Abstract
Mucormycosis is a devastating fungal infection that is usually seen in immunocompromised hosts. It is caused by fungi of the subphylum Mucoromycotina, order Mucorales, with most cases caused by Mucor, Rhizopus, or Rhizomucor species. It can involve any organ system and can disseminate in severe cases. Lately, there has been an increased number of reports for mucormycosis infection in immunocompetent patients. Gastrointestinal system involvement is rare compared to other organ systems but has been increasingly reported in the literature. Mucormycosis can affect any part of the gastrointestinal tract and lead to different presentations depending on the area of involvement. Due to the paucity of the condition, there has been no specific guidelines on how to treat gastrointestinal mucormycosis. In this review, we discuss the risk factors of gastrointestinal mucormycosis, clinical presentation, approach to diagnosis, and most recent treatment modalities for gastrointestinal mucormycosis.
Keywords: Risk factors, Gastrointestinal mucormycosis, Clinical presentation, Diagnosis, Treatment
Introduction
Mucormycosis is a life-threatening infection caused by fungi of the subphylum Mucoromycotina, order Mucorales, with most cases caused by Mucor, Rhizopus, or Rhizomucor species. It is most commonly reported in immunocompromised patients. However, there has been an increasing number of cases seen in immunocompetent hosts. Based on the organ system involved, it is further classified into pulmonary, rhinocerebral, cutaneous, gastrointestinal, central nervous system, and disseminated in that order [1]. Gastrointestinal mucormycosis is rare compared to other forms and reported incidence varies from 5% to 15% [2, 3]. In most instances, the diagnosis is made intraoperatively or postmortemly, making the accurate estimation of incidence challenging [4, 5].
Common risk factors for gastrointestinal mucormycosis in adults include diabetes, hemodialysis/peritoneal dialysis, malnutrition, alcohol use, corticosteroid therapy, and patients who have undergone organ transplantation (i.e., liver, renal) or surgery [4, 6, 7]. Another potential risk factor in immunocompetent patients is healthcare-associated mucormycosis infections secondary to intensive care unit (ICU) admission or intestinal digestive surgery [8]. Gastrointestinal mucormycosis can either be a primary infection or a secondary infection. Primary infection is related to the ingestion of contaminated food materials or use of contaminated instruments, while the secondary infection is through the spread of the fungi in disseminated forms [7]. Most gastrointestinal mucormycosis infections involve the stomach, intestine, and ileum with the presentation of gastrointestinal bleeding and abdominal pain with gastric ulcers [1]. A review of 176 cases including both immunocompetent and immunosuppressed patients showed that 64.1% of infections were presented in the intestine with 33% of cases in the stomach [4]. From the review, there appears to be a 2:1 incidence rate for the male to female from the 89 adult cases with age being around 47.7 ± 15.36 [4].
Clinical Presentation
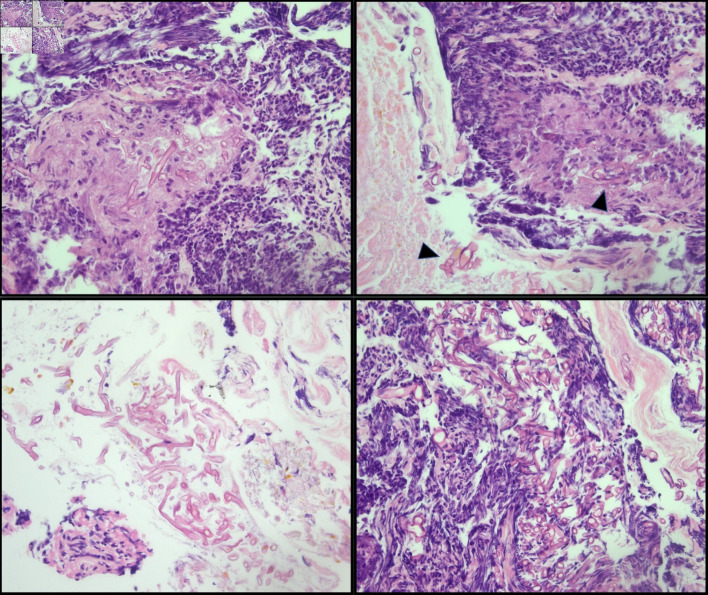
In the past, mucormycosis was usually reported in premature neonates but more recently there have been more reports of mucormycosis diagnosed in adult patients with known risk factors and rarely in immunocompetent patients [5]. Gastrointestinal mucormycosis can affect any part of the gastrointestinal tract with the stomach (57.5%) being the most commonly affected part followed by the colon (32.3%) (Fig. 1), small intestine (7%), and the esophagus (7%) [9]. However, a review of 31 cases of gastrointestinal mucormycosis in immunocompromised hosts showed an increasing incidence of mucormycosis in the intestine in the last two decades [10].
