Abstract
Background
The role of chemotherapy in the treatment of patients with non‐small cell lung cancer was not clear. A systematic review and quantitative meta‐analysis was therefore undertaken to evaluate the available evidence from all relevant randomised trials.
Objectives
To evaluate the effect of cytotoxic chemotherapy on survival in patients with non‐small cell lung cancer. To investigate whether or not pre‐defined patient sub‐groups benefit more or less from chemotherapy.
Search methods
MEDLINE and CANCERLIT searches (1963‐june 1992) were supplemented by information from trial registers and by hand searching relevant meeting proceedings and by discussion with relevant trialists and organisations.
Selection criteria
Trials comparing primary treatments of surgery, surgery + radiotherapy, radical radiotherapy or supportive care versus the same primary treatment, plus chemotherapy were eligible for inclusion provided that they randomised non‐small cell lung cancer patients using a method which precluded prior knowledge of treatment assignment.
Data collection and analysis
A quantitative meta‐analysis using updated information from individual patients from all available randomised trials was carried out. Data from all patients randomised in all eligible trials were sought directly from those responsible. Updated information on survival, and date of last follow up were obtained, as were details of treatment allocated, date of randomisation, age, sex, histological cell type, stage and performance status. To avoid potential bias, information was requested for all randomised patients including those who had been excluded from the investigators' original analyses. All analyses were done on intention to treat on the endpoint of survival. For trials using cisplatin‐based regimens, subgroup analyses by age, sex, histological cell type, tumour stage and performance status were also done.
Main results
Data from 52 trials and 9387 patients were included. The results for modern regimens containing cisplatin favoured chemotherapy in all comparisons and reached conventional levels of significance when used with radical radiotherapy and with supportive care. Trials comparing surgery with surgery plus chemotherapy gave a hazard ratio of 0.87 (13% reduction in the risk of death, equivalent to an absolute benefit of 5% at 5 years). Trials comparing radical radiotherapy with radical radiotherapy plus chemotherapy gave a hazard ratio 0.87 (13% reduction in the risk of death equivalent to an absolute benefit of 4% at 2 years), and trials comparing supportive care with supportive care plus chemotherapy gave a hazard ratio of 0.73 (27% reduction in the risk of death equivalent to a 10% improvement in survival at one year). The essential drugs needed to achieve these effects were not identified. No difference in the size of effect was seen in any subgroup of patients. In all but the radical radiotherapy setting, older trials using long term alkylating agents tended to show a detrimental effect of chemotherapy. This effect reached conventional significance in the adjuvant surgical comparison.
Authors' conclusions
At the outset of this meta‐analysis there was considerable pessimism about the role of chemotherapy in the treatment of non‐small cell lung cancer. These results offer hope of progress and suggest that chemotherapy may have a role in treating this disease.
Keywords: Humans; Antineoplastic Agents; Antineoplastic Agents/therapeutic use; Carcinoma, Non‐Small‐Cell Lung; Carcinoma, Non‐Small‐Cell Lung/drug therapy; Lung Neoplasms; Lung Neoplasms/drug therapy; Meta‐Analysis as Topic; Randomized Controlled Trials as Topic
Plain language summary
Chemotherapy can improve survival rates for non‐small cell lung cancer
Non‐small cell lung cancer is the most common type of lung cancer. The standard treatment for small tumours is surgery (operation to remove the tumour) or surgery and radiotherapy (x‐ray treatment). Where the tumour has spread within the chest, standard treatment is radiotherapy. Where the tumour has spread beyond the chest supportive (treatment to relieve symptoms) is given. Trials have tried giving chemotherapy (drugs) after these standard treatments to find out whether it can help people to live longer. This review found that giving chemotherapy after either radiotherapy or supportive care did seem to help patients live longer. Giving chemotherapy after radiotherapy to 1000 patients would mean that an extra 40 patients would be expected to be alive 2 years later, than if the chemotherapy was not given. Giving chemotherapy after supportive care to 1000 patients would mean that 100 more would be expected to be alive 2 years later, than if the chemotherapy was not given. Chemotherapy after surgery may also help patients live longer although the evidence to support this is less clear.
Background
More than half a million new cases of lung cancer are diagnosed each year (Parkin 1993). About 80% of these tumours are of non‐small cell histological type (Rankin 1986), including adenocarcinomas and squamous cell and large cell carcinomas. Non‐small cell lung cancer is the main cause of deaths related to cancer (Silverberg 1990), and five year survival across all stages of disease is about 12% (Boring 1993). Surgery is generally regarded as the best treatment option, but only about 20% of tumours are suitable for potentially curative resection (Rudd 1991). A further, small proportion of patients, usually those presenting with locally advanced disease, undergo radical thoracic radiotherapy. Most patients with late stage or metastatic disease are treated palliatively.
Although cytotoxic chemotherapy is used routinely in treating small cell lung cancer, its role in non‐small cell lung cancer remained controversial. This was despite over thirty years of research involving more than 10,000 patients in over 50 randomised clinical trials examining the efficacy of chemotherapy when combined with local treatment or best supportive care. With few exceptions, most trials were too small to reliably detect moderate treatment effects. Consequently, although a few trials reported significant results, both for and against chemotherapy, most trials were inconclusive. In 1991, an international consensus report concluded that post‐operative chemotherapy was of unproved benefit and should be considered experimental (Holmes 1991). In the same year, the British Medical Research Council's Cancer Trials Office, Cambridge; the Institut Gustave Roussy, Villejuif, France; and the Istituto Mario Negri, Milan, Italy initiated an individual patient data meta‐analysis to assess the role of chemotherapy in the treatment of non‐small cell lung cancer. This approach to meta‐analysis and systematic review involves the central collection, validation and analysis of the original trial data. It does not rely on data extracted from publications. At the outset, the secretariat contacted the investigators responsible for each trial and established the Non‐small Cell Lung Cancer Collaborative Group on whose behalf the meta‐analysis was carried out and published in the British Medical Journal in 1995 (NSCLCCG 1995). Since that time a number of further trials have been completed. However, the majority of these are not yet published and several large trials are still ongoing. Members of the secretariat met in 1999 and decided that the meta‐analysis should be updated when the results of these further trials become available. This is likely to be in 2000/2001.
Objectives
To compare, in terms of overall survival:
1. Surgery versus surgery plus adjuvant chemotherapy 2. Surgery plus radiotherapy versus surgery plus radiotherapy plus chemotherapy 3. Radical radiotherapy versus radical radiotherapy plus chemotherapy 4. Supportive care versus supportive care plus chemotherapy
in patients with histologically diagnosed non‐small cell lung cancer.
Trials where chemotherapy was given before surgery (neo‐adjuvant) were not included.
To investigate whether or not pre‐defined patient sub‐groups benefit more or less from cisplatin‐based chemotherapy in terms of survival. Quality of life was measured in only a few trials and so could not be reviewed in the meta‐analysis.
Methods
Criteria for considering studies for this review
Types of studies
Both published and unpublished trials were eligible for inclusion provided they randomised patients with non‐small cell lung cancer between one of the above four primary treatments and the same treatment plus an established form of cytotoxic chemotherapy. Each trial had to be unconfounded (i.e. differ only by the addition of chemotherapy to the treatment arm) and properly randomised. Trials allocating treatment by quasi random methods, e.g. by date of birth were not included. Trials were eligible if they started recruitment after 1 January 1965 and completed recruitment by 31 December 1991 (This upper date limit will be revised for the forthcoming update). Trials allowing patients to have received chemotherapy before randomisation were excluded. Trials in the early and locally advanced setting should not have permitted previous treatment for any other malignancy. Surgical trials were eligible only if they had randomised patients who had undergone a potentially curative resection and trials of neo‐adjuvant treatment were not included in this comparison as it was considered too early to evaluate the neo‐adjuvant approach. Trials of radical radiotherapy using orthovoltage radiotherapy or a total radiation dose of <30 Gy were excluded, as were trials in which drugs were used with the primary aim of sensitisation to radiation.
Types of participants
Eligible trials included individuals with histologically confirmed non‐small cell lung cancer. Individual data from all randomised patients were included in the meta‐analysis and where possible data were obtained for individuals who had been excluded from the original trial analyses. These individuals were included in the meta‐analysis. Patients with small cell lung cancer that were included in early trials that randomised all types of lung cancer were excluded from the meta‐analysis.
Types of interventions
1. Surgery vs surgery + adjuvant chemotherapy 2. Surgery + radiotherapy vs surgery + radiotherapy + chemotherapy 3. Radical radiotherapy vs radical radiotherapy + chemotherapy 4. Supportive care vs supportive care + chemotherapy
Trials investigating neo‐adjuvant chemotherapy, that is chemotherapy given before surgery were not included.
Trials were classified as belonging to one of four categories of chemotherapy: a) Regimens containing cisplatin b) Regimens using long‐term alkylating agents but not cisplatin c) Regimens containing etoposide or vinca alkaoids but not cisplatin d) Other regimens
Types of outcome measures
Mortality, death by any cause As is usual with cancer trials and meta‐analyses, results are discussed in terms of survival
Search methods for identification of studies
MEDLINE and CANCERLIT searches were carried out for the period 1963‐1990, and were updated to cover up to June 1992. Meetings abstracts of ASCO and the World Lung Cancer Conferences were hand searched as were bibliographies of books, reviews and specialist journals. Trial registers managed by the National Cancer Institute (PDQ, ClinProt), United Kingdom Coordinating Committee for Cancer Research and the Union Internationale Contre le Cancer were also consulted. All trialists who took part in the meta‐analysis were also asked to help identify trials.
At the time of the initial literature searches, the Cochrane Collaboration optimal search strategy was not yet established. Since the publication of the meta‐analysis (NSCLCCG 1995), all searches have been repeated using a modified version of the Cochrane Collaboration optimal search strategy.
Data collection and analysis
This review is based on individual patient data obtained directly from the responsible trialist or data centre. It does not use information extracted from published papers. All data were collected, checked and analysed centrally.
Trials were classified as belonging to one of four categories of chemotherapy:
a) Regimens containing cisplatin b) Regimens using long‐term alkylating agents but not cisplatin c) Regimens containing etoposide or vinca alkaoids but not cisplatin d) Other regimens
Note that the trials using oral alkylating agents (b) gave treatments for extended periods of more than one year as was considered best practice at the time, and that duration of treatment was not an exclusion criterium, but is rather purely descriptive.
Data were sought for all patients randomised in all eligible randomised trials (published or unpublished) and updated follow‐up requested. For all comparisons the following data were collected: patient identifier, treatment allocated, date of randomisation, survival status, date of last follow up or death and whether the individual was excluded from the original analyses. Data on age, sex, stage, histology and performance status were also collected. Collection and validation of data were carried out in two centres (Cancer Trials Office and Institut Gustave Roussy).
All data were checked thoroughly and a common database was agreed. The final database entries for each trial were verified by the responsible trialist or data centre. As stage was recorded using different classification systems, for the purposes of this meta‐analysis, all stage data were translated to a common staging system. Table 1
1. Common meta‐analysis stage scale.
| T | N | M | Meta‐analysis Stage | AJC Stage |
| 0,1,2,X,S | 0 | 0 | I | I |
| 0,1,2,X,S | 1 | 0 | II | II |
| Any | 2,3 | 0 | III | III non‐metastatic |
| 3,4 | Any | 0 | III | III non‐metastatic |
| Any | Any | 1 | IV | Any metastatic |
All analyses were based on intention to treat. Survival analyses were stratified by trial, and the log rank expected number of deaths and variance used to calculate individual and pooled hazard ratios (HRs) using the fixed effect model (Yusuf 1985). Thus, the times to death for individual patients were used within trials to calculate HRs representing the overall chance of dying when receiving chemotherapy in addition to primary treatment alone. HRs were also calculated for pre‐specified sub‐groups of patients using similar stratified methodology. Analyses were performed for each pre‐specified category, for example, for males and for females within each individual trial. These trial results were then combined to give overall HRs for males and females. Results are also presented as absolute differences at 2 years calculated using the HR and baseline event rate on the treatment alone arm; proportional hazards are assumed. Confidence intervals for absolute differences were similarly calculated from the baseline event rate and the HR at the 95% confidence interval boundary values. Chi‐squared tests were used to test for gross statistical heterogeneity over all trials in a comparison, between sub‐sets of trials, and between subgroups using the test for interaction or trend as appropriate (EBCTCG 1990). These tests are aimed primarily at detecting differences in effect size rather than direction and were chosen because qualitative differences were not anticipated.
Analyses of the "raw" individual patient data were done using an in‐house program (SCHARP). For transfer to the Cochrane Library, the log rank summary statistics of these analyses (o‐e and variance) were entered into RevMan under the individual patient data category. Survival curves were drawn as simple (non‐stratified) Kaplan Meier (Kaplan 1958) curves. These are not currently reproducible in the Cochrane Library but can be found in the original meta‐analysis publication (NSCLCCG 1995). All P values quoted are two‐sided.
Results
Description of studies
In total, 91 trials were identified as potentially eligible for the meta‐analysis. Thirty three of these were found to be ineligible and therefore excluded. Reasons for exclusions are listed in the table of excluded studies. Of the 58 eligible trials, data were not available from six as they had been lost, destroyed or were untraceable. These trials are also listed in the table of excluded studies. Data from 52 randomised trials and 9387 patients were therefore included in this meta‐analysis.
Risk of bias in included studies
Only trials with adequate methods of randomisation were included. Trials using quasi random methods such as birthdate were not included. All "raw" data received on individual patients were checked thoroughly to ensure both the accuracy of the meta‐analysis database and the quality of randomisation and follow‐up. Any queries were resolved and the final database entries verified by discussion with the responsible trial investigator or statistician.
Effects of interventions
EARLY DISEASE 1. Surgery vs surgery plus adjuvant chemotherapy Data were available from 14 trials (4357 patients,2574 deaths). Five early trials used long term alkylating agents , mainly cyclophosphamide and nitrosourea. Eight more recent trials used cisplatin based combination chemotherapy. Three of these used the regimen of cisplatin, doxorubicin and cyclophosphamide (CAP) and three used cisplatin with vindesine. The intended dose of cisplatin ranged from 40 mg/m² to 80 mg/m² per cycle and total dose from 50 mg/m² to 240 mg/m². A further three trials used other drug regimens, all of which included tegafur or UFT (tegafur plus uracil), a drug similar to fluorouracil. In all the trials chemotherapy was scheduled to start no later than six weeks after surgery.
There is considerable diversity of results across all trials and clear evidence of a difference in the direction of effect between the predefined categories of chemotherapy. The test for overall statistical heterogeneity is conventionally significant (P=0.02), as is the test for interaction (P=0.004). There is no evidence, however, of heterogeneity within each category of drugs(P=0.21). The results for each of the predefined chemotherapy categories should therefore be considered independently.
Trials using long‐term alkylating agents The results for trials using long term alkylating agents are consistent, all favour surgery alone. The combined hazard ratio is 1.15 (P=0.005) in favour of surgery alone. This 15% increase in the relative risk of death is equivalent to absolute detriments of chemotherapy of 4% at 2 years reducing survival from 70% to 66% and 5% at 5 years reducing survival from 50% to 45%.
Trials using cisplatin‐based regimens For regimens containing cisplatin, the results of most trials favour chemotherapy. There is no obvious statistical heterogeneity between the results of these trials (P=0.55). The overall hazard ratio of 0.87 (P=0.08), or 13% reduction in the risk of death, suggests an absolute benefit from chemotherapy of 3% at 2 years, improving survival from 70% to 73% and 5% at 5 years, improving survival from 50 to 55%. On their own these results are not conclusive such that the 95% confidence intervals for absolute difference in survival are consistent with a 0.5% detriment to a 7% benefit of chemotherapy at 2 years and similarly consistent with a 1% detriment to a 10% benefit at 5 years.
Other trials The trials that were classified as using other regimens give an estimated hazard ratio of 0.89 in favour of chemotherapy (P=0.30), but there was insufficient information to draw any reliable conclusions.
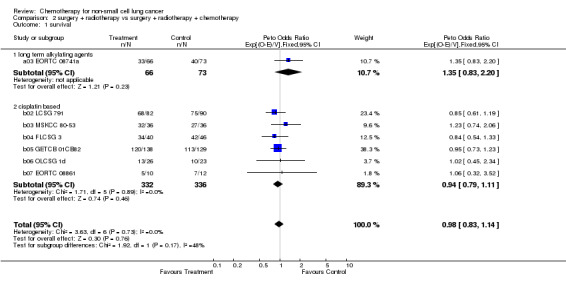
2. Surgery + radiotherapy vs surgery + radiotherapy + chemotherapy Data were available from all seven eligible trials (807 patients and 619 deaths), six of which used a cisplatin based regimen. Intended doses of cisplatin ranged from 40 mg/m² to 100 mg/m² per cycle and total dose from 80 mg/m² to 400 mg/m². Total planned doses of radiotherapy ranged from 40 Gy in 10 fractions to 65 Gy in 33 fractions. The delay between surgery and the first adjuvant treatment was scheduled to be no longer than seven weeks.
