Abstract
Laccase is a copper-containing phenoloxidase, involved in lignin degradation by white rot fungi. The laccase substrate range can be extended to include nonphenolic lignin subunits in the presence of a noncatalytic cooxidant such as 2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonate) (ABTS), with ABTS being oxidized to the stable cation radical, ABTS·+, which accumulates. In this report, we demonstrate that the ABTS·+ can be efficiently reduced back to ABTS by physiologically occurring organic acids such as oxalate, glyoxylate, and malonate. The reduction of the radical by oxalate results in the formation of H2O2, indicating the formation of O2·− as an intermediate. O2·− itself was shown to act as an ABTS·+ reductant. ABTS·+ reduction and H2O2 formation are strongly stimulated by the presence of Mn2+, with accumulation of Mn3+ being observed. Additionally, 4-methyl-O-isoeugenol, an unsaturated lignin monomer model, is capable of directly reducing ABTS·+. These data suggest several mechanisms for the reduction of ABTS·+ which would permit the effective use of ABTS as a laccase cooxidant at catalytic concentrations.
Lignin, the second most abundant renewable organic compound in the biosphere after cellulose, is highly recalcitrant, and therefore its biodegradation is a rate-limiting step in the global carbon cycle (9). White rot fungi have evolved a unique mechanism to accomplish this degradation, which utilizes extracellular enzymes to generate oxidative radical species (16). This degradative system is highly nonspecific, and as a consequence, these fungi can also oxidize a broad spectrum of structurally diverse environmental pollutants (4, 18). Three main groups of enzymes, i.e., lignin peroxidases (LiP), manganese peroxidases (MnP), and laccases, along with their low-molecular-weight cofactors, have been implicated in the lignin degradation process. LiP can oxidize the nonphenolic aromatic moieties that make up approximately 85% of the lignin polymer (21), while MnP uses the Mn2+/Mn3+ couple to oxidize phenolic subunits (19). Laccase, a copper-containing phenoloxidase, catalyzes the four-electron reduction of oxygen to water, and this is accompanied by the oxidation of a phenolic substrate (32).
In recent years, however, the laccase substrate range has been extended to include nonphenolic lignin subunits in the presence of readily oxidizable primary substrates. These cooxidants have been denoted mediators because they were previously speculated (but not proven) to act as electron transfer mediators. The most extensively investigated laccase mediator is 2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonate) (ABTS), a synthetic nitrogen-substituted aromatic compound which allows the oxidation of nonphenolic lignin model compounds (6) and the delignification of kraft pulp (8) by laccase. More recent work has also focused on an alternative compound, 1-hydroxybenzotriazole (7, 10). In the presence of these compounds, laccase can also catalyze the oxidation of polycyclic aromatic hydrocarbons (PAH) (12, 23), chemical synthesis (29), and textile dye bleaching (31). ABTS is oxidized by laccase to its corresponding cation radical. In the case of ABTS, the radical (ABTS·+) is highly stable, and it has been suggested that it may act as a diffusible oxidant of the enzyme (7). However, although the redox chemistry of ABTS (22) and its radical has been characterized, the mechanisms by which it interacts with laccase to “mediate” lignin oxidation are still unknown. Potthast et al. (28) have found evidence suggesting that ABTS acts as an activator or cooxidant of the enzyme. The observation that the laccase/ABTS couple can oxidize the nonphenolic veratryl alcohol, while ABTS·+ alone cannot (6), provides a further indication of this activator role for ABTS. If compounds such as ABTS do indeed act as cooxidants of the enzyme, it is necessary that some mechanism(s) exists for the recycling of their cation radicals back to their reduced forms so as to be available for subsequent catalytic cycles.
A number of low-molecular-weight compounds have been implicated in the catalysis of MnP during the oxidation of lignin. The most important of these is manganese, which is present in virtually all woody tissues (17). Divalent manganese (Mn2+) is oxidized by the enzyme to the trivalent form (Mn3+), which is capable of oxidizing an extensive range of phenolic compounds (19). To catalyze lignin oxidation, Mn3+ is chelated and stabilized by organic acids, which facilitate its diffusion to act as an oxidant at a distance from the MnP active site (19, 33). A range of these acids are produced by ligninolytic fungi (25, 30, 33), but the most ubiquitous is oxalate, whose production at levels as high as 28 mM by cultures of Pleurotus ostreatus has been observed (1). Oxalate can itself be oxidized by Mn3+, producing the formate anion radical (CO2·−), which can then reduce molecular oxygen to produce superoxide (O2·−) (24), and a role for these radicals as reducing agents in lignin degradation has been suggested (24).
