Abstract
Background
Although both levetiracetam and phenytoin are used for seizure prophylaxis in subdural hematomas (SDHs), there is little data on their comparative efficacies. We compared the efficacy and risk of using levetiracetam versus phenytoin for seizure prophylaxis following acute or subacute SDH diagnosis.
Methods
In this retrospective cohort study, the clinical data registry at a tertiary care hospital was searched for all cases of acute or subacute SDHs that were admitted to hospital in 2002, 2003, or 2011. Risk of clinical and/or electrographic seizures, and risk of adverse drug events were compared between the two exposure arms.
Results
124 subjects in the phenytoin arm and 164 subjects in the levetiracetam arm were included. There was no significant difference in clinical and/or electrographic seizure risk, though there was a decreased risk of adverse events in the levetiracetam arm (p < 0.001). In subjects with midline shift >0 mm, levetiracetam was associated with an increased risk of electrographic seizures during hospitalization (p = 0.028) and a decreased risk of adverse drug effects (p = 0.001), compared with phenytoin use.
Conclusions
Levetiracetam generally appears to have a similar efficacy to phenytoin in preventing clinical and/or electrographic seizures following acute/subacute SDH diagnosis, though patients with midline shift >0 mm may have associated with a higher risk of electrographic seizures on levetiracetam compared with patients on phenytoin. Levetiracetam is associated with a lower risk of adverse drug effects. A prospective, randomized study would more definitively determine any difference in efficacy and risk between phenytoin and levetiracetam.
Keywords: Antiepileptic drug, Seizure, Subdural hematoma
Introduction
Subdural hematomas (SDHs) are common neurosurgical ailments, and are frequently associated with the seizures [1]. The precise increase in risk of seizures following acute/subacute SDH diagnosis has been incompletely characterized in the literature, which has focused primarily on the risk of seizures and the role of seizure prophylaxis after traumatic brain injury (TBI) [2, 3], and also on the risk of seizures associated with chronic SDHs [4–6]. Patients with acute and subacute SDHs are at a particularly high seizure risk. In one study of 134 consecutive patients who underwent surgical evacuation of an acute SDH, clinical, and electrographic seizures occurred in 25 % in the postoperative period, and were associated with a poorer functional outcome on discharge [7].
It is current standard of care to treat patients diagnosed with an acute traumatic SDH from a severe TBI with anti-epileptic drugs (AEDs) throughout, at minimum, the first week following TBI, in order to prevent early post-traumatic seizures [2, 3]. Seizure prophylaxis has also been extended by some centers to more generalized usage, with indications including postoperative from supratentorial neurosurgery, diagnosis of aneurysmal subarachnoid hemorrhage (SAH) [8], and diagnosis of an intracranial tumor [9, 10].
Phenytoin is the historical gold standard for seizure prophylaxis, and has been shown to be associated with a 0.25 relative risk of seizures compared with placebo within the first 7 days following TBI [11]. Levetiracetam is a newer AED with a side-effect profile superior to phenytoin, fewer drug–drug interactions, a simple twice-daily dosing regimen, and without blood level monitoring requirements [12], making it easier to use for both prescribers and patients. However, levetiracetam is more expensive than phenytoin: the cost of a seven day course of levetiracetam is priced at $480.00, compared to $37.50 for phenytoin [13]. Nonetheless, in many centers, levetiracetam has effectively replaced phenytoin as the standard AED of choice for seizure prophylaxis in neurosurgical and neurological patients. To our knowledge, there are no published studies on the efficacy and risk profile of levetiracetam versus phenytoin for seizure prophylaxis in the acute and subacute SDH population.
The purpose of this study was to investigate the comparative efficacy and risk profile of levetiracetam versus phenytoin in this patient population. We hypothesized that there would be no difference in seizure risk during hospitalization between the two AEDs, and that phenytoin would be associated with a higher adverse event risk.
Methods
Study Design
This is a retrospective cohort study of patient with SDHs from a tertiary care facility treated during the years 2002, 2003, or 2011. Approval to conduct this retrospective cohort study was obtained by the institutional review board. Using a clinical data registry (Research Patient Data Registry or RPDR) [14], we retrospectively selected all admissions for acute or subacute SDHs in 2002, 2003, and 2011, using the following broadly inclusive search strategy: (1) subdural hematoma OR nontraumatic subdural hematoma AND BWH AND inpatient) and (2) Craniectomy or craniotomy for evacuation of hematoma, supratentorial; extradural or subdural OR craniectomy or craniotomy for evacuation of hematoma, infratentorial; extradural or subdural AND BWH AND inpatient. Inclusion criteria were: diagnosis of acute or subacute SDH during a hospital admission in 2002, 2003, or 2011. Exclusion criteria were: acute or subacute SDH was not the primary brain pathology on head CT, an AED other than phenytoin or levetiracetam was given for seizure prophylaxis, or no seizure prophylaxis was given. The years 2002, 2003 were chosen because at this time phenytoin was almost exclusively the first line AED used, while levetiracetam was almost exclusively used as the first line AED used in 2011. Furthermore, all 3 years were following the introduction of the electronic health record (EHR) at the hospital.