Figure 1.
Cecal biopsy. Large, non-septate fungal organisms consistent with Mucorales (Mucor/Rhizopus) in a background of ulceration and acute inflammation (arrowhead). The presence of fungal organisms in the ulcer bed is most likely indicative of infection.
Symptoms of gastrointestinal mucormycosis are nonspecific abdominal pain and distention, usually depending on the exact area affected of the gastrointestinal tract. Abdominal pain and gastrointestinal bleeding (either upper or lower) and fever are common presentations, especially in patients who have risk factors like immunosuppression [9]. For two of the three stomach mucormycosis case reports, there was evidence of hematemesis, which is one of the more common symptoms of an upper gastrointestinal bleed in these patients [11, 12]. In the other case, there were reports of acute onset of severe abdominal pain and progressive distension with failure to pass feces or flatulence [13]. With intestinal mucormycosis, all patients have been reported to experience either low- or high-grade fever with abdominal pain [6, 9, 14, 15]. There are reported cases where the patients were generally admitted due to other underlying causes and then found to have mucormycosis in the intestines [6]. The other case report illustrated incidental findings of colonic mucormycosis in a patient with high fever, shock, and hypoxic respiratory failure with generalized symptoms of shaking associated with Streptococcus infantarius endocarditis from colonic translocation [16]. Hepatic involvement has been reported in the literature mostly in association with the involvement of other organs; however, it can also happen as an isolated infection in immunosuppressed hosts, especially with the use of herbal and naturopathic products that are contaminated [17]. In one of the hepatic mucormycosis cases, there was associative small bowel mucormycosis [14]. Complications of intestinal mucormycosis include intestinal obstruction, bleeding, and perforation, which can be the presenting clinical feature as well.
Diagnosis
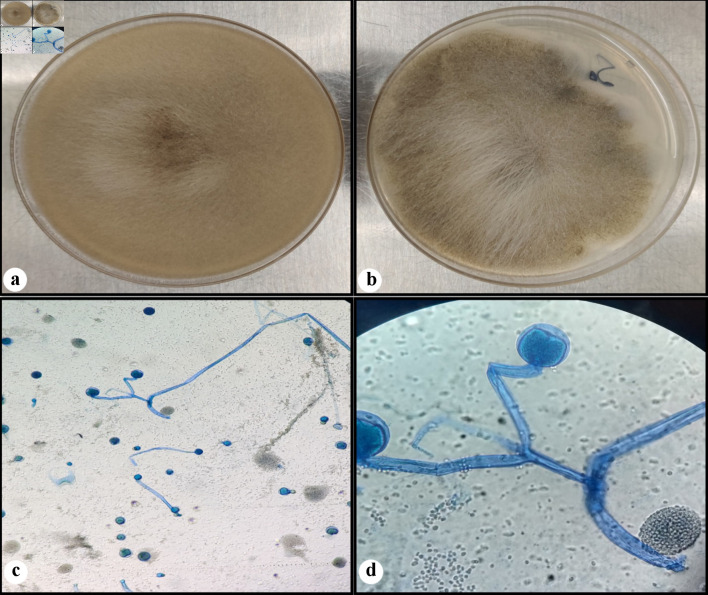
Diagnosis of gastrointestinal mucormycosis is usually delayed due to nonspecific presentation. Early endoscopic biopsy is key to diagnosis [18]. Endoscopic features include hemorrhagic and edematous changes of the mucosa which can mimic ischemic injuries, ulceration with sharply demarcated edges associated with necrosis, and thrombosis in the adjacent vasculature, and ulcers might be covered with black eschar [19]. Tissue diagnosis remains the gold standard currently and Mucorales polymerase chain reaction (PCR) of formalin-fixed paraffin-embedded tissue adds to the diagnostic yield [20]. In tissue, Mucorales are seen as broad (3 - 25 µm in diameter) thin-walled, aseptate, folded, twisted, or compressed hyphae with irregular branching [21] (Fig. 2). Mucormycosis lacks the cell wall components that can be detected on serum tests such as the 1,3-beta-D-glucan assay which make these tests not useful.
