Abstract
Background
Endoscopic mucosal resection is a frequently employed method for removing colonic polyps. Nonetheless, the recurrence of these polyps over a healed submucosal base can complicate the extraction of leftover lesions through standard procedures. EndoRotor®, a non-thermal device specifically designed for endoscopic mucosal resection, has recently been assessed for its utility in removing colonic polyps, non-dysplastic Barrett’s esophagus, and pancreatic necrosis. We conducted a systematic review and meta-analysis to ascertain the safety and efficacy of EndoRotor® in resecting scared or recurrence colonic polyps.
Methods
We conducted an exhaustive review of existing literature using databases such as Medline, Embase, Web of Science, and the Cochrane Library until January 2023. Our aim was to find all studies that assessed the safety of non-thermal endoscopic resection devices in removing colonic polyps. The primary outcome we focused on was the rate of technical success. Secondary outcomes that we considered included the frequency of remaining lesions and instances of adverse events. To analyze these data, we used comprehensive meta-analysis software.
Results
Our analysis incorporated three studies comprising 54 patients who underwent resection of 60 lesions. The combined technical success rate was 93.9% (95% confidence interval (CI): 77.7-98.6%, I2 = 25.5%). In patients who had another endoscopic examination, 20 were found to have a residual lesion. After the initial session, the combined rate of remaining lesions was 39.8% (95% CI: 15.3-70.8%, I2 = 74.5%). There were eight occurrences of intraoperative bleeding and four instances of bleeding post-procedure. The combined rate of intraoperative bleeding was 13.2% (95% CI: 6.7-24.3%, I2 = 0%), and post-procedure bleeding stood at 8.5% (95% CI: 3.4-19.8%, I2 = 0%). Only one major bleeding event was recorded, and no cases of perforation were reported.
Conclusion
Our research indicates that the EndoRotor® effectively removes scarred colonic polyps, though the rate of remaining lesions is significant, potentially necessitating several sessions for a thorough removal. There is a need for broader prospective studies, mainly randomized controlled trials, to further assess EndoRotor®’s efficiency and safety in eliminating colonic polyps.
Keywords: Colonic polyps, Efficiency, Safety, Endoscopic mucosal resection, Meta-analysis
Introduction
Colonoscopy with polypectomy is a critical procedure in preventing colorectal cancers (CRC), and its widespread application has led to a decrease in CRC cases in the United States [1, 2]. Endoscopic mucosal resection (EMR), a frequently used technique, is particularly effective in removing large colonic polyps, especially non-pedunculated lesions that are 20 mm or more significant [3, 4].
The Powered Non-Thermal Endoscopic Resection Device, designed for the removal of colonic polyps, offers an innovative approach to polypectomy without the application of heat. The primary indications for its use include the resection of benign, non-invasive colonic polyps that may pose a risk for malignancy over time. This device is particularly beneficial in situations where traditional thermal-based resection might present complications, such as in proximity to delicate structures or when there is an increased risk of thermal injury. By employing mechanical means of resection rather than heat, the device can minimize potential collateral tissue damage, leading to faster recovery times and reduced complications. The integration of this device into endoscopic practice aims to ensure safer and more efficient polyp removal [4].
The most common method for carrying out an EMR involves injecting a substance into the submucosa to create a “lift”, along with a dye to outline the lesion’s borders [5]. Compared to surgical resection, endoscopic resection of colorectal polyps, even large ones, is often favored due to its association with reduced morbidity, mortality, and cost. Recurrence rates are fairly low, at about 14% [6, 7]. However, the recurrence of local polyps over a healed submucosal base can complicate the removal of leftover lesions with standard EMR techniques. The removal of non-lifting fibrotic lesions also presents challenges when using traditional snares [8].
The EndoRotor® device emerged as a significant innovation in the realm of endoscopic resections. Originating in the late 2010s, this non-thermal tool was introduced to the medical community as a safer alternative to traditional thermal-based resections. Its debut in the medical field marked a substantial shift in approach to polypectomies, offering practitioners a novel method that reduced potential complications and improved efficacy in colonic polyp removal [6, 7, 9].