The overall hazard ratio of 0.98 (P=0.76) is marginally in favour of chemotherapy. There is no gross statistical heterogeneity between the trials (P=0.73). For the cisplatin based trials the hazard ratio of 0.94 (P=0.46), or 6% reduction in the risk of death, favours chemotherapy and is equivalent to a 2% absolute benefit at both 2 and 5 years improving from 50% to 52% and from 15% to 17% respectively. The results are however not conventionally significant, the 95% confidence intervals range from a 4% detriment to an 8% benefit at 2 years and from a 3% detriment to an 8% benefit at 5 years.
LOCALLY ADVANCED DISEASE 3. Radical radiotherapy vs radical radiotherapy plus chemotherapy Data were available from 22 trials (3033 patients and 2814 deaths). Five trials used long term alkylating agents, mainly cyclophosphamide or nitrosourea in combination with methotrexate. Three used vinca‐alkaloids or etoposide, and three used 'other' regimens, which in this comparison were mostly based on doxorubicin. Eleven trials used chemotherapy regimens containing cisplatin. Two used the regimen of cisplatin, doxorubicin and cyclophosphamide and seven used a combination of cisplatin plus a vinca‐alkaloid or etoposide. Intended doses of cisplatin ranged from 40 mg/m² to 120 mg/m² per cycle and total doses from 120 mg/m² to 800 mg/m². The intended radiation dose for cisplatin based trials ranged from 50 Gy in 20 fractions to 65 Gy in 30 fractions. Ten of these trials started chemotherapy before radiotherapy.
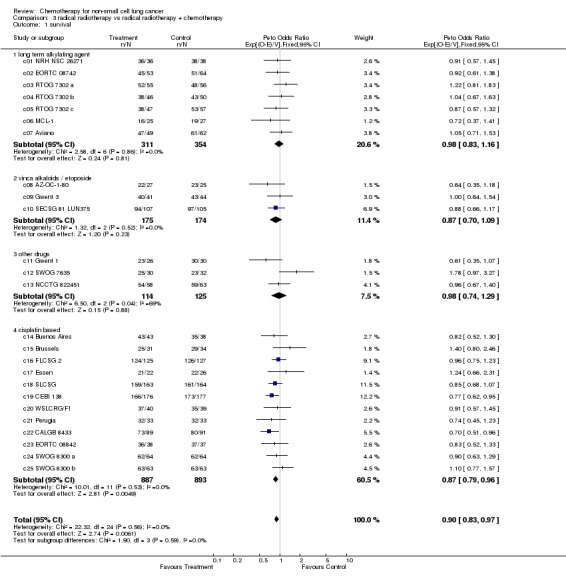
In this comparison there was no gross statistical heterogeneity between trials (P=0.56), nor such strong evidence of a difference between chemotherapy categories, reflected in the non‐significant test for interaction (P=0.59). The overall results show a significant overall benefit of chemotherapy. The hazard ratio of 0.90 (P=0.006), or 10% reduction in the risk of death, corresponds to absolute benefits of 3% at 2 years and 2% at 5 years. However it is useful to also consider each of the chemotherapies independently.
Trials using cisplatin‐based regimens Trials using cisplatin based chemotherapy provided the most information (more than 50%) and the strongest evidence for an effect in favour of chemotherapy. The hazard ratio of 0.87 (P=0.005), or 13% reduction in the risk of death, is equivalent to absolute benefits of 4% (95% confidence interval 1% to 7%) at 2 years improving survival from 15% to 19% and 2% (95% confidence interval 1% to 4%) at 5 years improving survival from 5% to 7%.
Other trials Trials using long term alkylating agents and 'other' regimens both give a hazard ratio of 0.98 (P=0.81 and P=0.88 respectively), both marginally in favour of chemotherapy, but inconclusive. Trials using regimens containing vinca alkaloids or etoposide also favour chemotherapy, with a hazard ratio of 0.87 (P=0.23) or 13% reduction in the risk of death, but no firm conclusions can be drawn. Furthermore, there was no firm evidence that the results of the trials using regimens containing vinca alkaloids or etoposide or of those using other regimens of modern drugs are any different from those using cisplatin based chemotherapy.
ADVANCED DISEASE 4. Supportive care vs supportive care plus chemotherapy Data were available from all 11 eligible trials (1190 patients and 1144 deaths). Two trials used long term alkylating agents and one used etoposide as a single agent. The remaining eight trials used cisplatin based chemotherapy, seven of which used a combination of cisplatin and vinca alkaloids or etoposide. The intended dose of cisplatin ranged from 40 mg/m² to 120 mg/m² per cycle, with total doses of 280 mg/m² upwards. This included several trials in which chemotherapy was given until the disease progressed or the toxicity was unacceptable. In this advanced disease setting, however, many patients would not have received the planned number of treatment cycles. One trial allowed entry of only patients with metastatic disease, the rest included patients with both locally advanced and advanced disease.
There is considerable overall statistical heterogeneity (P<0.0001) and a pronounced difference in the results for the different chemotherapy categories, (P=0.003) and again it is wise to focus on the results within each of the pre‐defined chemotherapy categories.
Trials using long‐term alkylating agents The result for trials using long term alkylating agents suggested a detriment of chemotherapy with a hazard ratio of 1.26 or 26% increase in the relative risk of death. However, with only two such trials, the confidence intervals are wide (0.96 to 1.66) and the result does not reach conventional levels of significance (P=0.095).
Trials using cisplatin‐based chemotherapy The cisplatin based trials show a clear benefit of chemotherapy with a hazard ratio of 0.73 (P<0.0001) or 27% reduction in the risk of death. This is equivalent to an absolute improvement in survival of 10% at one year, improving survival from 15% to 25%, or an increased median survival of 1.5 months, improving median survival from 4 months to 5.5 months. One trial (CEP‐85) showed an extreme result in favour of chemotherapy. When this trial is excluded from the analysis, the results are still significantly in favour of chemotherapy (hazard ratio 0.77 (0.63 to 0.85, P=0.001). When this trial is removed, there is no gross statistical heterogeneity within the cisplatin based category ( P=0.09).
TREATMENT EFFECT IN PATIENT SUBGROUPS Predefined subgroups of patients were analysed to determine whether we could identify particular types of patient or tumour that benefited more (or less) from chemotherapy. To minimise heterogeneity, only cisplatin based regimens were included in this analysis. Data on stage were available for 92% of patients, performance status for 94% of patients and age, sex and histological cell type for more than 99% of patients. There is no evidence that any group of patients specified by age, sex, histological cell type, performance status or stage benefit more or less from chemotherapy. This means, for example, that patients of all ages appear to gain the same relative benefit from chemotherapy. Note that these analyses do not compare the underlying survival of patients. It does not imply that old and young patients live for the same amount of time.
UPDATING RESULTS
As this systematic review is based on the original data from trials (not data taken from publications), updates are major projects taking many months of full time work and requiring the input of numerous individuals and groups. Standard practice for IPD meta‐analyses are to undertake full updates, when appropriate, depending on the maturity of data and the rate at which further trials are completed and published.
This systematic review has now been updated to include more patients from more randomised controlled trials published since 1995. Details of current numbers of patients and trials included and the most up to date citations are listed below. Several new Cochrane Reviews will be submitted.
1. Surgery vs. surgery + adjuvant chemotherapy Updated to include: 8447 patients, 34 trial comparisons
Most recent citation: NSCLC Meta‐analysis Collaborative Group. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non‐small‐cell lung cancer: two meta‐analyses of individual patient data. Lancet. 2010;375:1267‐77.
2. Surgery + radiotherapy vs. surgery + radiotherapy + chemotherapy Updated to include: 2660 patients, 13 trial comparisons
Most recent citation: NSCLC Meta‐analysis Collaborative Group. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non‐small‐cell lung cancer: two meta‐analyses of individual patient data. Lancet. 2010;375:1267‐77.
3. Radiotherapy vs. radiotherapy + sequential chemotherapy
Updated to include: 3839 patients, 22 RCTs Most recent citation: Le Pechoux C, Burdett S, Auperin A. Individual patient data (IPD) meta‐analysis (MA) of chemotherapy (CT) in locally advanced non‐small cell lung cancer (NSCLC). Journal of Thoracic Oncology. 2008; 3(Supplement 1):S20, 35IN.
4. Supportive care vs. supportive care + chemotherapy Updated to include: 2714 patients, 16 RCTs Most recent citations:
Non‐Small Cell Lung Cancer Collaborative Group. Chemotherapy and supportive care versus supportive care alone for advanced non‐small cell lung cancer. Cochrane Database of Systematic Reviews 2010, Issue 5. Art. No.: CD007309. DOI: 10.1002/14651858.CD007309.pub2
NSCLC Meta‐Analyses Collaborative Group. Chemotherapy in addition to supportive care improves survival in advanced non‐small cell lung cancer: a systematic review and meta‐analysis of individual patient data from 16 randomized trials. J Clin Oncol. 2008;26:4617‐25.
Three other comparisons of timing of chemotherapy have also been explored:
a) Radiotherapy vs. radiotherapy + concomitant chemotherapy
Includes: 2910 patients, 16 RCTs Most recent citation: Le Pechoux C, Burdett S, Auperin A. Individual patient data (IPD) meta‐analysis (MA) of chemotherapy (CT) in locally advanced non‐small cell lung cancer (NSCLC). Journal of Thoracic Oncology. 2008; 3(4, Supplement 1):S20, 35IN.
b) Radiotherapy + sequential chemotherapy vs. radiotherapy + concomitant chemotherapy
Includes: 1205 patients, 6 RCTs Most recent citation: Aupérin A, Le Péchoux C, Rolland E, et al on behalf of the NSCLC Collaborative Group. Concomitant versus sequential radiochemotherapy in locally advanced non‐small cell lung cancer: A meta‐analysis of individual data of 1205 patients. J Clin Oncol. 2010;28:2181‐90.
c) Surgery vs. surgery + neoadjuvant chemotherapy
Status: ongoing
Discussion
This meta‐analysis is based on an extensive dataset comprising individual data on 9387 patients from 52 randomised controlled trials that compared local surgical or radiotherapy treatment or best supportive care with the same treatment plus chemotherapy in non‐small cell lung cancer. A number of methods were employed to try to identify all trials and both published and unpublished data were included thereby minimising the influence of publication bias. Furthermore, at the time the meta‐analysis was completed only six other eligible trials were found, for which data were not available. These were mostly older trials using chemotherapy regimens based on the long term administration of oral alkylating agents, regimens that are no longer used. Only one of the unavailable trials used a cisplatin based regimen and data from approximately 99% of all patients ever entered into all known relevant trials of modern chemotherapies were analysed. Thus it is unlikely that the observed results would be changed by the unavailable data. Furthermore, for almost all trials the data on individual patient had been updated to the point of data collection, which was often many years after the publication of the trial's results. Although a number of trials have been completed since the meta‐analysis was published, most of these are not yet in the public domain. This meta‐analysis therefore currently provides a comprehensive and reliable assessment of the average treatment effect of broad categories of chemotherapy regimens among broad classes of patients with non‐small cell lung cancer. We aim to obtain data from the new trials including data from further large trials due to complete soon in the next update of the individual patient data meta‐analysis.
One of the most striking aspects of the results is the consistency in the direction, and indeed in the estimated hazard ratios, of the various chemotherapy categories among the different primary treatment comparisons. This consistency allows stronger conclusions to be drawn than perhaps could be inferred from each of the individual results.
In the early and advanced settings, older trials using long term alkylating agents tended to show a detrimental effect of chemotherapy. This effect was conventionally significant for the adjuvant surgical trials. Chemotherapies of the type used in the early 1970s based on long term administration of oral alkylating agents are therefore likely to be detrimental to patients with non‐small cell lung cancer. The mechanism for this is unknown, although some occurrences of leukaemia after treatment with busulphan have been described for non‐small cell lung cancer (Stott 1977), and a possible model for an observed detrimental effect of cyclophosphamide and other alkylating agents in non‐small cell lung cancer has been proposed (Stewart 1992). Clearly, such regimens are not used today, but the result could have implications for other disease sites, albeit that the administration of chemotherapy and the drugs used have changed considerably over the past twenty years.
In all comparisons, results for modern cisplatin‐containing regimens favour chemotherapy. These are conventionally significant in the locally advanced and supportive care settings. However, it should be stressed that this categorisation of drug regimens was chosen mainly as an objective way of classifying modern chemotherapy. Furthermore, several cisplatin based regimens were used and it is not possible to deduce to what extent the observed effects are due to the cisplatin or to the other drugs, in the combinations studied. Indeed cisplatin was used in combination with vinca alkaloids or etoposide in two thirds of trials. It is therefore not possible to recommend a particular regimen over another.
Trials using regimens containing vinca alkaloids or etoposide and those in the "other drug" category also tend to favour chemotherapy. For these categories the confidence intervals are relatively wide and no reliable conclusions can be drawn.
The meta‐analysis provides no evidence that modern cisplatin based chemotherapy is more or less effective in any particular subgroup of patients. Thus, no good evidence exists that the relative effect of chemotherapy is any smaller or larger for any particular type of patient. Nevertheless, as certain types of patient may have intrinsically different prognoses and consequently differing baseline survivals, the same hazard ratio or relative effect may provide different absolute differences in survival. For example, in the surgical setting, the hazard ratio of 0.87 would increase the 2‐year survival of patients with a good prognosis from a baseline of 80%, to 82%. For patients with a poor prognosis this same HR would improve survival from a 2‐year baseline of 40% to 45%. Similarly, the same observed hazard ratio of 0.87 in the locally advanced setting would increase the survival of patients with a good prognosis from a baseline of 30%, to 35% and patients with a poor prognosis from 5% to 7%. Thus the absolute benefit derived from the same relative risk and consequently the clinical decisions reached may be different for older or younger patients for example. It is also worth noting that the patients included in these trials are generally of better prognosis than those in the lung cancer population at large. For example few patients older than 75 years or with poor performance status were included in the trials.
The meta‐analysis suggests that modern chemotherapy regimens may provide absolute benefits of about 5% in the surgical and 2% in the radical radiotherapy setting at 5 years and 10% at one year in the supportive care setting. The confidence intervals are such, however, that the results are consistent with benefits of as much as 10%, 4% and 15% respectively or with as little as a 1% detriment and 1% and 5% benefits respectively. Although modest, such improvements could, given the high incidence of lung cancer, be important in public health terms, and studies of patients' opinions of treatments for cancer have shown that many patients accept considerable toxicity in return for small improvements in survival (Slevin 1990). However, patients are not uniform in their preferences, and the trade offs involved in choosing between more and less intensive therapy are not necessarily straightforward and warrant further study (Till 1992).
An important consideration when making such choices is the effect that chemotherapy may have on quality of life. Unfortunately, because few trials reported it, quality of life could not be addressed in the meta‐analysis.
Authors' conclusions
Implications for practice.
Although, inevitably, meta‐analyses give only average estimates of treatment effects, these are probably the best estimates on which to base treatment policy. At the outset of this meta‐analysis there was considerable pessimism about the treatment of non‐small cell lung cancer. Although the suggested benefits of modern chemotherapies are modest, these results offer hope of progress and show that chemotherapy may have a role in treating this disease. Some patients and clinicians would need to observe larger treatment effects than others before being convinced that chemotherapy is worthwhile, and undoubtedly these results will be applied differently by individual clinicians and patients around the world. Some groups may consider these results to be good enough evidence to use cisplatin based chemotherapy for certain patients. As essential drugs were not determined by this meta‐analysis, however, others may need further evidence to decide whether to use chemotherapy routinely in the treatment of non‐small cell lung cancer.
Implications for research.
Extended follow up on existing trials and the inclusion of further randomised trials will add to the evidence in the next update of this meta‐analysis. Continued research into screening new drugs and improving chemotherapy regimens is required as is measurement of quality of life.
What's new
| Date | Event | Description |
|---|---|---|
| 10 March 2015 | Review declared as stable | This review has been superseded by a new review "Adjuvant chemotherapy for resected early‐stage NSCLC" (DOI: 10.1002/14651858.CD011430). It will no longer be updated. |
History
Protocol first published: Issue 2, 2000 Review first published: Issue 2, 2000
| Date | Event | Description |
|---|---|---|
| 14 October 2010 | Amended | Some information about the update of this review has been included in the results section. |
| 7 September 2010 | Amended | Some information about the update of this review has been included. Contact person has changed. |
| 18 September 2008 | Amended | Converted to new review format. |
| 1 January 2000 | New citation required and conclusions have changed | Substantive amendment |
Notes
This review has been superseded by a new review from the same authors "Adjuvant chemotherapy for resected early‐stage NSCLC" (DOI: 10.1002/14651858.CD011430). It will therefore no longer be updated. Please go to that review for the most up to date information.
Updated Results
As this systematic review is based on the original data from trials (not data taken from publications), updates are major projects taking many months of full time work and requiring the input of numerous individuals and groups. Standard practice for IPD meta‐analyses are to undertake full updates, when appropriate, depending on the maturity of data and the rate at which further trials are completed and published.
