Abstract
BACKGROUND
The use of azithromycin reduces maternal infection in women during unplanned cesarean delivery, but its effect on those with planned vaginal delivery is unknown. Data are needed on whether an intrapartum oral dose of azithromycin would reduce maternal and offspring sepsis or death.
METHODS
In this multicountry, placebo-controlled, randomized trial, we assigned women who were in labor at 28 weeks’ gestation or more and who were planning a vaginal delivery to receive a single 2-g oral dose of azithromycin or placebo. The two primary outcomes were a composite of maternal sepsis or death and a composite of stillbirth or neonatal death or sepsis. During an interim analysis, the data and safety monitoring committee recommended stopping the trial for maternal benefit.
RESULTS
A total of 29,278 women underwent randomization. The incidence of maternal sepsis or death was lower in the azithromycin group than in the placebo group (1.6% vs. 2.4%), with a relative risk of 0.67 (95% confidence interval [CI], 0.56 to 0.79; P<0.001), but the incidence of stillbirth or neonatal death or sepsis was similar (10.5% vs. 10.3%), with a relative risk of 1.02 (95% CI, 0.95 to 1.09; P= 0.56). The difference in the maternal primary outcome appeared to be driven mainly by the incidence of sepsis (1.5% in the azithromycin group and 2.3% in the placebo group), with a relative risk of 0.65 (95% CI, 0.55 to 0.77); the incidence of death from any cause was 0.1% in the two groups (relative risk, 1.23; 95% CI, 0.51 to 2.97). Neonatal sepsis occurred in 9.8% and 9.6% of the infants, respectively (relative risk, 1.03; 95% CI, 0.96 to 1.10). The incidence of stillbirth was 0.4% in the two groups (relative risk, 1.06; 95% CI, 0.74 to 1.53); neonatal death within 4 weeks after birth occurred in 1.5% in both groups (relative risk, 1.03; 95% CI, 0.86 to 1.24). Azithromycin was not associated with a higher incidence in adverse events.
CONCLUSIONS
Among women planning a vaginal delivery, a single oral dose of azithromycin resulted in a significantly lower risk of maternal sepsis or death than placebo but had little effect on newborn sepsis or death. (Funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and others; A-PLUS ClinicalTrials.gov number, NCT03871491.)
Maternal infections, particularly sepsis, during the peripartum period account for 10% of maternal deaths and are among the top three causes of maternal death worldwide.1 The proportion of deaths that are caused by infection has increased over time, whereas deaths from causes such as hemorrhage and preeclampsia have remained stable or decreased.1 Neonatal sepsis, accounting for 16% of neonatal deaths, is the third most common cause of neonatal death.2 Furthermore, maternal infection increases the risk of neonatal sepsis.1,2
The World Health Organization (WHO) and others have prioritized the reduction of maternal sepsis to decrease the risk of maternal death.3 Such efforts have included the evaluation of the use of prophylactic antibiotics in women who are giving birth.1 In a randomized trial of adjunctive intravenous azithromycin prophylaxis for cesarean delivery performed during labor, investigators found a 50% lower incidence of maternal infection in the azithromycin group than in the placebo group, as well as lower costs.4,5 As a result, adjunctive azithromycin prophylaxis is now recommended in the United States and elsewhere for women undergoing cesarean delivery during labor.6 In another trial, a single intrapartum oral dose of 2 g of azithromycin reduced maternal and neonatal infection in women who were planning a vaginal delivery in Gambia.7
We performed the Azithromycin Prevention in Labor Use Study (A-PLUS) to test the two primary hypotheses that a single oral dose of azithromycin in women in labor who were planning a vaginal delivery would reduce maternal sepsis or death along with stillbirth or neonatal death or sepsis.
METHODS
TRIAL DESIGN AND OVERSIGHT
This multicountry, double-blind, placebo-controlled, randomized trial was conducted at eight sites in seven low- or middle-income countries of the Global Network for Women’s and Children’s Health Research of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). The institutional review board at each site and partner U.S. institution and the data coordinating center approved the protocol (available with the full text of this article at NEJM.org). A steering committee (including an NICHD program scientist) and an NICHD-appointed independent data and safety monitoring committee provided oversight. The first three authors plus the penultimate and next-to-penultimate authors vouch for the accuracy and completeness of the data and for the fidelity of the trial to the protocol.
TARGET POPULATION AND ENTRY CRITERIA
Pregnant women who had been admitted to health facilities for spontaneous or induced vaginal delivery were eligible. We included women with singleton and multiple gestations of at least 28 weeks who provided written informed consent. We excluded women with infection warranting the use of antibiotics, arrhythmia or known cardiomyopathy, allergy to azithromycin or other macrolide antibiotics or their use within 3 days, planned cesarean delivery before randomization, advanced stage of labor, and any medical condition that was considered to be a contraindication by the site investigator. Advanced labor was defined as complete cervical dilation or dilation of more than 6 cm. Details are provided in Table S1 in the Supplementary Appendix, available at NEJM.org.
INTERVENTIONS, MASKING, AND RANDOMIZATION
The intervention was a single 2-g oral dose of azithromycin or identical placebo. An independent drug distributor packaged the azithromycin and placebo. Each dose consisted of four pills of 500 mg of azithromycin or placebo and were labeled with a unique package identifier. Clinicians, research staff members, and patients were unaware of trial-group assignments. Trial packs of azithromycin and identical placebo were numbered sequentially with the use of a computer algorithm, which used a predetermined 1:1 randomization schedule for azithromycin and placebo, stratified according to site, and a permuted-block randomization with varied block sizes.
Research staff members administered the intervention pack and observed pill intake. All other procedures, including antibiotic use, followed standard practices.
