The coronavirus disease-2019 (COVID-19) pandemic has had wide-ranging impacts on American healthcare delivery, with myriad resources being diverted to prevention in vulnerable populations.1 The immunocompromised, notably solid organ transplant recipients (SOTRs), remains susceptible to severe COVID-19 disease and death.2 Accordingly, SOTRs have been prioritized for COVID-19 vaccination and, more recently, booster immunization. Indeed, chronic immunosuppression may blunt vaccine efficacy due to its deleterious effect on humoral immunity, which is necessary for production of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) neutralizing antibodies.3 With the constant threat of emerging variants containing mutations that render vaccine-elicited antibodies ineffective, those with low titers to begin with remain especially vulnerable. Despite an abundance of clinical data on COVID-19 in the general population and among those with comorbid conditions, granular data on COVID-19 in heart transplant (HTx) recipients remain limited. Herein, we report the risk factors associated with COVID-19 mortality in HTx recipients.
The United Network for Organ Sharing (UNOS) database was queried for all HTx recipients greater than or equal to 18 years of age who expired between January 31, 2020, and January 6, 2022. January 31, 2020 represents the date of the Public Health Emergency declaration.4 Recipients with COVID-19 listed as a primary, secondary, or tertiary cause of death were considered to have COVID-19–related death; those without were considered controls.
Demographics of cases and controls were compared using Wilcoxon rank-sum and χ2 tests for continuous and categorical variables, respectively. Univariate odds ratios with 95% confidence intervals were calculated for 37 recipient characteristics to assess the relationship between potential risk factors and COVID-19–related death. Variables with significant univariate odds ratios or those of biological interest were included in a multivariable logistic regression model. Analyses were performed in Stata version 17 (College Station, TX). A two-tailed p-value ≤0.05 was considered statistically significant. Given the UNOS database is publicly available and deidentified, this study was deemed exempt from Institutional Review Board approval.
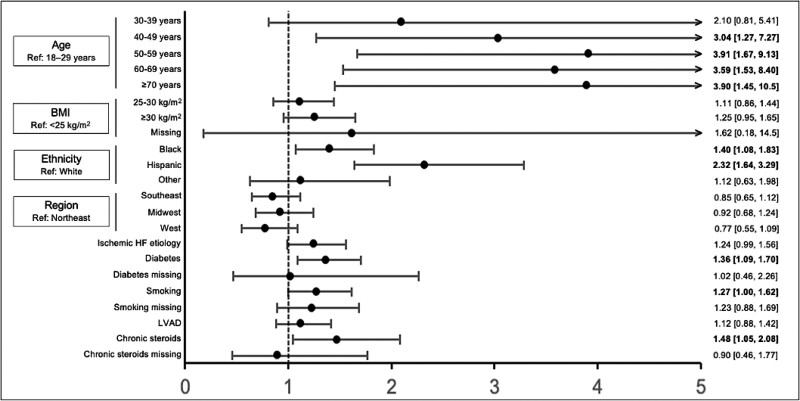
During the study period, 3,002 deceased HTx recipients were identified, of which 439 were cases. Cases and controls were similar regarding sex, blood type, and ventilator dependence. Cases were significantly older (p = 0.005), had a higher body mass index (BMI) (p = 0.005) more likely to be Black or Hispanic (p < 0.001), and were more likely to have a history of cigarette use (p = 0.01), diabetes (p < 0.001), and ischemic heart failure (p = 0.003) (Table 1). Seven characteristics including age, BMI, ethnicity, region, ischemic heart failure, diabetes, cigarette use, and bridging with left ventricular assist device were significantly associated with COVID-19–related death on univariate analysis and included in the final model. Compared with the Northeast region, the West region was significantly associated with decreased likelihood of COVID-19–related death on univariate analysis; therefore, region was included in the final multivariable model as well. Chronic steroid exposure at HTx was also included due to biological interest. In multivariable logistic regression, increasing age, identifying as Black and Hispanic, presence of diabetes, history of smoking, and chronic steroid use at HTx were independently associated with COVID-19–related death (Figure 1).
Table 1.