This systematic review has now been updated to include more patients from more randomised controlled trials published since 1995. Details of current numbers of patients and trials included and the most up to date citations are listed below. Several new Cochrane Reviews will be submitted.
1. Surgery vs. surgery + adjuvant chemotherapy Updated to include: 8447 patients, 34 trial comparisons
Most recent citation: NSCLC Meta‐analysis Collaborative Group. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non‐small‐cell lung cancer: two meta‐analyses of individual patient data. Lancet. 2010;375:1267‐77.
2. Surgery + radiotherapy vs. surgery + radiotherapy + chemotherapy Updated to include: 2660 patients, 13 trial comparisons
Most recent citation: NSCLC Meta‐analysis Collaborative Group. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non‐small‐cell lung cancer: two meta‐analyses of individual patient data. Lancet. 2010;375:1267‐77.
3. Radiotherapy vs. radiotherapy + sequential chemotherapy
Updated to include: 3839 patients, 22 RCTs Most recent citation: Le Pechoux C, Burdett S, Auperin A. Individual patient data (IPD) meta‐analysis (MA) of chemotherapy (CT) in locally advanced non‐small cell lung cancer (NSCLC). Journal of Thoracic Oncology. 2008; 3(Supplement 1):S20, 35IN.
4. Supportive care vs. supportive care + chemotherapy Updated to include: 2714 patients, 16 RCTs Most recent citations:
Non‐Small Cell Lung Cancer Collaborative Group. Chemotherapy and supportive care versus supportive care alone for advanced non‐small cell lung cancer. Cochrane Database of Systematic Reviews 2010, Issue 5. Art. No.: CD007309. DOI: 10.1002/14651858.CD007309.pub2
NSCLC Meta‐Analyses Collaborative Group. Chemotherapy in addition to supportive care improves survival in advanced non‐small cell lung cancer: a systematic review and meta‐analysis of individual patient data from 16 randomized trials. J Clin Oncol. 2008;26:4617‐25.
Three other comparisons of timing of chemotherapy have also been explored:
a) Radiotherapy vs. radiotherapy + concomitant chemotherapy
Includes: 2910 patients, 16 RCTs Most recent citation: Le Pechoux C, Burdett S, Auperin A. Individual patient data (IPD) meta‐analysis (MA) of chemotherapy (CT) in locally advanced non‐small cell lung cancer (NSCLC). Journal of Thoracic Oncology. 2008; 3(4, Supplement 1):S20, 35IN.
b) Radiotherapy + sequential chemotherapy vs. radiotherapy + concomitant chemotherapy
Includes: 1205 patients, 6 RCTs Most recent citation: Aupérin A, Le Péchoux C, Rolland E, et al on behalf of the NSCLC Collaborative Group. Concomitant versus sequential radiochemotherapy in locally advanced non‐small cell lung cancer: A meta‐analysis of individual data of 1205 patients. J Clin Oncol. 2010;28:2181‐90.
c) Surgery vs. surgery + neoadjuvant chemotherapy
Status: ongoing
Acknowledgements
The following investigators, groups and secretariat were members of the Non‐small Cell Lung Cancer Collaborative Group and participated in this meta‐analysis:
The investigators were: W Alberti, G Anderson, A Bartolucci, D Bell, J Blanco Villalba, O Brodin, C Cardiello, F Cartei, G Cartei, R Cellerino, C Chastang, Y Cormier, J Cox, L Crino, J Crowley, B Dautzenberg, A Depierre, A Dietemann, R Dillman, O Doi, R Feld, R Figlin, P Ganz, M Green, A Gregor, P Helle, J Herndon, S Hitomi, H Host, M Imaizumi, J Jett, D Johnson, S Kaasa, H Kimura, J Klastersky, H Kondo, H Kreisman, M Kris, K Kunishima, O Kuwahara, T Lad, A Laing, F Macbeth, A Masaoka, K Mattson, E Minatel, J Mira, T Mori, C Mountain, N Neiderle, A Niiranen, E Nou, W Page, J Pater, S Piantadosi, K Pisters, S Pyrhonen, E Quoix, E Rapp, N Rowell, T Sahmoud, K Sawamura, D Schallier, C Scott, J Simpson, M Stagg, T Teramatsu, M Trovo, R Tsuchiya, D Tummarello, P van Houtte, N Van Zandwijk, R Vincent, H Wada, J White, C Williams, R Wood, Y Yamaguchi
The organisations and groups that collaborated in the meta‐analysis were: Cancer and Leukemia Group B, Cercle d'Etudes Pneumologiques (Strasbourg), European Organisation for Research and Treatment of Cancer, Fédération Nationale des Centres de Lutte Contre le Cancer, Finnish Lung Cancer Study, Groupe d'Etude et de Traitement des Cancers Bronchiques, Japan Lung Cancer Surgical Study Group, Lung Cancer Study Group, Medical Research Council, National Cancer Institute of Canada Clinical Trials Group, North Central Cancer Treatment Group, Osaka Lung Cancer Study Group, Radiation Therapy Oncology Group, Southeastern Cancer Study Group, South West Oncology Group, Study Group of Adjuvant Chemotherapy for Lung Cancer (Chibu, Japan), Swedish Lung Cancer Study Group, Veterans Administration Surgical Adjuvant Group, Veterans Administration Surgical Oncology Group, West of Scotland Lung Cancer Research Group, West Japan Study Group for Lung Cancer Surgery.
The members of the secretariat were: R Arriagada, J Cartmell‐Davies, D Girling, T Le Chevalier, S Marsoni, M Parmar, JP Pignon, C Rekacewicz, R Souhami, L Stewart, M Tarayre, A Tinazzi, V Torri
We thank all the patients who took part in all the trials included in the meta‐analysis. We also thank the following institutions that funded the Non‐small Cell Lung Cancer Collaborative Group Meeting in September 1993, at which preliminary results of this meta‐analysis were presented and discussed: Bristol‐Myers Oncology, Bristol‐Myers Squibb, Amgen, Asta‐Medica Ltd, Asta‐Sarget, Beecham Sevigne, Roger Bellon, Cancer Research Campaign, Chugai, Ciba‐Geigy, Eurocetus, Farmitalia Carlo Erba, Glaxo France, Harold Hyam Wingate Foundation, Institut National de la Santé et de la Recherche Médicale, Ipsen Biotech, Lederle, Lilly France, Lilly Industries, Medical Research Council, Pfizer, Rhône‐Poulenc and Servier Medical. The Cancer Trials Office's secretariat thanks Richard Wood, Sarah Walker and John Machin, all the computing, data management and secretarial staff for their support throughout this project. The Institute Gustave Roussy's secretariat thanks Ben Affaied for programming support and Sylviane Iacobelli for secretarial help.
Data and analyses
Comparison 1. surgery vs surgery + chemotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 survival | 16 | 4457 | Peto Odds Ratio (95% CI) | 1.04 [0.96, 1.12] |
| 1.1 long term alkylating agents | 5 | 2145 | Peto Odds Ratio (95% CI) | 1.15 [1.04, 1.27] |
| 1.2 other drugs | 4 | 918 | Peto Odds Ratio (95% CI) | 0.89 [0.72, 1.11] |
| 1.3 cisplatin based | 8 | 1394 | Peto Odds Ratio (95% CI) | 0.87 [0.74, 1.02] |
| 2 subgroup for survival ‐ age | 4 | Peto Odds Ratio (95% CI) | Totals not selected | |
| 3 subgroup for survival ‐ sex | 2 | Peto Odds Ratio (95% CI) | Totals not selected | |
| 4 subgroup for survival ‐ performance status | 2 | Peto Odds Ratio (95% CI) | Totals not selected | |
| 5 subgroup for survival ‐ histology | 3 | Peto Odds Ratio (95% CI) | Totals not selected | |
| 6 subgroup for survival ‐ stage | 2 | Peto Odds Ratio (95% CI) | Totals not selected |
1.1. Analysis.
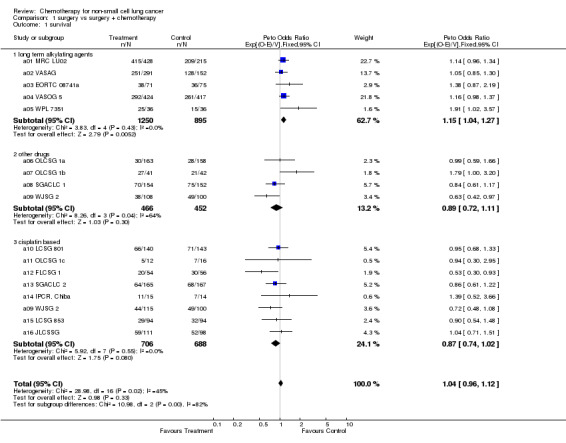
Comparison 1 surgery vs surgery + chemotherapy, Outcome 1 survival.
1.2. Analysis.
Comparison 1 surgery vs surgery + chemotherapy, Outcome 2 subgroup for survival ‐ age.
1.3. Analysis.
Comparison 1 surgery vs surgery + chemotherapy, Outcome 3 subgroup for survival ‐ sex.
1.4. Analysis.
Comparison 1 surgery vs surgery + chemotherapy, Outcome 4 subgroup for survival ‐ performance status.
1.5. Analysis.
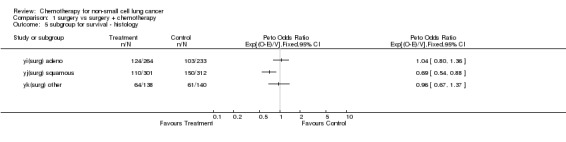
Comparison 1 surgery vs surgery + chemotherapy, Outcome 5 subgroup for survival ‐ histology.
1.6. Analysis.
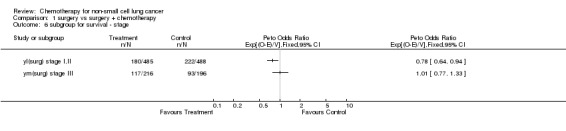
Comparison 1 surgery vs surgery + chemotherapy, Outcome 6 subgroup for survival ‐ stage.
Comparison 2. surgery + radiotherapy vs surgery + radiotherapy + chemotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 survival | 7 | 807 | Peto Odds Ratio (95% CI) | 0.98 [0.83, 1.14] |
| 1.1 long term alkylating agents | 1 | 139 | Peto Odds Ratio (95% CI) | 1.35 [0.83, 2.20] |
| 1.2 cisplatin based | 6 | 668 | Peto Odds Ratio (95% CI) | 0.94 [0.79, 1.11] |
| 2 subgroup for survival ‐ age | 4 | Peto Odds Ratio (95% CI) | Totals not selected | |
| 3 subgroup for survival ‐ sex | 2 | Peto Odds Ratio (95% CI) | Totals not selected | |
| 4 subgroup for survival ‐ performance status | 2 | Peto Odds Ratio (95% CI) | Totals not selected | |
| 5 subgroup for survival ‐ histology | 3 | Peto Odds Ratio (95% CI) | Totals not selected | |
| 6 subgroup for survival ‐ stage | 2 | Peto Odds Ratio (95% CI) | Totals not selected |
2.1. Analysis.
Comparison 2 surgery + radiotherapy vs surgery + radiotherapy + chemotherapy, Outcome 1 survival.
2.2. Analysis.
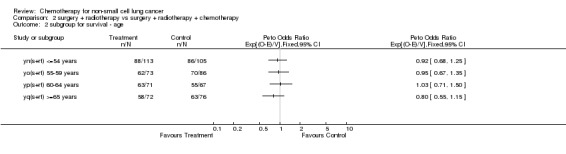
Comparison 2 surgery + radiotherapy vs surgery + radiotherapy + chemotherapy, Outcome 2 subgroup for survival ‐ age.
2.3. Analysis.
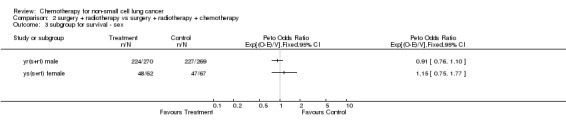
Comparison 2 surgery + radiotherapy vs surgery + radiotherapy + chemotherapy, Outcome 3 subgroup for survival ‐ sex.
2.4. Analysis.
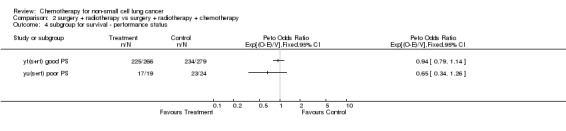
Comparison 2 surgery + radiotherapy vs surgery + radiotherapy + chemotherapy, Outcome 4 subgroup for survival ‐ performance status.
2.5. Analysis.
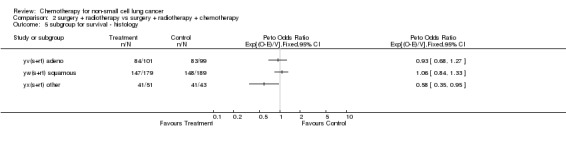
Comparison 2 surgery + radiotherapy vs surgery + radiotherapy + chemotherapy, Outcome 5 subgroup for survival ‐ histology.
2.6. Analysis.
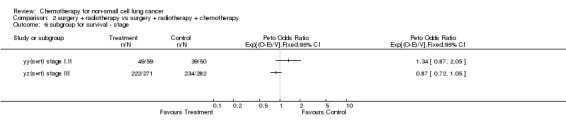
Comparison 2 surgery + radiotherapy vs surgery + radiotherapy + chemotherapy, Outcome 6 subgroup for survival ‐ stage.
Comparison 3. radical radiotherapy vs radical radiotherapy + chemotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 survival | 25 | 3033 | Peto Odds Ratio (95% CI) | 0.90 [0.83, 0.97] |
| 1.1 long term alkylating agent | 7 | 665 | Peto Odds Ratio (95% CI) | 0.98 [0.83, 1.16] |
| 1.2 vinca alkaloids / etoposide | 3 | 349 | Peto Odds Ratio (95% CI) | 0.87 [0.70, 1.09] |
| 1.3 other drugs | 3 | 239 | Peto Odds Ratio (95% CI) | 0.98 [0.74, 1.29] |
| 1.4 cisplatin based | 12 | 1780 | Peto Odds Ratio (95% CI) | 0.87 [0.79, 0.96] |
| 2 subgroup for survival ‐ age | 4 | Peto Odds Ratio (95% CI) | Totals not selected | |
| 3 subgroup for survival ‐ sex | 2 | Peto Odds Ratio (95% CI) | Totals not selected | |
| 4 subgroup for survival ‐ performance status | 2 | Peto Odds Ratio (95% CI) | Totals not selected | |
| 5 subgroup for survival ‐ histology | 3 | Peto Odds Ratio (95% CI) | Totals not selected | |
| 6 subgroup for survival ‐ stage | 2 | Peto Odds Ratio (95% CI) | Totals not selected |
3.1. Analysis.
Comparison 3 radical radiotherapy vs radical radiotherapy + chemotherapy, Outcome 1 survival.
3.2. Analysis.
Comparison 3 radical radiotherapy vs radical radiotherapy + chemotherapy, Outcome 2 subgroup for survival ‐ age.
3.3. Analysis.
Comparison 3 radical radiotherapy vs radical radiotherapy + chemotherapy, Outcome 3 subgroup for survival ‐ sex.
3.4. Analysis.
Comparison 3 radical radiotherapy vs radical radiotherapy + chemotherapy, Outcome 4 subgroup for survival ‐ performance status.
3.5. Analysis.
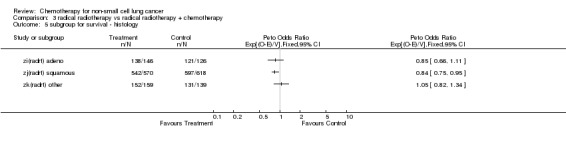
Comparison 3 radical radiotherapy vs radical radiotherapy + chemotherapy, Outcome 5 subgroup for survival ‐ histology.
3.6. Analysis.
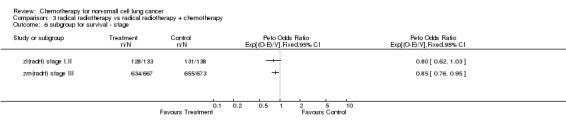
Comparison 3 radical radiotherapy vs radical radiotherapy + chemotherapy, Outcome 6 subgroup for survival ‐ stage.