OUTCOMES
The two primary outcomes were a composite of maternal sepsis or death within 6 weeks after delivery and a composite of stillbirth or neonatal death or sepsis within 4 weeks. Maternal sepsis was defined according to WHO criteria as suspected or confirmed infection including fever (>100.4°F or 38°C) or hypothermia (<96.8°F or 36°C) plus one or more signs of organ dysfunction: tachycardia (≥120 beats per minute), low systolic blood pressure (<90 mm Hg), tachypnea (>24 breaths per minute), altered mental status or confusion, reduced urinary output (<500 ml over 24 hours), jaundice, or renal failure (creatinine level, >1.2 mg per deciliter).8–10 Neonatal sepsis was defined as a proven or possible serious bacterial infection on the basis of the following WHO criteria: severe chest in-drawing, fever (≥100.4°F or 38.0°C), hypothermia (<95.9°F or 35.5°C), no movement or movement only on stimulation, poor or no feeding, convulsions, pneumonia, or meningitis.11
Secondary maternal outcomes were the components of the primary outcome; specific infections, including chorioamnionitis, endometritis, wound infections, abdominal or pelvic abscess, mastitis or breast abscess, pneumonia, or pyelonephritis; and therapeutic use of antibiotics, duration of hospital stay, readmission, admission to a special care unit, and unscheduled health care visits. Key secondary neonatal outcomes were the components of the primary outcome, other infections, the duration of hospital stay, readmission, admission to a special care unit, unscheduled health care visits, and safety outcomes. We examined the results of bacterial growth and antimicrobial resistance from clinical cultures, including blood samples. Safety outcomes were reported as maternal or neonatal adverse events, including medication side effects (nausea, vomiting, and diarrhea) and allergy (anaphylaxis, liver failure, arrhythmias, and infant pyloric stenosis). Details regarding the definitions for secondary outcomes are provided in Table S2.
PROCEDURES
We implemented guidelines for monitoring patients’ temperatures (Table S3). All the patients were educated about signs and symptoms of infection and instructed to call the research team or go to the health facility or health care provider with any issues. Trained research staff members collected data from medical records or directly from the patients. Outcomes were identified before discharge; during visits at postpartum days 3, 7, and 42; during visits or telephone contacts at postpartum days 14 and 28; and as part of record review for any health care visits through day 42. Centralized, masked adjudication of primary and key secondary outcomes by the first two authors supplemented ascertainment.
STATISTICAL ANALYSIS
We estimated that a sample size of 34,000 patients would provide the trial with more than 90% power to detect a relative difference of 20% between the azithromycin group and the placebo group in the maternal primary outcome on the basis of a baseline incidence of 3% across sites, assuming a two-sided alpha level of 0.05 and a loss to follow-up of 2 to 3%. The trial was also designed to ensure more than 90% power to detect a relative difference of at least 25% in the neonatal primary outcome on the basis of an incidence of at least 8% at baseline at a two-sided alpha level of 0.05 overall and separately for the African and Asian sites. We also determined that a sample size of 5500 women at high risk for infection would provide a power of at least 80% to detect a relative risk difference of 30 to 35% in the maternal primary outcome, assuming a baseline risk of 5 to 6% and a loss to follow-up of 2 to 3%. (At the time of randomization, high risk was defined as a labor duration of ≥18 hours, the rupture of membranes ≥8 hours before randomization, or both.)
The primary analyses were performed in the intention-to-treat population. We compared the primary outcomes in the two groups using generalized linear models after adjustment for trial site with imputation for missing variables to estimate the relative risks and 95% confidence intervals. Models for neonatal outcomes accounted for correlation among multiple births. We calculated P values to test each of the primary hypotheses at an alpha level of 0.05 overall, with a nominal alpha level of 0.0001 at the interim analysis. We also assessed primary outcomes post hoc using a P value of less than 0.025 to account for the two primary outcomes. Secondary analyses of the primary outcomes included subgroup analyses according to region (Africa or Asia) and the presence or absence of a high risk of infection. We performed additional prespecified subgroup analyses of the primary outcomes according to the prophylactic use of any antibiotic during labor and delivery mode (cesarean or vaginal) and post hoc analyses that examined additional potential effect modifiers, including gestational age and type of labor. Exploratory analyses included an alternative definition of being at high risk for infection as assessed before delivery rather than at randomization. Secondary maternal and neonatal outcomes were analyzed in the intention-to-treat population with corresponding relative risk or mean difference and 95% confidence intervals.
With oversight from the data and safety monitoring committee, we performed one planned interim analysis of efficacy and futility for both primary outcomes. We determined cutoff P values for testing for efficacy using a Bonferroni-type correction for multiple comparisons to ensure an overall alpha level of 0.05, which was controlled with a nominal alpha level of 0.0001 for each outcome at the interim analysis and a 0.0499 level for the final analysis. Futility assessment was based on an analysis of conditional power. The data and safety monitoring committee could recommend stopping for efficacy only on two conditions: if the results for both primary outcomes were significant in all patients and the direction and magnitude of effect in subgroups (risk or region) were consistent or if efficacy was observed in one outcome or subgroup and there was no conditional power to draw conclusions in the other outcome or subgroup. After the interim analysis in which both primary outcomes had been evaluated in approximately 70% of the patients, the data and safety monitoring committee recommended stopping the trial because of maternal benefit.
RESULTS
PATIENTS
From September 9, 2020, through August 18, 2022, a total of 44,078 women underwent screening and 29,278 underwent randomization: 14,590 women (with 14,687 neonates or stillbirths) to the azithromycin group and 14,688 women (14,782 neonates or stillbirths) to the placebo group (Fig. 1). Advanced labor and planned cesarean delivery were the most common reasons for exclusion.
Figure 1. Enrollment and Outcomes.
