Cardiovascular Diseases, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, A 167 Beilishi Rd, Xicheng District, Beijing 100037, China; Department of Cardiology, National Clinical Research Center for Cardiovascular Diseases, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, A 167 Beilishi Rd, Xicheng District, Beijing 100037, China; State Key Laboratory of Cardiovascular Disease, A 167 Beilishi Rd, Xicheng District, Beijing 100037, China.
Rui Zhang, Cardiometabolic Medicine Center, National Clinical Research Center for Cardiovascular Diseases, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, A 167 Beilishi Rd, Xicheng District, Beijing 100037, China; Department of Cardiology, National Clinical Research Center for Cardiovascular Diseases, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, A 167 Beilishi Rd, Xicheng District, Beijing 100037, China; State Key Laboratory of Cardiovascular Disease, A 167 Beilishi Rd, Xicheng District, Beijing 100037, China.
Kefei Dou, Cardiometabolic Medicine Center, National Clinical Research Center for Cardiovascular Diseases, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, A 167 Beilishi Rd, Xicheng District, Beijing 100037, China; Department of Cardiology, National Clinical Research Center for Cardiovascular Diseases, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, A 167 Beilishi Rd, Xicheng District, Beijing 100037, China; State Key Laboratory of Cardiovascular Disease, A 167 Beilishi Rd, Xicheng District, Beijing 100037, China; National Clinical Research Center for Cardiovascular Diseases, Fuwai Hospital Chinese Academy of Medical Sciences, A 12 Langshan Rd, Nanshan District, Shenzhen 518057, China.
Yunfei Huang, Department of Cardiology, National Clinical Research Center for Cardiovascular Diseases, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, A 167 Beilishi Rd, Xicheng District, Beijing 100037, China.
Lihua Xie, Department of Cardiology, National Clinical Research Center for Cardiovascular Diseases, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, A 167 Beilishi Rd, Xicheng District, Beijing 100037, China.
Zheng Qiao, Cardiometabolic Medicine Center, National Clinical Research Center for Cardiovascular Diseases, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, A 167 Beilishi Rd, Xicheng District, Beijing 100037, China; Department of Cardiology, National Clinical Research Center for Cardiovascular Diseases, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, A 167 Beilishi Rd, Xicheng District, Beijing 100037, China; State Key Laboratory of Cardiovascular Disease, A 167 Beilishi Rd, Xicheng District, Beijing 100037, China.
Tongqiang Zou, Department of Cardiology, National Clinical Research Center for Cardiovascular Diseases, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, A 167 Beilishi Rd, Xicheng District, Beijing 100037, China.
Changdong Guan, Department of Cardiology, National Clinical Research Center for Cardiovascular Diseases, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, A 167 Beilishi Rd, Xicheng District, Beijing 100037, China.
Lei Song, Department of Cardiology, National Clinical Research Center for Cardiovascular Diseases, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, A 167 Beilishi Rd, Xicheng District, Beijing 100037, China.
Weixian Yang, Department of Cardiology, National Clinical Research Center for Cardiovascular Diseases, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, A 167 Beilishi Rd, Xicheng District, Beijing 100037, China.
Yongjian Wu, Department of Cardiology, National Clinical Research Center for Cardiovascular Diseases, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, A 167 Beilishi Rd, Xicheng District, Beijing 100037, China.
Shengxian Tu, Biomedical Instrument Institute, School of Biomedical Engineering, Shanghai Jiao Tong University, Shanghai, China.
William Wijns, The Lambe Institute for Translational Medicine and Curam, University of Galway, Galway, Ireland.
Bo Xu, Department of Cardiology, National Clinical Research Center for Cardiovascular Diseases, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, A 167 Beilishi Rd, Xicheng District, Beijing 100037, China; National Clinical Research Center for Cardiovascular Diseases, Fuwai Hospital Chinese Academy of Medical Sciences, A 12 Langshan Rd, Nanshan District, Shenzhen 518057, China.
Declarations
Data Availability
The data that supports the findings of this study are available from the corresponding author upon reasonable request. All such requests should be submitted to Prof. Bo Xu (Email: bxu@citmd.com) or Kefei Dou (Email: drdoukefei@126.com).