The EndoRotor® device, developed by Interscope Medical, Inc., based in Worcester, Massachusetts, United States, is a non-thermal EMR tool designed for removing diseased tissue within the gastrointestinal tract. The device works by drawing tissue into a catheter that contains an internal cannula with a rotating blade. This blade resects the tissue, which is then suctioned into a collection trap for easy retrieval and subsequent pathological examination [9]. This combined suction and rotating blade functionality might enable the resection of non-pedunculated colonic lesions without necessitating submucosal lift or cauterization. It has been recently assessed for treating non-dysplastic Barrett’s esophagus and pancreatic necrosis and has been suggested as a potential alternative to EMR and snare-based resections for colonic polyps. However, the existing literature on its efficacy and outcomes is limited. Hence, we conducted a systematic review and meta-analysis to assess the safety and effectiveness of EndoRotor® in resecting colonic polyps.
Materials and Methods
An in-depth search strategy employing truncated keywords and phrases was executed in Embase by a seasoned health sciences librarian to identify studies related to EndoRotor®. This approach was then applied to other databases, including MEDLINE (via the PubMed platform, NCBI), Cochrane Central Register of Controlled Trials (Wiley) [10], Web of Science Core Collection (through the Web of Science platform, Clarivate), and Google Scholar (utilizing Harzing’s Publish or Perish software), with all searches conducted in January 2023 (Supplementary Material 1, www.gastrores.org). Neither publication dates nor language constraints were applied. All findings were transferred to EndNote 20 citation management software (Clarivate, Philadelphia, PA, USA). Duplicates were eliminated through successive runs of EndNote’s duplicate detection algorithms, complemented by a manual review (Supplementary Material 1, www.gastrores.org). Our meta-analysis was conducted in accordance with the PRISMA guidelines [11].
Inclusion/exclusion criteria
The criteria we established for including studies were as follows: 1) studies involving patients who underwent non-thermal endoscopic resection for the extraction of colonic polyps either scared from previous resection or recurrent polyp, and 2) studies that documented clinical results and any adverse events associated with the EndoRotor® procedure performed on patients with colonic polyps.
Screening and data collection
The studies were screened by two independent reviewers (ZA and NM). The initial screening was based on titles and abstracts, with the full-text screening of relevant publications following. Next, two independent reviewers extracted the data (ZA and NM). Discrepancy in study selection and data extraction was resolved through mutual discussion. Finally, data on study characteristics, demographics (age and male sex), and outcomes of interest were collected and summarized using Microsoft Excel (Microsoft, Redmond, Washington, USA).
Data synthesis and statistical analysis
All statistical analyses were conducted using the comprehensive meta-analysis software. To allow for heterogeneity, a random-effects model was used as a priori to pool and compare outcomes. Odds ratios (ORs) with 95% confidence intervals (CIs) and P-values were determined for dichotomous outcomes. An I2 test was used to evaluate the heterogeneity of the studies, and an I2 of > 50% represented significant heterogeneity.
Bias assessment
The risk of bias within each study was determined using the Methodological Index for Non-Randomized Studies (MINORS) scale [12] (Table 1 [13-15]).
Table 1. Quality Assessment of Studies via MINORS Scale.
| Study | Clearly stated aim | Inclusion of consecutive patients | Prospective collection of data | Endpoints appropriate to the aim of the study | Unbiased assessment of the study endpoint | Follow-up period appropriate to the aim of the study | Loss to follow-up less than 5% | Prospective calculation of the study size | An adequate control group | Contemporary groups | Baseline equivalence of groups | Adequate statistical analyses | Total score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kandiah et al (2019) [15] | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 2 | 14 |
| Kaul et al (2021) [14] | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 2 | 2 | 1 | 2 | 19 |
| Knabe et al (2019) [13] | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | 2 | 2 | 0 | 1 | 15 |
Quality assessment
MINORS was also used to assess the quality of the studies. Non-comparative studies were graded on eight MINORS criteria, with each item ranging from 0 to 2 (0 if not reported, 1 if reported but inadequate, and 2 if reported and adequate). A global score of 16 for non-comparative studies and 24 for comparative analyses are ideal. Two authors (ZA and NM) independently completed the quality assessment, and discrepancies were handled by a third reviewer (SFA).