Data on the following subject characteristics were extracted from the EHR of each subject that met inclusion and exclusion criteria: total number of days of continuous in hospital follow-up, age, sex, mechanism of injury, or whether the SDH was spontaneous, taking anticoagulants or thrombocytopenic with a platelet count of <50 on admission, past history of seizures, past history of intracranial infection, past or current history of alcohol use/abuse, past history of previous TBI, history or evidence of prior neurosurgery or other brain pathology on brain CT or MRI, the number of millimeters of midline shift and the maximal thickness of the largest SDH on computed tomography (CT) of the head, age, and side of SDH on CT head, Glasgow Coma Scale (GCS) at hospital presentation, presence of new focal neurological findings including hemiparesis, visual field cut, aphasia/dysphasia, dysarthria, gaze preference, hemisensory loss, dilated/unreactive pupils on admission, other injuries on admission, occurrence of any hypotension or hypoxia events during hospitalization, and surgical procedures underwent during hospitalization.
The primary outcome measures for which data were also extracted were “clinical or electrographic seizures during hospitalization” and “documented AED related adverse drug events” (defined as the discontinuation of the initial AED prophylaxis agent used, either to change to a new AED for unexplained reasons, or because of a documented adverse event attributed to the AED). The secondary outcome measures were “clinical seizures during hospitalization” and “electrographic seizures during hospitalization.”
Phenytoin and levetiracetam were used for SDH seizure prophylaxis based on standardized hospital-wide clinical protocols. Phenytoin was typically given as an intravenous (IV) loading dose of 15–20 mg/kg 1 h, followed by a dose of 15–20 mg/kg (IV or oral) divided in three doses per day, titrated to achieve therapeutic blood phenytoin levels. Levetiracetam was typically given as a 1,000 mg IV loading dose, followed by a dose of 500–1,000 mg IV or orally twice daily.
Continuous and routine EEGs were recorded digitally using 21 standard scalp electrodes placed, according to the international 10–20 system. Subjects were selected for long-term monitoring (LTM) at the discretion of the medical team. The presence of clinical and electrographic seizures, interictal epileptiform discharges (spikes, sharp waves), including periodic epileptiform patterns such as lateralized periodic discharges and generalized periodic discharges deemed to be epileptiform were recorded in the EHR.
Statistical Methods
Baseline characteristics were compared between the two exposure arms (subjects treated with phenytoin for seizure prophylaxis compared with subjects treated with levetiracetam). Continuous variables were compared using a two-tailed Student’s t test, or Wilcoxon rank sum test depending on data normality, and categorical variables were compared using Chi square (χ2) test or, if the χ2 assumptions did not hold, the Fisher’s exact test. A crude analysis was then undertaken to examine for associations between the exposure variable (phenytoin vs. levetiracetam for AED prophylaxis) and each of the outcome measures, using the Chi square or Fisher’s exact test as appropriate. Multivariable logistic regression was then used to obtain adjusted odds ratios for the associations between the choices of AED used for seizure prophylaxis and every outcome measure, controlling for all baseline characteristics that were significantly different between the two exposure arms, to reduce the impact of selection bias and confounding on the exposure/outcome associations.
Subgroup analysis was completed on subjects with (1) midline shift of CT head >0 mm, and (2) a traumatic etiology of SDH and a GCS ≤8, using the same general principles outlined above for the general analysis except for the following: the secondary outcome measure “clinical seizures in the first 7 days” was added to the subgroup analysis for “traumatic etiology of SDH and a GCS ≤8.” This was because looking for the association between AED choice and this outcome measure lies within the uncontroversial standard of care in managing severe traumatic brain injuries with seizure prophylaxis for 7 days to prevent early post-traumatic seizures [2, 3, 11], specific to only this subgroup. The data were analyzed using STATA version 11.2, and the threshold for statistical significance was set at α = 0.05.
Results
Selection Process (Fig. 1)
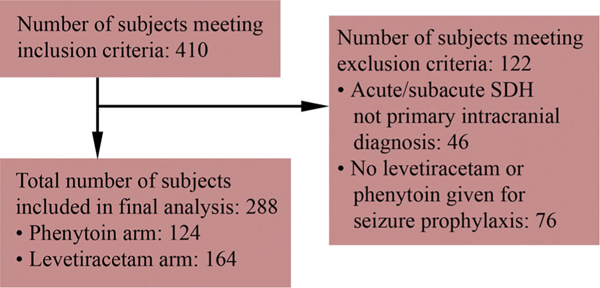
Fig. 1.
Flow chart demonstrating the selection process for this study
Though application of the search strategy to the clinical data registry, 410 subjects were identified. Of these, 46 subjects were excluded for not having a primary intracranial diagnosis of acute or subacute SDH, leaving a total of 364 subjects (166 in 2002/2003 and 198 in 2011). Another 76 subjects were then excluded for not having received either levetiracetam or phenytoin for seizure prophylaxis, leaving a total of 124 subjects who received phenytoin and 164 subjects who received levetiracetam for prophylaxis.