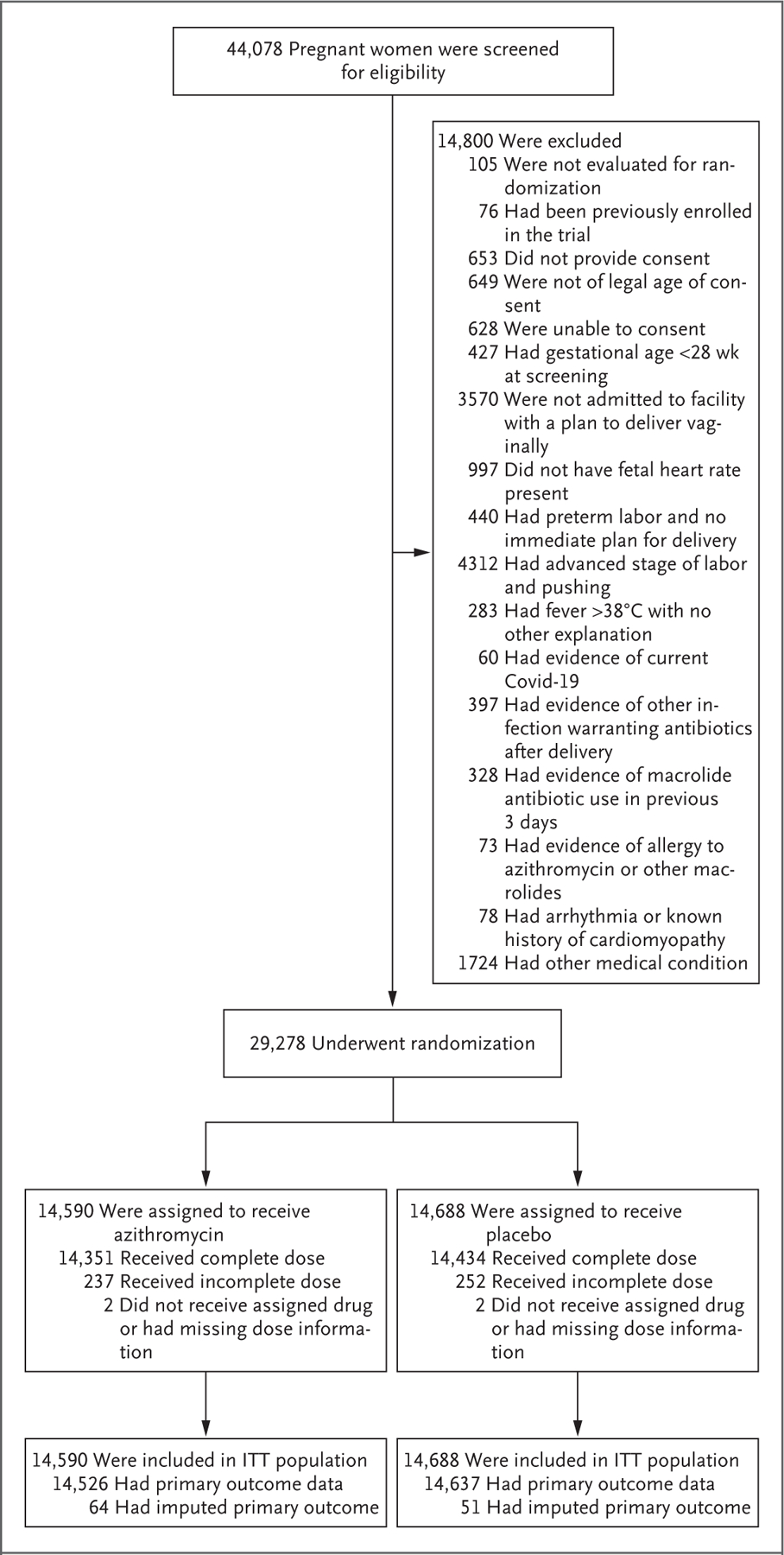
A total of 44,078 pregnant women who had been admitted to health facilities for spontaneous or induced vaginal delivery were screened for eligibility. After all exclusions, including evidence of coronavirus disease 2019 (Covid-19) and other infections, 14,590 women were assigned to receive azithromycin and 14,688 to receive placebo. ITT denotes intention to treat.
The characteristics of the two groups were similar at baseline (Table 1). The majority of patients (55%) were enrolled in Asia; at randomization, 18.4% had induced labor, and 8.6% were at high risk for infection.
Table 1.
Maternal Characteristics at Baseline.*
| Characteristic | Azithromycin (N = 14,590) | Placebo (N = 14,688) |
| Region of residence — no. (%) | ||
| Africa | 5,779 (39.6) | 5,801 (39.5) |
| Asia | 8,017 (54.9) | 8,084 (55.0) |
| Latin America | 794 (5.4) | 803 (5.5) |
| Median age (IQR) — yr | 24.0 (21.0–28.0) | 24.0 (21.0–28.0) |
| Married — no./total no. (%) | 13,729/14,589 (94.1) | 13,834/14,687 (94.2) |
| Maternal education — no./total no. (%) | ||
| No formal schooling | 3,457/14,565 (23.7) | 3,476/14,665 (23.7) |
| 1–6 yr of schooling | 2,002/14,565 (13.7) | 2,022/14,665 (13.8) |
| 7–12 yr of schooling | 7,308/14,565 (50.2) | 7,325/14,665 (49.9) |
| ≥13 yr of schooling | 1,798/14,565 (12.3) | 1,842/14,665 (12.6) |
| Primiparous — no./total no. (%) | 6,311/14,588 (43.3) | 6,376/14,687 (43.4) |
| Multiple birth — no./total no. (%) | 99/14,588 (0.7) | 95/14,687 (0.6) |
| Any infection during pregnancy — no./total no. (%)† | 797/14,589 (5.5) | 821/14,687 (5.6) |
| Any medical condition during pregnancy — no./total no. (%)‡ | 1,017/14,589 (7.0) | 955/14,687 (6.5) |
| Gestational age <37 wk — no./total no. (%) | 1,841/14,583 (12.6) | 1,895/14,684 (12.9) |
| Labor induction — no./total no. (%) | 2,651/14,581 (18.2) | 2,724/14,677 (18.6) |
| High risk for sepsis before randomization — no./total no. (%) | 1,247/14,588 (8.5) | 1,283/14,687 (8.7) |
| Prolonged labor ≥18 hr before randomization | 670/14,588 (4.6) | 698/14,687 (4.8) |
| Prolonged rupture of membranes ≥8 hours before randomization | 615/14,588 (4.2) | 632/14,687 (4.3) |
Percentages may not total 100 because of rounding. IQR denotes interquartile range.
