Abstract
Aberrant threat reactivity has been implicated in the pathophysiology of posttraumatic stress disorder (PTSD); however, the literature on this association is mixed. One factor that may contribute to this inconsistent association is differences in severity of posttraumatic stress symptoms (PTSSs) across studies, but no studies have tested this hypothesis. The relation between PTSD and threat reactivity may also differ between unpredictable threats (U-threats) and predictable threats (P-threats), given burgeoning evidence to support a particular role for aberrant responding to U-threat in PTSD. The present study examined how PTSS severity relates to startle potentiation to U-threat and P-threat in a trauma-exposed community sample (N = 258). There was a negative linear, but not quadratic, relation between PTSS severity and startle potentiation to U-threat, but not P-threat. Blunted defensive responding to U-threat may therefore contribute to higher levels of PTSSs and may represent a novel treatment target for higher levels of PTSSs.
Keywords: Posttraumatic stress symptoms, startle potentiation, unpredictable threat
Posttraumatic stress disorder (PTSD) is highly impairing (Panagioti et al., 2009; Rapaport et al., 2005), with even subsyndromal PTSD being associated with significant distress (Marshall et al., 2001). It is therefore important to identify specific neurobehavioral processes that underlie the development and maintenance of PTSD. Heightened threat reactivity is a core feature of PTSD that has been implicated in the pathophysiology of PTSD (Lissek and van Meurs, 2015; Norrholm and Jovanovic, 2018). For example, individuals with PTSD often experience hypervigilance and exaggerated physiologic responses to trauma reminders (American Psychiatric Association [APA], 2013). Relative to trauma-exposed controls, individuals with PTSD display heightened startle potentiation to threat (Grillon et al., 2009; Lissek and van Meurs, 2015; Morgan et al., 1995), and PTSD has been characterized by heightened responding of the amygdala, a limbic region that mediates fear and anxiety, to threatening facial expressions (Bryant et al., 2008; El Khoury-Malhame et al., 2011). Taken together, multiple studies utilizing diverse measurement modalities support the role of heightened threat reactivity as an underlying feature of PTSD (Jovanovic et al., 2010).
However, several studies also have found evidence for blunted threat reactivity in PTSD. Katz et al. (2017) found that the presence of comorbid PTSD moderated the association between panic disorder and startle potentiation to unpredictable threat, such that startle to unpredictable threat was blunted among individuals with comorbid PTSD and panic disorder relative to those with panic disorder alone. Others have found that individuals with PTSD evidence blunted neural reactivity to aversive stimuli, such as fearful faces (Britton et al., 2005; Macnamara et al., 2013) and blunted cortisol responding (Zaba et al., 2015). Responding to threats in the environment is adaptive in certain contexts, and so additional research is needed to better understand this discrepant pattern of findings.
Heterogeneity in clinical characteristics across samples may be one factor leading to an inconsistent relationship between PTSD and threat reactivity. For example, differences in the levels/severity of PTSD symptoms (PTSSs) across samples may explain some of the discrepant findings on threat reactivity in PTSD. Indeed, individuals with more severe PTSSs have been found to display blunted threat reactivity relative to those with less severe PTSSs (D’Andrea et al., 2013; McTeague et al., 2010). Thus, severe PTSSs may be characterized by hyporeactivity of the defensive motivational system, whereas less severe PTSSs may be characterized by heightened reactivity of the defensive motivational system. That is, there may be a quadratic relation (inverted U) between PTSS severity and threat reactivity, which could lead some studies to detect a negative relation and others to detect a positive relation between PTSD and threat reactivity.
In addition to variability in the severity of PTSSs across samples, differences in the type of threat used to index reactivity across studies may also lead to discrepant findings across studies. Differences in the predictability of a threat may be particularly important given that responses to unpredictable threat (U-threat) and predictable threat (P-threat) are mediated by overlapping, but distinguishable, neural circuitry (Davis, 2006; Shackman and Fox, 2016). Importantly, responses to U-threat and P-threat display distinct relationships with internalizing symptom dimensions (e.g., Gorka et al., 2017), suggesting that PTSD may have different associations with P-threat relative to U-threat. Despite this, only two studies to our knowledge have separately examined reactivity to U-threat and P-threat among those with PTSD. Grillon et al. (2009) found that individuals with PTSD displayed heightened startle potentiation to U-threat, whereas Katz et al. (2017), as noted above, found that PTSD was associated with blunted startle potentiation to U-threat. Neither study found an association between PTSD and reactivity to P-threat. These studies provide initial evidence that aberrant U-threat reactivity in particular may play a role in PTSD. A unique relation between PTSD and U-threat reactivity would also be consistent with animal studies showing that exposure to chronic unpredictable stress contributes to behavioral outcomes akin to PTSD in rats (e.g., Algamal et al., 2018).