Comparing Subject Characteristics Between 2002/2003 and 2011
In 2002/2003, 79.5 % (132 out of 166) of subjects diagnosed with acute/subacute SDH received seizure prophylaxis, compared with 87.4 % (173 out of 198) in 2011 (p = 0.043). Also, subjects in the 2002/2003 arm were more likely to have undergone at least one neurosurgical procedure (p = 0.003), to have received a craniotomy (p = 0.007), and to have received burr hole drainage of their SDH during their hospital admission (p = 0.019), and were less likely to have received a minicraniotomy (p < 0.001) compared with subjects in the 2011 arm, suggesting a change in management of patients with acute/subacute SDHs over time. Subjects in the 2002/2003 arm were also, in general, more acutely ill than subjects in the 2011 arm, being more likely to have spent more days admitted to hospital (p = 0.003), to be younger (p = 0.008), to have had a thicker SDH collection (p = 0.003), to have had more millimeters of midline shift (p = 0.001), to have had a lower GCS on admission (p = 0.028), to have a history of a brain tumor (p = 0.020), to have had a past history of seizures or epilepsy (p = 0.008), and to have 1 or 2 fixed and dilated pupils on admission (0.008) compared with subjects in the 2011 arm. It is unclear whether the difference in illness levels of patients between 2002/2003 and 2011 is because of changing admission criteria over time, or because of differences in selection of patients for addition to the EHR in 2011 compared to 2002/2003, when the EHR was in its early days of utilization.
Of subjects admitted with acute/subacute SDH in 2002/2003, 72.9 % (121/166), 1.8 % (3/166), and 4.8 % (8/166) were treated with phenytoin, levetiracetam, or a different AED, respectively, compared to 1.5 % (3/198), 81.3 % (161/198), and 4.5 % (9/198) in 2011.
Comparing Subject Characteristics Between the Levetiracetam and Phenytoin Exposure Arms (Table 1)
Table 1.
A comparison of baseline characteristics and hospital course factors that could be associated with either seizure risk or adverse drug event risk, for subjects who were treated with phenytoin prophylaxis compared with subjects who were treated with levetiracetam prophylaxis
| Characteristics | Phenytoin arm [mean ± SD or number with characteristic/total number with data available for analysis (%)] | Levetiracetam arm [mean ± SD or number with characteristic/total number with data available for analysis (%)] | p value |
|---|---|---|---|
|
| |||
| Number of days spent continuously in hospital after SDH diagnosis [mean ± SD] | 13.0 ± 13.7 | 9.8 ± 17.1 | <0.001 |
| Age on admission (years) (mean ± SD) | 62.00 ± 18.51 | 65.96 ± 18.19 | 0.062 |
| Sex [n (%) male] | 85/124 (68.5) | 98/164 (59.8) | 0.125 |
| Mechanism of injury (traumatic vs. spontaneous) [n (%) traumatic] | 100/119 (84.0) | 126/160 (78.8) | 0.266 |
| Taking anticoagulants or thrombocytopenic with platelets <50 (yes/no) [n (%) yes] | 56/119 (47.1) | 100/163a (61.3) | 0.017 |
| Widest thickness of largest SDH collection in mm (mean ± SD) | 14.0 ± 10.6 | 11.4 ± 7.6 | 0.026 |
| Number of mm of midline shift [mean ± SD] | 5.1 ± 5.3 | 3.6 ± 5.1 | 0.007 |
| Focal neurological signs (yes/no) [n (%) yes] | 39/121 (32.2) | 40/164 (24.4) | 0.144 |
| GCS on admission (3–15) [mean ± SD] | 12.7 ± 3.4 | 13.5 ± 2.7 | 0.009 |
| Any neurosurgical procedures during hospital admission? (yes/no) [n (%) yes] | 74/124 (59.7) | 69/164 (42.1) | 0.003 |
| Craniotomy during hospital admission (yes/no) [n (%) yes] | 40/124 (32.3) | 32/164 (19.5) | 0.013 |
| Minicraniotomy during hospital admission (yes/no) [n (%) yes] | 2/124 (1.6) | 19/164 (11.6) | 0.001 |
| Burr hole drainage of SDH during hospital admission (yes/no) [n (%) yes] | 17/124 (13.7) | 11/164 (6.7) | 0.047 |
| Bilateral surgery done during hospital admission (yes/no) [n (%) yes] | 8/124 (6.5) | 10/164 (6.1) | 0.902 |