In this report, evidence is presented indicating that physiologically occurring organic acids can directly reduce ABTS·+. The rate of reduction is highly stimulated by the presence of manganese, and the results indicate a mechanism involving O2·−.
MATERIALS AND METHODS
Chemicals and enzyme preparations.
All chemicals used are commercially available and were used without further purification. ABTS, KO2, and MnSO4 were obtained from Sigma-Aldrich (Poole, United Kingdom). Organic acids were obtained from either Sigma-Aldrich or Merck (Darmstadt, Germany), and 4-methyl-O-isoeugenol was obtained from Acros Chimica (Geel, Belgium). The laccase preparation used was laccase isozyme I purified from cultures of Trametes versicolor 290 as previously described (12). Manganese-dependent peroxidase in a semipurified form was obtained from Tienzyme, Inc. (State College, Pa.). Horseradish peroxidase was purchased from Boehringer GmbH (Mannheim, Germany).
ABTS·+ preparation.
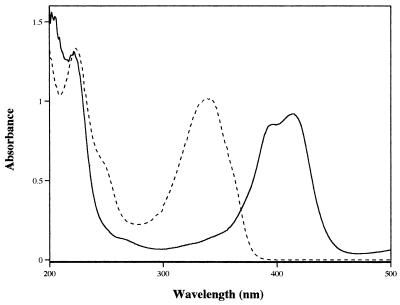
Reaction mixtures contained 25 mM sodium acetate (pH 4.5), ABTS (500 μM), and laccase (0.1 U ml−1) and were incubated for at least 1 h at room temperature. ABTS·+ was then separated from the enzyme by ultrafiltration through an Amicon YM10 membrane followed by vigourous boiling for 5 min to inactivate any trace levels of laccase still remaining. The spectra of ABTS and its cation radical, ABTS·+, are shown in Fig. 1.
FIG. 1.
Absorbance spectra of 25 μM ABTS (–––) and its cation radical, ABTS·+ (——).
ABTS·+ reduction assays.
Assay mixtures in experiments to determine the rates of ABTS·+ reduction contained 25 μM ABTS·+ (ɛ420 = 36,000). In reaction mixtures (1-ml total volume) containing oxalate, KO2, or 4-methyl-O-isoeugenol as the reductant, 10 mM sodium acetate (pH 4.5) was used as the buffer. In one experiment, the reducing effect of a range of organic acids (all at 50 mM [pH 4.5]) was determined. For determination of reduction rates under anaerobic conditions, 1.8-ml screw-cap chromatography vials with butyl rubber PTFE grey blue seals (PhaseSep, Waddinxveen, The Netherlands) were used and the reaction mixtures were flushed with 100% nitrogen gas for 3 min before incubation. ABTS·+ reduction was monitored spectrophotometrically at 30°C with a Perkin-Elmer (Norwalk, Conn.) 550A UV-Vis spectrophotometer. Rates were measured during the initial 3 to 5 min, and in some experiments the extent of reduction was measured after incubation for 1 h. All data represent the mean values of at least triplicate samples.
Mn3+ determination.
The product of Mn2+ oxidation, Mn3+, was measured as a Mn3+-malonate complex at 270 nm. To confirm that this absorbance was due to Mn3+ production, the absorbance spectrum in the UV range was compared with that of a standard Mn3+-malonate solution. Mn3+ accumulation determinations were corrected for background absorbance changes at 270 nm as a result of increased absorbance at this wavelength due to the reduction of ABTS·+ to ABTS.
H2O2 production assay.
H2O2 produced as a product of oxalate oxidation by ABTS·+ was measured by a method modified from that of Pick and Keisari (27) based on the H2O2-dependent oxidation of ABTS. Triplicate samples (1 ml) were first incubated at 95°C for 1 h to completely reduce any remaining ABTS·+. Volumes of 100 μl from each sample were then added to a reaction mixture providing final concentrations of the following components: 100 mM sodium phosphate (pH 6.0), 500 μM ABTS, and 1 U of horseradish peroxidase ml−1. The absorbance at 420 nm was immediately measured, and the maximum value was taken. A standard curve was constructed with known H2O2 concentrations, and this was used for the conversion of A420 values to H2O2 concentrations. Data presented are means from triplicate reactions.
Carbon dioxide analysis.