Of note, however, is that neither Grillon et al. (2009) nor Katz et al. (2017) examined how PTSS severity related to U-threat or P-threat reactivity. Furthermore, no studies to date have evaluated Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) PTSD in relation to U-threat, limiting applicability to the current definition of PTSD (which changed substantially from DSM-IV). The present study thus expanded upon the extant literature by examining how severity of DSM-5-defined PTSSs relates to startle potentiation to U-threat and P-threat during the widely used no-threat, predicable-threat, unpredictable threat-task (NPU-threat task; Schmitz and Grillon, 2012). Given the focus on the dimension of PTSSs in the present study, participants were not required to be full-syndromal for PTSSs but were required to endorse a Criterion A traumatic event (i.e., traumatic event as defined by the DSM-5). Individuals who endorse PTSSs but do not meet criteria for PTSD represent a clinically significant subgroup, as subsyndromal PTSS is associated with impairment, comorbid internalizing conditions, and heightened suicide risk (Jakupcak and Varra, 2011; Marshall et al., 2001). Additionally, examining PTSSs as a continuous dimension in the present study is valid as a) taxometric studies have shown that PTSD is a dimensional construct indicative a stress-response continuum rather than a discrete clinical syndrome and b) individuals with PTSSs display comparable neurobiologic abnormalities to those with PTSD (e.g., Costanzo et al., 2016; Forbes et al., 2005; Peres et al., 2007; Roy et al., 2012).
Based on preliminary evidence that less severe PTSD is associated with heightened threat reactivity, whereas more severe PTSD may be associated with blunted threat reactivity (D’Andrea et al., 2013; McTeague et al., 2010), we hypothesized that there would be a quadratic relation (inverted U) between PTSS severity and startle potentiation to U-threat, such that individuals with severe PTSSs would display the lowest startle potentiation to U-threat and those with moderate PTSSs would display the highest startle potentiation to U-threat. Given evidence to suggest that reactivity to U-threat may play a unique role in the pathophysiology of PTSD, it was anticipated that this quadratic effect would be specific to U-threat, relative to P-threat.
METHODS
Participants and Procedure
Participants were drawn from two larger studies that were funded through the National Institute of Mental Health’s Research Domain Criteria initiative and designed to examine individual differences in threat reactivity across internalizing psychopathologies (Burkhouse et al. 2018; Shankman et al., 2018). Both studies were conducted at the University of Illinois at Chicago, used similar recruitment techniques, and had identical laboratory protocols, making them well suited for combined analyses. Participants were recruited via advertisements posted in the Chicago community, local psychiatric clinics, and nearby college campuses. Both protocols were approved by the university institutional review board and participants provided written informed consent. In both studies, participants completed a set of laboratory tasks, a battery of questionnaires, and a semistructured clinical interview and received cash as payment for participation.
Consistent with Research Domain Criteria (Shankman and Gorka, 2015), these two larger investigations focused on identifying transdiagnostic affective and physiologic abnormalities that may cut across psychopathologies. The inclusion criteria for these two studies were therefore largely agnostic toward DSM boundaries and thus did not focus on individuals with specific diagnoses but rather aimed to recruit individuals with a broad range of internalizing and externalizing symptoms. A major aim of the first study was to examine threat reactivity and internalizing symptoms within families and so participants were recruited in biologic sibling pairs. Participants were required to be between 18 and 30 years old and have at least one biologic sibling willing to participate. Participants were screened for the following exclusion criteria: personal or family history of mania or psychosis (as assessed by the Structured Clinical Interview for DSM-5 [SCID-5]; First et al., 2014]), major medical or neurologic illness, history of serious head trauma, and left-handedness.
A major aim of the second study was to examine changes in threat reactivity after psychotherapy versus pharmacotherapy. Participants were either a) seeking treatment for anxiety or depressive symptoms (i.e., patients were required to meet DSM-5 criteria for an anxiety or depressive disorder) or b) endorsing no lifetime history of psychopathology (i.e., healthy controls). Participants were required to be between age 18 and 65 years old. Exclusion criteria included an inability to provide consent and read and write in English, a major active medical or neurologic problem that could impact psychophysiologic and brain function, lifetime history of mania or psychosis, any contraindication to receiving selective serotonin reuptake inhibitors, being already engaged in any form of psychiatric treatment, psychoactive medication use within the past 4 months, history of traumatic brain injury, and current pregnancy. All analyses described below focused on baseline (pretreatment data) for study 1.