Results
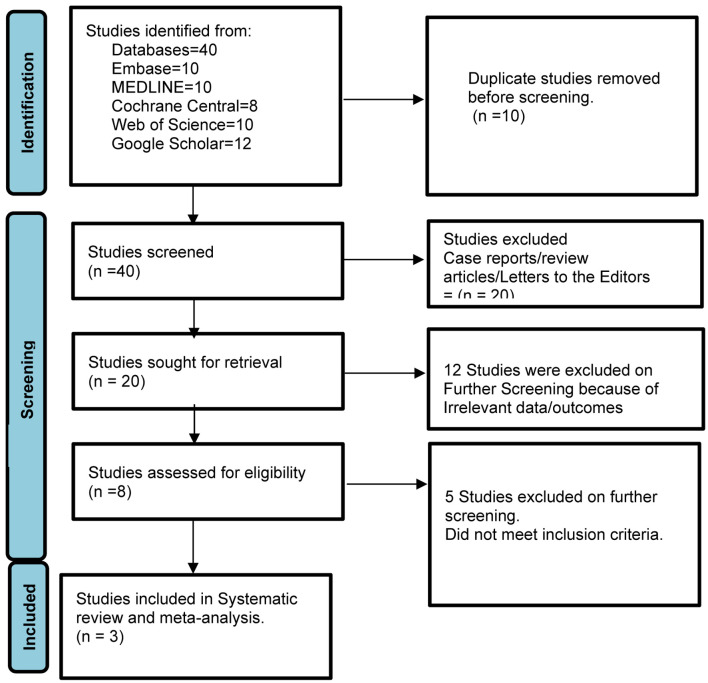
In our analysis, we included data from three studies [13-15], covering a total of 61 patients. These patients underwent the resection of 60 lesions, had a mean age of 70, and 45% of them were male (Table 2 [13-15]). Figure 1 elaborates the systematic literature search of our study.
Table 2. Study Characteristics.
| Study | Study design | Total number | Age, mean (years) | Male sex, % | Technical success rate | Rate of residual lesions |
|---|---|---|---|---|---|---|
| Kandiah et al (2019) [15] | Prospective | 19 | 71 | 58% | 84% | 47.4% |
| Kaul et al (2021) [14] | Retrospective | 28 | 68 | 50% | 100% | 11.8% |
| Knabe et al (2019) [13] | Prospective | 14 | NR | 21% | 83% | 64% |
NR: not reported.
Figure 1.
Prisma flow diagram of literature review process.
Technical success
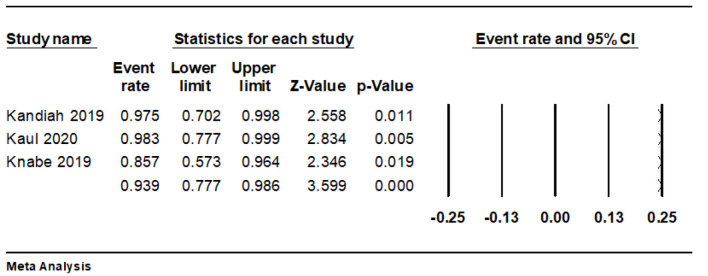
The pooled technical success rate was 93.9% (95% CI: 77.7-98.6%, I2 = 25.5%) (Fig. 2).
Figure 2.
Forest plot of technical success rate.
Adverse events
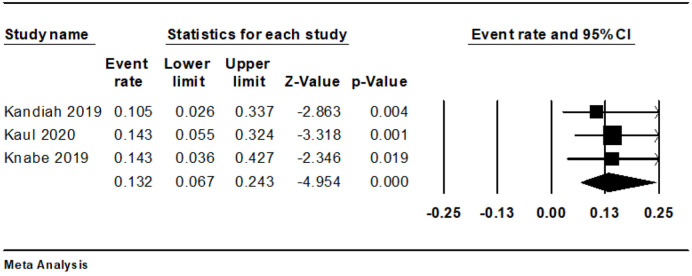
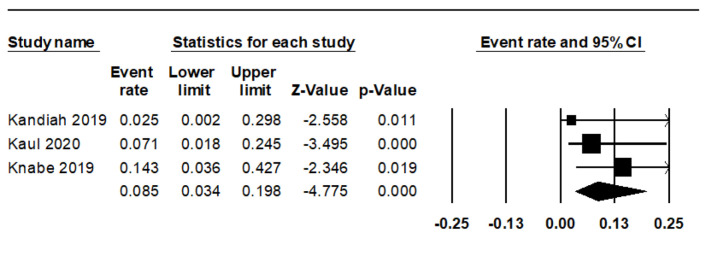
We observed eight cases of bleeding during and four instances after the procedure. The combined rate of intraoperative bleeding was 13.2% (95% CI: 6.7-24.3%, I2 = 0%) (Fig. 3), while the rate of post-procedure bleeding stood at 8.5% (95% CI: 3.4-19.8%, I2 = 0%) (Fig. 4). There was only a single instance of significant bleeding, and no cases of perforation were reported (Table 3 [13-15]).