Funding
The study was supported by research grants from the Capital's Funds for Health Improvement and Research (CFH) (grant number 2022-2-4033), the National High Level Hospital Clinical Research Funding (grant number 2023-GSP-QN-34, 2023-GSP-RC-05, 2022-GSP-GG-20), and the CAMS Innovation Fund for Medical Sciences (CIFMS) (grant number 2021-I2M-1-008). Dr. Wijns is supported by Science Foundation Ireland Research Professorship (grant number RSF15/RP/2765).
Ethical Approval
The present study was approved by the Institutional Review Committee of Fuwai Hospital and performed in accordance with the Declaration of Helsinki. All patients provided written informed consent for use of their clinical data in clinical research.
Pre-registered Clinical Trial Number
None supplied.
References
- 1. Armstrong PW, Bates ER, Gaudino M. Left main coronary disease: evolving management concepts. Eur Heart J 2022;43:4635–4643. 10.1093/eurheartj/ehac542 [DOI] [PubMed] [Google Scholar]
- 2. Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J 2019;40:87–165. 10.1093/eurheartj/ehy394 [DOI] [PubMed] [Google Scholar]
- 3. Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: executive summary: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. J Am Coll Cardiol 2022;79:197–215. 10.1016/j.jacc.2021.09.005 [DOI] [PubMed] [Google Scholar]
- 4. Park S, Park SJ, Park DW. Percutaneous coronary intervention for left main coronary artery disease: present status and future perspectives. JACC Asia 2022;2:119–138. 10.1016/j.jacasi.2021.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sabatine MS, Bergmark BA, Murphy SA, O'Gara PT, Smith PK, Serruys PW, et al. Percutaneous coronary intervention with drug-eluting stents versus coronary artery bypass grafting in left main coronary artery disease: an individual patient data meta-analysis. Lancet 2021;398:2247–2257. 10.1016/s0140-6736(21)02334-5 [DOI] [PubMed] [Google Scholar]
- 6. Lee HS, Kim U, Yang S, Murasato Y, Louvard Y, Song YB, et al. Physiological approach for coronary artery bifurcation disease: position statement by Korean, Japanese, and European bifurcation clubs. JACC Cardiovasc Interv 2022;15:1297–1309. 10.1016/j.jcin.2022.05.002 [DOI] [PubMed] [Google Scholar]
- 7. Ding D, Huang J, Westra J, Cohen DJ, Chen Y, Andersen BK, et al. Immediate post-procedural functional assessment of percutaneous coronary intervention: current evidence and future directions. Eur Heart J 2021;42:2695–2707. 10.1093/eurheartj/ehab186 [DOI] [PubMed] [Google Scholar]
- 8. Lee CH, Choi SW, Hwang J, Kim IC, Cho YK, Park HS, et al. 5-year outcomes according to FFR of left circumflex coronary artery after left main crossover stenting. JACC Cardiovasc Interv 2019;12:847–855. 10.1016/j.jcin.2019.02.037 [DOI] [PubMed] [Google Scholar]
- 9. Chen SL, Ye F, Zhang JJ, Xu T, Tian NL, Liu ZZ, et al. Randomized comparison of FFR-guided and angiography-guided provisional stenting of true coronary bifurcation lesions: the DKCRUSH-VI trial (Double Kissing Crush Versus Provisional Stenting Technique for Treatment of Coronary Bifurcation Lesions VI). JACC Cardiovasc Interv 2015;8:536–546. 10.1016/j.jcin.2014.12.221 [DOI] [PubMed] [Google Scholar]
- 10. Kogame N, Ono M, Kawashima H, Tomaniak M, Hara H, Leipsic J, et al. The impact of coronary physiology on contemporary clinical decision making. JACC Cardiovasc Interv 2020;13:1617–1638. 10.1016/j.jcin.2020.04.040 [DOI] [PubMed] [Google Scholar]
- 11. Tu S, Ding D, Chang Y, Li C, Wijns W, Xu B. Diagnostic accuracy of quantitative flow ratio for assessment of coronary stenosis significance from a single angiographic view: a novel method based on bifurcation fractal law. Catheter Cardiovasc Interv 2021;97:1040–1047. 10.1002/ccd.29592 [DOI] [PubMed] [Google Scholar]