Eligible participants from the two studies (N = 503 and N = 168, respectively) were included in the current analyses if they endorsed a lifetime history of Criterion A trauma exposure (n = 255 and n = 75 for the two studies, respectively). Four participants from the first study and one from the second study were excluded from startle analyses because of missing item-level responses on the SCID-5 (see below), the measure used to index PTSSs. An additional 23 participants did not complete the NPU-threat task, 39 were excluded because of excessive artifacts in the electromyographic (EMG) data, and 5 participants were excluded from startle analyses because of outlier startle amplitude values (z-scores > ±3; Hoaglin and Iglewicz, 1987). The final sample size for startle analyses was 258 (n = 197 from study 1 and n = 61 from study 2). See Table 1 for demographic and clinical characteristics.
TABLE 1.
Demographics and Clinical Characteristics of Participants in Studies 1 and 2
| Study 1 (N =219) | Study 2 (N = 61) | Merged Study 1 and 2 (N = 258) | |
|---|---|---|---|
|
| |||
| Lifetime PTSD | 13.20% | 57.37% | 22.87% |
| Current PTSD | 2.30% | 31.14% | 9.30% |
| Current PTSS severity, mean (SD) | 22.97 (5.69) | 30.18 | 24.67 (7.29) |
| Age, mean (SD), yrs | 22.72 (3.22) | 26.57 (7.96) | 23.57 (5.06) |
| Sex (% female) | 63% | 75.4% | 66.3% |
| Caucasian | 42% | 59.01% | 47.80% |
| African American | 20.10% | 11.50% | 14.34% |
| Asian | 6.80% | 16.40% | 14.34% |
| Middle Eastern | 1.40% | - | 1.40% |
| Mixed race or unknown | 8.22% | 9.80% | 7.75% |
| Hispanic | 21.50% | 18% | 20.93% |
| Current comorbid internalizing disorders | |||
| Generalized anxiety disorder | 4.60% | 70.49% | 20.16% |
| Social anxiety disorder | 13.70% | 52.46% | 22.09% |
| Panic disorder | 4.10% | 21.31% | 8.53% |
| Major depressive disorder | 7.80% | 57.38% | 19.76% |
| Specific phobia | 17.80% | 16.39% | 17.83% |
| Current alcohol use disorder | 6.80% | 1.64% | 5.81% |
| Current substance use disorder | 5.90% | 1.64% | 5.43% |
| GAF (impairment), mean (SD) | 72.19(12.92) | 62.54 (15.55) | 74.78 (14.87) |
GAF, Global Assessment of Functioning.
Measure of PTSSs
For studies 1 and 2, current and lifetime axis I psychopathology was assessed using the SCID-5 (APA, 2013). The SCID-5 was modified in the present studies (Shankman et al., 2018) to ignore interview skip-out rules so that every symptom was given a dimensional rating (1 = not present, 2 = subthreshold, or 3 = present). Of note is that the development of PTSSs immediately after a trauma is common and may be part of a natural recovery response from trauma or reflect acute stress disorder. It is for this reason that the SCID-5 was only used to assess PTSSs for those individuals who denied a Criterion A trauma in the past 30 days, thus reducing risk of mistaking PTSSs for normal traumatic recovery or acute stress disorder. The overall PTSS dimension was calculated by summing the dimensional ratings of all PTSSs. Research staff were trained to criterion on the SCID-5. The overall PTSS symptom severity dimension yielded from this modified SCID was found to exhibit significant retest reliability (current symptoms, r = 0.87, p < 0.05) and excellent internal consistency (Cronbach’s alpha values of 0.89 and 0.94 for current and lifetime symptoms, respectively; Nunnally, 1978; Shankman et al., 2018).