| Postoperative recurrence of SDH (yes/no) [n (%) yes] | 5/124 (4.0) | 11/164 (6.7) | 0.326 |
| Tentorial/falx vs convexity SDH [n (%) convexity] | 115/124 (92.7) | 150/164 (91.4) | 0.692 |
| Age of the hemorrhage (acute, subacute, acute on chronic) [n (%) acute, subacute, acute on chronic] | 74/124 (59.7), 22/124 (17.7), 28/124 (22.6) | 107/164 (65.2), 22/164 (13.4), 35/164 (21.3) | 0.532 |
| Other, nonhemorrhagic/traumatic brain pathology on history or imaging (yes/no) [n (%) yes] | 33/124 (26.6) | 45/164 (27.4) | 0.876 |
| History of or evidence of a chronic SDH or hygroma (yes/no) [n (%) yes] | 7/124 (5.6) | 7/164 (4.3) | 0.591 |
| History of or evidence of an old stroke (yes/no) [n (%) yes] | 15/124 (12.1) | 18/164 (11.0) | 0.767 |
| History or imaging evidence of an intracranial tumor (yes/no) [n (%) yes] | 8/124 (6.5) | 5/164 (3.0) | 0.168 |
| Congenital lesion on imaging (yes/no) [n (%) yes] | 1/124 (0.8) | 2/164 (1.2) | 1.000 |
| Evidence of concurrent traumatic SAH on initial CT head (yes/no) [n (%) yes] | 37/124 (29.8) | 53/164 (32.3) | 0.653 |
| Evidence of concurrent ICH on initial CT head (yes/no) [n (%) yes] | 30/124 (24.2) | 40/164 (24.4) | 0.969 |
| Evidence of concurrent EDH on initial CT head (yes/no) [n (%) yes] | 6/124 (4.8) | 2/164 (1.2) | 0.079 |
| Evidence of concurrent IVH on initial CT head (yes/no) [n (%) yes] | 11/124 (8.9) | 7/164 (4.3) | 0.110 |
| Evidence of concurrent depressed skull fracture on initial CT head (yes/no) [n (%) yes] | 3/124 (2.4) | 3/164 (1.8) | 1.000 |
| Evidence of TBI in other compartments than subdural (yes/no) [n (%) yes] | 54/124 (43.5) | 67/164 (40.9) | 0.646 |
| Evidence of hydrocephalus (yes/no) [n (%) yes] | 10/124 (8.1) | 5/164 (3.0) | 0.058 |
| History of previous cranial neurosurgery (yes/no) [n (%) yes] | 8/124 (6.5) | 18/164 (11.0) | 0.185 |
| History of intracranial infection (yes/no) [n (%) yes] | 1/124 (0.8) | 1/164 (0.6) | 1.000 |
| History of previous TBI (yes/no) [n (%) yes] | 8/124 (6.5) | 9/164 (5.5) | 0.731 |
| Evidence of new infarct following TBI (yes/no) [n (%) yes] | 3/124 (2.4) | 3/164 (1.8) | 1.000 |
| History of seizures or epilepsy (yes/no) [n (%) yes] | 15/124 (12.1) | 9/164 (5.5) | 0.045 |
| Unreactive/dilated pupils on admission | |||
| n (%) single pupil affected | 5/123 (4.1) | 4/163a (2.5) | 0.392 |
| n (%) bilateral pupils affected | 1/123 (0.8) | 0/163a (0.0) | |
| History of alcohol abuse/dependence (yes/no) [n (%) yes] | 31/121 (25.6) | 37/164 (22.6) | 0.549 |
| Other traumatic injuries other than cranial (yes/no) [n (%) yes] | 24/124 (19.4) | 35/164 (21.3) | 0.679 |
| Any hypoxia/hypotension before subject admitted to hospital (yes/no) [n (%) yes] | 6/124 (4.8) | 1/164 (0.6) | 0.045 |
| Any seizure risk factors present on admission? (yes/no) [n (%) yes] | 57/124 (46.0) | 70/164 (42.7) | 0.578 |
Data on one patient not available
Subjects in the phenytoin arm were more likely to have spent more days admitted to hospital (p < 0.001), to have had a thicker SDH collection (p = 0.026), to have had more millimeters of midline shift (p = 0.007), to have had a lower GCS on admission (p = 0.009), and were less likely to have been taking anticoagulants or been thrombocytopenic with platelets <50 (p = 0.017) than subjects in the levetiracetam arm. There were differences in surgical treatment; subjects in the phenytoin arm were more likely to have an underwent at least one neurosurgical procedure (p = 0.003), to have received a craniotomy (p = 0.013), and to have received burr hole drainage (p = 0.047), and were less likely to have received a minicraniotomy (p = 0.001). Subjects in the phenytoin arm also were more likely to have had a past history of seizures or epilepsy (p = 0.045), and have episodes of hypotension or hypoxia during their hospital admission (p = 0.045). All other baseline characteristics were approximately equally distributed between the two exposure arms.
Analyses (Table 2): Complete Dataset
Table 2.