Comparison 4. supportive care vs supportive care + chemotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 survival | 11 | 1190 | Peto Odds Ratio (95% CI) | 0.84 [0.74, 0.95] |
| 1.1 long term alkylating agents | 2 | 226 | Peto Odds Ratio (95% CI) | 1.26 [0.96, 1.66] |
| 1.2 vinca alkaloids / etoposide | 1 | 186 | Peto Odds Ratio (95% CI) | 0.87 [0.64, 1.20] |
| 1.3 cisplatin based | 8 | 778 | Peto Odds Ratio (95% CI) | 0.73 [0.63, 0.85] |
| 2 subgroup for survival ‐ age | 4 | Peto Odds Ratio (95% CI) | Totals not selected | |
| 3 subgroup for survival ‐ sex | 2 | Peto Odds Ratio (95% CI) | Totals not selected | |
| 4 subgroup for survival ‐ performance status | 2 | Peto Odds Ratio (95% CI) | Totals not selected | |
| 5 subgroup for survival ‐ histology | 3 | Peto Odds Ratio (95% CI) | Totals not selected | |
| 6 subgroup for survival ‐ stage | 2 | Peto Odds Ratio (95% CI) | Totals not selected |
4.1. Analysis.
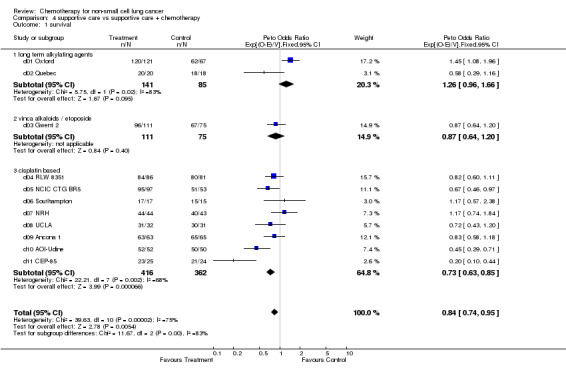
Comparison 4 supportive care vs supportive care + chemotherapy, Outcome 1 survival.
4.2. Analysis.
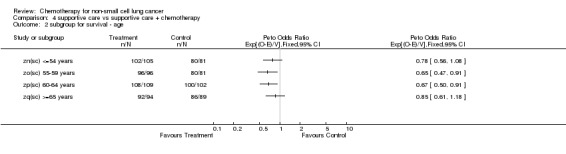
Comparison 4 supportive care vs supportive care + chemotherapy, Outcome 2 subgroup for survival ‐ age.
4.3. Analysis.

Comparison 4 supportive care vs supportive care + chemotherapy, Outcome 3 subgroup for survival ‐ sex.
4.4. Analysis.
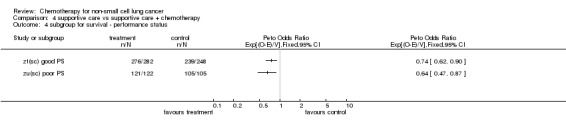
Comparison 4 supportive care vs supportive care + chemotherapy, Outcome 4 subgroup for survival ‐ performance status.
4.5. Analysis.
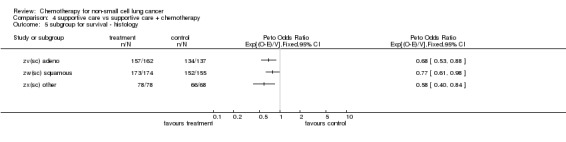
Comparison 4 supportive care vs supportive care + chemotherapy, Outcome 5 subgroup for survival ‐ histology.
4.6. Analysis.
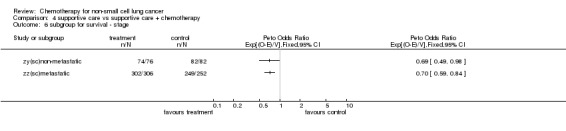
Comparison 4 supportive care vs supportive care + chemotherapy, Outcome 6 subgroup for survival ‐ stage.
Comparison 5. RT + C vs Rt.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Survival | 25 | 3055 | Peto Odds Ratio (99% CI) | 0.90 [0.83, 0.97] |
| 1.1 Adjuvant Chemo | 24 | 1235 | Peto Odds Ratio (99% CI) | 0.94 [0.83, 1.06] |
| 1.2 Neoadjuvant chemo | 12 | 1820 | Peto Odds Ratio (99% CI) | 0.88 [0.80, 0.97] |
5.1. Analysis.
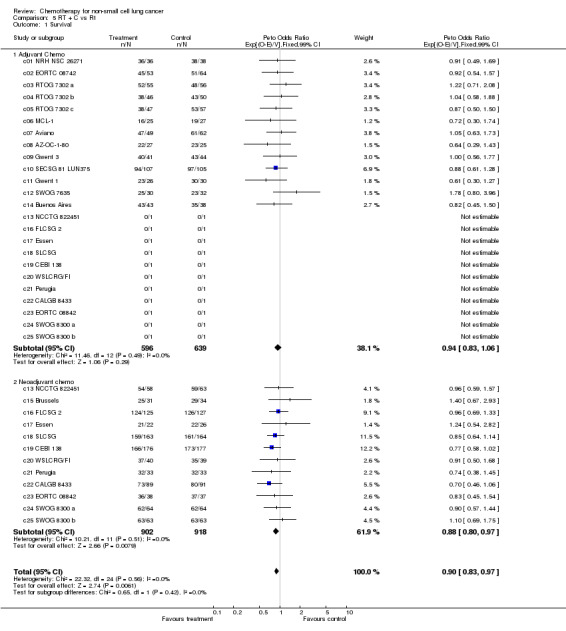
Comparison 5 RT + C vs Rt, Outcome 1 Survival.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
a01 MRC LU02.
| Methods | RCT ‐ 1965‐68 | |
| Participants | 643 patients | |
| Interventions | surgery vs surgery + chemotherapy (i) cyclophosphamide* 200/75† (ii) busulphan 4*/1.5 | |
| Outcomes | survival | |
| Notes | daily treatment 2 years | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
a02 VASAG.
| Methods | RCT ‐ 1968‐73 | |
| Participants | 443 patients | |
| Interventions | surgery vs surgery + chemotherapy (i) cyclophosphamide 40‡ (ii) cyclophosphamide 40‡ methotrexate 50§ | |
| Outcomes | survival | |
| Notes | 15 cycles of chemotherapy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
a03 EORTC 08741a.
| Methods | RCT ‐ 1973‐79 | |
| Participants | 146 patients | |
| Interventions | surgery vs surgery + chemotherapy lomustine* 70 cyclophosphamide 1000 methotrexate 40 | |
| Outcomes | survival | |
| Notes | 13 cycles of chemotherapy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
a04 VASOG 5.
| Methods | RCT ‐ 1973‐79 | |
| Participants | 841 patients | |
| Interventions | surgery vs surgery + chemotherapy lomustine* 70 nitrogen mustard* 2000 | |
| Outcomes | survival | |
| Notes | lomustine in 9 cycles nitrogen mustard in 52 cycles | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
a05 WPL 7351.
| Methods | RCT ‐ 1974‐76 | |
| Participants | 72 patients | |
| Interventions | surgery vs surgery + chemotherapy lomustine* 130 | |
| Outcomes | survival | |
| Notes | 17 cycles of chemotherapy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
a06 OLCSG 1a.
| Methods | RCT ‐ 1982‐87 | |
| Participants | 321 patients | |
| Interventions | surgery vs surgery + chemotherapy tegafur* 600‐800§ | |
| Outcomes | survival | |
| Notes | daily treatment >1 year | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
a07 OLCSG 1b.
| Methods | RCT ‐ 1982‐86 | |
| Participants | 83 patients | |
| Interventions | surgery vs surgery + chemotherapy doxorubicin 100§ mitomycin C 20§ tegafur(a)* 600‐800§ tegafur(b)* 600‐800§ | |
| Outcomes | survival | |
| Notes | doxorubicin given in 3 cycles tegafur (a) daily treatment tegafur (b) daily treatment > 1 year | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
a08 SGACLC 1.
| Methods | RCT ‐ 1982‐85 | |
| Participants | 306 patients | |
| Interventions | surgery vs surgery + chemotherapy mitomycin C 0.08‡ cyclophosphamide 2‡ tegafur* 12‡ | |
| Outcomes | survival | |
| Notes | mitomycin C given in 10 cycles tegafur daily treatment > 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
a09 WJSG 2.
| Methods | RCT ‐ 1985‐88 | |
| Participants | 323 patients | |
| Interventions | surgery vs surgery + chemotherapy (i) cisplatin 50 vindesine 6‐9§ UFT* 400§ (ii) UFT* 400§ | |
| Outcomes | survival | |
| Notes | 1 cycle of cisplatin / vindesine given UFT daily treatment 1 year | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
a10 LCSG 801.
| Methods | RCT ‐ 1980‐86 | |
| Participants | 283 patients | |
| Interventions | surgery vs surgery + chemotherapy cisplatin 60 doxorubincin 40 cyclophosphamide 400 | |
| Outcomes | survival | |
| Notes | 4 cycles of chemotherapy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
a11 OLCSG 1c.
| Methods | RCT ‐ 1982‐87 | |
| Participants | 28 patients | |
| Interventions | surgery vs surgery + chemotherapy cisplatin 80 tegafur* 600‐800§ | |
| Outcomes | survival | |
| Notes | cisplatin given in 1 cycle tegafur daily treatment > 1 year | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
a12 FLCSG 1.
| Methods | RCT ‐ 1982‐87 | |
| Participants | 110 patients | |
| Interventions | surgery vs surgery + chemotherapy cisplatin 40 doxorubicin 40 cyclophosphamide 400 | |
| Outcomes | survival | |
| Notes | 6 cycles of chemotherapy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
a13 SGACLC 2.
| Methods | RCT ‐ 1985‐87 | |
| Participants | 332 patients | |
| Interventions | surgery vs surgery + chemotherapy cisplatin 66 doxorubicin 26 UFT* 8‡ | |
| Outcomes | survival | |
| Notes | Unpublished UFT daily treatment > 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
a14 IPCR, Chiba.
| Methods | RCT ‐ 1985‐91 | |
| Participants | 29 patients | |
| Interventions | surgery vs surgery + chemotherapy cisplatin 80 vindesine 3 mitomycin c¶ 8 | |
| Outcomes | survival | |
| Notes | >2 cycles of chemotherapy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
a15 LCSG 853.
| Methods | RCT ‐ 1985‐89 | |
| Participants | 188 patients | |
| Interventions | surgery vs syrgery + chemotherapy cisplatin 60 doxorubicin 40 cyclophosphamide 400 | |
| Outcomes | survival | |
| Notes | Unpublished 4 cycles of chemotherapy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
a16 JLCSSG.
| Methods | RCT ‐ 1986‐88 | |
| Participants | 209 patients | |
| Interventions | surgery vs surgery + chemotherapy cisplatin 80 vindesine 6 | |
| Outcomes | survival | |
| Notes | 2‐3 cycles of chemotherapy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
b01 EORTC 08741b.
| Methods | RCT ‐ 1973‐79 | |
| Participants | 139 patients | |
| Interventions | surgery + radiotherapy vs surgery + radiotherapy + chemotherapy lomustine* 70 cyclophosphamide 1000 methotrexate 40 | |
| Outcomes | survival | |
| Notes | 13 cycles of chemotherapy radiotherapy 45 Gy in 14‐25 fractions complete resection | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
b02 LCSG 791.
| Methods | RCT ‐ 1979‐85 | |
| Participants | 172 patients | |
| Interventions | surgery + radiotherapy vs surgery + radiotherapy + chemotherapy cyclophosphamide 400 doxorubicin 40 cisplatin 40 | |
| Outcomes | survival | |
| Notes | 6 cycles of chemotherapy radiotherapy 40 Gy in 10 fractions** incomplete resection | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
b03 MSKCC 80‐53.
| Methods | RCT ‐ 1981‐87 | |
| Participants | 72 patients | |
| Interventions | surgery + radiotherapy vs surgery + radiotherapy + chemotherapy cisplatin 120 vindesine 9 | |
| Outcomes | survival | |
| Notes | 4 cycles of chemotherapy radiotherapy 46 Gy complete and incomplete resections | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
b04 FLCSG 3.
| Methods | RCT ‐ 1982‐87 | |
| Participants | 86 patients | |
| Interventions | surgery + radiotherapy vs surgery + radiotherapy + chemotherapy cyclophoshamide 400 doxorubicin 40 cisplatin 40 | |
| Outcomes | survival | |
| Notes | unpublished 8 cycles of chemotherapy, 2 given before radiotherapy radiotherapy 55 Gy in 20 fractions** incomplete resection | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
b05 GETCB 01CB82.
| Methods | RCT ‐ 1982‐86 | |
| Participants | 267 patients | |
| Interventions | surgery + radiotherapy vs surgery + radiotherapy + chemotherapy doxorubicin 40 vincristine 1.2 cisplatin 75 lomustine 80§ alternating with cyclophosphamide 600 | |
| Outcomes | survival | |
| Notes | 3 cycles of chemotherapy given before radiotherapy radiotherapy 60‐65 Gy in 30‐33 fractions complete and incomplete resection | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
b06 OLCSG 1d.
| Methods | RCT ‐ 1983‐87 | |
| Participants | 49 patients | |
| Interventions | surgery + radiotherapy vs surgery + radiotherapy + chemotherapy cisplatin 80 tegafur* 600‐800 | |
| Outcomes | survival | |
| Notes | cisplatin given once tegafur daily treatment radiotherapy 40 Gy in 20 fractions complete resection | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
b07 EORTC 08861.
| Methods | RCT ‐ 1986‐90 | |
| Participants | 22 patients | |
| Interventions | surgery + radiotherapy vs surgery + radiotherapy + chemotherapy cisplatin 100 vindesine 6 | |
| Outcomes | survival | |
| Notes | unpublished 4 cycles of chemotherapy, 2 given before radiotherapy radiotherapy 56 Gy in 28 fractions complete resection | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
c01 NRH NSC 26271.
| Methods | RCT ‐ 1968‐71 | |
| Participants | 74 patients | |
| Interventions | radical radiotherapy vs radical radiotherapy + chemotherapy cyclophosphamide 400†† cyclophosphamide* 100 | |
| Outcomes | survival | |
| Notes | oral cyclophosphamide given as daily treatment until tumour progression or toxicity radiotherapy 50 Gy in 25‐31 fractions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
c02 EORTC 08742.
| Methods | RCT ‐ 1973‐80 | |
| Participants | 117 patients | |
| Interventions | radical radiotherapy vs radical radiotherapy + chemotherapy cyclophosphamide 1000 lomustine* 100 methotrexate 40 | |
| Outcomes | survival | |
| Notes | 12 cycles of chemotherapy radiotherapy 50‐60 Gy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
c03 RTOG 7302 a.
| Methods | RCT ‐ 1973‐78 | |
| Participants | 111 patients | |
| Interventions | radical radiotherapy vs radical radiotherapy + chemotherapy cyclophosphamide 1000 | |
| Outcomes | survival | |
| Notes | chemotherapy given until tumour progression or toxicity radiotherapy 40 Gy in 10 fractions** | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
c04 RTOG 7302 b.
| Methods | RCT ‐ 1973‐78 | |
| Participants | 96 patients | |
| Interventions | radical radiotherapy vs radical radiotherapy + chemotherapy cyclophosphamide 1000 | |
| Outcomes | survival | |
| Notes | chemotherapy given until tumour progression or toxicity radiotherapy 30 Gy in 10 fractions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
c05 RTOG 7302 c.
| Methods | RCT ‐ 1973‐78 | |
| Participants | 104 patients | |
| Interventions | radical radiotherapy vs radical radiotherapy + chemotherapy cyclophosphamide 1000 | |
| Outcomes | survival | |
| Notes | chemotherapy given until tumour progression or toxicity radiotherapy 40 Gy in 20 fractions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
c06 MCL‐1.
| Methods | RCT ‐ 1980‐84 | |
| Participants | 52 patients | |
| Interventions | radical radiotherapy vs radical radiotherapy + chemotherapy doxorubicin 40 lomustine* 30 cyclophosphamide 400 methotrexate 30 | |
| Outcomes | survival | |
| Notes | chemotherapy given until tumour progression or toxicity radiotherapy 55 Gy in 25 fractions** | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
c07 Aviano.
| Methods | RCT ‐ 1980‐84 | |
| Participants | 111 patients | |
| Interventions | radical radiotherapy vs radical radiotherapy + chemotherapy doxorubicin 40 cyclophosphamide 600 methotrextate 30 procarbazine* 1000 | |
| Outcomes | survival | |
| Notes | 12 cycles of chemotherapy radiotherapy 45 Gy in 15 fractions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
c08 AZ‐OC‐1‐80.
| Methods | RCT ‐ 1981‐85 | |
| Participants | 52 patients | |
| Interventions | radical radiotherapy vs radical radiotherapy + chemotherapy vinblastine 6 | |
| Outcomes | survival | |
| Notes | chemotherapy given until tumour progression or toxicity radiotherapy 55 Gy in 28 fractions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
c09 Gwent 3.
| Methods | RCT ‐ 1981‐85 | |
| Participants | 85 patients | |
| Interventions | radical radiotherapy vs radical radiotherapy + chemotherapy etoposide* 1000 | |
| Outcomes | survival | |
| Notes | unpublished 7 cycles of chemotherapy given radiotherapy 32 Gy in 8 fractions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
c10 SECSG 81 LUN375.
| Methods | RCT ‐ 1981‐85 | |
| Participants | 212 patients | |
| Interventions | radical radiotherapy vs radical radiotherapy + chemotherapy vindesine(a) 3 vindesine(b) 6 | |
| Outcomes | survival | |
| Notes | 5 cycles of vindesine(a) then 10 cycles of vindesine(b) radiotherapy 60 Gy in 33 fractions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
c11 Gwent 1.