NPU-Threat Task
All participants in studies 1 and 2 completed the same modified version of the original NPU-threat task that was developed by Grillon and colleagues (Schmitz and Grillon, 2012) and has been used repeatedly in our laboratory (e.g., Gorka et al., 2017; Lieberman et al., 2017a). The task includes three within-subject conditions—no shock (N), predictable shock (P), and unpredictable shock (U). Text at the bottom of the computer monitor informs participants of the current threat condition by displaying “no shock” (N), “shock at 1” (P), or “shock at any time” (U). Each condition lasted 145 seconds, during which a 4-second visual countdown (CD) was presented six times. The interstimulus intervals (ISIs; i.e., time between CDs) ranged from 15 to 21 seconds, during which only the text describing the condition was on the screen. During the N condition, no shocks were delivered. During the P condition, participants received a shock every time the CD reached 1. During the U condition, shocks were administered at any time (i.e., during the CD or ISI). Startle probes were presented both during the CD (1–2 seconds after CD onset) and ISI (4–13 seconds after ISI onset). The time interval between a shock (and a startle probe) and the following startle probe was always greater than 10 seconds to ensure that the subsequent startle response was not significantly affected by an immediately preceding stimulus. Consistent with previous studies from our group and others that have used the NPU-threat paradigm (e.g., Grillon et al., 2004; Lieberman et al., 2017b; Shankman et al., 2013), shock levels were ideographic to each participant to ensure equality in perceived shock aversiveness (Rollman and Harris, 1987). Each condition was presented two times in a randomized order (counterbalanced across participants). All participants received 24 total electric shocks (12 in P and 12 in U) and 60 total startle probes (20 in N, 20 in P, and 20 in U).
EMG Startle Data Recording and Processing
EMG startle data in both studies were acquired using BioSemi Active Two Version 7.01 system (BioSemi, Amsterdam, the Netherlands), and stimuli were administered using Presentation version 20.3 (Albany, CA). Acoustic startle probes were 40 millisecond–duration, 103-dB bursts of white noise with near-instantaneous rise time presented binaurally through headphones. Electric shocks lasted 400 milliseconds. Startle responses were recorded from two 4 mm Ag/AgCl electrodes placed over the orbicularis oculi muscle below the left eye—one 1 cm below the pupil and the other 1 cm lateral of that electrode. The ground electrode was located at the frontal pole of an electroencephalography cap that participants were wearing as part of the larger studies. Data were collected using a bandpass filter of DC-500 Hz at a sampling rate of 2000 Hz.
Startle blinks were scored according to published guidelines (Blumenthal et al., 2005). Data processing included applying a 28 Hz high-pass filter, rectifying, and then smoothing using a 40 Hz low-pass filter. Blink response was defined as the peak amplitude of EMG activity within the period of 20 to 150 milliseconds after the startle probe onset relative to baseline (i.e., the average baseline EMG level for the 50 milliseconds preceding the startle probe). Each peak was identified by software but examined by hand to ensure acceptability. Blinks were scored as nonresponses if EMG activity during the post-stimulus time frame of 20 to 150 milliseconds did not produce a blink peak that was visually differentiated from baseline activity. Blinks that were scored as nonresponses were included as missing responses. Blinks were also scored as missing if the baseline period was contaminated with noise or movement artifact or if a spontaneous or voluntary blink began before minimal onset latency and thus interfered with the startle probe-elicited blink response.
Data Analysis Plan
To test the relation between PTSSs and startle potentiation to P-threat and U-threat, data from the two studies were merged to provide the maximal sample size for analyses (N = 258). We conducted a series of flexible multilevel mixed models to test the relation between PTSS severity and startle potentiation to P-threat and U-threat. Multilevel modeling allowed for the shared variance between biologic siblings (who were recruited for the first study) to be modeled and statistically accounted for by including “family” as a random factor for all models. Continuous independent variables were Z-transformed, and sex was entered as a fixed factor for all analyses. Before conducting analyses with the P-threat and U-threat startle variables, we first conducted all models with NCD and NISI as the dependent variables to confirm that there was no association between current PTSSs and baseline startle amplitude (p values > 0.86).
To test the linear association between PTSS severity and startle potentiation, we first conducted two separate models (one for P-threat, one for U-threat) in which the PTSS severity dimension was included as a fixed factor. We then tested the quadratic relationship between PTSS severity and startle potentiation by conducting models in which the PTSS severity dimension (linear effect) and the squared PTSS severity dimension (quadratic effect) were included as fixed factors. For P-threat models, the standardized residual of startle amplitude during PCD, statistically adjusting for startle amplitude during NCD, was used as the dependent variable. For U-threat models, the standardized residual of startle amplitude during U (i.e., averaged across UCD and UISI), statistically adjusting for average startle amplitude during N (i.e., averaged across NCD and NISI), was used as the dependent variable.