Among the maternal infections during pregnancy were group B streptococcus, pneumonia, pyelonephritis, rubella, chlamydia, herpes, syphilis, gonorrhea, human immunodeficiency virus, hepatitis B, malaria, and urinary tract infection.
Among the maternal conditions during pregnancy were diabetes, chronic hypertension, and hypertensive disorders of pregnancy.
The groups were also well balanced with respect to labor and delivery characteristics (incidence of cesarean delivery, receipt of prophylactic antibiotics, high-risk status at delivery, and the median time between randomization and delivery [3 hours]) (Table S4). The frequencies of prophylactic antibiotic use (mainly cephalosporins) and cesarean delivery varied according to site, with higher occurrences at non-African sites (Tables S5 and S6). Complete intake of azithromycin or placebo was high in both groups (>98%), and vomiting within 15 minutes after ingestion was rare (Table S7).
PRIMARY OUTCOMES
Maternal sepsis or death (the composite primary outcome) occurred in 227 of 14,526 patients (1.6%) in the azithromycin group and in 344 of 14,637 (2.4%) in the placebo group (adjusted relative risk, 0.67; 95% confidence interval [CI], 0.56 to 0.79; P<0.001) (Table 2). This finding remained clearly significant according to the more conservative criterion of a P value of less than 0.025. Maternal sepsis occurred in 219 women (1.5%) in the azithromycin group and in 339 (2.3%) in the placebo group (relative risk, 0.65; 95% CI, 0.55 to 0.77); death from sepsis occurred in less than 0.1% of the women in each group.
Table 2.
Maternal and Neonatal Primary Outcomes and Their Components.
| Outcome | Azithromycin | Placebo | Relative Risk (95% CI) * | P Value † | |
| no./total no. (%) | |||||
| Maternal | |||||
| Death or sepsis within 6 wk after birth | 227/14,526 (1.6) | 344/14,637 (2.4) | 0.67 (0.56–0.79) | <0.001 | |
| Sepsis | 219/14,558 (1.5) | 339/14,662 (2.3) | 0.65 (0.55–0.77) | ||
| Death | |||||
| From any cause | 11/14,526 (0.1) | 9/14,635 (0.1) | 1.23 (0.51–2.97) | ||
| From sepsis | 4/14,526 (<0.1) | 1/14,635 (<0.1) | 4.04 (0.45–36.14) | ||
| Neonatal | |||||
| Stillbirth or neonatal death or sepsis within 4 wk after birth | 1,540/14,658 (10.5) | 1,526/14,756 (10.3) | 1.02 (0.95–1.09) | 0.56 | |
| Stillbirth | 59/14,687 (0.4) | 56/14,782 (0.4) | 1.06 (0.74–1.53) | ||
| Death | |||||
| Within 4 wk after birth | 222/14,598 (1.5) | 219/14,700 (1.5) | 1.03 (0.86–1.24) | ||
| From sepsis | 64/14,598 (0.4) | 62/14,700 (0.4) | 1.04 (0.73–1.47) | ||
| Sepsis | 1,433/14,570 (9.8) | 1,407/14,652 (9.6) | 1.03 (0.96–1.10) | ||
Relative risks and 95% confidence intervals were calculated with the use of generalized linear models that included terms for group assignment and site. Models for neonatal outcomes account for correlation among multiple births on the assumption of an exchangeable covariance structure.
P values were calculated with the use of multiple imputation for missing outcomes implemented separately for mothers and neonates by means of logistic regression imputation with the use of site and high-risk status before randomization as covariates.
Stillbirth or neonatal death or sepsis within 4 weeks after delivery (the neonatal composite primary outcome) occurred in 1540 of 14,658 infants (10.5%) in the azithromycin group and in 1526 of 14,756 infants (10.3%) in the placebo group (relative risk, 1.02; 95% CI, 0.95 to 1.09; P = 0.56). Neonatal sepsis occurred in 1433 infants (9.8%) and in 1407 infants (9.6%), respectively (relative risk, 1.03; 95% CI, 0.96 to 1.10). In each of the two groups, the incidence of stillbirth was 0.4% and the incidence of neonatal death was 1.5%.
SUBGROUP ANALYSES
Results of prespecified and post hoc subgroup analyses of the primary outcomes are shown in Figure 2. Findings in subgroups were generally consistent with the overall results, with the exception that there appeared to be greater maternal benefit with azithromycin in Africa (relative risk, 0.47; 95% CI, 0.36 to 0.61) than in Asia (relative risk, 0.88; 95% CI, 0.70 to 1.10).
Figure 2. Specified and Post Hoc Subgroup Analyses.