| Methods | RCT ‐ 1974‐76 | |
| Participants | 56 patients | |
| Interventions | radical radiotherapy vs radical radiotherapy + chemotherapy doxorubicin 50 fluorouracil 1200§ | |
| Outcomes | survival | |
| Notes | 4 cycles of chemotherapy radiotherapy 32 Gy in 8 fractions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
c12 SWOG 7635.
| Methods | RCT ‐ 1977‐79 | |
| Participants | 62 patients | |
| Interventions | radical radiotherapy vs radical radiotherapy + chemotherapy doxorubicin 50 | |
| Outcomes | survival | |
| Notes | 8 cycles of chemotherapy radiotherapy 60 Gy in 20 fractions** | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
c13 NCCTG 822451.
| Methods | RCT ‐ 1983‐87 | |
| Participants | 121 patients | |
| Interventions | radical radiotherapy vs radical radiotherapy + chemotherapy doxorubicin 40 cyclophosphamide 400 methotrexate 40 lomustine* 30 | |
| Outcomes | survival | |
| Notes | 4 cycles of chemotherapy, 2 given before radiotherapy radiotherapy 60 Gy in 30 fractions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
c14 Buenos Aires.
| Methods | RCT ‐ 1981‐85 | |
| Participants | 81 patients | |
| Interventions | radical radiotherapy vs radical radiotherapy + chemotherapy cisplatin 40 doxorubicin 40 cyclophosphamide 400 | |
| Outcomes | survival | |
| Notes | 6 cycles of chemotherapy radiotherapy 55 Gy in 22 fractions** | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
c15 Brussels.
| Methods | RCT ‐ 1981‐84 | |
| Participants | 65 patients | |
| Interventions | radical radiotherapy vs radical radiotherapy + chemotherapy cisplatin 60 etoposide 360 vindesine 3 | |
| Outcomes | survival | |
| Notes | 3 cycles of chemotherapy given before radiotherapy radiotherapy 55 Gy in 28 fractions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
c16 FLCSG 2.
| Methods | RCT ‐ 1982‐84 | |
| Participants | 252 patients | |
| Interventions | radical radiotherapy vs radical radiotherapy + chemotherapy cisplatin 40 doxorubicin 40 cyclophosphamide 400 | |
| Outcomes | survival | |
| Notes | 6 cycles of chemotherapy, 3 given before radiotherapy radiotherapy 55 Gy in 20 fractions** | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
c17 Essen.
| Methods | RCT ‐ 1983‐87 | |
| Participants | 48 patients | |
| Interventions | radical radiotherapy vs radical radiotherapy + chemotherapy cisplatin 80 vindesine 6 | |
| Outcomes | survival | |
| Notes | 3 cycles of chemotherapy given before radiotherapy radiotherapy 52‐56 Gy in 13‐14 fractions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
c18 SLCSG.
| Methods | RCT ‐ 1983‐89 | |
| Participants | 327 patients | |
| Interventions | radical radiotherapy vs radical radiotherapy vs chemotherapy cisplatin 120 etoposide 300 | |
| Outcomes | survival | |
| Notes | 3 cycles of chemotherapy given before radiotherapy radiotherapy 56 Gy in 28 fractions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
c19 CEBI 138.
| Methods | RCT ‐ 1983‐89 | |
| Participants | 353 patients | |
| Interventions | radical radiotherapy vs radical radiotherapy + chemotherapy cisplatin 100 cyclophosphamide 600 vindesine 3 lomustine* 75 | |
| Outcomes | survival | |
| Notes | 6 cycles of chemotherapy, 3 given before radiotherapy radiotherapy 65 Gy in 26 fractions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
c20 WSLCRG/FI.
| Methods | RCT ‐ 1984‐89 | |
| Participants | 79 patients | |
| Interventions | radical radiotherapy vs radical radiotherapy + chemotherapy cisplatin 100 vindesine 6 | |
| Outcomes | survival | |
| Notes | 8 cycles of chemotherapy, 2 given before radiotherapy radiotherapy 50 Gy in 20 fractions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
c21 Perugia.
| Methods | RCT ‐ 1984‐88 | |
| Participants | 66 patients | |
| Interventions | radical radiotherapy vs radical radiotherapy + chemotherapy cisplatin 100 etoposide 360 | |
| Outcomes | survival | |
| Notes | 3 cycles of chemotherapy given before radiotherapy radiotherapy 56 Gy in 30 fractions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
c22 CALGB 8433.
| Methods | RCT ‐ 1984‐87 | |
| Participants | 180 patients | |
| Interventions | radical radiotherapy vs radical radiotherapy + chemotherapy cisplatin 100 vinblastine 5 | |
| Outcomes | survival | |
| Notes | 2 cycles of cisplatin given before radiotherapy 5 cycles of vinblastine given before radiotherapy radiotherapy 60 Gy in 30 fractions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
c23 EORTC 08842.
| Methods | RCT ‐ 1984‐89 | |
| Participants | 75 patients | |
| Interventions | radical radiotherapy vs radical radiotherapy + chemotherapy cisplatin 100 vindesine 6 | |
| Outcomes | survival | |
| Notes | unpublished 3 cycles of chemotherapy, 2 given before radiotherapy radiotherapy 55 Gy in 20 fractions** | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
c24 SWOG 8300 a.
| Methods | RCT ‐ 1984‐88 | |
| Participants | 128 patients | |
| Interventions | radical radiotherapy vs radical radiotherapy + chemotherapy fluorouracil 1200 vincristine 2 mitomycin C 10 alternating with cisplatin 40 doxorubicin 40 cyclophosphamide 400 | |
| Outcomes | survival | |
| Notes | unpublished 6 cycles of chemotherapy, 2 given before radiotherapy radiotherapy 58 Gy in 29 fractions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
c25 SWOG 8300 b.
| Methods | RCT ‐ 1984‐88 | |
| Participants | 126 patients | |
| Interventions | radical radiotherapy vs radical radiotherapy + chemotherapy fluorouracil 1200 vincristine 2 mitomycin C 10 cisplatin 40 doxorubicin 40 cyclophosphamide 400 | |
| Outcomes | survival | |
| Notes | unpublished 6 cycles of chemotherapy, 2 given before radiotherapy radiotherapy 58 Gy in 29 fractions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
d01 Oxford.
| Methods | RCT ‐ 1970‐73 | |
| Participants | 188 patients | |
| Interventions | supportive care vs supportive care + chemotherapy (i) procarbazine* 2.5‡ ‡‡ (ii) nitrogen mustard 0.3‡ vinblastine 0.5‡ procarbazine* 35‡ prednisolone 560‡ | |
| Outcomes | survival | |
| Notes | (i) daily treatment for 1 year (ii) 11 cycles of chemotherapy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
d02 Quebec.
| Methods | RCT ‐ 1978‐79 | |
| Participants | 38 patients | |
| Interventions | supportive care vs supportive care + chemotherapy methotrexate 40 doxorubicin 40§§ cyclophosphamide 400 lomustine* 30 | |
| Outcomes | survival | |
| Notes | chemotherapy given until tumour progression or toxicity | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
d03 Gwent 2.
| Methods | RCT ‐ 1982‐84 | |
| Participants | 186 patients | |
| Interventions | supportive care vs supportive care + chemotherapy etoposide* 600 | |
| Outcomes | survival | |
| Notes | 6 cycles of chemotherapy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
d04 RLW 8351.
| Methods | RCT ‐ 1982‐86 | |
| Participants | 167 patients | |
| Interventions | supportive care vs supportive care + chemotherapy cisplatin 120 vindesine 3 | |
| Outcomes | survival | |
| Notes | chemotherapy given until tumour progression or toxicity | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
d05 NCIC CTG BR5.
| Methods | RCT ‐ 1983‐86 | |
| Participants | 150 patients | |
| Interventions | supportive care vs supportive care + chemotherapy (i) cisplatin 120 vindesine 3 (ii) cisplatin 40 doxorubicin 40 cyclophosphamide 400 | |
| Outcomes | survival | |
| Notes | chemotherapy given until tumour progression or toxicity | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
d06 Southampton.
| Methods | RCT ‐ 1983‐86 | |
| Participants | 32 patients | |
| Interventions | supportive care vs supportive care + chemotherapy cisplatin 120 vindesine 3 | |
| Outcomes | survival | |
| Notes | Same ref as RLW 8351 cisplatin given in 6 cycles vindesine given in 15 cycles | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
d07 NRH.
| Methods | RCT ‐ 1983‐87 | |
| Participants | 87 patients | |
| Interventions | supportive care vs supportive care + chemotherapy cisplatin 70 etoposide 100 etoposide* 400 | |
| Outcomes | survival | |
| Notes | 4 cycles of chemotherapy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
d08 UCLA.
| Methods | RCT ‐ 1984‐86 | |
| Participants | 63 patients | |
| Interventions | supportive care vs supportive care + chemotherapy cisplatin 120 vinblastine 6 | |
| Outcomes | survival | |
| Notes | chemotherapy given until tumour progression or toxicity | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
d09 Ancona 1.
| Methods | RCT ‐ 1985‐88 | |
| Participants | 128 patients | |
| Interventions | supportive care vs supportive care + chemotherapy cisplatin 80 cyclophosphamide 500 epirubicin 50 methotrexate 30 etoposide 200 lomustine* 70 | |
| Outcomes | survival | |
| Notes | chemotherapy given until tumour progression or toxicity | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
d10 AOI‐Udine.
| Methods | RCT ‐ 1984‐86 | |
| Participants | 102 patients | |
| Interventions | supportive care vs supportive care + chemotherapy cisplatin 75 cyclophosphamide 400 mitomycin C 10 | |
| Outcomes | survival | |
| Notes | 6 cycles of chemotherapy given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
d11 CEP‐85.
| Methods | RCT ‐ 1985‐88 | |
| Participants | 49 patients | |
| Interventions | supportive care vs supportive care + chemotherapy cisplatin 120 vindesine 3 | |
| Outcomes | survival | |
| Notes | cisplatin given in 8 cycles vindesine given in 18 cycles | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
ya(surg) <=54 years.
| Methods | Subgroup analysis for age | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | Stratified analysis of data from a number of trials | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
yb(surg) 55‐59 years.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
yc(surg) 60‐64 years.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
yd(surg) >=65 years.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
ye(surg) male.
| Methods | Subgroup analysis for sex | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | Stratified analysis of data from a number of trials | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
yf(surg) female.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
yg(surg) good PS.
| Methods | Subgroup analysis for performance status | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | Stratified analysis of data from a number of trials | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
yh(surg) poor PS.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
yi(surg) adeno.
| Methods | Subgroup analysis for histology | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | Stratified analysis of data from a number of trials | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
yj(surg) squamous.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
yk(surg) other.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
yl(surg) stage I,II.
| Methods | Subgroup analysis for stage | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | Stratified analysis of data from a number of trials | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
ym(surg) stage III.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
yn(s+rt) <=54 years.
| Methods | Subgroup analysis for age | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | Stratified analysis of data from a number of trials | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
yo(s+rt) 55‐59 years.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
yp(s+rt) 60‐64 years.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
yq(s+rt) >=65 years.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
yr(s+rt) male.
| Methods | Subgroup analysis for sex | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | Stratified analysis of data from a number of trials | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
ys(s+rt) female.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
yt(s+rt) good PS.
| Methods | Subgroup analysis for performance status | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | Stratified analysis of data from a number of trials | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
yu(s+rt) poor PS.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
yv(s+rt) adeno.
| Methods | Subgroup analysis for histology | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | Stratified analysis of data from a number of trials | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
yw(s+rt) squamous.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
yx(s+rt) other.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
yy(s+rt) stage I,II.
| Methods | Subgroup analysis for stage | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | Stratified analysis of data from a number of trials | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
yz(s+rt) stage III.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
za(radrt) <=54 years.
| Methods | Subgroup analysis for age | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | Stratified analysis of data from a number of trials | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
zb(radrt) 55‐59years.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
zc(radrt) 60‐64years.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
zd(radrt) >=65 years.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
ze(radrt) male.
| Methods | Subgroup analysis for sex | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | Stratified analysis of data from a number of trials | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
zf(radrt) female.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
zg(radrt) good PS.
| Methods | Subgroup analysis for performance status | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | Stratified analysis of data from a number of trials | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
zh(radrt) poor PS.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
zi(radrt) adeno.
| Methods | Subgroup analysis for histology | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | Stratified analysis of data from a number of trials | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
zj(radrt) squamous.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
zk(radrt) other.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
zl(radrt) stage I,II.
| Methods | Subgroup analysis for stage | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | Stratified analysis of data from a number of trials | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
zm(radrt) stage III.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
zn(sc) <=54 years.
| Methods | Subgroup analysis for age | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | Stratified analysis of data from a number of trials | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
zo(sc) 55‐59 years.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
zp(sc) 60‐64 years.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
zq(sc) >=65 years.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
zr(sc) male.
| Methods | Subgroup analysis for sex | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | Stratified analysis of data from a number of trials | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
zs(sc) female.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
zt(sc) good PS.
| Methods | Subgroup analysis for performance status | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | Stratified analysis of data from a number of trials | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
zu(sc) poor PS.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
zv(sc) adeno.
| Methods | Subgroup analysis for histology | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | Stratified analysis of data from a number of trials | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
zw(sc) squamous.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
zx(sc) other.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
zy(sc)non‐metastatic.
| Methods | Subgroup analysis for stage | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | Stratified analysis of data from a number of trials | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
zz(sc)metastatic.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
drug doses given in mg/m² unless specified
* given orally † after 10 days patients switched to maintenance chemotherapy. For first year only, drug doses were cyclophosphamide 150mg and busulphan 3mg ‡ dose in mg/kg § total dose ¶ mitomycin c was added to regimen from 1990 ** split course of radiotherapy †† daily during radiotherapy ‡‡ dose escalating to 5mg/kg during weeks 3‐6 then reduced to starting dose §§ stopped at total dose 450 mg/m²
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aliev 1985 | non‐random use of radiotherapy |
| Bergsagel 1972 | ELIGIBLE ‐ IPD not available from trialist |
| Blaha 1973 | mopidamol not established cytotoxic chemotherapy |
| Brunner 1971 | ELIGIBLE ‐ IPD not available from trialist |
| Buyze 1973 | pre 1965 and pseudo‐random (birthdate) |
| Byar 1978 | prior chemotherapy allowed |
| Carr 1972 | pre 1965, includes small cell lung cancer |
| Castberg 1976 | pseudo‐random (birthdate) |
| Crosbie 1966 | pre 1965, pseudo‐random |
| Dolton 1970 | pre 1965, historical control |
| Durrant 1971 | pre 1965 |
| Hall 1967 | pre 1965, prior chemotherapy allowed |
| Hansen 1973 | RSV (1,2‐diphenyl‐ab‐dicetone) not established cytotoxic chemotherapy |
| Helsper 1962 | pre 1965, historical control |
| Holsti 1971 | pre 1965, pseudo‐random (birthdate) |
| Hosley 1962 | pre 1965, used orthovoltage RT |
| Karageorgis 1991 | confounded, RT dose not equal on each arm |
| Karp | confounded, RT dose not equal on each arm |
| Karrer 1978 | ELIGIBLE ‐ IPD not available from trialist |
| Kaung 1974 | ELIGIBLE ‐ IPD not available from trialist |
| NCCTG852451 | Trial closed with no patients recruited |
| Newman 1985 | razoxane not established cytotoxic chemotherapy |
| Okawa 1992 | SPG (sizofiran) not established cytotoxic chemotherapy |
| Osterlind 1985 | RSV (1,2‐diphenyl‐ab‐dicetone) not established cytotoxic chemotherapy |
| Petrovic 1978 | ELIGIBLE ‐ IPD not available from trialist |
| Pirogov 1976 | pseudo randomised |
| Privitera 1987 | Ionidamine not established cytotoxic chemotherapy |
| Scheer 1974 | randomised all lung cancer, no histology |
| Selawry 1977 | prior chemotherapy allowed |
| Slack 1970 | pre 1965 |
| Spatti 1985 | used histological control |
| Spittle 1980 | razoxane not established cytotoxic chemotherapy |
| Vincent 1975 | pre 1965, only partly randomised |
| Wils 1984 | confounded, RT dose different on each arm |
| Wingfield 1970 | non‐randomised |
| Wolf 1991 | confounded, cisplatin used as a sensitiser on each arm |
Characteristics of ongoing studies [ordered by study ID]
Ancona.
| Trial name or title | |
| Methods | |
| Participants | 47 |
| Interventions | supportive care vs supportive care + chemotherapy |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Ongoing at time of review to be included in update |
ANITA.
| Trial name or title | |
| Methods | |
| Participants | 800 projected |
| Interventions | surgery versus surgery + chemotherapy |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Ongoing, to be included in update |
BLT.
| Trial name or title | |
| Methods | |
| Participants | 3300 projected |
| Interventions | (1)surgery + radiotherapy vs surgery + radiotherapy + chemotherapy (2) radiotherapy vs radiotherapy + chemotherapy (3)supportive care vs supportive care + chemotherapy |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Ongoing, to be included in update |
CAN NCIC BR10.