Figure 3.
Forest plot of intraoperative bleeding.
Figure 4.
Forest plot of post-procedure bleeding.
Table 3. Adverse Outcomes.
Residual lesions
The combined rate of remaining lesions after the initial session was 39.8% (95% CI: 15.3-70.8%, I2 = 74.5%). Among patients who underwent a second endoscopic evaluation, 20 had a residual lesion. Kandiah et al reported that 10 out of 19 patients (52.6%) were successfully treated after one attempt, while six patients (31.5%) required two attempts for successful treatment. Of the three remaining, two were referred for endoscopic submucosal dissection and one for surgery.
Discussion
In our study, we assessed the safety and effectiveness of EndoRotor® in removing colonic polyps particularly scared. As far as we know, this is the first in-depth systematic review and meta-analysis carried out on this subject. Our findings indicate that EndoRotor® has a commendable success rate in removing colonic polyps. However, the rate of residual lesions is high in cases where recurring polyps appear over healed mucosal bases, potentially necessitating several removal sessions. Nevertheless, the rate of adverse events parallels those seen in traditional EMRs.
Existing literature generally reports high success rates for EMR, though these rates inversely correlate with the size of the polyp. A meta-analysis by Russo et al, which encompassed 49 studies, exhibited a complete endoscopic resection rate of 99.5% using EMR for laterally spreading colorectal tumors, generally referring to non-polypoid lesions that are 10 mm or more significant [4, 16]. In a retrospective study by Chaoui et al of 165 sessile and flat colorectal lesions ≥ 15 mm treated with EMR, the technical success rate was 95.2% [17]. Another meta-analysis by Hassan et al, including 50 studies and 6,442 patients, reported a success rate of 90.3% with EMR for the resection of large colonic polyps (≥ 20 mm) [7]. These rates are comparable to our study, which found that the pooled technical success rate with EndoRotor® was 93.9% (95% CI: 77.7-98.6%, I2 = 25.5%).
In the literature, polyp recurrence rates after traditional EMR are approximately 12.6-16.2% [7, 15, 16, 18]. Our pooled residual lesion rate after endoscopic intervention with EndoRotor® was calculated to be much higher at 39.8% (95% CI: 15.3-70.8%, I2 = 74.5%). Knabe et al and Kandiah et al included patients with recurrent polyps over a scarred base. On the other hand, in the study by Kaul et al, 25/28 patients (89%) had a history of prior resection, but it was not reported whether the resected lesions had developed scarred bases [13]. If not all the lesions were scarred, this could explain why the rate of residual lesions was 11.8% in the Kaul et al study, which is comparable to recurrence rates after EMR, and is much lower than the 64% rate in the Knabe et al study which included only scarred lesions [13, 14].
The most common complications of EMR are bleeding and, less frequently, perforation. Literature reports estimate bleeding rates to be around 3-10% and perforation rates between 0.7% and 1.5%. In our meta-analysis, the combined rate of intraoperative bleeding was 13.2% (95% CI: 6.7-24.3%, I2 = 0%), while post-procedural bleeding was 8.5% (95% CI: 3.4-19.8%, I2 = 0%). Interestingly, no perforations were reported. The higher rate of intraoperative bleeding in comparison to traditional EMR could be attributed to the patient population in our study, which primarily included individuals with scarred mucosal bases or a history of previous resection. These intervention areas may have been more susceptible to bleeding than areas untouched by previous interventions [19].
The strengths of this meta-analysis include the comprehensive and systematic literature search we carried out, including all studies that met the clearly defined inclusion criteria and the careful exclusion of redundant studies. However, a few limitations to our meta-analysis should be noted. Our analysis did not include direct comparative studies between the Endorotor-assisted removal and other established techniques such as conventional EMR and underwater EMR. Therefore, while we can provide insights into the potential efficacy and safety of the Endorotor technique based on the available data, we cannot assert its superiority or comparability to other prevalent techniques. The meta-analysis was based on pooling outcomes from three studies with small sample sizes. This poses challenges in terms of the generalizability of the findings, as larger sample sizes often offer more robust and reliable results. Given that the Endorotor technique is relatively novel, there is a paucity of studies available, which might mean that certain complications or outcomes specific to the procedure have not yet been thoroughly documented.
In our meta-analysis research study, delivering a subgroup analysis was not feasible due to the inclusion of a limited number of available published studies to date.