CO2 produced from the oxidation of various concentrations of oxalate by ABTS·+ was measured in reaction mixtures containing the components described above. These values were corrected for background levels of oxalate decarboxylation at each concentration. Reaction mixtures (1 ml) were incubated at 30°C for 1 h in 1.8-ml screw-cap chromatography vials with butyl rubber PTFE grey blue seals. CO2 levels in headspaces were then determined by gas chromatography with a no. 427 (Packard, Delft, The Netherlands) apparatus fitted with a thermal conductivity detector (140°C). The Hayesep Q column (Chrompack, Middelburg, The Netherlands) was maintained at 110°C, and helium was used as the carrier gas (30 ml min−1). The injection volume was 100 μl. A standard curve was used for the determination of CO2 concentrations present in the reaction headspaces. Values represent the total CO2 concentration present in reaction vials, i.e., the sum of the CO2 present in both the liquid and gaseous phases. The results are presented as means and standard deviations from triplicate reactions.
HPLC analysis.
For the analysis of oxidation products from 4-methyl-O-isoeugenol, 50 μl of the incubation mixtures was injected into a high-pressure liquid chromatography diode array detector system (Chemstation Pascal series; Hewlett-Packard, Waldbronn, Germany) with a column (200 by 3 mm) filled with ChromSpher C18-PAH (5-μm particles) (Chrompack). Aromatic metabolites were analyzed with the following gradient (0.4 ml min−1 at 30°C): 90:10, 0:100, and 0:100 H2O-CH3CN at 0, 15, and 20 min, respectively. The UV absorbance was monitored at 2-nm wavelength intervals from 200 to 400 nm. Compound identification was attempted by matching UV spectra and the retention times of the observed products with those of their standards.
RESULTS
Reduction of ABTS·+ by organic acids.
A range of physiologically occurring organic acids were tested for their ability to reduce ABTS·+, and several were found to act as effective reductants (Table 1). When no buffer was added to the ABTS·+ preparation, the initial basal rate of reduction was low. Tartrate did not act as a reducing agent of the radical, while acetate and succinate appeared to be poor reductants. In contrast, the presence of glyoxylate, oxalate, or malonate resulted in much higher rates, with malonate having the greatest reducing effect on the radical. However, oxalate is produced more abundantly and at higher levels by ligninolytic fungi than is malonate and was therefore chosen for use in subsequent experiments to investigate the role of organic acids in ABTS·+ reduction.
TABLE 1.
Rate of ABTS·+ reduction by different organic acidsa
| Organic acidb | Concn (μM) of ABTS·+ reduced min−1 |
|---|---|
| None | 0.056 ± 0.003 |
| Acetate | 0.077 ± 0.009 |
| Tartrate | 0.056 ± 0.007 |
| Succinate | 0.092 ± 0.011 |
| Malonate | 0.750 ± 0.043 |
| Glyoxylate | 0.347 ± 0.062 |
| Oxalate | 0.463 ± 0.031 |
The ABTS·+ concentration was 25 μM.
All acids were added to final concentration of 50 mM.
CO2 production.
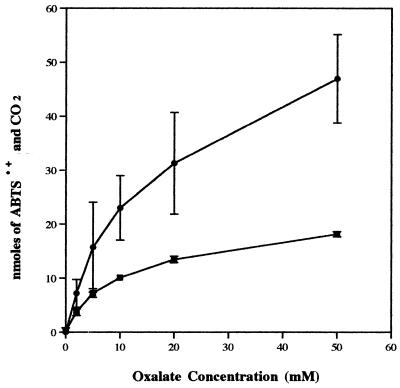
To demonstrate that ABTS·+ reduction by oxalate corresponds to the oxidation of the acid, we measured the formation of the reaction product, CO2. Each mole of oxalate should be oxidatively decarboxylated to finally produce 2 mol of CO2. As the concentration of oxalate was increased from 0 to 50 mM, increasing levels of ABTS·+ reduction were observed after the 1-h incubation period (Fig. 2). These corresponded to increases in the total amount of CO2 present in reaction vials, and, as expected, approximately 2 nmol of CO2 was produced per nmol of ABTS·+ reduced.
FIG. 2.
Reduction of 25 μM ABTS·+ (▪) and corresponding production of CO2 (•) in the presence of various concentrations of oxalate after 1 h in a 1-ml reaction volume.
Reduction of ABTS·+ by O2·−.
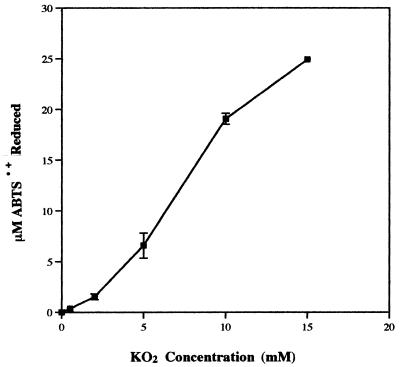
The ability of O2·− to directly reduce ABTS·+ was investigated by adding it to reaction mixtures in the form of KO2. The buffer used in these reactions was the poorly oxidized acid sodium acetate. As the O2·− concentration was increased from 0 to 15 mM, a corresponding increase in ABTS·+ reduction was observed (Fig. 3). This reduction was instantaneous (less than 3 min) upon addition of the O2·−, and in the reaction mixture to which 15 mM O2·− was added, all the ABTS·+ was immediately converted to the colorless reduced form (ABTS).