| Trial name or title | |
| Methods | |
| Participants | 600 projected |
| Interventions | surgery vs surgery + chemotherapy |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Ongoing, to be included in update |
CLB 9633.
| Trial name or title | |
| Methods | |
| Participants | 500 projected |
| Interventions | surgery vs surgery + chemotherapy |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Ongoing, to be included in update |
CNR ALPI.
| Trial name or title | |
| Methods | |
| Participants | 1200 |
| Interventions | surgery vs surgery + chemotherapy |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Closed, to be included in update |
CRC‐TU‐LU3001.
| Trial name or title | |
| Methods | |
| Participants | 466 projected |
| Interventions | radiotherapy vs radiotherapy + chemotherapy |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Ongoing at time of review to be included in update |
CRC‐TU‐LU3002.
| Trial name or title | |
| Methods | |
| Participants | 359 projected |
| Interventions | supportive care vs supportive care + chemotherapy |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Ongoing at time of review to be included in update |
EORTC 08922.
| Trial name or title | |
| Methods | |
| Participants | 34 |
| Interventions | surgery vs surgery + chemotherapy |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Ongoing at time of review, now closed, to be included in update |
FRE IALT.
| Trial name or title | |
| Methods | |
| Participants | 3300 projected |
| Interventions | surgery vs surgery + chemotherapy |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Ongoing, to be included in update |
GAP.
| Trial name or title | |
| Methods | |
| Participants | 200 |
| Interventions | surgery vs surgery + chemotherapy |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Ongoing at time of review,closed 92 to be included in update |
Helsing.
| Trial name or title | |
| Methods | |
| Participants | 48 |
| Interventions | supportive care vs supportive care + chemotherapy |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Reported in 1998 after review published. To be included in update. |
Ichinose 1991.
| Trial name or title | |
| Methods | |
| Participants | 86 patients |
| Interventions | surgery vs surgery + chemotherapy |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Identified after meta‐analysis published. To be included in update |
Kim 1993.
| Trial name or title | |
| Methods | |
| Participants | 101 |
| Interventions | Radical RT versus radical RT + chemotherapy |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Ongoing at the time of the review. To be included in update |
NCCTG‐822451.
| Trial name or title | |
| Methods | |
| Participants | 120 projected |
| Interventions | radiotherapy vs radiotherapy + chemotherapy |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Completed in 1993 (outside time boundary of review). To be included in update. |
RICUM.
| Trial name or title | |
| Methods | |
| Participants | 242 173 |
| Interventions | (1)surgery vs surgery + chemotherapy (2)surgery + radiotherapy vs surgery + radiotherapy + chemotherapy |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Ongoing at time of review,closed 92 to be included in update |
RTOG 8808.
| Trial name or title | |
| Methods | |
| Participants | 327 |
| Interventions | radiotherapy vs radiotherapy + chemotherapy |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Ongoing at time of review,closed 92 to be included in update |
TELCVIS 1999.
| Trial name or title | |
| Methods | |
| Participants | 191 |
| Interventions | supportive care versus supportive care + chemotherapy |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Reported in 1999 after review published. To be included in update |
Thongprasert 1999.
| Trial name or title | |
| Methods | |
| Participants | 288 |
| Interventions | supportive care vs supportive care + chemotherapy |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Reported in 1996 after review published. To be included in update. |
Sources of support
Internal sources
Medical Research Council, UK.
Institute Gustave Roussy, France.
External sources
INSERM 921204, France.
ARC 2025, France.
Declarations of interest
There is no known conflict of interest
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
a01 MRC LU02 {unpublished data only}
- Girling DJ, Stott H, Stephens RJ, Fox W. Fifteen‐year follow‐up of all patients in a study of post‐operative chemotherapy for bronchial carcinoma. British Journal of Cancer 1985;52:867‐873. [DOI] [PMC free article] [PubMed] [Google Scholar]
a02 VASAG {unpublished data only}
- Shields TW, Humphrey EW, Eastridge CE, Keehn RJ. Adjuvant cancer chemotherapy after resection of carcinoma of the lung. Cancer 1977;40:2057‐2062. [DOI] [PubMed] [Google Scholar]
a03 EORTC 08741a {unpublished data only}
- Israel L, Bonadonna G, Sylvester R. Controlled study with adjuvant radiotherapy, chemotherapy, immunotherapy and chemoimmunotherapy in operable squamous carcinoma of the lung. In: Muggia FM, Rozencweig M editor(s). Lung Cancer: Progress in therapeutic Research. New York: Raven Press, 1979:443‐452. [Google Scholar]
a04 VASOG 5 {unpublished data only}
- Shields TW, Higgins GA, Humphrey EW, Matthews MJ, Keehn RJ. Prolonged intermittent adjuvant chemotherapy with CCNU and hydroxyurea after resection of carcinoma of the lung. Cancer 1982;50:1713‐1721. [DOI] [PubMed] [Google Scholar]
a05 WPL 7351 {unpublished data only}
- Mountain CF, Vincent RG, Sealy R, et al. A clinical trial of CCNU as surgical adjuvant treatment for patients with surgical stage I and stage II non‐small cell lung cancer: Preliminary findings.. In: Muggia FM, Rozencweig M editor(s). Lung Cancer: Progress in Therapeutic Research.. New York: Raven Press, 1979:421‐431. [Google Scholar]
a06 OLCSG 1a {unpublished data only}
- Sawamura K, Mori T, Doi O, Yasumitsu T, Kuwahara O, Kuwabara M, Nakahara K, Kurata M, Sagara N. A prospective randomized controlled study of the postoperative adjuvant therapy for non‐small cell lung cancer. Lung Cancer 1988;4:A166. [Google Scholar]
a07 OLCSG 1b {unpublished data only}
- Sawamura K, Mori T, Doi O, Yasumitsu T, Kuwahara O, Kuwabara M, Nakahara K, Kurata M, Sagara N. A prospective randomized controlled study of the postoperative adjuvant therapy for non‐small cell lung cancer. Lung Cancer 1988;4:A166. [Google Scholar]
a08 SGACLC 1 {unpublished data only}
- Study Group for Adjuvant Chemotherapy for Lung Cancer. A randomised controlled trial of postoperative adjuvant chemotherapy in non‐small cell lung cancer (in Japanese.). Hai‐gan 1992;32:481‐486. [Google Scholar]
a09 WJSG 2 {unpublished data only}
- Teramatsu T on behalf of the Society of adjuvant chemotherapy for lung cancer surgery in West Japan. Assessment of postoperative adjuvant chemotherapy on non‐small cell lung cancer (abstract). Lung Cancer 1991;7(Suppl):124. [Google Scholar]
- Wada H, Hitomi S, Takashi T, and the West Japan Study Group for Lung Cancer Surgery. Adjuvant chemotherapy after complete resection in non‐small cell lung cancer (full publication). Journal of Clinical Oncology 1996;14:1048‐1054. [DOI] [PubMed] [Google Scholar]
a10 LCSG 801 {unpublished data only}
- Feld R, Rubinstein L, Thomas PA. Adjuvant chemotherapy with cyclophosphamide, doxorubicin and cisplatin in patients with completely resected stage I non‐small‐cell lung cancer. Journal of the National Cancer Institute 1993;85(4):299‐306. [DOI] [PubMed] [Google Scholar]
a11 OLCSG 1c {unpublished data only}
- Sawamura K, Mori T, Doi O, Yasumitsu T, Kawahara O, Kuwabara M, Nakahara K, Kurata M, Sagara N. A prospective randomized controlled study of the postoperative adjuvant therapy for non‐small cell lung cancer. Lung Cancer 1988;4:A166. [Google Scholar]
a12 FLCSG 1 {unpublished data only}
- Niiranen A, Niitamo‐Korhonen S, Kouri M, Assendelft A, Mattson K, Pyrhönen S. Adjuvant chemotherapy after radical surgery for non‐small cell lung cancer: A randomized study. Journal of Clinical Oncolcology 1992;10(12):1927‐1932. [DOI] [PubMed] [Google Scholar]
a13 SGACLC 2 {unpublished data only}
- Study Group for Adjuvant Chemotherapy for Lung Cancer. A randomized trial of postoperative adjuvant chemotherapy in non‐small cell lung cancer (the second cooperative study). European Journal of Surgical Oncology 1995;21:69‐77. [PubMed] [Google Scholar]
a14 IPCR, Chiba {unpublished data only}
- Kimura H, Yamaguchi Y, Fujisawa T, Baba M, Shiba M. A randomized controlled study of postoperative adjuvant chemoimmunotherapy of resected non‐small cell lung cancer with IL2 and LAK cells. Lung Cancer 1991;7(Suppl):113. [Google Scholar]
a15 LCSG 853 {unpublished data only}
- Lung Cancer Study Group. A clinical trial in patients with stage II and III completely resected non‐small cell cancer of the lung comparing chemotherapy (CAP) versus no therapy following surgery.
a16 JLCSSG {unpublished data only}
- Ohta M, Tsuchiya R, Shimoyama M, Sawamura K, Mori T, Miyazawa N, Suemasu K, Watanabe Y, Tomita M, Terashima M. Adjuvant chemotherapy for completely resected stage III non‐small cell lung cancer. Journal of Thoracic and Cardiovascular Surgery 1993;106:703‐708. [PubMed] [Google Scholar]
b01 EORTC 08741b {unpublished data only}
- Israel L, Bonadonna G, Sylvester R and members of the EORTC Lung Cancer Group. Controlled study with adjuvant radiotherapy, chemotherapy, immunotherapy and chemoimmunotherapy in operable squamous carcinoma of the lung. In: Muggia FM, Rozencweig M editor(s). Lung Cancer: Progress in therapeutic research. New York: Raven Press, 1979:443‐452. [Google Scholar]
b02 LCSG 791 {unpublished data only}
- Lad T. The comparison of CAP chemotherapy and radiotherapy to radiotherapy alone for resected lung cancer with positive margin or involved highest sampled paratracael node (stage IIIa). Chest 1994;106(6):303S. [PubMed] [Google Scholar]
- Lad T, Rubinstein L, Sadeghi A. The benefit of adjuvant treatment for resected locally advanced non‐small cell lung cancer. Journal of Clinical Oncology 1988;6:9‐17. [DOI] [PubMed] [Google Scholar]
b03 MSKCC 80‐53 {unpublished data only}
- Pisters KMW, Kris MG, Gralla RT, Hilaris B, McCormack PM, Bains MS. Randomized trial comparing post‐operative chemotherapy with vindesine and cisplatin plus thoracic irradiation with irradiation alone in stage III (N2) non‐small cell lung cancer. Journal of Surgical Oncology 1994;56:236‐241. [DOI] [PubMed] [Google Scholar]
b04 FLCSG 3 {unpublished data only}
- Niiranen, Kouri M, Pyrhonen S, Mattson K. Postsurgical radiotherapy versus postsurgical radiotherapy plus chemotherapy for non‐small cell lung cancer.
b05 GETCB 01CB82 {unpublished data only}
- Chastang CI, Dautzenberg B, Arriagada R, Chevalier T, Belpomme D, Hurdebourg J, the GETCB. Results of a randomized clinical trial of a postoperative chemotherapy (COPAC) in stage II and III resected bronchogenic carcinoma. European Journal of Cancer 1991;27(Suppl 2):A1017. [Google Scholar]
b06 OLCSG 1d {unpublished data only}
- Sawamura K, Mori T, Doi O, Yasumitsu T, Kawahara O, Kuwabara M, Nakahara K, Kurata M, Sagara N. A prospective randomized controlled study of the postoperative adjuvant therapy for non‐small cell lung cancer. Lung Cancer 1988;4:A166. [Google Scholar]
b07 EORTC 08861 {unpublished data only}
- EORTC Lung Cancer Cooperative Group. Phase III randomized trial of adjuvant radiotherapy vs radiotherapy plus chemotherapy with DDP/VDS vs no adjuvant therapy in patients with completely resected non‐small cell lung cancer.
c01 NRH NSC 26271 {unpublished data only}
- Høst H. Cyclophosphamide as adjuvant to radiotherapy in the treatment of unresectable bronchogenic carcinoma. Cancer Chemotherapy Reports 1973;4(Part 3):161‐164. [PubMed] [Google Scholar]
c02 EORTC 08742 {unpublished data only}
- Israel L, Depierre A, Dalesio O. Interim results of EORTC protocol 08742: comparison, after irradiation of locally advanced squamous cell bronchial carcinoma, of abstention, immunotherapy, combination chemotherapy or chemoimmunotherapy. Recent Results on Cancer Research 1982;80:214‐218. [DOI] [PubMed] [Google Scholar]
c03 RTOG 7302 a {unpublished data only}
- Simpson JR, Francis ME, Perez‐Tamayo R, Mark RD, Rao DV. Palliative radiotherapy for inoperable carcinoma of the lung: Final report of a RTOG multi‐institutional trial. International Journal of Radiation, Oncology, Biology and Physics 1985;11(4):751‐758. [DOI] [PubMed] [Google Scholar]
c04 RTOG 7302 b {unpublished data only}
- Simpson JR, Francis ME, Perez‐Tamayo R, Mark RD, Rao DV. Palliative radiotherapy for inoperable carcinoma of the lung: Final report of a RTOG multi‐institutional trial. International Journal of Radiation, Oncology, Biology and Physics 1985;11(4):751‐758. [DOI] [PubMed] [Google Scholar]
c05 RTOG 7302 c {unpublished data only}
- Simpson JR, Francis ME, Perez‐Tamayo R, Mark RD, Rao DV. Palliative radiotherapy for inoperable carcinoma of the lung: Final report of a RTOG multi‐institutional trial. International Journal of Radiation, Oncology, Biology and Physics 1985;11(4):751‐758. [DOI] [PubMed] [Google Scholar]
c06 MCL‐1 {unpublished data only}
- Sidorowicz E, Hirsch V, Hand R, Guerra J, Margolese R, Thirlwell M, Colman N, Frank H, Kreisman H. Comparison of radiotherapy (RT) and RT‐MACC chemotherapy in limited non‐small cell lung cancer (NSCLC). Proceedings of the IVth world conference on lung cancer 1985:A277. [Google Scholar]
c07 Aviano {unpublished data only}
- Trovo MG, Minatel E, Veronesi A, Roncadin M, Paoli A, Franchin G, Magri DM, Tirelli U, Carboma A, Grigoletto E. Combined radiotherapy and chemotherapy versus radiotherapy alone in locally advanced epidermoid bronchogenic carcinoma. Cancer 1990;65:400‐404. [DOI] [PubMed] [Google Scholar]
c08 AZ‐OC‐1‐80 {unpublished data only}
- Schallier DC, Neve WJ, Greve JL, Belle SP, Wasch GJ, Dotremont G, Storme GA. Is adjuvant treatment with vinblastine effective in reducing the occurrence of distant metastasis in limited squamous cell lung cancer? A randomized study. Clinical and Experimental Metastasis 1988;6(1):39‐48. [DOI] [PubMed] [Google Scholar]
c09 Gwent 3 {unpublished data only}
- Anderson G. Study of radiotherapy versus radiotherapy + VP‐16‐213 in carcinoma of the bronchus.