An evaluation of the associations between AED choice and the outcome variables (clinical and/or electrographic seizures in the hospitalization period, in the first 7 days, and the risk of adverse drug events)
| Subgroup | Outcome measure | Crude analysis: number with outcome/total number with data available for analysis (%) |
Unadjusted OR of outcome in the levetiracetam arm compared to in the phenytoin arm | p value | Adjusted OR of outcome in the levetiracetam arm compared to in the phenytoin armb | p value | |
|---|---|---|---|---|---|---|---|
| Phenytoin arm [number experiencing outcome event/total number with data available for analysis (%)] | Levetiracetam arm [number experiencing outcome event/total number with data available for analysis (%)] | ||||||
|
| |||||||
| Complete dataset | Clinical or electrographic seizures during hospitalization (yes/no) [n (%) yes] | 21/123 (17.1) | 23/164 (14.0) | 0.79 | 0.478 | 1.00c | 0.919 |
| Clinical seizures during hospitalization (yes/no) [n (%) yes] | 21/123 (17.1) | 19/164 (11.6) | 0.64 | 0.184 | 1.00 | 0.979 | |
| Electrographic seizures during hospitalization (yes/no) [n (%) yes] | 1/23 (4.3) | 9/48 (18.8) | 5.08 | 0.151 | 1.18c | 0.130 | |
| Adverse drug events from AED (yes/no) [n (%) yes] | 26/122 (21.3) | 6/163a (3.7) | 0.14 | <0.001 | 0.83 | <0.001 | |
| Midline shift on initial CT head >0 mm | Clinical or electrographic seizures during hospitalization (yes/no) [n (%) yes] | 14/76 (18.4) | 16/78 (20.5) | 1.14 | 0.743 | 0.97c | 0.693 |
| Clinical seizures during hospitalization (yes/no) [n (%) yes] | 14/76 (18.4) | 12/78 (15.4) | 0.81 | 0.615 | 0.96 | 0.561 | |
| Electrographic seizures during hospitalization (yes/no) [n (%) yes] | 0/13 (0.0) | 8/30 (26.7) | Infinity | 0.082 | 1.34c | 0.028 | |
| Adverse drug events from AED (yes/no) [n (%) yes] | 16/75 (21.3) | 3/77 (3.9) | 0.15 | 0.001 | 0.84 | 0.001 | |
| Traumatic etiology and GCS ≤8 | Clinical or electrographic seizures during hospitalization (yes/no) [n (%) yes] | 2/13 (15.4) | 2/17 (11.8) | 0.73 | 1.000 | 0.94c | 0.676 |
| Clinical seizures during hospitalization (yes/no) [n (%) yes] | 2/13 (15.4) | 1/17 (5.9) | 0.34 | 0.565 | 0.91 | 0.407 | |
| Electrographic seizures during hospitalization (yes/no) [n (%) yes] | 0/3 (0.0) | 1/9 (11.1) | Infinity | 1.000 | 1.12c | 0.588 | |
| Adverse drug events from AED (yes/no) [n (%) yes] | 5/13 (38.5) | 1/17 (5.9) | 0.10 | 0.061 | 0.72 | 0.027 | |
| Clinical seizures in the first 7 days | 1/13 (7.7) | 0/17 (0.0) | 0.00 | 0.433 | 0.93 | 0.260 | |
Associations are listed for the complete dataset and for two subgroup analyses
Data on one patient not available
Odds ratios adjusted for all baseline characteristics that significantly differed between the two AED exposure arms within each subgroup
Odds ratio also adjusted for whether the subjects had EEG/LTM testing during hospitalization in addition to significantly different baseline characteristics
Comparing outcome measurements across the two study arms, there was no significant association between choice of AED and risk of clinical and/or electrographic seizures during hospitalization. There was a statistically significant difference in adverse drug reaction risk between the two study arms, with 21.3 % of the phenytoin arm compared with 3.7 % of the levetiracetam arm experiencing adverse drug reactions requiring a change in medications (p < 0.001) (see Table 3 for a summary of adverse drug events in the two exposure arms).
Table 3.
The different types of adverse drug events experienced by subjects taking phenytoin and levetiracetam who did not have missing electronic health record information, and the incidence proportions of subjects who experienced each type of event
| Adverse drug effect | Phenytoin arm (n = 122) | Levetiracetam arm (n = 164) |
|---|---|---|
|
| ||
| Decreased level of consciousness | 5 (4.1) | 0 (0.0) |
| Liver dysfunction | 4 (3.3) | 1 (0.6) |
| Rash | 4 (3.3) | 0 (0.0) |
| Persistent fevers | 3 (2.5) | 0 (0.0) |
| Neutropenia | 1 (0.8) | 0 (0.0) |
| Vertigo | 2 (1.6) | 0 (0.0) |
| Drug switch | 7 (5.7) | 1 (0.6) |
| Cognitive problems | 0 (0.0) | 0 (0.0) |
| Fatigue | 0 (0.0) | 1 (0.6) |
| Vomiting | 0 (0.0) | 1 (0.6) |
| Decreased appetite | 0 (0.0) | 1 (0.6) |
| Death | 0 (0.0) | 1 (0.6) |
| Total | 26 (21.3) | 6 (3.7) |
On multivariable logistic regression analysis, there was a significantly lower adjusted odds of adverse drug effects in the levetiracetam arm compared with the phenytoin arm (OR 0.83, p < 0.001). There continued to be no significant association between AED choice and clinical and/or electrographic seizures during hospitalization between the two treatment arms.
Other Analyses: Midline Shift on CT Head >0 mm
In this subgroup, there were 77 subjects who received phenytoin and 78 subjects who received levetiracetam for AED prophylaxis. The baseline characteristics that significantly differed between the groups included the following: patients receiving phenytoin had a greater number of days spent continuously in hospital after SDH diagnosis (mean 13.0 ± 13.7 vs 9.8 ± 17.1 days, p = 0.007), and a greater chance of treatment with intracranial neurosurgical procedures during hospitalization (81.8 vs 65.4 %, p = 0.020).