RESULTS
NPU-Threat Task Effect
To confirm the efficacy of the NPU-threat task in eliciting threat-potentiated startle, a manipulation check was first conducted. There was a main effect of condition, F(2, 258.01) = 40.30, p < 0.01, and a main effect of cue, F(1, 258) = 77.67, p < 0.01 (see Fig. 1). There was also a condition cue interaction, F(2, 516.02) = 16.44, p < 0.01. Specifically, startle differed across conditions during the cue, such that NCD < PCD < UCD (p values < 0.01). Startle also differed across conditions during the ISI, such that startle during UISI was greater than startle during PISI and NISI (p values < 0.01), but PISI did not differ from NISI (p = 0.62). The task effect of condition × cue was not moderated by study site (p = 0.35).
FIGURE 1.
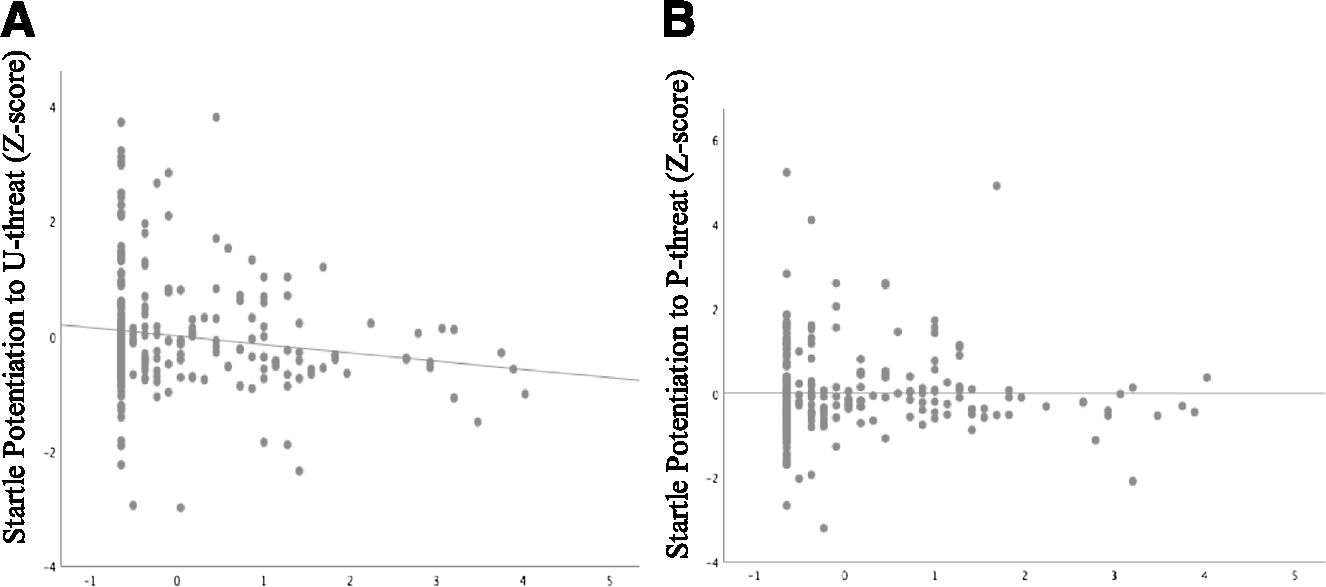
(A) Relation between startle potentiation to U-threat and severity of posttraumatic stress symptoms (PTSSs). (B) Relation between startle potentiation to P-threat and severity of PTSSs.
Relation Between PTSS Severity and Startle Potentiation to Threat
There was no quadratic relationship between PTSS severity and startle potentiation to U-threat, b = −0.02, SE = 0.05, t(254) = −0.33, ns. However, there was a negative linear relation between PTSS severity and startle potentiation to U-threat, b = −0.16, SE = 0.06, t(255) = −2.62, p < 0.05 (see Table 2 and Fig. 1A). There was neither a linear, b = −0.03, SE = 0.06, t(255) = −0.42, ns, nor quadratic, b = −0.08, SE = 0.05, t(254) = −1.62, ns, relationship between PTSS severity and startle potentiation to P-threat (see Table 2). That is, the negative linear relation between PTSS severity and startle potentiation was specific to U-threat, relative to P-threat (see Fig. 1B). Analyses to further test the specificity of the relation between PTSS severity and startle potentiation revealed that the negative association between overall PTSS severity and startle potentiation to U-threat remained even when controlling for startle potentiation to P-threat, b = −0.15, SE = 0.06, t(254) = −2.75, p < 0.05. Study site did not moderate the effects of PTSSs across startle analyses (p > 0.25), suggesting that the pattern of results did not differ between the two samples. Sex also did not moderate the effects of PTSSs (p > 0.88).