Figure 2.
Fungal culture. Rapidly growing white fluffy colony that overfills agar plate on the Sabouraud dextrose agar (SDA) (a), and almost completely filling the potato dextrose agar (PDA) (b). A lactophenol cotton blue (LCB) preparation (c, d) showing wide hyphae, rare septations, branched sporangiophores, with round sporangia (sac-like structure). (d) Seen in the background are some spores of ruptured sporangium.
Radiologic features of mucormycosis are also nonspecific. Diffuse bowel wall thickening with areas of intense and poor contrast enhancement on computed tomography (CT) scans which correlate with the congestive and necrotic changes caused by the fungal infection has been reported [22].
Treatment
In addition to addressing the underlying risk factors for mucormycosis such as long-term high-dose steroids, hyperglycemia, metabolic acidosis, deferoxamine, immunosuppressive drugs, and neutropenia, treatment of gastrointestinal infection requires a combination of surgical debridement of the involved tissues and antifungal therapy [23]. However, considering the adversity of infection and the risk of poor outcome, early empirical antifungal therapy should be considered based on compatible clinical syndrome and predisposing factors in an immunocompromised host. This is supported by a retrospective analysis of 70 patients with hematologic malignancy and concomitant mucormycosis, showing delay (> 6 days after diagnosis) inappropriate antifungal therapy was associated with an almost two-fold increase in mortality at 12 weeks after diagnosis [24]. The empirical antifungal therapy should be a polyene antifungal agent, as this drug class is the most effective agent against mucormycosis. In this regard, the intravenous lipid formulation of amphotericin B is preferred over amphotericin B deoxycholate due to less nephrotoxicity at a higher dose [23]. Since the disease is rare, there is no randomized trial to assess the efficacy of various antifungal regimens for gastrointestinal mucormycosis.
Once the diagnosis is confirmed, the treatment therapy is based on initial intravenous antifungal therapy followed by a step-down consolidation therapy. Lipid formulation of amphotericin B is the drug of choice and starting intravenous dose is 5 mg/kg/day. If tolerated, the dose can be increased to 10 mg/kg/day in order to control the infection. There are limited data regarding the use of combination therapy with echinocandins for improving clinical outcome based on concordant retrospective data in humans [23]. The duration of therapy depends upon the clinical course and disease burden. It is equally important that immunosuppressive medications, particularly corticosteroids, should be dose-reduced or stopped during the treatment therapy if possible [5].
Various treatment regimens are described in Table 1.
Table 1. Primary and Combination Antifungal Therapy for the Treatment of Systemic Mucormycosis Including Gastrointestinal Involvement.
| Primary antifungal therapy | Recommended dosage | Comments |
|---|---|---|
| Liposomal amphotericin B | 5 - 10 mg/kg/day | Expensive, less nephrotoxic, better CNS penetration than other formulations. |
| Amphotericin B deoxycholate | 1.0 - 1.5 mg/kg/day | Highly toxic, inexpensive, only licensed agent. |
| Amphotericin B lipid complex | 5 - 7.5 mg/kg/day | More nephrotoxic than liposomal amphotericin B, murine data suggest possible benefits in combination with echinocandins. |
CNS: central nervous system.
However, antifungal therapy alone is typically insufficient in controlling gastrointestinal disease, and debulking surgery is often required to decrease the disease burden and resect all infected tissue for an effective cure. Meanwhile, it is equally possible that the patients who underwent nonsurgical management likely differed in disease severity and burden of comorbidities from those who had surgical management, but surgery remains an independent variable for favorable outcomes in patients with mucormycosis [5]. Certain adjunctive treatment therapies including non-siderophore iron chelators, hyperbaric oxygen, or cytokine therapy with either γ-interferon or colony-stimulating factor have been under study, but no proven methods have been published to establish the effectiveness of these regimens as compared to standard treatment with surgery and intravenous amphotericin B and require randomized controlled trials to establish treatment benefits [25].