On unadjusted analysis, in the levetiracetam arm compared with the phenytoin arm, there was a nonsignificantly higher odds of electrographic seizures during hospitalization (OR = infinity, p = 0.082), and a significantly lower odds of adverse drug effects (OR = 0.15, p = 0.001). On multivariable logistic regression analysis, in the levetiracetam arm compared with the phenytoin arm, there was a significantly higher odds of electrographic seizures during hospitalization (OR = 1.34, p = 0.028), and a significantly lower odds of adverse drug effects (OR = 0.84, p = 0.001).
Subgroup: Traumatic Etiology of SDH and GCS ≤8
In this subgroup, there were 13 subjects who received phenytoin and 17 subjects who received levetiracetam for AED prophylaxis. There were no baseline characteristics that significantly differed between the exposure arms. On unadjusted analysis, in the levetiracetam arm compared with the phenytoin arm, there was a nonsignificantly lower odds of adverse drug effects (OR = 0.10, p = 0.061). On multivariable logistic regression analysis, in the levetiracetam arm compared with the phenytoin arm, there was a significantly lower odds of adverse drug effects (OR = 0.72, p = 0.027).
Discussion
No significant difference in clinical and/or electrographic seizure risk during hospitalization were found for patients who received phenytoin for seizure prophylaxis following diagnosis of an acute or subacute SDH, compared with patients who received levetiracetam. This remained true even, when controlling for differences in baseline characteristics between the two exposure arms, suggesting that phenytoin and levetiracetam are equally efficacious at preventing seizures following diagnosis of acute or subacute subdural in the general acute/subacute SDH population. These findings are in agreement with prior studies that have compared the efficacy of these two AEDs in preventing early post-traumatic clinical seizures post severe TBI [15], and in different types of brain injury settings, including a 2012 meta-analysis [16].
On the other hand, subgroup analysis for subjects with >0 mm of midline shift reveals some suggestion that levetiracetam may in fact be associated with an increased risk of electrographic seizure activity during hospitalization compared with phenytoin, which has been shown in previously published studies [17]. This association only became stronger on multivariable regression analyses, which controlled for differences in percentage of patients receiving EEG/LTM between the two exposure arms. This suggests that the increase in electrographic seizure risk in the levetiracetam arm is not due to the increased probability of receiving an EEG/LTM during admission in the levetiracetam arm compared with the phenytoin arm. Potentially, the levetiracetam doses used for prophylaxis could be subtherapeutic, since levels could not be followed as opposed to while using phenytoin.
Nonetheless, even if levetiracetam is associated with a higher electrographic seizure risk than phenytoin, the clinical significance of this is unclear. This is because it is unclear whether treating clinically silent epileptiform discharges on EEG/LTM leads to reduced risk of clinical seizures or improved outcomes, and also whether LTM in general leads to improved outcomes [18].
This study confirms previous findings of favorable side effect profile of levetiracetam over phenytoin. Phenytoin treatment is associated with a significant risk of adverse events requiring change of medication or cancelation of therapy, including cutaneous hypersensitivity reactions, fever of unknown origin, induction of the hepatic cytochrome P450 system leading to adverse medication interactions, hypotension, and reduced level of consciousness [17]. Furthermore, the use of phenytoin may be independently associated with poor outcome in patients with some types of intracranial hemorrhage, including intracerebral hemorrhage (ICH) [19, 20] and SAH [8, 21].
Though centers may vary in their indication criteria for starting seizure prophylaxis in patients presenting with traumatic and spontaneous subacute and acute SDHs of varying clinical severity, generally it is standard of care to start all patients presenting with severe TBI (including those with primarily an acute SDH) on at least a 7 day course of seizure prophylaxis to reduce the risk of early post traumatic seizures [2, 3, 11]. Therefore, a subgroup analysis was also carried out on all subjects with both a traumatic etiology of their SDH and with a GCS ≤8 to determine which AED was more efficacious or risky in this particular clinical situation where starting seizure prophylaxis would universally be the standard of care. In this subgroup, there was no difference in clinical or electrographic seizure risk between the exposure arms, suggesting that it may be equally efficacious to use either AED in this clinical situation. However, as a cautionary note, the number of subjects in each exposure arm was small (13 subjects in the phenytoin arm compared with 17 subjects in the levetiracetam arm), which would have greatly reduced the chance of finding an AED exposure/clinical or electrographic seizure association, unless it was very strong. It is therefore noteworthy that there was still a reduced risk of adverse drug events in the levetiracetam arm compared with the phenytoin arm, which supports the strength of this particular association.
Limitations
Limitations of this study include those associated with the retrospective reviews, including in particular an increased risk of selection bias. Efforts were made to reduce this risk by screening and including every eligible case within 2002/2003 and 2011 using a broad search strategy. However, the significantly lower number of cases selected in 2002/2003 compared to 2011 suggests that either there were many fewer cases of acute/subacute SDH presenting to the BWH in 2002/2003, or admission criteria were more stringent in 2002/2003. Also, a smaller proportion of all admitted acute/subacute SDH cases may have been documented in the EHR in 2002/2003, compared to 2011. Either way, this introduces selection bias to our study.