FIG. 3.
Reduction of 25 μM ABTS·+ by various concentrations of KO2. The extent of reduction was measured after 3 min.
Reduction of ABTS·+ in the presence of Mn2+.
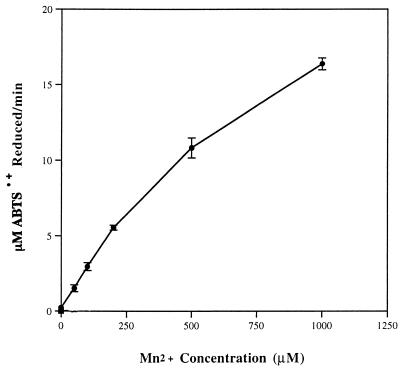
The presence of Mn2+ was found to have a strong stimulatory effect on the reduction of ABTS·+ by either malonate or oxalate. In the absence of any reactive acids, Mn2+ alone had no effect on the rate of ABTS·+ reduction. However, concentrations of Mn2+ as low as 50 μM had an enhancing effect on ABTS·+ reduction by malonate and a very marked effect on ABTS·+ reduction by oxalate (Table 2). As the Mn2+ concentration was increased, this stimulatory effect also increased (Fig. 4), with a very high rate (16.38 μM ABTS·+ reduced per min) being observed in the presence of 1,000 μM Mn2+. It is notable, however, that for this stimulation to occur, the ABTS·+ had to be preincubated with the oxalate alone before the Mn2+ addition. Furthermore, this enhancement by Mn2+ of ABTS·+ reduction did not occur when reaction mixtures were incubated under anaerobic conditions.
TABLE 2.
Effect of manganese on the rate of ABTS·+ reduction by organic acids
| Reaction mixturea | Concn (μM) of ABTS·+ reduced min−1 |
|---|---|
| ABTS·+ + malonate | 0.54 ± 0.05 |
| ABTS·+ + malonate + Mn2+ | 0.67 ± 0.12 |
| ABTS·+ + oxalate | 0.36 ± 0.02 |
| ABTS·+ + oxalate + Mn2+ | 2.96 ± 0.20 |
| ABTS·+ + oxalate + Mn2+ + MnP | 3.11 ± 0.24 |
| ABTS·+ + oxalate + Mn2+ + MnP + H2O2 | 3.87 ± 0.33 |
Concentrations: 25 μM ABTS·+, 10 mM oxalate or malonate, 50 μM Mn2+, 20 mU of MnP ml−1, and 200 μM H2O2.
FIG. 4.
Initial rates of reduction of 25 μM ABTS·+ by 10 mM oxalate at various concentrations of Mn2+.
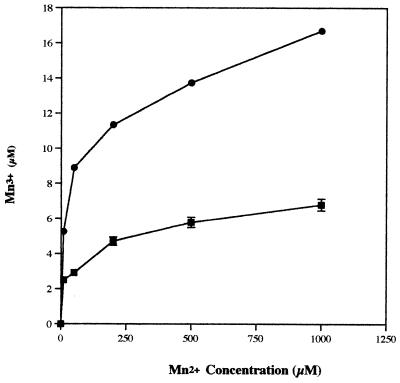
The accumulation of the reaction product, Mn3+, was measured after incubation for 1 h in reaction mixtures containing the same range of Mn2+ concentrations. These reaction mixtures contained either malonate (20 mM) alone or both malonate and oxalate (10 mM each) as the ABTS·+ reductant. Increasing Mn2+ concentrations corresponded to increased levels of Mn3+ accumulation in both cases, but a much higher level of Mn3+ could be measured when oxalate was present (Fig. 5).
FIG. 5.
Accumulation of Mn3+ produced from various concentrations of Mn2+, as a result of ABTS·+ (25 μM) reduction after 1 h of incubation in the presence of either 20 mM malonate (▪) or 10 mM each oxalate and malonate combined (•).
The addition of MnP together with H2O2 had a stimulatory effect on ABTS·+ reduction (Table 2). The main function of MnP is to cause the H2O2-dependent oxidation of Mn2+ to Mn3+ (19). Mn3+ is known to oxidize oxalate, which would contribute to an enhanced rate of superoxide formation (24).