- 12. Ding DX, Tu SX, Chang YX, Li CM, Xu B, Wijns W. Quantitative flow ratio based on Murray fractal law: accuracy of single versus two angiographic views. J Soc Cardiovasc Angiogr Interv 2022;1:100399. 10.1016/j.jscai.2022.100399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang J, Li C, Ding D, Zhang M, Wu Y, Xu R, et al. Functional comparison of different jailed balloon techniques in treating non-left main coronary bifurcation lesions. Int J Cardiol 2022;364:20–26. 10.1016/j.ijcard.2022.05.036 [DOI] [PubMed] [Google Scholar]
- 14. Xu B, Tu S, Qiao S, Qu X, Chen Y, Yang J, et al. Diagnostic accuracy of angiography-based quantitative flow ratio measurements for online assessment of coronary stenosis. J Am Coll Cardiol 2017;70:3077–3087. 10.1016/j.jacc.2017.10.035 [DOI] [PubMed] [Google Scholar]
- 15. Xu B, Tu S, Song L, Jin Z, Yu B, Fu G, et al. Angiographic quantitative flow ratio-guided coronary intervention (FAVOR III China): a multicentre, randomised, sham-controlled trial. Lancet 2021;398:2149–2159. 10.1016/s0140-6736(21)02248-0 [DOI] [PubMed] [Google Scholar]
- 16. Wang HY, Xu B, Dou K, Guan C, Song L, Huang Y, et al. Implications of periprocedural myocardial biomarker elevations and commonly used MI definitions after left main PCI. JACC Cardiovasc Interv 2021;14:1623–1634. 10.1016/j.jcin.2021.05.006 [DOI] [PubMed] [Google Scholar]
- 17. Wang HY, Dou KF, Guan C, Xie L, Huang Y, Zhang R, et al. New insights into long- versus short-term dual antiplatelet therapy duration in patients after stenting for left main coronary artery disease: findings from a prospective observational study. Circ Cardiovasc Interv 2022;15:e011536. 10.1161/circinterventions.121.011536 [DOI] [PubMed] [Google Scholar]
- 18. Lunardi M, Louvard Y, Lefèvre T, Stankovic G, Burzotta F, Kassab GS, et al. Definitions and standardized endpoints for treatment of coronary bifurcations. J Am Coll Cardiol 2022;80:63–88. 10.1016/j.jacc.2022.04.024 [DOI] [PubMed] [Google Scholar]
- 19. Fearon WF, Yong AS, Lenders G, Toth GG, Dao C, Daniels DV, et al. The impact of downstream coronary stenosis on fractional flow reserve assessment of intermediate left main coronary artery disease: human validation. JACC Cardiovasc Interv 2015;8:398–403. 10.1016/j.jcin.2014.09.027 [DOI] [PubMed] [Google Scholar]
- 20. Hwang D, Koo BK, Zhang J, Park J, Yang S, Kim M, et al. Prognostic implications of fractional flow reserve after coronary stenting: a systematic review and meta-analysis. JAMA Netw Open 2022;5:e2232842. 10.1001/jamanetworkopen.2022.32842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim HY, Lim HS, Doh JH, Nam CW, Shin ES, Koo BK, et al. Physiological severity of coronary artery stenosis depends on the amount of myocardial mass subtended by the coronary artery. JACC Cardiovasc Interv 2016;9:1548–1560. 10.1016/j.jcin.2016.04.008 [DOI] [PubMed] [Google Scholar]
- 22. Emori H, Kubo T, Shiono Y, Ino Y, Shimamura K, Terada K, et al. Comparison of optical flow ratio and fractional flow ratio in stent-treated arteries immediately after percutaneous coronary intervention. Circ J 2020;84:2253–2258. 10.1253/circj.CJ-20-0661 [DOI] [PubMed] [Google Scholar]
- 23. Xu B, Redfors B, Yang Y, Qiao S, Wu Y, Chen J, et al. Impact of operator experience and volume on outcomes after left main coronary artery percutaneous coronary intervention. JACC Cardiovasc Interv 2016;9:2086–2093. 10.1016/j.jcin.2016.08.011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that supports the findings of this study are available from the corresponding author upon reasonable request. All such requests should be submitted to Prof. Bo Xu (Email: bxu@citmd.com) or Kefei Dou (Email: drdoukefei@126.com).