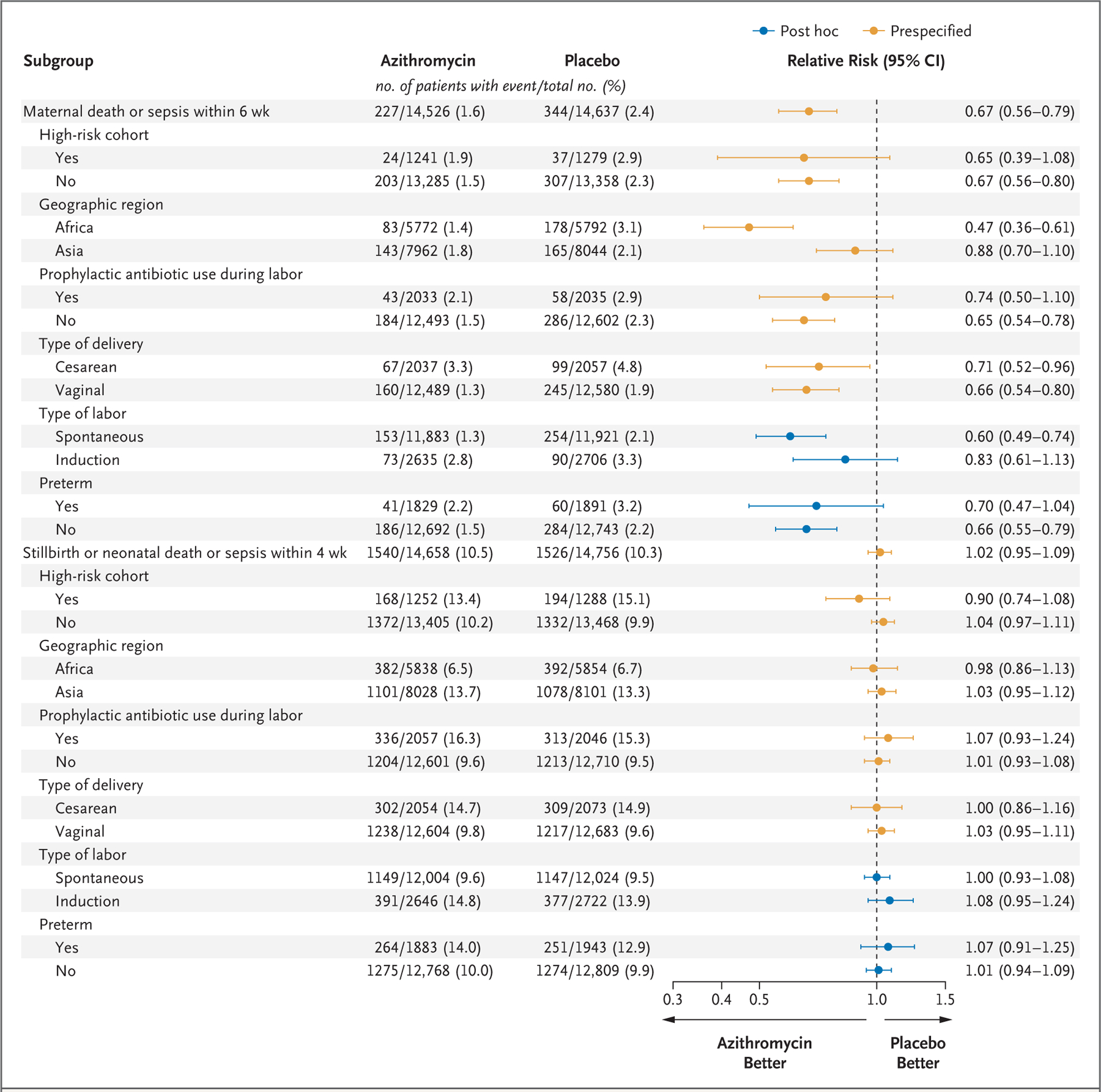
Relative risks and 95% confidence intervals were calculated from generalized linear models that included terms for assigned group, site, subgroup, and an interaction term for the assigned group according to subgroup. Models for neonatal outcomes account for correlation among multiple births on the assumption of an exchangeable covariance structure. If model-convergence problems occurred, the generalized linear model was fit without the adjustment for correlation among multiple births. Relative risks and 95% confidence intervals have not been adjusted for multiplicity and should not be used in place of hypothesis testing.
SECONDARY MATERNAL AND NEONATAL OUTCOMES
Endometritis occurred in 1.3% of the women in the azithromycin group and in 2.0% of those in the placebo group (relative risk, 0.66; 95% CI, 0.55 to 0.79), wound infections (cesarean and perineal) in 1.6% and 2.2%, respectively (relative risk, 0.71; 95% CI, 0.60 to 0.84), and other infections in 1.0% and 1.5%, respectively (relative risk, 0.69; 95% CI, 0.56 to 0.85); chorioamnionitis was rare in the two groups. These and other secondary outcomes, including hospital readmissions and unscheduled visits, are shown in Table 3.
Table 3.