Prognosis
A case series of 66 reported cases of gastrointestinal mucormycosis revealed that 85% of the patients with the gastrointestinal involvement died. This high mortality rate was primarily due to bowel perforation. Low birth weight infants, patients with diarrhea and malnutrition, and patients receiving peritoneal dialysis were at high risk of gastrointestinal involvement of the infection. Meanwhile, systemic lupus erythematosus, immunosuppression/bone marrow transplant, malnutrition, and renal failure are the predictors of worse outcomes with the overall mortality of 89%, 91%, 88%, and 89%, respectively [3]. In one study, liposomal amphotericin B treatment was associated with a 67% survival rate (16 of 24 patients), compared with 39% survival (24 of 62 patients) with amphotericin B deoxycholate (P = 0.02) in cancer patients with mucormycosis [26].
Overall, a review of 929 cases of zygomycosis revealed that the survival rate was 61% for cases that were treated with amphotericin B deoxycholate, 57% for cases treated with surgery alone, and 70% for cases treated with antifungal therapy and surgery [3].
Acknowledgments
None to declare.
Funding Statement
None to declare.
Conflict of Interest
None to declare.
Author Contributions
YA, AN, MV, FA, RR, and SA: conceptualization, methodology, writing - original draft. SA, YA, MV, AN: data curation, and writing. YA, MV, FA, RR: supervision, and project administration.
Data Availability
The authors declare that data supporting the findings of this study are available within the article. Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
References
- 1.Guddati H, Andrade C, Muscarella P, Hertan H. An unusual cause of massive upper gastrointestinal bleeding-gastric mucormycosis. Oxf Med Case Reports. 2019;2019(2):omy135. doi: 10.1093/omcr/omy135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chakrabarti A, Singh R. Mucormycosis in India: unique features. Mycoses. 2014;57(Suppl 3):85–90. doi: 10.1111/myc.12243. [DOI] [PubMed] [Google Scholar]
- 3.Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, Sein M. et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41(5):634–653. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- 4.Kaur H, Ghosh A, Rudramurthy SM, Chakrabarti A. Gastrointestinal mucormycosis in apparently immunocompetent hosts-A review. Mycoses. 2018;61(12):898–908. doi: 10.1111/myc.12798. [DOI] [PubMed] [Google Scholar]
- 5.Spellberg B. Gastrointestinal mucormycosis: an evolving disease. Gastroenterol Hepatol (N Y) 2012;8(2):140–142. [PMC free article] [PubMed] [Google Scholar]
- 6.Antony SJ, Parikh MS, Ramirez R, Applebaum B, Friedman G, Do J. Gastrointestinal mucormycosis resulting in a catastrophic outcome in an immunocompetent patient. Infect Dis Rep. 2015;7(3):6031. doi: 10.4081/idr.2015.6031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shankaralingappa S. Unsuspected invasive gastrointestinal mucormycosis masquerading as inflammatory bowel disease: A pathologist's perspective. Indian J Pathol Microbiol. 2019;62(2):332–334. doi: 10.4103/IJPM.IJPM_240_18. [DOI] [PubMed] [Google Scholar]
- 8.Rammaert B, Lanternier F, Zahar JR, Dannaoui E, Bougnoux ME, Lecuit M, Lortholary O. Healthcare-associated mucormycosis. Clin Infect Dis. 2012;54(Suppl 1):S44–54. doi: 10.1093/cid/cir867. [DOI] [PubMed] [Google Scholar]
- 9.Ha TS, Park C-M, Yang JH. et al. Disseminated gastrointestinal mucormycosis in immunocompromised disease [Internet] Korean J Crit Care Med. 2015;30:323–328. [Google Scholar]
- 10.Dioverti MV, Cawcutt KA, Abidi M, Sohail MR, Walker RC, Osmon DR. Gastrointestinal mucormycosis in immunocompromised hosts. Mycoses. 2015;58(12):714–718. doi: 10.1111/myc.12419. [DOI] [PubMed] [Google Scholar]