Demographic Characteristics Among Adult HTx Recipients and Donors Included in the Study
| Recipient | Cases (n = 439) | Controls (n = 2,563) | p |
|---|---|---|---|
| Age, years | 56.7 ± 9.4 | 54.3 ± 12.1 | 0.005 |
| Female sex | 98 (22.3) | 606 (23.6) | 0.55 |
| BMI, kg/m2 | 28.1 ± 4.6 | 27.4 ± 5.0 | 0.005 |
| Blood type O | 165 (37.6) | 1,023 (39.9) | 0.36 |
| Ethnicity | |||
| White | 265 (60.4) | 1,727 (67.4) | <0.001 |
| Black | 105 (23.9) | 556 (21.7) | |
| Hispanic | 54 (12.3) | 172 (6.7) | |
| Other | 15 (3.4) | 108 (4.2) | |
| Region | |||
| Northeast | 110 (25.1) | 545 (21.3) | 0.24 |
| Southeast | 159 (36.2) | 944 (36.8) | |
| Midwest | 105 (23.9) | 623 (24.3) | |
| West | 65 (14.8) | 451 (17.6) | |
| Ischemic HF etiology | 194 (44.2) | 917 (35.8) | 0.003 |
| Diabetes mellitus | 181 (41.2) | 763 (29.8) | <0.001 |
| Cerebrovascular accident | 29 (6.6) | 146 (5.7) | 0.50 |
| Cigarette use | 202 (46.0) | 984 (38.4) | 0.010 |
| Chronic steroid use | 47 (10.7) | 200 (7.8) | 0.066 |
| LVAD at HTx | 151 (34.4) | 748 (29.2) | 0.028 |
| IABP at HTx | 37 (8.4) | 252 (9.8) | 0.36 |
| Independent (functional status) | 40 (9.1) | 239 (9.3) | 0.89 |
| Ventilator dependent | 10 (2.3) | 55 (2.2) | 0.86 |
| Inotrope dependent | 185 (42.1) | 1,011 (39.5) | 0.29 |
| Retransplant | 15 (3.4) | 73 (2.9) | 0.51 |
| Multiorgan transplant | 28 (6.4) | 142 (5.5) | 0.48 |
| Kidney | 26 (5.9) | 125 (4.9) | |
| Liver | 2 (0.5) | 17 (0.7) | |
| Years from transplant to death | 9.5 ± 7.4 | 9.7 ± 8.1 | 0.60 |
| COVID as cause of death (cases only) | |||
| Primary | 402 (91.6) | - | |
| Secondary | 26 (5.9) | - | |
| Tertiary | 11 (2.5) | - | |
| Donor | |||
| Age, years | 32.3 ± 11.5 | 32.2 ± 11.7 | 0.75 |
| Female sex | 126 (28.7) | 734 (28.6) | 0.98 |
| Sex mismatch | 98 (22.3) | 620 (24.2) | 0.40 |
| BMI, kg/m2 | 27.7 ± 6.3 | 27.3 ± 5.9 | 0.24 |
| Blood type O | 207 (47.2) | 1,319 (51.5) | 0.10 |
| Ethnicity | |||
| White | 304 (69.3) | 1,683 (65.7) | 0.49 |
| Black | 64 (14.6) | 411 (16.0) | |
| Hispanic | 60 (13.7) | 385 (15.0) | |
| Other | 11 (2.5) | 84 (3.3) | |
| Diabetes mellitus | 13 (3.0) | 81 (3.3) | 0.82 |
| Cocaine abuse | 72 (16.4) | 425 (16.6) | 0.77 |
| Alcohol abuse | 61 (17.3) | 326 (16.3) | 0.46 |
| Smoking | 74 (17.2) | 415 (16.7) | 0.33 |
| Hypertension | 55 (12.8) | 369 (14.8) | 0.50 |
| Baseline LVEF | 61.7 ± 6.9 | 61.6 ± 7.2 | 0.91 |
| Ischemic time ≥4 hours | 69 (15.7) | 499 (19.5) | 0.11 |
BMI, body mass index; HF, heart failure; HTx, heart transplant; IABP, intra-aortic balloon pump; LVAD, left ventricular assist device; LVEF, left ventricular ejection fraction.
Bold indicates significance of p<0.05.
Figure 1.
Multivariable predictors of COVID-19–related death among adult HTx recipients. Point estimates are displayed with associated 95% confidence intervals. Regions are comprised of the following UNOS regions: Northeast: 1,2,9; Southeast: 3, 4, 11; Midwest: 7, 8, 10; West: 5, 6. BMI, body mass index; COVID-19, Coronavirus Disease-2019; HF, heart failure; HTx, heart transplant; LVAD, left ventricular assist device; UNOS, United Network for Organ Sharing.
In this study, we identify increasing age, minority racial status, history of diabetes, cigarette use, and chronic steroids before HTx as risk factors for COVID-19–related death in HTx recipients. While several studies have also associated age, diabetes, tobacco use, and immunosuppression with increased risk of COVID-19 mortality in the general population,5,6 our results extend these risk factors to the heart transplant community specifically. Increasing age up to 59 years appears to be associated with a linear increase in odds of COVID-19–related death, after which odds plateau. This attenuation is likely related to selection bias, as recipients transplanted greater than or equal to 60 years of age tend to be less critically ill than their younger counterparts, as evidenced by lower rates of intensive care requirement.7 Notably, recipients greater than or equal to 70 years display lower rates of chronic obstructive pulmonary disease compared with those aged 18–59 years.7 Although COVID-19 vaccination rates are highest among older adults nationwide, the applicability of these results to HTx recipients remains unclear given COVID-19 vaccine prioritization of SOTRs.