Selection bias was accounted for in this study by looking at a broad range of factors that by could conceivably influence seizure risk, narrowing the pool down to those that were also significantly different between the exposure groups (and hence were likely influenced by the selection process), and controlling for them. The cases exposed to phenytoin for seizure prophylaxis were in general more acutely ill patients than those exposed to levetiracetam, with a longer hospital admission, a thicker SDH, a greater degree of midline shift, a lower GCS on admission, a higher likelihood of neurosurgical procedures during hospitalization (in particular craniotomy), a higher likelihood of a history of seizures, and a greater likelihood of episodes of hypoxia or hypotension before being admitted to hospital. Given the greater clinical severity of brain injury in the phenytoin arm, if anything, the risk of clinical and electrographic seizures should be higher in the phenytoin arm compared to the levetiracetam arm in the crude analysis. Yet, there was no significant difference in seizure risks in the general analysis in between the two exposure arms, on crude or multivariable logistic regression analysis. Indeed, in the subgroup analyses, it were often the levetiracetam arm that had a higher risk of clinical and electrographic seizures compared to the phenytoin arm, despite selection factors likely biasing the association in the other direction toward the null.
Changing management practices over time may have also influenced the association between AED exposure and seizure risk. The cases in 2002/2003 had a lower chance of being started on seizure prophylaxis despite being more acutely ill, and had a higher chance of undergoing craniotomy and burr hole drainage, and a lower risk of undergoing minicraniotomy drainage of their SDHs, compared with the cases in 2011. This may also have affected the association between AED exposure and seizure risk, although the patients who were not started on seizure prophylaxis were eliminated from the main analysis, and differences between the AED exposure arms with respect to clinical severity and the chances of receiving a craniotomy, a minicraniotomy and/or a burr hole were controlled for in the multivariable regression analysis, and were found not to affect the statistical significance of the exposure/outcome associations.
Another limitation of this study is that there was extensive loss to follow-up after discharge from hospital, and so it was not possible to study long-term clinical outcomes, including Glasgow Outcome Scale, seizure, and epilepsy risk. Ideally, it would have been useful and important to discover whether there were differences in long-term outcome measures as well between the exposure arms.
Ultimately, though most of the evidence from the literature and this analysis suggests that phenytoin and levetiracetam are equally efficacious at preventing clinical seizures following acute/subacute SDH diagnosis, there is some suggestion that levetiracetam may be associated with a generally increased risk of electrographic seizures, as well as, in this analysis, an increased risk of status epilepticus and clinical seizures in patients with >0 mm of midline shift, compared with phenytoin. Therefore, despite the improved side effect profile and improved ease of administration of levetiracetam compared with phenytoin, caution must be undertaken before enacting the widespread usage of levetiracetam as a primary agent for seizure prophylaxis in cases of acute SDH with midline shift. Centers that currently use phenytoin as the first line agent in these clinical situations, either because of its much lower cost, or because of its proven efficacy, or because only a short seven day course of seizure prophylaxis is their standard practice, and the absolute risk of adverse drug effects is felt to be low, are likely justified in continuing to do so. Certainly however, levetiracetam appears to have a better adverse drug effect profile, and if the risk of adverse events from phenytoin use is felt to be high, then it could be justified to use levetiracetam instead.
Conclusion
In this retrospective cohort study, levetiracetam generally appears to have similar efficacy to phenytoin in preventing clinical and/or electrographic seizures following acute/subacute SDH diagnosis, though patients with midline shift >0 mm may have associated with a higher risk of electrographic seizures on levetiracetam compared with patients on phenytoin for seizure prophylaxis. Levetiracetam is associated with a lower risk of adverse drug effects in patients with acute and subacute SDHs. As this is a retrospective study, generalizability is limited, and a prospective, randomized study would more definitively determine any difference in efficacy and risk between phenytoin and levetiracetam as seizure prophylaxis agents in traumatic acute/subacute SDH.
Acknowledgments
Jong Woo Lee has received research grants from UCB, Inc. and Sunovion, Inc.
Footnotes
Conflict of interest Julia A. E. Radic declares that she has no conflict of interest. Rose Du declares that she has no conflict of interest. Sherry H-Y. Chou declares that she has no conflict of interest.
Contributor Information
Julia Anne Elisabeth Radic, The Edward B. Bromfield Epilepsy Program, Department of Neurology, Brigham and Women’s Hospital, 75 Francis Street, 02115 Boston, MA, USA; Division of Neurosurgery, DalhousieUniversity, Halifax, Nova Scotia, Canada.
Sherry H. -Y. Chou, The Edward B. Bromfield Epilepsy Program, Department of Neurology, Brigham and Women’s Hospital, 75 Francis Street, 02115 Boston, MA, USA Neurocritical Care, Department of Neurology, Boston, MA, USA.
Rose Du, The Edward B. Bromfield Epilepsy Program, Department of Neurology, Brigham and Women’s Hospital, 75 Francis Street, 02115 Boston, MA, USA; Department of Neurosurgery, Boston, MA, USA.
Jong Woo Lee, The Edward B. Bromfield Epilepsy Program, Department of Neurology, Brigham and Women’s Hospital, 75 Francis Street, 02115 Boston, MA, USA.