Secondary Maternal and Neonatal Outcomes.*
| Outcome | Azithromycin | Placebo | Relative Risk (95% CI) † |
| Maternal | |||
| Infection — no./total no. (%) | |||
| Chorioamnionitis | 5/14,590 (<0.1) | 8/14,688 (<0.1) | NA |
| Endometritis | 191/14,558 (1.3) | 294/14,659 (2.0) | 0.66 (0.55– 0.79) |
| Wound according to method of delivery | |||
| Cesarean | 77/2,038 (3.8) | 134/2,060 (6.5) | 0.57 (0.43–0.75) |
| Perineal | 149/12,519 (1.2) | 188/12,595 (1.5) | 0.80 (0.65–0.99) |
| Other | 149/14,558 (1.0) | 217/14,657 (1.5) | 0.69 (0.56–0.85) |
| Abdominal or pelvic abscess | 4/14,558 (<0.1) | 6/14,657 (<0.1) | 0.67 (0.19–2.37) |
| Mastitis or breast abscess | 38/14,558 (0.3) | 57/14,655 (0.4) | 0.67 (0.44–1.01) |
| Pyelonephritis | 11/14,558 (0.1) | 43/14,655 (0.3) | 0.26 (0.13–0.50) |
| Pneumonia | 29/14,558 (0.2) | 31/14,657 (0.2) | 0.95 (0.57–1.57) |
| Other bacterial infection | 68/14,558 (0.5) | 81/14,657 (0.6) | 0.85 (0.61–1.17) |
| Antibiotic therapy from randomization to 42 days postpartum — no./total no. (%) | |||
| For any reason | 7,937/14,567 (54.5) | 8,180/14,672 (55.8) | 0.98 (0.95–1.01) |
| For treatment | 458/14,558 (3.1) | 730/14,660 (5.0) | 0.63 (0.56–0.71) |
| Time from drug administration until initial discharge after delivery — days | 1.4±1.8 | 1.4±1.9 | NA |
| Postpartum care — no./total no. (%) | |||
| Readmission ≤42 days after delivery | 124/14,552 (0.9) | 192/14,646 (1.3) | 0.65 (0.52–0.82) |
| Admission to special care unit | 116/14,560 (0.8) | 130/14,662 (0.9) | 0.90 (0.70–1.15) |
| Unscheduled visit for care | 1,397/14,554 (9.6) | 1,790/14,649 (12.2) | 0.79 (0.73–0.84) |
| Neonatal | |||
| Other infection — no./total no. (%)‡ | 763/14,573 (5.2) | 798/14,657 (5.4) | 0.97 (0.88–1.07) |
| Initial length of hospital stay — days | 1.5±2.4 | 1.5±2.1 | NA |
| Postnatal care — no./total no. (%) | |||
| Readmission ≤42 days after delivery | 553/14,420 (3.8) | 518/14,514 (3.6) | 1.08 (0.96–1.21) |
| Admission to special care unit | 951/14,499 (6.6) | 927/14,585 (6.4) | 1.03 (0.94–1.12) |
| Unscheduled visit for care | 3,223/14,458 (22.3) | 3,366/14,565 (23.1) | 0.96 (0.93–1.00) |
Plus–minus values are means ±SD.
Relative risks and 95% confidence intervals have not been adjusted for multiplicity and should not be used in place of hypothesis testing. NA denotes that the calculation of relative risk was not applicable because models were not constructed for this category.
Other neonatal infections included eye infection with swelling and drainage, skin infection with 10 or more pustules or bullae, omphalitis, urinary tract infection, pyelonephritis or kidney infection, pneumonia or lung infection with a respiratory rate of 60 breaths per minute or more, meningitis, and other infections that had been documented in the clinical record.
ADVERSE EVENTS
At least one maternal side effect was reported in 7.1% of mothers in the azithromycin group and in 7.6% in the placebo group; none of the side effects were substantively more frequent in the azithromycin group (Table S8). Pyloric stenosis was diagnosed in 8 infants in the azithromycin group and in 3 in the placebo group.
SENSITIVITY ANALYSES
The frequency of at least one protocol deviation was similar in the two groups (Table S9). Results of sensitivity analyses that considered alternative outcomes for those patients who were lost to follow-up and survival analyses for the primary outcomes were not materially different from those in the primary analyses (Table S10 and Figs. S1 and S2).
DISCUSSION
In this multicountry, randomized trial involving pregnant women in labor who were planning a vaginal delivery, azithromycin prophylaxis led to a significantly lower frequency of maternal sepsis or death than placebo but had little effect on stillbirth or neonatal sepsis or death. Maternal deaths were infrequent in both groups; findings were driven by the effects of azithromycin on maternal sepsis. The frequencies of selected maternal infections that cause sepsis (including endometritis, cesarean or perineal wound infections, and pyelonephritis) maternal readmissions, and unscheduled health care visits were consistent with the primary maternal results. Findings for individual neonatal outcomes mirrored those for the primary neonatal outcome. The number of women who would need to be treated to prevent one case of maternal death or sepsis was 125; the same number would need to be treated to prevent one maternal sepsis event. In addition, apart from a potential greater benefit in Africa than in Asia, the benefit did not appear to vary according to subgroup, including risk status for infection.
Our results are consistent with findings from a large U.S. trial and other studies involving the use of azithromycin in women who had undergone a cesarean delivery and received usual antibiotics.4,12–14 In the U.S. trial, the use of azithromycin resulted in a lower incidence of maternal infections (including a 50% lower risk of endometritis and wound infections) than the use of placebo and was associated with fewer readmissions or unscheduled care visits but did not affect newborn outcomes.4 Our finding of maternal benefit was also consistent with the results of two small trials involving women in labor who were planning a vaginal delivery: one trial involving high-risk women in Cameroon and the other involving women regardless of risk in Gambia.7,15 In contrast to other trials, the Gambian trial also suggested potential benefit of azithromycin in preventing neonatal infections.7 This discrepancy could be due to the inclusion in the Gambian trial of neonatal skin infections among key outcomes and less frequent use of antibiotics in usual care than in our trial. Although chorioamnionitis was rare in our population, the incidences of maternal and neonatal infections that we observed were consistent with estimates from previous trials and global surveys.7,15,16
The strengths of our trial include the large enrollment in multiple countries, the use of WHO clinical definitions of maternal and neonatal sepsis, and blinded adjudication of key outcomes. These factors represent an improvement over previous studies of azithromycin to prevent maternal and neonatal infection in low- and middle-income countries. Staff members received frequent training on key protocol features, including temperature monitoring and criteria for infection outcomes. The trial population broadly reflected the general population of women who were giving birth in these countries on the basis of our ongoing population-based registry of largely rural clusters (Table S13).