In concordance with studies in the general population, we recognize minority race as a significant risk factor for mortality in HTx recipients. While the disproportionate impact of COVID-19 among minorities in the United States is likely mediated by higher rates of risk factors such as diabetes, cardiovascular disease, and pulmonary disease, the effect is compounded by lower vaccination rates and decreased access to care among disadvantaged populations.8
We also report chronic corticosteroid use before HTx as a significant risk factor for COVID-19–related death. While corticosteroids have proven effective in mitigating COVID-19 severity,9 they may compromise COVID-19 vaccine efficacy by blunting humoral immunity.10 Further study is required to understand the interplay between immunosuppression, vaccine efficacy, and severe disease prevention.
As a retrospective analysis of UNOS data, it is important to note the limitations of this study. There are several variables potentially relevant to this analysis, which are not available in the UNOS database. Given that vaccination is shown to improve mortality rates in the general population, knowledge of vaccination status likely adds insight to this analysis. Notably, COVID-19 vaccination status is unavailable in the UNOS database. While these data are not concretely available, several transplant organizations strongly recommend that SOTRs be vaccinated. Second, granular data regarding immunosuppressive regimen, or the decision of treatment used during COVID-19 infection, were not available for analysis. Both variables may have a significant effect on a patient’s outcome, especially, earlier in the pandemic when the effects of treatment regimens were more uncertain. As such, our analysis is limited by these unknown variables.
Identifying risk factors for COVID-19 mortality in HTx recipients may prove critical as social distancing and masking regulations are lifted. Specifically, transplant centers may seek to leverage this data to ensure pre-op vaccination in individuals with noted risk factors. Furthermore, assessing the risk of mortality from COVID-19 may inform the intensity of follow-up and careful consideration of patient management postoperatively. While the threat of emerging SARS-CoV-2 variants persists, HTx recipients with increased COVID-19 mortality risk should also be duly informed.
Acknowledgments
The data reported here have been supplied by the United Network for Organ Sharing as the contractor for the Organ Procurement and Transplantation Network. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the OPTN or the U.S. Government.
Footnotes
Disclosure: The authors have no conflicts of interest to report.
References
- 1.Blumenthal D, Fowler EJ, Abrams M, Collins SR: Covid-19 — implications for the health care system. N Engl J Med 383: 1483–1488, 2020. [DOI] [PubMed] [Google Scholar]
- 2.Hadi YB, Naqvi SFZ, Kupec JT, Sofka S, Sarwari A: Outcomes of COVID-19 in solid organ transplant recipients: a propensity-matched analysis of a large research network. Transplantation 105: 1365–1371, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grupper A, Rabinowich L, Schwartz D, et al. Reduced humoral response to mRNA SARS‐CoV‐2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transplant. 2021:ajt.16615. doi:10.1111/ajt.16615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azar AM. Renewal of determination that a public health emergency exists. https://www.phe.gov/emergency/news/healthactions/phe/Pages/COVID-15April2021.aspx.
- 5.Kompaniyets L, Pennington AF, Goodman AB, et al.: Underlying medical conditions and severe illness among 540,667 adults hospitalized with COVID-19, March 2020–March 2021. Prev Chronic Dis 2021: 23, 2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jordan RE, Adab P, Cheng KK: Covid-19: risk factors for severe disease and death. BMJ 368, 2020. doi: 10.1136/BMJ.M1198. [DOI] [PubMed] [Google Scholar]
- 7.Cooper LB, Lu D, Mentz RJ, et al.: Cardiac transplantation for older patients: Characteristics and outcomes in the septuagenarian population. J Hear Lung Transplant 35: 362–369, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alcendor DJ: Racial Disparities-Associated COVID-19 Mortality among Minority Populations in the US. J Clin Med 9, 2442, 2022. doi: 10.3390/jcm9082442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The RECOVERY. Dexamethasone in hospitalized patients with Covid-19 — Preliminary report. N Engl J Med. 2020. doi:10.1056/nejmoa2021436. [Google Scholar]
- 10.Garcia-Beltran WF, Lam EC, Astudillo MG, et al. : COVID-19-neutralizing antibodies predict disease severity and survival. Cell 184: 476–488.e11, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]