TABLE 2.
Results From Flexible Multilevel Mixed Models Assessing the Relation between Posttraumatic Stress Symptom Severity and Startle Potentiation to Threat
|
B
|
t Score |
P
|
||||
|---|---|---|---|---|---|---|
| U-Threat | P-Threat | U-Threat | P-Threat | U-Threat | P-Threat | |
|
| ||||||
| Biological sex | −0.22 | −0.27 | −1.67 | −2.01 | 0.10 | 0.05* |
| Overall PTSS severity | −0.16 | −0.03 | −2.62 | −0.42 | 0.01* | 0.68 |
p < 0.05.
Given that PTSSs often cooccur with symptoms of depression (Kilpatrick et al., 2003), we conducted a post hoc analysis to assess whether this negative relation remained when depression symptoms were entered into the model as a covariate. Depression symptoms were measured using the General Depression Scale of the Inventory for Depression and Anxiety Symptoms-II (IDAS-II; Watson et al., 2012), a 99-item self-report measure of symptoms of anxiety and depression during the previous 2 weeks. Participants are asked to respond to each item using a 5-point Likert-type scale ranging from 1 (not at all) to 5 (extremely). IDAS-II scores were available for 254 of the 258 participants in the larger sample. The negative relation between overall PTSS severity and startle potentiation to U-threat remained when general depression scores were entered into the model alongside sex as a covariate, b = −0.19, SE = 0.07, t(250) = −2.73, p < 0.05.
DISCUSSION
The literature on the relation between PTSD and threat reactivity is mixed, such that some studies have found a negative relation between PTSD and threat reactivity and others found a positive relation between PTSD and threat reactivity (D’Andrea et al., 2013; Grillon et al., 2009; Katz et al., 2017; Lieberman et al., 2017a; Morgan et al., 1995). Given preliminary evidence to suggest that more severe PTSSs are associated with blunted threat reactivity, we hypothesized that there would be a quadratic relation between PTSS severity and threat reactivity (inverted U) and sought to test this in a sample of trauma-exposed individuals with a range of PTSS severity. Furthermore, we examined whether the relation between PTSS severity and threat reactivity differed between two qualitatively distinct types of threat—unpredictable threat (U-threat) and predictable threat (P-threat). Although we did not find the hypothesized quadratic relationship between PTSS severity and startle potentiation to U-threat, there was a negative linear association between PTSS severity and startle potentiation to U-threat. This negative relation was unique to U-threat, relative to P-threat, and remained when statistically adjusting for depression symptoms.
This pattern of results is strikingly consistent with a recent study from our group that was conducted in a different sample, which found that individuals with comorbid panic disorder and PTSD displayed blunted startle potentiation to U-threat, but not P-threat, relative to individuals with panic disorder only (Katz et al., 2017). Similarly, D’Andrea et al. (2013) found that more severe PTSS was associated with blunted skin conductance responding to unpredictable startle probes, and Blechert et al. (2007) found that individuals with PTSD evidenced blunted skin conductance responding to unpredictable threat of shock. It is therefore possible that individuals with higher levels of PTSSs experience blunted reactivity to U-threat in particular. Although speculative, given previous studies showing blunted hypothalamic-pituitary-adrenal (HPA) axis functioning in PTSD (Zaba et al., 2015), blunted startle potentiation to U-threat in those with high levels of PTSSs may reflect hyposensitivity of the HPA axis to certain types of stressors (i.e., unpredictable, but not predictable). The unique association between PTSS severity and startle potentiation to U-threat could also suggest that severe PTSS is associated with blunted reactivity of the bed nucleus of the stria terminalis (BNST) to unpredictable aversive stimuli but not aberrant reactivity of central nucleus of the amygdala to predictable aversive stimuli (Davis, 1998, 2006; Lieberman et al., 2017b). Blunted BNST reactivity to U-threat may therefore reflect an important pharmacologic treatment target for individuals with high levels of PTSSs. The finding of blunted reactivity to U-threat may also suggest that individuals with high levels of PTSSs are particularly avoidant of uncertainty, and so psychotherapeutic interventions that focus on facing and tolerating uncertainty may be beneficial for those with high levels of PTSSs.