Among the trial limitations, the incidence of maternal sepsis or death was 2% in Asia, as compared with the projected incidence of 3% or more, a factor that limited the statistical power for this subgroup. Furthermore, the frequencies of prophylactic use of antibiotics (which partially reflects increased screening for group B streptococcus) and cesarean birth varied according to site and were particularly high in several non-African sites. Although these factors probably blunted the effect of the intervention, maternal benefits were still observed in the overall population and in subgroups according to mode of delivery. The use of azithromycin is postulated to reduce infections because of its broad antimicrobial coverage, including for ureaplasmas or mycoplasmas and some anaerobes that may not be covered by other common antibiotics.12,17,18 However, we did not perform cultures for these specific microorganisms, a factor that limits our ability to evaluate this mechanism.
Potential harms of adding routine azithromycin for vaginal deliveries include increased antimicrobial resistance, effects of changes to the maternal or neonatal microbiome, and drug side effects and costs. The high prevalence of antibiotic use in non-African sites increases these concerns. Although available studies have not shown significant associations between a single azithromycin dose and sustained carriage of resistant organisms or an increase in resistant infections,4,17,19–21 more long-term data are needed to inform the association between the routine use of oral azithromycin prophylaxis for vaginal delivery and macrolide resistance patterns and subsequent effects on the microbiome.
Adverse effects were similar in the two trial groups. No safety signal was observed regarding sudden cardiac death, which was consistent with the results of other studies of a single dose of azithromycin and those involving women of reproductive age.4,19,22,23 More cases of pyloric stenosis were observed in the azithromycin group, a finding that warrants further surveillance, but these cases were rare in both groups, with incidences that were lower than the expected background rates.24 Previous data have suggested an increased risk with postnatal but not prenatal use of azithromycin.25,26 Studies of factors that influence the effects of azithromycin and of its implementation may further inform the generalizability of our findings.
In this multicenter trial, the use of intrapartum oral azithromycin among women who were planning a vaginal delivery resulted in a lower risk of maternal sepsis or death than placebo, an outcome that was driven by a reduction in sepsis. However, the intervention did not reduce the risk of sepsis or death in newborns.
The views expressed in this article are those of the authors and do not necessarily represent the views of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institutes of Health, the Department of Health and Human Services, or the Bill and Melinda Gates Foundation.
Supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and a grant from the Foundation for the National Institutes of Health through the Maternal, Newborn, and Child Health Discovery and Tools initiative of the Bill and Melinda Gates Foundation.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Supplementary Material
Footnotes
This is the New England Journal of Medicine version of record, which includes all Journal editing and enhancements. The Author Accepted Manuscript, which is the author’s version after external peer review and before publication in the Journal, is available at PubMed Central.
Contributor Information
A.T.N. Tita, University of Alabama at Birmingham, Birmingham
W.A. Carlo, University of Alabama at Birmingham, Birmingham
E.M. McClure, RTI International, Durham, North Carolina
M. Mwenechanya, University Teaching Hospital, Lusaka, Zambia
E. Chomba, University Teaching Hospital, Lusaka, Zambia
J.J. Hemingway-Foday, RTI International, Durham, North Carolina
A. Kavi, Women’s and Children’s Health Research Unit, KLE Academy of Higher Education and Research, Jawaharlal Nehru Medical College, Belagavi, India
M.C. Metgud, Women’s and Children’s Health Research Unit, KLE Academy of Higher Education and Research, Jawaharlal Nehru Medical College, Belagavi, India
S.S. Goudar, Women’s and Children’s Health Research Unit, KLE Academy of Higher Education and Research, Jawaharlal Nehru Medical College, Belagavi, India
R. Derman, Thomas Jefferson University, Philadelphia
A. Lokangaka, Kinshasa School of Public Health, Kinshasa, Democratic Republic of Congo
A. Tshefu, Kinshasa School of Public Health, Kinshasa, Democratic Republic of Congo
M. Bauserman, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina
C. Bose, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina
P. Shivkumar, Mahatma Gandhi Institute of Medical Sciences, Sewagram, India
M. Waikar, Government Medical College, Nagpur, India
A. Patel, Lata Medical Research Foundation, Nagpur, India; Datta Meghe Institute of Medical Sciences, Wardha, India
P.L. Hibberd, Boston University School of Public Health, Boston
P. Nyongesa, Moi University School of Medicine, Eldoret, Kenya
F. Esamai, Moi University School of Medicine, Eldoret, Kenya
O.A. Ekhaguere, Indiana School of Medicine, University of Indiana, Indianapolis
S. Bucher, Indiana School of Medicine, University of Indiana, Indianapolis
S. Jessani, Aga Khan University, Karachi, Pakistan
S.S. Tikmani, Aga Khan University, Karachi, Pakistan
S. Saleem, Aga Khan University, Karachi, Pakistan
R.L. Goldenberg, Columbia University School of Medicine, New York
S.M. Billah, International Center for Diarrheal Disease research, Dhaka, Bangladesh; University of Sydney, Sydney
R. Lennox, LAMB Hospital, Parbattipur, Bangladesh
R. Haque, International Center for Diarrheal Disease research, Dhaka, Bangladesh
W. Petri, University of Virginia, Charlottesville
L. Figueroa, Instituto de Nutrición de Centroamérica y Panamá, Guatemala City, Guatemala
M. Mazariegos, Instituto de Nutrición de Centroamérica y Panamá, Guatemala City, Guatemala
N.F. Krebs, University of Colorado–Anschutz Medical Campus, Denver
J.L. Moore, RTI International, Durham, North Carolina
T.L. Nolen, RTI International, Durham, North Carolina
M. Koso-Thomas, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD
REFERENCES
- 1.World Health Organization. WHO recommendations for the prevention and treatment of maternal peripartum infections September 28, 2015. (https://www.who.int/publications/i/item/9789241549363). [PubMed]
- 2.Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet 2015;385:430–40. [DOI] [PubMed] [Google Scholar]
- 3.reinhart K, Daniels R, Kissoon N, Machado FR, Schachter RD, Finfer S. Recognizing sepsis as a global health priority — a WHO resolution. N Engl J Med 2017; 377:414–7. [DOI] [PubMed] [Google Scholar]
- 4.Tita AT, Szychowski JM, Boggess K, et al. Adjunctive azithromycin prophylaxis for cesarean delivery. N Engl J Med 2016; 375:1231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harper LM, Kilgore M, Szychowski JM, Andrews WW, Tita ATN. Economic evaluation of adjunctive azithromycin prophylaxis for cesarean delivery. Obstet Gynecol 2017;130:328–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Committee on Practice Bulletins-Obstetrics. ACOG practice bulletin no. 199: use of prophylactic antibiotics in labor and delivery. Obstet Gynecol 2018; 132(3):e103–e119. [DOI] [PubMed] [Google Scholar]
- 7.Oluwalana C, Camara B, Bottomley C, et al. Azithromycin in labor lowers clinical infections in mothers and newborns: a double-blind trial. Pediatrics 2017;139(2): e20162281. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. Statement on maternal sepsis May 31, 2017. (https://www.who.int/publications/i/item/WHO-rHr-17.02).