References
- 1.Wiedemayer H, Triesch K, Schafer H, Stolke D. Early seizures following non-penetrating traumatic brain injury in adults: risk factors and clinical significance. Brain Inj. 2002;16:323–30. [DOI] [PubMed] [Google Scholar]
- 2.Chang BS, Lowenstein DH. Practice parameter: antiepileptic drug prophylaxis in severe traumatic brain injury: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2003;60:10–6. [DOI] [PubMed] [Google Scholar]
- 3.Schierhout G, Roberts I. Anti-epileptic drugs for preventing seizures following acute traumatic brain injury. Cochrane Database Syst Rev. 2001:CD000173. [DOI] [PubMed] [Google Scholar]
- 4.Chen CW, Kuo JR, Lin HJ, et al. Early post-operative seizures after burr-hole drainage for chronic subdural hematoma: correlation with brain CT findings. J Clin Neurosci. 2004;11: 706–9. [DOI] [PubMed] [Google Scholar]
- 5.Ohno K, Maehara T, Ichimura K, Suzuki R, Hirakawa K, Monma S. Low incidence of seizures in patients with chronic subdural haematoma. J Neurol Neurosurg Psychiatry. 1993;56:1231–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sabo RA, Hanigan WC, Aldag JC. Chronic subdural hematomas and seizures: the role of prophylactic anticonvulsive medication. Surg Neurol. 1995;43:579–82. [DOI] [PubMed] [Google Scholar]
- 7.Rabinstein AA, Chung SY, Rudzinski LA, Lanzino G. Seizures after evacuation of subdural hematomas: incidence, risk factors, and functional impact. J Neurosurg. 2010;112:455–60. [DOI] [PubMed] [Google Scholar]
- 8.Szaflarski JP, Sangha KS, Lindsell CJ, Shutter LA. Prospective, randomized, single-blinded comparative trial of intravenous levetiracetam versus phenytoin for seizure prophylaxis. Neurocrit Care. 2010;12:165–72. [DOI] [PubMed] [Google Scholar]
- 9.Kern K, Schebesch KM, Schlaier J, et al. Levetiracetam compared to phenytoin for the prevention of postoperative seizures after craniotomy for intracranial tumours in patients without epilepsy. J Clin Neurosci. 2012;19:99–100. [DOI] [PubMed] [Google Scholar]
- 10.Wu AS, Trinh VT, Suki D, et al. A prospective randomized trial of perioperative seizure prophylaxis in patients with intraparenchymal brain tumors. J Neurosurg. 2013;118:873–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Temkin NR, Dikmen SS, Wilensky AJ, Keihm J, Chabal S, Winn HR. A randomized, double-blind study of phenytoin for the prevention of post-traumatic seizures. N Engl J Med. 1990; 323:497–502. [DOI] [PubMed] [Google Scholar]
- 12.Ramael S, Daoust A, Otoul C, et al. Levetiracetam intravenous infusion: a randomized, placebo-controlled safety and pharmacokinetic study. Epilepsia. 2006;47:1128–35. [DOI] [PubMed] [Google Scholar]
- 13.Cotton BA, Kao LS, Kozar R, Holcomb JB. Cost-utility analysis of levetiracetam and phenytoin for posttraumatic seizure prophylaxis. J Trauma. 2011;71:375–9. [DOI] [PubMed] [Google Scholar]
- 14.Nalichowski R, Keogh D, Chueh HC, Murphy SN. Calculating the benefits of a Research Patient Data Repository. Bethesda: annual symposium proceedings/AMIA symposium AMIA symposium; 2006. p. 1044. [PMC free article] [PubMed] [Google Scholar]
- 15.Jones KE, Puccio AM, Harshman KJ, et al. Levetiracetam versus phenytoin for seizure prophylaxis in severe traumatic brain injury. Neurosurg Focus. 2008;25:E3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zafar SN, Khan AA, Ghauri AA, Shamim MS. Phenytoin versus leviteracetam for seizure prophylaxis after brain injury—a meta analysis. BMC Neurol. 2012;12:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones GL, Wimbish GH, McIntosh WE. Phenytoin: basic and clinical pharmacology. Med Res Rev. 1983;3:383–434. [DOI] [PubMed] [Google Scholar]
- 18.Varelas PN, Corry J, Rehman M, et al. Management of status epilepticus in neurological versus medical intensive care unit: does it matter? Neurocrit Care. 2013;19:4–9. [DOI] [PubMed] [Google Scholar]
- 19.Messe SR, Sansing LH, Cucchiara BL, Herman ST, Lyden PD, Kasner SE. Prophylactic antiepileptic drug use is associated with poor outcome following ICH. Neurocrit Care. 2009;11:38–44. [DOI] [PubMed] [Google Scholar]
- 20.Naidech AM, Garg RK, Liebling S, et al. Anticonvulsant use and outcomes after intracerebral hemorrhage. Stroke. 2009;40:3810–5. [DOI] [PubMed] [Google Scholar]
- 21.Naidech AM, Kreiter KT, Janjua N, et al. Phenytoin exposure is associated with functional and cognitive disability after subarachnoid hemorrhage. Stroke. 2005;36:583–7. [DOI] [PubMed] [Google Scholar]