- 11.Huang H, Xie L, Zheng Z, Yu H, Tu L, Cui C, Yu J. Mucormycosis-induced upper gastrointestinal ulcer perforation in immunocompetent patients: a report of two cases. BMC Gastroenterol. 2021;21(1):311. doi: 10.1186/s12876-021-01881-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanchez Velazquez P, Pera M, Gimeno J, Zapatero A, Nolla J, Pera M. Mucormycosis: an unusual cause of gastric perforation and severe bleeding in immunocompetent patients. Rev Esp Enferm Dig. 2017;109(3):223–225. doi: 10.17235/reed.2016.4269/2016. [DOI] [PubMed] [Google Scholar]
- 13.Hameed T, Jain SK, Ansari FM, Nizam A, Dua A. Spontaneous gastric necrosis: a rare presentation of invasive mucormycosis in an immunocompetent adult. Case Rep Infect Dis. 2020;2020:7514051. doi: 10.1155/2020/7514051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vikum D, Nordoy I, Torp Andersen C, Fevang B, Line PD, Kolrud FK, Aukrust P. et al. A Young, Immunocompetent Woman with Small Bowel and Hepatic Mucormycosis Successfully Treated with Aggressive Surgical Debridements and Antifungal Therapy. Case Rep Infect Dis. 2017;2017:4173246. doi: 10.1155/2017/4173246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wotiye AB, Ks P, Ayele BA. Invasive intestinal mucormycosis in a 40-year old immunocompetent patient - a rarely reported clinical phenomenon: a case report. BMC Gastroenterol. 2020;20(1):61. doi: 10.1186/s12876-020-01202-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah C, Zimmerman S, McKinney J, Ebers A. Colonic mucormycosis in an immunocompetent patient with endocarditis. IDCases. 2020;20:e00773. doi: 10.1016/j.idcr.2020.e00773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karigane D, Kikuchi T, Sakurai M, Kato J, Yamane Y, Hashida R, Abe R. et al. Invasive hepatic mucormycosis: A case report and review of the literature. J Infect Chemother. 2019;25(1):50–53. doi: 10.1016/j.jiac.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 18.Park YS, Lee JD, Kim TH, Joo YH, Lee JH, Lee TS, Kim EK. Gastric mucormycosis. Gastrointest Endosc. 2002;56(6):904–905. doi: 10.1067/mge.2002.128699. [DOI] [PubMed] [Google Scholar]
- 19.Sakorafas GH, Tsolakides G, Grigoriades K, Bakoyiannis CN, Peros G. Colonic mucormycosis: an exceptionally rare cause of massive lower gastrointestinal bleeding. Dig Liver Dis. 2006;38(8):616–617. doi: 10.1016/j.dld.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 20.Hammond SP, Bialek R, Milner DA, Petschnigg EM, Baden LR, Marty FM. Molecular methods to improve diagnosis and identification of mucormycosis. J Clin Microbiol. 2011;49(6):2151–2153. doi: 10.1128/JCM.00256-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.A colour atlas, textbook of the histopathology of mycotic disease [Internet]. Postgrad Med J. 1981;57:471–471. doi: 10.1136/pgmj.57.669.471. [DOI] [Google Scholar]
- 22.Rupani A, Shah V, Lad S. et al. Isolated gastrointestinal mucormycosis mimicking peptic ulcer disease [Internet] Internet J Gastroenterol. 2007;6(2):1. [Google Scholar]
- 23.Spellberg B, Walsh TJ, Kontoyiannis DP, Edwards J Jr, Ibrahim AS. Recent advances in the management of mucormycosis: from bench to bedside. Clin Infect Dis. 2009;48(12):1743–1751. doi: 10.1086/599105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chamilos G, Lewis RE, Kontoyiannis DP. Delaying amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin Infect Dis. 2008;47(4):503–509. doi: 10.1086/590004. [DOI] [PubMed] [Google Scholar]
- 25.Agha FP, Lee HH, Boland CR, Bradley SF. Mucormycoma of the colon: early diagnosis and successful management. AJR Am J Roentgenol. 1985;145(4):739–741. doi: 10.2214/ajr.145.4.739. [DOI] [PubMed] [Google Scholar]
- 26.Gleissner B, Schilling A, Anagnostopolous I, Siehl I, Thiel E. Improved outcome of zygomycosis in patients with hematological diseases? Leuk Lymphoma. 2004;45(7):1351–1360. doi: 10.1080/10428190310001653691. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors declare that data supporting the findings of this study are available within the article. Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.