While our meta-analysis highlighted discrepancies in the outcomes presented by Kaul et al [14] compared to other studies, the available dataset did not provide specific details on patient selection, polyp characteristics, or procedural differences for this particular study. This lack of granularity prevents us from drawing definitive conclusions about the reasons for the observed variation in outcomes.
Lastly, the notable heterogeneity found in the pooled residual lesion rate (I2 = 74.5%) could be traced back to the inclusion criteria of two of the three studies in our meta-analysis, which focused solely on polyps with scarred bases, leading to variability.
EndoRotor® presents a promising instrument for the removal of recurrent polyps with healed bases, demonstrating considerable safety and high success rates. More research, particularly randomized controlled trials, is required to compare the rate of residual lesions following EndoRotor® application versus traditional EMR for the removal of recurrent polyps with scarred bases. It is also important to conduct additional studies comparing the effectiveness of EndoRotor® with traditional EMR for the initial removal of polyps that have not yet undergone any intervention. Further exploration is needed into whether EndoRotor® could provide enhanced visualization of tissue during resection, as polyps can appear in areas that are difficult to see or reach, such as the appendiceal orifice, ileocecal valve, dentate line, and areas of inflammation or colitis. Additionally, not all gastroenterologists currently have access to EndoRotor® in their practice, and it remains to be seen what obstacles might exist to the widespread adoption of EndoRotor® into regular clinical practice, possibly including cost concerns, training requirements, or technical maintenance issues.
In conclusion, EndoRotor®, as a non-thermal resection device, may provide a feasible alternative to current methods for recurrent colonic polyp removal. The device seems effective in removing scarred polyps, although direct comparisons with conventional EMR have not yet been published. The risk of adverse events linked to the EndoRotor® procedure appears to be comparatively low and similar to EMR. More extensive prospective studies, particularly randomized controlled trials, are needed to further clarify the role of EndoRotor® in the resection of colonic polyps.
Supplementary Material
Full Search Strategies (All Searched Performed in January 2023).
Acknowledgments
None to declare.
Funding Statement
This study required no funding.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
A meta-analysis does not require informed consent because it is a type of research that primarily analyzes and synthesizes already published data from multiple studies rather than directly involving human participants. Since individual patient data are generally anonymized and public, no new or additional information from patients is being collected or utilized. Hence, there is no direct interaction with patients and no potential for harm, making informed consent unnecessary.
Author Contributions
Zohaib Ahmed, Nooraldin Merza, Joyce Badal, and Daryl Ramai: manuscript writing, an overview of the previously published works on a topic. Joyce Badal, Als Alsadiq Al-Hillan, Tony Varughese, Umair Iqbal, and Syeda F. Arif: database extraction, results, and statistics. Wade Lee-Smith, Ali Nawras, Yaseen Alastal, Harshit S. Khara, Bradley D. Confer, David L. Diehl, and Douglas G. Adler: manuscript preparation and editing. Joyce Badal: data collection, manuscript preparation, and editing content. Ali Nawras, Yaseen Alastal, Harshit S. Khara, Bradley D. Confer, David L. Diehl, and Douglas G. Adler: drafted and approved the final version, manuscript review, and critical manuscript revision for the manuscript’s important intellectual content.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
- CI
confidence interval
- CRC
colorectal cancer
- EMR
endoscopic mucosal resection
- MINORS
Methodological Index for Non-Randomized Studies
References
- 1.Brenner H, Chang-Claude J, Seiler CM, Rickert A, Hoffmeister M. Protection from colorectal cancer after colonoscopy: a population-based, case-control study. Ann Intern Med. 2011;154(1):22–30. doi: 10.7326/0003-4819-154-1-201101040-00004. [DOI] [PubMed] [Google Scholar]