Although a negative linear relationship was detected between PTSS severity and startle potentiation to U-threat, it is of note that the hypothesized quadratic relation between PTSS severity and startle potentiation to U-threat was not found. The absence of a quadratic association between PTSS severity and startle potentiation to U-threat may indicate that PTSSs of moderate severity are not characterized by heightened threat reactivity. It also is possible that differences in trauma type and number of lifetime traumatic events could influence the relation between PTSD and threat reactivity (McTeague et al., 2010). For example, PTSSs that are secondary to repeated trauma might be associated with blunted reactivity to U-threat, whereas PTSSs that are secondary to a single trauma may be associated with heightened reactivity to U-threat. The present study was a tertiary aim of two larger investigations and so comprehensive information about the participants’ trauma histories was not obtained. Because of this, the moderating effect of trauma type/severity or count could not be tested. Relatedly, it would be important for future studies to test whether the relationship displayed between PTSS severity and startle potentiation to U-threat is specific to unpredictable threats that are not trauma related.
Another important limitation of the present study is that study participants were not recruited on the basis of trauma exposure, and so few individuals (9.3%) met full criteria for current PTSD. Future studies of PTSS severity and startle potentiation to U-threat would benefit from recruiting participants on the basis of traumatic exposures with high conditional probability for subthreshold and full-threshold PTSD to ensure greater range in PTSSs, such as interpersonal traumatic events. Lastly, although the SCID-5 is a well-validated instrument for the assessment of the presence or absence of PTSSs, the Clinician-Administered PTSD Scale for DSM-5 (Weathers et al., 2018) is the gold standard tool for assessing PTSSs and may therefore provide a more comprehensive index of PTSS severity.
IMPLICATIONS AND CONCLUSIONS
In sum, the present study found support for a negative linear relation between PTSS severity and reactivity to U-threat but did not yield evidence to support a quadratic relation between PTSS severity and threat reactivity. Higher levels of PTSSs may therefore be characterized by blunted startle potentiation to U-threat, but not P-threat. Given that repeated trauma exposure has been associated with more severe PTSSs than exposure to a single trauma (McTeague et al., 2010), blunted responding to U-threat may represent a biologic adaptation to repeated/severe trauma exposure. Blunted responding to U-threat may reflect avoidant behavior in PTSSs and could point toward a mechanistic target for PTSS interventions.
Sources of funding:
National Institute of Mental Health grants awarded to Dr Shankman and Dr Phan (R01 MH098093, primary investigator: Dr Shankman; and R01 MH101497, primary investigator: Dr Phan).
Footnotes
DISCLOSURE
The authors declare no conflict of interest.
REFERENCES
- Algamal M, Ojo JO, Lungmus CP, Muza P, Cammarata C, Owens MJ, Mouzon BC, Diamond DM, Mullan M, Crawford F (2018) Chronic hippocampal abnormalities and blunted HPA axis in an animal model of repeated unpredictable stress. Front Behav Neurosci. 12:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders (DSM 5®). Arlington, VA: American Psychiatric Association. [Google Scholar]
- Blechert J, Michael T, Grossman P, Lajtman M, Wilhelm FH (2007) Autonomic and respiratory characteristics of posttraumatic stress disorder and panic disorder. Psychosom Med. 69:935–943. [DOI] [PubMed] [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, Van Boxtel A (2005) Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology. 42:1–15. [DOI] [PubMed] [Google Scholar]
- Britton JC, Phan KL, Taylor SF, Fig LM, Liberzon I (2005) Corticolimbic blood flow in posttraumatic stress disorder during script-driven imagery. Biol Psychiatry. 57:832–840. [DOI] [PubMed] [Google Scholar]