H2O2 production from oxalate.
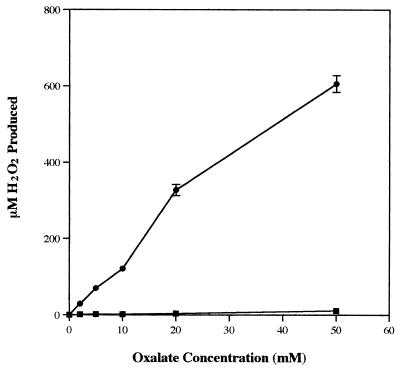
H2O2 production results indirectly from the oxidative decarboxylation of oxalate. Increasing the oxalate concentration (and thus the ABTS·+ reduction rate [Fig. 2]) corresponded to increased H2O2 accumulation (Fig. 6). However, the amount of accumulating H2O2 was between 22- and 82-fold greater when 500 μM Mn2+ was included in the reaction mixtures. No H2O2 production was detected in either the absence or presence of Mn2+ when the reaction mixtures were incubated in the absence of oxygen.
FIG. 6.
Production of H2O2 after 1 h, as a result of 25 μM of ABTS·+ reduction by various oxalate concentrations in the absence (▪) or presence (•) of 500 μM Mn2+.
Reduction of ABTS·+ by 4-methyl-O-isoeugenol.
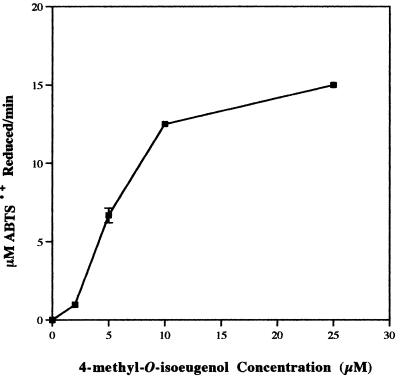
As well as organic acids, the unsaturated lignin subunit analog, 4-methyl-O-isoeugenol, was found to be a very efficient ABTS·+ reductant. When present in reaction mixtures at concentrations as low as 2 μM, 4-methyl-O-isoeugenol reduced 0.973 μM ABTS·+ per min while 25 μM, 4-methyl-O-isoeugenol had an initial ABTS·+ reduction rate of 15 μM per min (Fig. 7). The putative product of this reaction eluted from a high-performance liquid chromatography column as one peak, and its levels increased as the initial ABTS·+ concentration (and so the level of reduced ABTS·+) was increased. The compound represented by this peak has not yet been identified, but it was confirmed not to represent veratryl aldehyde or coniferyl alcohol.
FIG. 7.
Reduction of 25 μM ABTS·+ by various concentrations of 4-methyl-O-isoeugenol.
DISCUSSION
Laccases are widely distributed in nature, and their involvement in both lignin synthesis (13) and lignin degradation (32, 35) has been recently reviewed. The enzyme catalyzes the one-electron oxidation of phenolic substrates or aromatic amines to form different products via various pathways. In the past, the laccase substrate range was thought to be limited to these classes of compounds and the enzyme was considered to be inactive with nonphenolics. In recent years, however, a number of synthetic compounds have been identified (6, 7, 10) which allow the oxidation of nonphenolic substrates by laccases. In the presence of ABTS, the most extensively studied of these synthetic compounds, laccase can oxidize lignin model dimers (6), polymeric lignin (8), and other nonphenolic aromatics such as veratryl alcohol (6) and PAHs (12, 23). These compounds have been proposed to function as electron transfer mediators between the enzyme and its substrate (6, 7, 11). However, no direct evidence for such a mediated process exists. In contrast, a number of findings suggest that the role of ABTS is not that of a redox mediator. Bourbonnias and Paice (6) have reported that although the laccase/ABTS couple oxidizes veratryl alcohol, ABTS·+ itself cannot. Similarly, we have found that laccase/ABTS oxidizes the PAH anthracene (12) but that ABTS·+ in the absence of the enzyme has no effect (data not presented). Instead, Potthast et al. (29) have proposed an alternative role for ABTS in laccase catalysis. Data from experiments investigating the oxidation of benzyl alcohol by the laccase/ABTS couple has led these workers to conclude that ABTS functions to transfer one electron to the enzyme, thus initiating the ability of the enzyme to accomplish electron transfer from the substrate to dioxygen in a two-electron transfer process. In this way, ABTS would act as a cooxidant which activates the enzyme rather than as an electron mediator of the substrate.