- 9.Albright CM, Has P, rouse DJ, Hughes BL. Internal validation of the Sepsis in Obstetrics Score to identify risk of morbidity from sepsis in pregnancy. Obstet Gynecol 2017;130:747–55. [DOI] [PubMed] [Google Scholar]
- 10.Bowyer L, robinson HL, Barrett H, et al. SOMANZ guidelines for the investigation and management of sepsis in pregnancy. Aust N Z J Obstet Gynaecol 2017; 57:540–51. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. Guideline: managing possible serious bacterial infection in young infants when referral is not feasible 2015. (http://apps.who.int/iris/bitstream/handle/10665/181426/9789241509268_eng.pdf). [PubMed]
- 12.Andrews WW, Hauth JC, Cliver SP, Savage K, Goldenberg rL. randomized clinical trial of extended spectrum antibiotic prophylaxis with coverage for Ureaplasma urealyticum to reduce post-cesarean delivery endometritis. Obstet Gynecol 2003;101:1183–9. [DOI] [PubMed] [Google Scholar]
- 13.Tita ATN, Hauth JC, Grimes A, Owen J, Stamm AM, Andrews WW. Decreasing incidence of postcesarean endometritis with extended-spectrum antibiotic prophylaxis. Obstet Gynecol 2008;111:51–6. [DOI] [PubMed] [Google Scholar]
- 14.Tita AT, Owen J, Stamm AM, Grimes A, Hauth JC, Andrews WW. Impact of extended-spectrum antibiotic prophylaxis on incidence of postcesarean surgical wound infection. Am J Obstet Gynecol 2008;199(3):303.e1–303.e3. [DOI] [PubMed] [Google Scholar]
- 15.Subramaniam A, Ye Y, Mbah r, et al. Single dose of oral azithromycin with or without amoxicillin to prevent peripartum infection in laboring, high-risk women in Cameroon: a randomized controlled trial. Obstet Gynecol 2021;138:703–13. [DOI] [PubMed] [Google Scholar]
- 16.WHO Global Maternal Sepsis Study (GLOSS) research Group. Frequency and management of maternal infection in health facilities in 52 countries (GLOSS): a 1-week inception cohort study. Lancet Glob Health 2020;8(5):e661–e671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tita ATN, Rouse DJ, Blackwell S, Saade GR, Spong CY, Andrews WW. Emerging concepts in antibiotic prophylaxis for cesarean delivery: a systematic review. Obstet Gynecol 2009;113:675–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Subramaniam A, Waites KB, Jauk VC, et al. Azithromycin-based extended-spectrum antibiotic prophylaxis for cesarean: role of placental colonization with genital ureaplasmas and mycoplasmas. Am J Perinatol 2019;36:1002–8. [DOI] [PubMed] [Google Scholar]
- 19.roca A, Oluwalana C, Bojang A, et al. Oral azithromycin given during labour decreases bacterial carriage in the mothers and their offspring: a double-blind randomized trial. Clin Microbiol Infect 2016;22(6):565.e1–565.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bojang A, Camara B, Jagne Cox I, et al. Long-term impact of oral azithromycin taken by Gambian women during labor on prevalence and antibiotic susceptibility of Streptococcus pneumoniae and Staphylococcus aureus in their infants: follow-up of a randomized clinical trial. Clin Infect Dis 2018;67:1191–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bojang A, Baines SL, Camara B, et al. Impact of intrapartum oral azithromycin on the acquired macrolide resistome of infants’ nasopharynx: a randomized controlled trial. Clin Infect Dis 2020;71:3222–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ray WA, Murray KT, Hall K, Arbogast PG, Stein CM. Azithromycin and the risk of cardiovascular death. N Engl J Med 2012;366:1881–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Svanström H, Pasternak B, Hviid A. Use of azithromycin and death from cardiovascular causes. N Engl J Med 2013; 368:1704–12. [DOI] [PubMed] [Google Scholar]
- 24.Lin KJ, Mitchell AA, Yau W-P, Louik C, Hernández-Díaz S. Safety of macrolides during pregnancy. Am J Obstet Gynecol 2013;208(3):221.e1–221.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooper WO, ray WA, Griffin Mr. Prenatal prescription of macrolide antibiotics and infantile hypertrophic pyloric stenosis. Obstet Gynecol 2002;100:101–6. [DOI] [PubMed] [Google Scholar]
- 26.Abdellatif M, Ghozy S, Kamel MG, et al. Association between exposure to macrolides and the development of infantile hypertrophic pyloric stenosis: a systematic review and meta-analysis. Eur J Pediatr 2019;178:301–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