c10 SECSG 81 LUN375 {unpublished data only}
- Johnson DH, Einhorn LH, Bartolucci A, Birch R, Omura G, Perez CA, Greco FA. Thoracic radiotherapy does not prolong survival in patients with locally advanced unresectable non‐small cell lung cancer. Annals of Internal Medicine 1990;113:33‐38. [DOI] [PubMed] [Google Scholar]
c11 Gwent 1 {unpublished data only}
- Anderson G, Deeley TJ, Smith C, Jani J. Comparison of radiotherapy alone and radiotherapy with chemotherapy using adriamycin and 5‐fluorouracil in bronchogenic carcinoma. Thorax 1981;36:190‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]
c12 SWOG 7635 {unpublished data only}
- White JE, Chen T, Reed R, Mira J, Stuckey WJ, Weatherall T, O'Bryan R, Samson MK, Seydel HG. Limited squamous cell carcinoma of the lung: A Southwest Oncology Group randomised study of radiation with or without doxorubicin chemotherapy and with or without levasmisole immunotherapy. Cancer Treatment Reports 1982;66(5):1113‐1120. [PubMed] [Google Scholar]
c13 NCCTG 822451 {unpublished data only}
- Morton RF, Jett JR, McGinnis WL, Earle JD, Thernea TM, Krook JE, Elliott TE, Mailliard JA, Nelimark RA, Maksymiuk AW, et al. Thoracic radiation therapy alone compared with combined chemoradiotherapy for locally unresectable non‐small cell lung cancer. Annals of Internal Medicine 1991;115:681‐686. [DOI] [PubMed] [Google Scholar]
c14 Buenos Aires {unpublished data only}
- Cardiello C, Blanco Villalba J, Anac S, Francheri Wilson C, Trodler C, Hunis A, Alvarez Bermudez C. Combined radiochemotherapy (RtCt) versus radiotherapy (Rt) in limited inoperable non‐small cell carcinoma of the lung (NSCLC). Proceedings of the American Society of Clinical Oncology 1985;4:117. [Google Scholar]
c15 Brussels {unpublished data only}
- Houtte P, Klastersky J, Renauld A, Michel J, Vandermoten G, Nguyen H, Sculier JP, Devriendt J, Mommen P. Induction chemotherapy with cisplatin etoposide and vindesine before radiation therapy for non‐small cell lung cancer. Antibiotic Chemotherapy 1988;41:131‐137. [DOI] [PubMed] [Google Scholar]
c16 FLCSG 2 {unpublished data only}
- Mattson K, Holsti LR, Holsti P, Jakobsson M, Kajanti M, Liippo K, Mäntylä M, Niitamo‐Korhonen S, Nikkanen V, Nordman E, et al. Inoperable non‐small cell lung cancer: radiation with or without chemotherapy. European Journal of Clinical Oncology 1988;24:477‐482. [DOI] [PubMed] [Google Scholar]
c17 Essen {unpublished data only}
- Alberti W, Niederle N, Budach V, Konietzko N, Sack H. Prospective randomized study comparing immediate radiotherapy, chemo‐ plus radiotherapy or delayed radiotherapy in non‐small cell lung cancer. Journal of Cancer Research and Clinical Oncology 1990;116(part 1):503. [Google Scholar]
c18 SLCSG {unpublished data only}
- Brodin O, Nou E, on behalf of the Swedish Multicentric Group for the study of squamous cell carcinoma of the lung. Patients with non‐resectable squamous cell carcinoma of the lung. A prospective randomised study. Lung Cancer 1991;7:A615. [Google Scholar]
c19 CEBI 138 {unpublished data only}
- Chevalier T, Arriagada R, Quoix E, Ruffie P, Martin M, Tarayre M, Lacombe‐Terrier M‐J, Douillard J‐Y, Laplanche A. Radiotherapy alone versus combined chemotherapy and radiotherapy in nonresectable non‐small cell lung cancer: First analysis of a randomized trial in 353 patients. Journal of the National Cancer Institute 1991;83(6):417‐423. [DOI] [PubMed] [Google Scholar]
c20 WSLCRG/FI {unpublished data only}
- Gregor A, Macbeth FR, Paul J, Cram L, Hansen HH. Radical radiotherapy in localized inoperable non‐small cell lung cancer: A randomized trial. Journal of the National Cancer Institute 1993;85(12):997‐999. [DOI] [PubMed] [Google Scholar]
c21 Perugia {unpublished data only}
- Crinò L, Latini P, Meacci M, Corgna E, Maranzano E, Darwish S, Minotti V, Santucci A, Tonato M. Induction chemotherapy plus high‐dose radiotherapy versus radiotherapy alone in locally advanced unresectable non‐small cell lung cancer. Annals of Oncology 1993;4:847‐851. [DOI] [PubMed] [Google Scholar]
c22 CALGB 8433 {unpublished data only}
- Dillman RO, Seagren SL, Propert KJ, Guerra J, Eaton WL, Perry MC, Carey R, Frei EF, Green MR. A randomized trial of induction chemotherapy plus high‐dose radiation versus radiation alone in stage III non‐small cell lung cancer. New England Journal of Medicine 1990;323:940‐945. [DOI] [PubMed] [Google Scholar]
c23 EORTC 08842 {unpublished data only}
- Planting A, Helle P, Drings P, Dalesio O, Kirkpatrick A, McVie G, Giaccone G. A randomized study of high‐dose split course radiotherapy preceeded by high‐dose chemotherapy versus high‐dose radiotherapy only in locally advanced non‐small cell lung cancer. Annals of Oncology 1996;7:139‐144. [DOI] [PubMed] [Google Scholar]
c24 SWOG 8300 a {unpublished data only}
- Miller TP, Mira JG, Horn B. Treatment of limited non‐small cell lung cancer: Radiation versus radiation plus chemotherapy.
c25 SWOG 8300 b {unpublished data only}
- Miller TP, Mira JG, Horn B. Treatment of non‐small cell lung cancer: Radiation versus radiation plus chemotherapy.
d01 Oxford {unpublished data only}
- Laing AH, Berry RJ, Newman CR, Peto J. Treatment of inoperable carcinoma of bronchus. The Lancet 1975;1161‐1164. [DOI] [PubMed] [Google Scholar]
d02 Quebec {unpublished data only}
- Cormier Y, Bergeron D, Forge J, Lavindier M, Founier M, Chenard J, Desmeules M. Benefits of polychemotherapy in advanced non‐small cell bronchogenic carcinoma. Cancer 1982;50:845‐849. [DOI] [PubMed] [Google Scholar]
d03 Gwent 2 {unpublished data only}
- Anderson G, Payne H. Response rate and toxicity of etoposide (VP‐16) in squamous carcinoma of the lung: Report from the Lung Cancer Treatment Study Group. Seminars of Oncology 1985;12(1 Suppl 2):21‐22. [PubMed] [Google Scholar]
d04 RLW 8351 {unpublished data only}
- Woods RL, Williams CJ, Levi J, Page J, Bell D, Byrne M, Kerestes ZL. A randomised trial of cisplatin and vindesine versus supportive care only in advanced non‐small cell lung cancer. British Journal of Cancer 1990;61:608‐611. [DOI] [PMC free article] [PubMed] [Google Scholar]
d05 NCIC CTG BR5 {unpublished data only}
- Rapp E, Pater JL, Willan A, Cormier Y, Murray N, Evans WK, Hodson DI, Clark DA, Feld R, Arnold AM, et al. Chemotherapy can prolong survival in patients with advanced non‐small cell lung cancer‐Report of a Canadian multicenter randomized trial. Journal of Clinical Oncology 1988;6(4):633‐641. [DOI] [PubMed] [Google Scholar]
d06 Southampton {unpublished data only}
- Woods RL, Williams CJ, Levi J, Page J, Bell D, Byrne M, Kerestes ZL. A randomised trial of cisplatin and vindesine versus supportive care only in advanced non‐small cell lung cancer. British Journal of Cancer 1990;61:608‐611. [DOI] [PMC free article] [PubMed] [Google Scholar]
d07 NRH {unpublished data only}
- Kaasa S, Lund E, Thorud E, Hatlevoll R, Høst H. Symptomatic treatment versus combination chemotherapy for patients with extensive non‐small cell lung cancer. Cancer 1991;67:2443‐2447. [DOI] [PubMed] [Google Scholar]
d08 UCLA {unpublished data only}
- Ganz PA, Figlin RA, Haskell CM, Soto N, Siau J. Supportive care versus supportive care and combination chemotherapy in metastatic non‐small cell lung cancer. Does chemotherapy make a difference?. Cancer 1989;63:1271‐1278. [DOI] [PubMed] [Google Scholar]
d09 Ancona 1 {unpublished data only}
- Cellerino R, Tummarello D, Guidi F, Isidori P, Raspugli M, Biscottini B, Fatati G. A randomized trial of alternating chemotherapy versus best supportive care in advanced non‐small cell lung cancer. Journal of Clinical Oncology 1991;9(8):1453‐1461. [DOI] [PubMed] [Google Scholar]
d10 AOI‐Udine {unpublished data only}
- Cartei G, Cartei F, Cantone A, Causarano D, Genco G, Tobaldin A, Interlandi G, Giraldi T. Cisplatin‐cyclophosphamide‐mitomycin combination chemotherapy with supportive care versus supportive care alone for treatment of metastatic non‐small cell lung cancer. Journal of the National Cancer Institute 1993;85(10):794‐800. [DOI] [PubMed] [Google Scholar]
d11 CEP‐85 {unpublished data only}
- Quoix É, Dietemann A, Charbonneau J, Boutin C, Meurice JC, Orlando JP, Ducolone A, Pauli G, Roegel É. Is cisplatinum‐based chemotherapy useful in disseminated non small cell lung cancer? Report of a French multicenter randomized trial [La chimiothérapie comportant du cisplatine est‐elle utile dans le cancer bronchique non microcellulaire au stade IV? Résultants d'une étude randomisée]. Bulletin du Cancer (Paris) 1991;78:341‐346. [PubMed] [Google Scholar]
ya(surg) <=54 years {unpublished data only}
- Subgroup analysis by age for cisplatin‐based trials in the surgical setting.
yb(surg) 55‐59 years {unpublished data only}
- Subgroup analysis by age for cisplatin‐based trials in the surgical setting.
yc(surg) 60‐64 years {unpublished data only}
- Subgroup analysis by age for cisplatin‐based trials in the surgical setting.
yd(surg) >=65 years {unpublished data only}
- Subgroup analysis by age for cisplatin‐based trials in the surgical setting.
ye(surg) male {unpublished data only}
- Subgroup analysis by sex for cisplatin‐based trials in the surgical setting.
yf(surg) female {unpublished data only}
- Subgroup analysis by sex for cisplatin‐based trials in the surgical setting.
yg(surg) good PS {unpublished data only}
- Subgroup analysis by performance status for cisplatin‐based trials in the surgical setting.
yh(surg) poor PS {unpublished data only}
- Subgroup analysis by performance status for cisplatin‐based trials in the surgical setting.
yi(surg) adeno {unpublished data only}
- Subgroup analysis by histology for cisplatin‐based trials in the surgical setting.
yj(surg) squamous {unpublished data only}
- Subgroup analysis by histology for cisplatin‐based trials in the surgical setting.
yk(surg) other {unpublished data only}
- Subgroup analysis by histology for cisplatin‐based trials in the surgical setting.
yl(surg) stage I,II {unpublished data only}
- Subgroup analysis by stage for cisplatin‐based trials in the surgical setting.
ym(surg) stage III {unpublished data only}
- Subgroup analysis by stage for cisplatin‐based trials in the surgical setting.
yn(s+rt) <=54 years {unpublished data only}
- Subgroup analysis by age for cisplatin‐based trials in the surgery + RT setting.
yo(s+rt) 55‐59 years {unpublished data only}
- Subgroup analysis by age for cisplatin‐based trials in the surgery + RT setting.
yp(s+rt) 60‐64 years {unpublished data only}
- Subgroup analysis by age for cisplatin‐based trials in the surgery + RT setting.
yq(s+rt) >=65 years {unpublished data only}
- Subgroup analysis by age for cisplatin‐based trials in the surgery + RT setting.
yr(s+rt) male {unpublished data only}
- Subgroup analysis by sex for cisplatin‐based trials in the surgery + RT setting.
ys(s+rt) female {unpublished data only}
- Subgroup analysis by sex for cisplatin‐based trials in the surgery + RT setting.
yt(s+rt) good PS {unpublished data only}
- Subgroup analysis by performance status for cisplatin‐based trials in the surgery + RT setting.
yu(s+rt) poor PS {unpublished data only}
- Subgroup analysis by performance status for cisplatin‐based trials in the surgery + RT setting.
yv(s+rt) adeno {unpublished data only}
- Subgroup analysis by histology for cisplatin‐based trials in the surgery + RT setting.
yw(s+rt) squamous {unpublished data only}
- Subgroup analysis by histology for cisplatin‐based trials in the surgery + RT setting.
yx(s+rt) other {unpublished data only}
- Subgroup analysis by histology for cisplatin‐based trials in the surgery + RT setting.
yy(s+rt) stage I,II {unpublished data only}
- Subgroup analysis by stage for cisplatin‐based trials in the surgery + RT setting.
yz(s+rt) stage III {unpublished data only}
- Subgroup analysis by stage for cisplatin‐based trials in the surgery + RT setting.
za(radrt) <=54 years {unpublished data only}
- Subgroup analysis by age for cisplatin‐based trials in the radical RT setting.
zb(radrt) 55‐59years {unpublished data only}
- Subgroup analysis by age for cisplatin‐based trials in the radical RT setting.
zc(radrt) 60‐64years {unpublished data only}
- Subgroup analysis by age for cisplatin‐based trials in the radical RT setting.
zd(radrt) >=65 years {unpublished data only}
- Subgroup analysis by age for cisplatin‐based trials in the radical RT setting.
ze(radrt) male {unpublished data only}
- Subgroup analysis by sex for cisplatin‐based trials in the radical RT setting.
zf(radrt) female {unpublished data only}
- Subgroup analysis by sex for cisplatin‐based trials in the radical RT setting.
zg(radrt) good PS {unpublished data only}
- Subgroup analysis by performance status for cisplatin‐based trials in the radical RT setting.
zh(radrt) poor PS {unpublished data only}
- Subgroup analysis by performance status for cisplatin‐based trials in the radical RT setting.
zi(radrt) adeno {unpublished data only}
- Subgroup analysis by histology for cisplatin‐based trials in the radical RT setting.
zj(radrt) squamous {unpublished data only}
- Subgroup analysis by histolohy for cisplatin‐based trials in the radical RT setting.
zk(radrt) other {unpublished data only}
- Subgroup analysis by histology for cisplatin‐based trials in the radical RT setting.
zl(radrt) stage I,II {unpublished data only}
- Subgroup analysis by stage for cisplatin‐based trials in the radical RT setting.
zm(radrt) stage III {unpublished data only}
- Subgroup analysis by stage for cisplatin‐based trials in the radical RT setting.
zn(sc) <=54 years {unpublished data only}
- Subgroup analysis by age for cisplatin‐based trials in the supportive care setting.
zo(sc) 55‐59 years {unpublished data only}
- Subgroup analysis by age for cisplatin‐based trials in the supportive care setting.
zp(sc) 60‐64 years {unpublished data only}
- Subgroup analysis by age for cisplatin‐based trials in the supportive care setting.
zq(sc) >=65 years {unpublished data only}
- Subgroup analysis by age for cisplatin‐based trials in the supportive care setting.
zr(sc) male {unpublished data only}
- Subgroup analysis by sex for cisplatin‐based trials in the supportive care setting.
zs(sc) female {unpublished data only}
- Subgroup analysis by sex for cisplatin‐based trials in the supportive care setting.
zt(sc) good PS {unpublished data only}
- Subgroup analysis by performance status for cisplatin‐based trials in the supportive care setting.
zu(sc) poor PS {unpublished data only}
- Subgroup analysis by performance status for cisplatin‐based trials in the supportive care setting.
zv(sc) adeno {unpublished data only}
- Subgroup analysis by histology for cisplatin‐based trials in the supportive care setting.
zw(sc) squamous {unpublished data only}
- Subgroup analysis by histology for cisplatin‐based trials in the supportive care setting.
zx(sc) other {unpublished data only}
- Subgroup analysis by histology for cisplatin‐based trials in the supportive care setting.
zy(sc)non‐metastatic {unpublished data only}
- Subgroup analysis by stage for cisplatin‐based trials in the supportive care setting.
zz(sc)metastatic {unpublished data only}
- Subgroup analysis by stage for cisplatin‐based trials in the supportive care setting.