In all in vitro studies to date (6, 11), at least 1 to 2 mM cooxidant concentrations have been required for the effective laccase-mediated oxidation of nonphenolic compounds. Such high concentrations would probably not be necessary if these compounds were indeed electron mediators, since their oxidized intermediates (cation radicals) would be redox cycled to the reduced form after each electron transfer step. The likely consequence of this recycling would be that the electron transfer species would be required at catalytic (micromolar) concentrations only. The necessity for millimolar concentrations of these compounds does not mimic natural systems in which natural cooxidants are actually present at catalytic concentrations. Eggert et al. (15) have identified a fungal metabolite, 3-hydroxyanthranillate, from Pycnoporus cinnabarinus which enabled the oxidation of nonphenolic lignin model compounds by laccase. The maximum concentration of 3-hydroxyanthranillate detected in fungal cultures was 20 μM (14). Under in vivo conditions, a range of potential reductants could be present, in the form of the other lignin degradation system components, to act as cooxidant recycling factors. In this work, we have used ABTS as a model cooxidant to investigate the effects of some of these components, namely, organic acids, manganese, and O2·−, in this reduction process.
The stable cation radical, ABTS·+, can be efficiently reduced by oxalate, glyoxylate, and malonate (Table 1), organic acids which have been detected in culture fluids of lignin-degrading fungi (30, 33). One major role for these acids is thought to be the binding of Mn3+ ions to facilitate Mn3+-mediated polymeric lignin oxidation (30, 33). It is well established, however, that oxalate can be directly oxidized by Mn3+ (24), and this reaction has also been demonstrated for glyoxylate (26). The oxidation of these acids leads to free radical formation, and these radicals decompose, yielding CO2·− as a product (24, 26), which can then be oxidized to CO2. For oxalate, the other product of this reaction is an additional molecule of CO2, so that the net production of 2 mol of CO2 results from the oxidation of 1 mol of oxalate (24). Our results are consistent with this, with approximately 2 nmol of accumulating CO2 being measured per nmol of ABTS·+ reduced (Fig. 2). This provides evidence that ABTS·+ is directly reduced by oxalate, which is itself oxidized to CO2.
Under aerobic conditions, CO2·− produced from organic acid oxidation donates an electron to oxygen, reducing it to O2·− (24). Dismutation of this O2·− results in the production of H2O2 (34). A consequence of this is that oxalate (25) and glyoxylate (14) can support MnP-catalyzed reactions in the absence of exogenous H2O2. In this study, the reduction of ABTS·+ by oxalate led to corresponding increases in H2O2 production, indicating that under the conditions used, CO2·− was indeed oxidized by O2 to produce O2·−. Furthermore, it was demonstrated that O2·− itself could reduce ABTS·+ back to ABTS. The addition of Mn2+ at various concentrations had a marked effect on both the rates of ABTS·+ reduction (Table 2; Fig. 4) and the corresponding levels of H2O2 accumulation (Fig. 6). The fact that O2·− can oxidize Mn2+ to Mn3+ (2, 5) provides a possible explanation for this observation. Oxidation of oxalate by ABTS·+ would result in the production of O2·−, which could then oxidize Mn2+ to Mn3+. This Mn3+ could then oxidize another molecule of oxalate, leading to further O2·− production. The effect of this would be accelerated O2·− production, resulting in accelerated Mn3+ production (Fig. 6) and ABTS·+ reduction (Fig. 4). Although Mn3+ production could be detected after incubation of reaction mixtures for 1 h (Fig. 5), high levels were not measured, probably because Mn3+ did not accumulate but was reduced to Mn2+ by oxalate. For Mn2+ to have this effect on the ABTS·+ reduction rate, it was observed that the radical should be preincubated with oxalate for a short period. The probable explanation is that during this preincubation time, ABTS·+ reduction would result in the formation of a pool of O2·− for subsequent reaction with Mn2+. Producing Mn3+ enzymatically with MnP/H2O2 also stimulated ABTS·+ reduction (Table 2), indicating a synergy between laccase and MnP in ligninolysis.
In conclusion, we have demonstrated that a number of ligninolytic system components may have an involvement in the redox cycle of the ABTS·+/ABTS couple, a putative cooxidant of laccase. Oxalate, as well as O2·− produced as a result of oxalate oxidation, can directly reduce ABTS·+ to ABTS, making it available for laccase reactivation. Although oxalate alone can support slow reduction of ABTS·+, the addition of Mn2+ greatly stimulates this process by increasing the rate of O2·− production. It is indeed likely that O2·−, which may have a number of other functions within the ligninolytic system (3, 20), is the critical factor involved in rapid cooxidant reduction. It is possible that the ABTS·+ reductants identified in this work function in vivo as agents of natural cooxidant recycling. The application of reductants in commercial laccase treatments of nonphenolic compounds, such as pulp-lignin or PAHs, may therefore reduce the requirement for high concentrations of environmentally detrimental compounds such as ABTS and HBT. However, additional research is necessary to further characterize the mechanisms involved in laccase-catalyzed oxidation reactions before the use of such reductants can be considered.