- 2.Zauber AG, Winawer SJ, O'Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, Shi W. et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366(8):687–696. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soetikno RM, Gotoda T, Nakanishi Y, Soehendra N. Endoscopic mucosal resection. Gastrointest Endosc. 2003;57(4):567–579. doi: 10.1067/mge.2003.130. [DOI] [PubMed] [Google Scholar]
- 4.Kaltenbach T, Anderson JC, Burke CA, Dominitz JA, Gupta S, Lieberman D, Robertson DJ. et al. Endoscopic removal of colorectal lesions-recommendations by the US multi-society task force on colorectal cancer. Gastrointest Endosc. 2020;91(3):486–519. doi: 10.1016/j.gie.2020.01.029. [DOI] [PubMed] [Google Scholar]
- 5.Yandrapu H, Desai M, Siddique S, Vennalganti P, Vennalaganti S, Parasa S, Rai T. et al. Normal saline solution versus other viscous solutions for submucosal injection during endoscopic mucosal resection: a systematic review and meta-analysis. Gastrointest Endosc. 2017;85(4):693–699. doi: 10.1016/j.gie.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Keswani RN, Law R, Ciolino JD, Lo AA, Gluskin AB, Bentrem DJ, Komanduri S. et al. Adverse events after surgery for nonmalignant colon polyps are common and associated with increased length of stay and costs. Gastrointest Endosc. 2016;84(2):296–303.e291. doi: 10.1016/j.gie.2016.01.048. [DOI] [PubMed] [Google Scholar]
- 7.Hassan C, Repici A, Sharma P, Correale L, Zullo A, Bretthauer M, Senore C. et al. Efficacy and safety of endoscopic resection of large colorectal polyps: a systematic review and meta-analysis. Gut. 2016;65(5):806–820. doi: 10.1136/gutjnl-2014-308481. [DOI] [PubMed] [Google Scholar]
- 8.Shichijo S, Takeuchi Y, Uedo N, Ishihara R. Management of local recurrence after endoscopic resection of neoplastic colonic polyps. World J Gastrointest Endosc. 2018;10(12):378–382. doi: 10.4253/wjge.v10.i12.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim HG, Thosani N, Banerjee S, Chen A, Friedland S. Effect of prior biopsy sampling, tattoo placement, and snare sampling on endoscopic resection of large nonpedunculated colorectal lesions. Gastrointest Endosc. 2015;81(1):204–213. doi: 10.1016/j.gie.2014.08.038. [DOI] [PubMed] [Google Scholar]
- 10. www.cochranelibrary.com .
- 11.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L. et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906. doi: 10.1016/j.ijsu.2021.105906. [DOI] [PubMed] [Google Scholar]
- 12.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 13.Knabe M. et al. Prospektive Multicenter Studie zur Evaluation der Sicherheit bei der Anwendung des Interscope EndoRotor® Resectionssystems im Colon. Zeitschrift fur Gastroenterologie. 2019;57(09):KV478. [Google Scholar]
- 14.Kaul V, Diehl D, Enslin S, Infantolino A, Tofani C, Bittner K, Tariq R. et al. Safety and efficacy of a novel powered endoscopic debridement tissue resection device for management of difficult colon and foregut lesions: first multicenter U.S. experience. Gastrointest Endosc. 2021;93(3):640–646. doi: 10.1016/j.gie.2020.06.068. [DOI] [PubMed] [Google Scholar]
- 15.Kandiah K, Subramaniam S, Chedgy F, Thayalasekaran S, Venetz D, Aepli P, Bhandari P. A novel non-thermal resection tool in endoscopic management of scarred polyps. Endosc Int Open. 2019;7(8):E974–E978. doi: 10.1055/a-0838-5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russo P, Barbeiro S, Awadie H, Libanio D, Dinis-Ribeiro M, Bourke M. Management of colorectal laterally spreading tumors: a systematic review and meta-analysis. Endosc Int Open. 2019;7(2):E239–E259. doi: 10.1055/a-0732-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaoui I, Demedts I, Roelandt P, Willekens H, Bisschops R. Endoscopic mucosal resection of colorectal polyps: results, adverse events and two-year outcome. Acta Gastroenterol Belg. 2022;85(1):47–55. doi: 10.51821/85.1.9207. [DOI] [PubMed] [Google Scholar]
- 18.Belderbos TD, Leenders M, Moons LM, Siersema PD. Local recurrence after endoscopic mucosal resection of nonpedunculated colorectal lesions: systematic review and meta-analysis. Endoscopy. 2014;46(5):388–402. doi: 10.1055/s-0034-1364970. [DOI] [PubMed] [Google Scholar]
- 19.Fujihara S, Mori H, Kobara H, Nishiyama N, Matsunaga T, Ayaki M, Yachida T. et al. Management of a large mucosal defect after duodenal endoscopic resection. World J Gastroenterol. 2016;22(29):6595–6609. doi: 10.3748/wjg.v22.i29.6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Full Search Strategies (All Searched Performed in January 2023).
Data Availability Statement
The authors declare that data supporting the findings of this study are available within the article.