References to studies excluded from this review
Aliev 1985 {published data only}
- Aliev BM, Starichkov MS, Mikhina ZP, Petrukhin OD, Bychkov MB. Radiotherapy and combined chemotherapy and radiotherapy of patients with squamous‐cell lung cancer (a randomized study). Voprosy Onkologii 1985;31(10):71‐6. [PubMed] [Google Scholar]
Bergsagel 1972 {published data only}
- Bergsagel DE, Jenkin RDT, Pringle JF, White DM, Fetterly JCM, Klaassen DJ. [Lung Cancer: clinical trial of radiotherapy alone vs radiotherapy plus cyclophosphamide]. Cancer 1972;30:621‐627. [DOI] [PubMed] [Google Scholar]
Blaha 1973 {published data only}
- Blaha H, Dierkesmann R, Feuerer W, et al. Adjuvant therapy of non‐small cell bronchial cancer with mopidamol [Adjuvante therapie des nicht‐kleinzellingen bronchialkarzinoms mit mopidamol]. Pneumologie 1973;43:299‐304. [PubMed] [Google Scholar]
Brunner 1971 {published data only}
- Brunner KW, Marthaler TH, Muller W. Unfavourable effects of long‐term adjuvant chemotherapy with endoxan in radically operated bronchogenic carcinoma. European Journal of Cancer 1971;7:285‐294. [DOI] [PubMed] [Google Scholar]
Buyze 1973 {published data only}
- Buyze EAC, Nelemans FA. A study of post‐operative cytostatic medication in patients with operable carcinoma of the lung. Arzneim. Forsh (Drug Research) 1973;23(6):860‐862. [PubMed] [Google Scholar]
Byar 1978 {published data only}
- Byar D, Kenis Y, Andel JG, et al. Results of a E.O.R.T.C. randomized trial of cyclophosphamide and radiotherapy in inoperable lung cancer: Prognostic factors and treatment results. European Journal of Cancer 1978;14:919‐930. [DOI] [PubMed] [Google Scholar]
Carr 1972 {published data only}
- Carr DT, Childs DS, Lee RE. Radiotherapy plus 5‐FU compared to radiotherapy alone for inoperable and unresectable bronchogenic carcinoma. Cancer 1972;29(2):375‐380. [DOI] [PubMed] [Google Scholar]
Castberg 1976 {published data only}
- Castberg T, Kruse‐Blinkenberg HO. Cancer chemotherapy as a supplement to operation in bronchogenic carcinoma [Cancerkemoterapi som supplement til operation ved bronkogent karcinom]. Ugestr Laeg 1976;138:1449‐1455. [PubMed] [Google Scholar]
Crosbie 1966 {published data only}
- Crosbie WA, Kamdar HH, Belcher JR. Controlled trial of vinblastine sulphate in the treatment of cancer of the lung. British Journal of Diseases of the Chest 1966;60:28. [DOI] [PubMed] [Google Scholar]
Dolton 1970 {published data only}
- Dolton EG. Combined surgery and chemotherapy for carcinoma of bronchus. Lancet 1970;Jan 3:40‐41. [DOI] [PubMed] [Google Scholar]
Durrant 1971 {published data only}
- Durrant KR, Ellis F, Black JM, Berry RJ, Ridehalgh FR, Hamilton WS. Comparison of treatment policies in inoperable bronchial carcinoma. Lancet 1971;1(7702):715‐719. [DOI] [PubMed] [Google Scholar]
Hall 1967 {published data only}
- Hall TC, Dederick MM, Chalmers TC, et al. A clinical pharmacologic study of chemotherapy and x‐ray therapy in lung cancer. American Journal of Medicine 1943;2:186‐193. [DOI] [PubMed] [Google Scholar]
Hansen 1973 {published data only}
- Hansen JL. Clinical evaluation of an innoxious antineoplastic agent: Preliminary study of 106 patients treated with or without RSV after pulmonary resection for bronchogenic carcinoma. Cancer Chemotherapy Reports 1973;4(Part 3):261‐264. [PubMed] [Google Scholar]
Helsper 1962 {published data only}
- Helsper JT, Sharpe GS. Combination therapy with 5‐fluorouracil and colbalt‐60 for inoperable carcinoma of the lungs. Cancer Chemotherapy Reports 1962;20:103‐105. [PubMed] [Google Scholar]
Holsti 1971 {published data only}
- Holsti LR. Adjuvant Chemotherapy. In: Deeley editor(s). Modern Radiotherapy. 222‐240. [Google Scholar]
Hosley 1962 {published data only}
- Hosley HF, Marangoudakis S, Ross CA, Murray WT, Holland JF. Combination radiation‐chemotherapy for bronchogenic carcinoma ‐ pilot study. Cancer Chemotherapy Reports 1962;16:467‐471. [PubMed] [Google Scholar]
Karageorgis 1991 {published data only}
- Karageorgis P, Koliarakis N, Paraskevaidis M. NSCLC st. IIIb patients survival after chemotherapy, radiotherapy and combination of the above modalities. Preliminary report. Proceedings of ECCO 1991;6:S180. [Google Scholar]
Karp {published data only}
- Karp DD, Daly BDT, Diehl JT, et al. A pilot study of radiation therapy with or without concomitant cisplatin and etoposide as adjuvant therapy in non‐small cell lung cancer. In: Bernal SD, Hesketh PJ editor(s). Lung Biology in Health and Disease. 58. 1992:325‐334. [Google Scholar]
Karrer 1978 {published data only}
- Karrer K, Prindun N, Denck H. Chemotherapy as an adjuvant to surgery in lung cancer. Cancer Chemotherapy and Pharmacology 1978;1:145‐159. [DOI] [PubMed] [Google Scholar]
Kaung 1974 {published data only}
- Kaung DT, Wolf J, Hyde L, Zelen M. [Preliminary report on the treatment of nonresectable cancer of the lung]. Cancer Chemotherapy Reports 1974;58(Part 1):359‐364. [PubMed] [Google Scholar]
NCCTG852451 {unpublished data only}
- NCCTG. Phase III randomized comparison of surgical adjuvant combination chemotherapy with CAP (CTX/ADR/CDDR) vs observation in patients with totally resected stage I, II or III.
Newman 1985 {published data only}
- Newman CE, Cox R, Ford CHJ, Johnson JR, Jones DR, Wheaton M. Reduced survival with radiotherapy and razoxane compared with radiotherapy alone for inoperable lung cancer in a randomised double‐blind trial. British Journal of Cancer 1985;51:731‐732. [DOI] [PMC free article] [PubMed] [Google Scholar]
Okawa 1992 {published data only}
- Okawa T, Hashimoto S, Niibe H, et al. Effect of sizofiran (SPG) combined with radiotherapy on non‐small cell lung cancer (NSCLC), results of a prospective randomized controlled study, cooperative study on SPG lung cancer. Proceedings of the American Society of Clinical Oncology 1992;11:288. [Google Scholar]
Osterlind 1985 {published data only}
- Osterlind K. Hansen M. Hansen HH. Dombernowsky P. Controlled trial of RSV, herbs or placebo as adjuvants to complete resection of squamous cell lung cancer. European Journal of Surgical Oncology 1985;11(4):349‐351. [PubMed] [Google Scholar]
Petrovic 1978 {published data only}
- Petrovich Z, Ohanian M, Cox J. [Clinical research on the treatment of locally advanced lung cancer. Final report of VALG protocol 13 limited]. Cancer 1978;42:1129‐1134. [DOI] [PubMed] [Google Scholar]
Pirogov 1976 {published data only}
- Pirogov AI, Traktenburg AKh. Results and prospects for combined surgery and antitumour chemotherapy for lung cancer. Cancer Treatment Reports 1976;60(10):1489‐1491. [PubMed] [Google Scholar]
Privitera 1987 {published data only}
- Privitera G, Ciottoli GB, Patanè C, et al. Phase II double‐blind randomized study of lonidamine and radiotherapy in epidermoid carcinoma of the lung. Radiotherapy and Oncology 1987;10:285‐90. [DOI] [PubMed] [Google Scholar]
Scheer 1974 {published data only}
- Scheer AC, Wilson RF, Kalisher L. Combined radiotherapy and hydroxyurea in the management of lung cancer. Clinical Radiology 1974;25:415‐418. [DOI] [PubMed] [Google Scholar]
Selawry 1977 {published data only}
- Selawry O, Krant M, Scotto J, et al. Methotrexate compared with placebo in lung cancer. Cancer 1977;40:4‐8. [DOI] [PubMed] [Google Scholar]
Slack 1970 {published data only}
- Slack NH. Bronchogenic carcinoma: nitrogen mustard as a surgical adjuvant and factors influencing survival. University surgical adjuvant lung project. Cancer 25;5:987‐1002. [DOI] [PubMed] [Google Scholar]
Spatti 1985 {published data only}
- Spatti G, Regazzoni M, Koronel R, Zecchini AM, Palo G. Adjuvant treatment with melphalan in ovarian carcinoma with no residual disease following surgery. Tumori 1987;73:157‐162. [DOI] [PubMed] [Google Scholar]
Spittle 1980 {published data only}
- Spittle M, Newton KA, White W, et al. Razoxane as a radiopotentiator for lung cancer.. Second World Conference on Lung Cancer, Copenhagen. 1980; Vol. 220.
Vincent 1975 {published data only}
- Vincent RG, Pickren JW, Fergen TB, Takita H. Evaluation of methotrexate in the treatment of bronchogenic carcinoma. Cancer 1975;36:873‐880. [DOI] [PubMed] [Google Scholar]
Wils 1984 {published data only}
- Wils JA, Utama I, Naus A, Verschueren TA. Phase II randomized trial of radiotherapy alone vs the sequential use of chemotherapy and radiotherapy in stage II non‐small cell lung cancer. Phase II trial of chemotherapy alone in stage IV non‐small cell lung cancer. European Journal of Clinical Oncology 1984;20(7):911‐914. [DOI] [PubMed] [Google Scholar]
Wingfield 1970 {published data only}
- Wingfield HV. Combined surgery and chemotherapy for carcinoma of the bronchus. Lancet 1970;1(7644):470‐471. [DOI] [PubMed] [Google Scholar]
Wolf 1991 {published data only}
- Wolf K, Havemann K, Hans K, et al. Radiotherapy versus chemotherapy followed by radiotherapy in inoperable limited stage non‐small cell lung cancer (NSCLC). Lung Cancer 1991;7s:A611. [Google Scholar]
References to ongoing studies
Ancona {unpublished data only}
- Gasparini S, Zuccatosta L, Massei V. Lonidamine and chemotherapy plus lonidamine vs the best supportive care in the treatment of advanced non‐small cell lung cancer.
ANITA {unpublished data only}
- Adjuvant Navelbine International Trialist Association (ANITA). Phase III randomized trial of adjuvant chemotherapy following curative resection for non‐small cell lung cancer.
BLT {published and unpublished data}
- MRC CTU. Phase III randomized study of cisplatin‐based chemotherapy in patients with non‐small cell lung cancer.
CAN NCIC BR10 {published and unpublished data}
- NCIC Clinical Trials Group. Phase III randomized study of adjuvant chemotherapy with VNB/CDDP vs no adjuvant chemotherapy for completely resected non‐small cell lung cancer.
CLB 9633 {published and unpublished data}
- Cancer and Leukemia Group B. Phase III randomized study of carboplatin / paclitaxel vs no adjuvant chemotherapy after resection of stage IB non‐small cell lung cancer.
CNR ALPI {published and unpublished data}
- Consiglio Nazinale Ricerche, Italy / EORTC. Phase III randomized study of adjuvant MVP vs no adjuvant chemotherapy (with or without adjuvant radiotherapy) for radically resected stage I/II/IIIa non‐small cell lung cancer.
CRC‐TU‐LU3001 {published and unpublished data}
- Cullen MH, Billingham LJ, Woodroffe CM, et al. Mitomycin, Ifosphamide, and Cisplatin in unresectable non‐small cell lung cancer: effect on survival and quality of live. Journal of Clinical Oncology 1999;17:3188‐3194. [DOI] [PubMed] [Google Scholar]
CRC‐TU‐LU3002 {published data only}
- Cullen MH, Billingham LJ, Woodroffe CM, et al. Mitomycin, Ifosphamide, and Cisplatin in unresectable non‐small cell lung cancer: effect on survival and quality of live. Journal of Clinical Oncology 1999;17:3188‐3194. [DOI] [PubMed] [Google Scholar]
EORTC 08922 {published and unpublished data}
- EORTC. Phase III randomised trial of adjuvant MICE (Mesna/IFF/CBDCA/VP‐16) or EP (VP‐16/CDDP) vs no adjuvant therapy in patients with completely resected stage I/II/IIIa non‐small cell lung cancer containing the activated oncogene K‐ras.
FRE IALT {published and unpublished data}
- Institut Gustave Roussy. Phase III randomized study of adjuvant chemotherapy following curative resection for non‐small cell lung cancer.
GAP {published data only}
- Clerici M, Barni S, Cantaluppi G, et al. Adjuvant chemotherapy in non‐small cell lung cancer: a randomised trial. European Journal of Cancer 1991;27(Suppl 2):S173. [Google Scholar]
Helsing {published and unpublished data}
- Helsing M, Bergman B, Thaning L, Hero U. Quality of life and survival in patients with advanced non‐small cell lung cancer receiving supportive care plus chemotherapy with carboplatin and etoposide or supportive care only. A multicentre randomised phase III trial. European Journal of Cancer 1998;34(7):1036‐1044. [DOI] [PubMed] [Google Scholar]
Ichinose 1991 {published data only}
- Ichinose Y, Hara N, Ohta M, Motohiro A, Kuda T, Aso H. Postoperative adjuvant chemotherapy in non‐small cell lung cancer: Prognostic value of DNA ploidy and postrecurrent survival. Journal of Surgical Oncology 1991;46:15‐20. [DOI] [PubMed] [Google Scholar]
Kim 1993 {published data only}
- Kim NK, Yang SH, Im YH, et al. A phase III randomized comparison of neoadjuvant chemotherapy with cisplatin, etoposide and vinblastine plus radiation vs. radiation alone in stage III non‐small cell lung cancer. Proceeding of the American Society for Clinical Oncology 1993;12:330. [Google Scholar]
NCCTG‐822451 {published data only}
- North Central Cancer Treatment Group. Phase III randomized comparison of surgical adjuvant combination chemotherapy with CAP vs observation in patients with totally resected stage I, II or III non‐small cell lung cancer.
RICUM {published data only}
- Ayoub J, Duranceau A, Lorange G, et al. Effectiveness of adjuvant chemotherapy in operable non‐small cell lung cancer. In: Salmon SE editor(s). Adjuvant therapy of cancer. New York: and Stratton, 1987:157‐164. [Google Scholar]
RTOG 8808 {unpublished data only}
- Sause WT, Scott C, Taylor S, et al. Radiation Therapy Oncology Group (RTOG) 88‐08 and Eastern Cooperative Oncology Group (ECOG) 4588: Preliminary results of a phase III trial in regionally advanced unresectable non‐small cell lung cancer. Journal of the National Cancer Institute 1995;87:198‐205. [DOI] [PubMed] [Google Scholar]
TELCVIS 1999 {published data only}
- The Elderly Lung Cancer Vinorelbine Italian Study. Effects of Vinorelbine on quality of live and survival of elderly patients with advanced non‐small celllung cancer. Journal of the National Cancer Institute 1999;91:66‐72. [DOI] [PubMed] [Google Scholar]
Thongprasert 1999 {published and unpublished data}
- Thongprasert S, Sanguanmitra P, Juthapan W, Clinch J. Relationship between quality of live and clinical outcomes in advanced non‐small cell lung cancer: best supportive care (BSC) versus BSC plus chemotherapy. Lung Cancer 1999;24:17‐24. [DOI] [PubMed] [Google Scholar]
Additional references
Asola 1991
- Asola R, Suovuori A, Karnani P. Peroral tegafur and calcium folinate in the treatment of advanced colorectal cancer: FINN‐SIC phase I trial. European Journal of Cancer 1991;2(suppl):S91, A529. [Google Scholar]
Boring 1993
- Boring CC, Squires TS, Tong T. Cancer statistics. Ca: a Cancer Journal for Clinicians 1993;43:7‐26. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
EBCTCG 1990
- Early Breast Cancer Trialists Collaborative Group. Treatment of Early Breast Cancer Vol 1. Worldwide evidence 1985‐1990. Oxford University Press, 1990. [Google Scholar]
Holmes 1991
- Holmes EC, Bleehen NM, Chevalier T, Ettinger D, Jett J, Johnson D. Post‐operative adjuvant treatments for non‐small cell lung cancers: a consensus report. Lung Cancer 1991;7:11‐3. [Google Scholar]
Kaplan 1958
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observation. Journal of the American Statistical Association 1958;53:457‐81. [Google Scholar]
Parkin 1993
- Parkin DM, Sasco AJ. Lung cancer: Worldwide variation in occurence and proportion attributable to tobacco use. Lung Cancer 1993;9:1‐16. [Google Scholar]
Rankin 1986
- Rankin EM. Non‐small cell lung cancer. In: Slevin ML, Staquet MJ editor(s). Randomised clinical trials in cancer: A critical review by site. New York: Raven Press, 1986:447‐92. [Google Scholar]
Rudd 1991
- Rudd R. Chemotherapy in the treatment of non‐small cell lung cancer. Respiratory Disease in Practice 1991;7(6):12‐4. [Google Scholar]
Silverberg 1990
- Silverberg E, Boring CC, Squires TS. Cancer Statistics. Ca: a Cancer Journal for Clinicians 1990;40:9‐26. [PubMed] [Google Scholar]
Slevin 1990
- Slevin ML, Stubbs L, Plant WJ, Wilson P, Gregory WP, Armes PJ. Attitudes to chemotherapy: comparing views of patients with cancer with those of doctors, nurses and the general public. British Medical Journal 1990;300:1458‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Squires 1993
- Squires TS, Tong T. Cancer Statistics. Ca: a Cancer Journal for Clinicians 1993;43:7‐26. [DOI] [PubMed] [Google Scholar]
Stewart 1992
- Stewart THM, Raman S. Adjuvant treatment of non‐small cell lung cancer, a meta‐analysis. In: Stewart THM, Wheelock EF editor(s). Cellular immune mechanisms and tumour dormancy. Boca Raton, Florida: CRC Press, 213‐38. [Google Scholar]
Stott 1977
- Stott H, Fox W, Girling DJ, Stephens RJ, Dalton DAG. Acute leukaemia after busulphan. British Medical Journal 1977;2:1513‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Till 1992
- Till JE, Sutherland HJ, Meslin EM. Is there a role for preference assessments in research on quality of life in oncology?. Quality of Life Research 1992;1:31‐40. [DOI] [PubMed] [Google Scholar]
Yusuf 1985
- Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta‐blockade during and after myocardial infarction: An overview of the randomised trials. Progress in Cardiovascular Diseases 1985;27(5):335‐71. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
NSCLCCG 1995
- Non‐small Cell Lung Cancer Collaborative Group. Chemotherapy in non‐small cell lung cancer: a meta‐analysis using updated data on individual patients from 52 randomised clinical trials. British Medical Journal 1995;311:899‐909. [PMC free article] [PubMed] [Google Scholar]