REFERENCES
- 1.Akamatsu Y, Takahashi M, Shimada M. Production of oxalic acid by wood-rotting basidiomycetes grown on low and high nitrogen culture media. Mater Org. 1994;28:251–264. [Google Scholar]
- 2.Archibald F S, Fridovich I. The scavenging of superoxide radical by manganous complexes in vitro. Arch Biochem Biophys. 1982;214:452–463. doi: 10.1016/0003-9861(82)90049-2. [DOI] [PubMed] [Google Scholar]
- 3.Barr D P, Aust S D. Effect of superoxide and superoxide dismutase on lignin peroxidase. Arch Biochem Biophys. 1994;311:378–382. doi: 10.1006/abbi.1994.1251. [DOI] [PubMed] [Google Scholar]
- 4.Barr D P, Aust S D. Pollution degradation by white-rot fungi. Rev Environ Contam Toxicol. 1994;138:49–72. doi: 10.1007/978-1-4612-2672-7_3. [DOI] [PubMed] [Google Scholar]
- 5.Bono J J, Goulas P, Boe J F, Portet N, Seris J L. Effect of Mn(II) on reactions catalyzed by lignin peroxidase from Phanerochaete chrysosporium. Eur J Biochem. 1990;192:189–193. doi: 10.1111/j.1432-1033.1990.tb19213.x. [DOI] [PubMed] [Google Scholar]
- 6.Bourbonnais R, Paice M G. Oxidation of non-phenolic substrates. An expanded role for laccase in lignin biodegradation. FEBS Lett. 1990;267:99–102. doi: 10.1016/0014-5793(90)80298-w. [DOI] [PubMed] [Google Scholar]
- 7.Bourbonnais R, Paice M G, Leech D, Freiermuth B. 1997 Biological Sciences Symposium. Atlanta, Ga: TAPPI Press; 1997. Reactivity and mechanism of laccase mediators for pulp delignification; pp. 335–338. [Google Scholar]
- 8.Bourbonnais R, Paice M G, Reid I D, Lanthier P, Yaguchi M. Lignin oxidation by laccase enzymes from Trametes versicolor and role of the mediator in 2,2′azinobis(3-ethylbenzthiazoline-6-sulfonate) kraft lignin depolymerization. Appl Environ Microbiol. 1995;61:1876–1880. doi: 10.1128/aem.61.5.1876-1880.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bumpus J A, Aust S D. Biodegradation of environmental pollutants by the white rot fungus Phanerochaete chrysosporium: involvement of the lignin degrading system. Bioessays. 1987;6:166–170. [Google Scholar]
- 10.Call H P. World patent application WO 94/29510. 1994. [Google Scholar]
- 11.Call H P, Mücke I. History, overview and applications of mediated lignolytic systems, especially laccase-mediator-systems (Lignozym-process) J Biotechnol. 1997;53:163–202. [Google Scholar]
- 12.Collins P J, Kotterman M J J, Field J A, Dobson A D W. Oxidation of anthracene and benzo[a]pyrene by laccases from Trametes versicolor 290. Appl Environ Microbiol. 1996;62:4563–4567. doi: 10.1128/aem.62.12.4563-4567.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dean J F D, Eriksson K-E L. Laccase and the deposition of lignin in vascular cells. Holzforzchung. 1994;48:21–33. [Google Scholar]
- 14.Eggert C, Temp U, Dean J F D, Eriksson K-E L. Laccase mediated transformation of the phenoxazinone derivative cinnabarinic acid. FEBS Lett. 1995;376:202–206. doi: 10.1016/0014-5793(95)01274-9. [DOI] [PubMed] [Google Scholar]
- 15.Eggert C, Temp U, Dean J F D, Eriksson K-E L. A fungal metabolite mediates degradation of non-phenolic lignin structures and synthetic lignin by laccase. FEBS Lett. 1996;391:144–148. doi: 10.1016/0014-5793(96)00719-3. [DOI] [PubMed] [Google Scholar]
- 16.Evans C S, Dutton M V, Guillen F, Veness R G. Enzymes and small molcular mass agents involved with lignocellulose degradation. FEMS Microbiol Rev. 1994;13:235–240. [Google Scholar]
- 17.Fengel D, Wegner G. Wood, chemistry, ultrastructure and reactions. Berlin, Germany: Walter de Gruyter; 1989. p. 159. [Google Scholar]
- 18.Field J A, de Jong E, Feijoo-Costa G, de Bont J A M. Screening for ligninolytic fungi applicable to the biodegradation of xenobiotics. Trends Biotechnol. 1993;11:44–49. [Google Scholar]
- 19.Glenn J K, Akileswaran L, Gold M H. Mn(II) oxidation is the principal function of the extracellular Mn-peroxidase from Phanerochaete chrysosporium. Arch Biochem Biophys. 1986;251:688–696. doi: 10.1016/0003-9861(86)90378-4. [DOI] [PubMed] [Google Scholar]
- 20.Guillén F, Martínez M J, Muñoz C, Martínez A T. Quinone redox cycling in the ligninolytic fungus Pleurotus eryngii leading to extracellular production of superoxide anion radical. Arch Biochem Biophys. 1997;339:190–199. doi: 10.1006/abbi.1996.9834. [DOI] [PubMed] [Google Scholar]
- 21.Hammel K E, Jensen K A, Jr, Mozuch M D, Landucci L L, Tien M, Pease E A. Ligninolysis by a purified lignin peroxidase. J Biol Chem. 1993;268:12274–12281. [PubMed] [Google Scholar]
- 22.Hünig S, Balli H, Conrad H, Schoot A. Polarographie von 2,2′-Azinen Aromatischer Heterocyclen. Justus Liebigs Ann Chem. 1964;676:52–65. [Google Scholar]
- 23.Johannes C, Majcherczyk A, Hüttermann A. Degradation of anthracene by laccase of Trametes versicolor in the presence of different mediator compounds. Appl Microbiol Biotechnol. 1996;46:313–317. doi: 10.1007/s002530050823. [DOI] [PubMed] [Google Scholar]
- 24.Khindaria A, Grover T A, Aust S D. Oxalate-dependent reductive activity of manganese peroxidase from Phanerochaete chrysosporium. Arch Biochem Biophys. 1994;314:301–306. doi: 10.1006/abbi.1994.1446. [DOI] [PubMed] [Google Scholar]
- 25.Kuan I C, Tien M. Stimulation of Mn peroxidase activity: a possible role for oxalate in lignin biodegradation. Proc Natl Acad Sci USA. 1993;90:1242–1246. doi: 10.1073/pnas.90.4.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuan I C, Tien M. Glyoxylate-supported reactions catalyzed by Mn peroxidase of Phanerochaete chrysosporium: activity in the absence of added hydrogen peroxide. Arch Biochem Biophys. 1993;302:447–454. doi: 10.1006/abbi.1993.1238. [DOI] [PubMed] [Google Scholar]
- 27.Pick E, Keisari Y. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J Immunol Methods. 1980;38:161–170. doi: 10.1016/0022-1759(80)90340-3. [DOI] [PubMed] [Google Scholar]
- 28.Potthast A, Rosenau T, Chen C-L, Gratzl J S. Selective enzymatic oxidation of aromatic methyl groups to aldehydes. J Org Chem. 1995;60:4320–4321. [Google Scholar]
- 29.Potthast A, Rosenau T, Chen C-L, Gratzl J S. A novel method for the conversion of benzyl alcohols to benzaldehydes by laccase-catalysed oxidation. J Mol Catal Ser A. 1996;108:5–9. [Google Scholar]
- 30.Roy B P, Archibald F S. Effects of kraft pulp and lignin on Trametes versicolor carbon metabolism. Appl Environ Microbiol. 1993;59:1855–1863. doi: 10.1128/aem.59.6.1855-1863.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schneider P, Pedersen A H. PCT. World patent application WO 95/01426. 1995. [Google Scholar]
- 32.Thurston C F. The structure and function of fungal laccases. Microbiology. 1994;140:19–26. [Google Scholar]
- 33.Wariishi H, Valli K, Gold M H. Mn(II) oxidation by manganese from the basidiomycete Phanerochaete chrysosporium: kinetic mechanisms and role of chelators. J Biol Chem. 1991;267:23688–23695. [PubMed] [Google Scholar]
- 34.Yokota A, Kitaoka S, Miura K, Wadano A. reactivity of glyoxylate with hydrogen peroxide and simulation of the glycolate pathway of C3 plants and euglena. Planta. 1985;165:59–67. doi: 10.1007/BF00392212. [DOI] [PubMed] [Google Scholar]
- 35.Youn H-D, Hah Y C, Kang S-O. Role of laccase in lignin degradation by white-rot fungi. FEMS Microbiol Lett. 1995;132:183–188. [Google Scholar]