- Bryant RA, Kemp AH, Felmingham KL, Liddell B, Olivieri G, Peduto A, Williams LM (2008) Enhanced amygdala and medial prefrontal activation during nonconscious processing of fear in posttraumatic stress disorder: An fMRI study. Hum Brain Mapp. 29:517–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhouse KL, Gorka SM, Klumpp H, Kennedy AE, Karich S, Francis J, Ajilore O, Craske MG, Langenecker SA, Shankman SA, Hajcak G, Phan KL (2018) Neural responsiveness to reward as an index of depressive symptom change following cognitive-behavioral therapy and SSRI treatment. J Clin Psychiatry. 79. pii: 17m11836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo M, Jovanovic T, Norrholm SD, Ndiongue R, Reinhardt B, Roy MJ (2016) Psychophysiological investigation of combat veterans with subthreshold posttraumatic stress disorder symptoms. Mil Med. 181:793–802. [DOI] [PubMed] [Google Scholar]
- D’Andrea W, Pole N, DePierro J, Freed S, Wallace DB (2013) Heterogeneity of defensive responses after exposure to trauma: Blunted autonomic reactivity in response to startling sounds. Int J Psychophysiol. 90:80–89. [DOI] [PubMed] [Google Scholar]
- Davis M (1998) Are different parts of the extended amygdala involved in fear versus anxiety? Biol Psychiatry. 44:1239–1247. [DOI] [PubMed] [Google Scholar]
- Davis M (2006) Neural systems involved in fear and anxiety measured with fearpotentiated startle. Am Psychol. 61:741–756. [DOI] [PubMed] [Google Scholar]
- El Khoury-Malhame Myriam M, Lanteaume L, Beetz EM, Roques J, Reynaud E, Samuelian JC, Khalfa S (2011) Attentional bias in post-traumatic stress disorder diminishes after symptom amelioration. Behav Res Ther. 49:796–801. [DOI] [PubMed] [Google Scholar]
- First MB, Williams JBW, Karg RS, Spitzer RL (2014) Structured Clinical Interview for DSM-5 Disorders—Research version (SCID-5-RV). Arlington, VA: American Psychiatric Association. [Google Scholar]
- Forbes D, Haslam N, Williams BJ, Creamer M (2005) Testing the latent structure of posttraumatic stress disorder: A taxometric study of combat veterans. J Traumatic Stress. 18:647–656. [DOI] [PubMed] [Google Scholar]
- Gorka SM, Lieberman L, Shankman SA, Phan KL (2017) Startle potentiation to uncertain threat as a psychophysiological indicator of fear-based psychopathology: An examination across multiple internalizing disorders. J Abnorm Psychol. 126:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Baas JP, Lissek S, Smith K, Milstein J (2004) Anxious responses to predictable and unpredictable aversive events. Behav Neurosci. 118:916. [DOI] [PubMed] [Google Scholar]
- Grillon C, Pine DS, Lissek S, Rabin S, Bonne O, Vythilingam M (2009) Increased anxiety during anticipation of unpredictable aversive stimuli in posttraumatic stress disorder but not in generalized anxiety disorder. Biol Psychiatry. 66:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoaglin DC, Iglewicz B (1987) Fine-tuning some resistant rules for outlier labeling. J Am Stat Assoc. 82:1147–1149. [Google Scholar]
- Jakupcak M, Varra EM (2011) Treating Iraq and Afghanistan war veterans with PTSD who are at high risk for suicide. Cogn Behav Pract. 18:85–97. [Google Scholar]
- Jovanovic T, Norrholm SD, Blanding NQ, Davis M, Duncan E, Bradley B, Ressler KJ (2010) Impaired fear inhibition is a biomarker of PTSD but not depression. Depress Anxiety. 27:244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz AC, Weinberg A, Gorka SM, Auerbach RP, Shankman SA (2017) Effect of Comorbid Post-Traumatic Stress Disorder and Panic Disorder on Defensive Responding. Journal of Psychophysiology. 32:43–52. [Google Scholar]
- Kilpatrick DG, Ruggiero KJ, Acierno R, Saunders BE, Resnick HS, Best CL (2003) Violence and risk of PTSD, major depression, substance abuse/dependence, and comorbidity: Results from the National Survey of Adolescents. J Consult Clin Psychol. 71:692. [DOI] [PubMed] [Google Scholar]
- Lieberman L, Gorka SM, Funkhouser CJ, Shankman SA, Phan KL (2017a) Impact of posttraumatic stress symptom dimensions on psychophysiological reactivity to threat and reward. J Psychiatr Res. 92:55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman L, Gorka SM, Shankman SA, Phan KL (2017b) Impact of panic on psychophysiological and neural reactivity to unpredictable threat in depression and anxiety. Clin Psychol Sci. 5:52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Van Meurs B (2015) Learning models of PTSD: Theoretical accounts and psychobiological evidence. Int J Psychophysiol. 98:594–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNamara A, Post D, Kennedy AE, Rabinak CA, Phan KL (2013) Electrocortical processing of social signals of threat in combat-related post-traumatic stress disorder. Biol Psychol. 94:441–449. [DOI] [PubMed] [Google Scholar]
- Marshall RD, Olfson M, Hellman F, Blanco C, Guardino M, Struening EL (2001) Comorbidity, impairment, and suicidality in subthreshold PTSD. AmJ Psychiatry. 158:1467–1473. [DOI] [PubMed] [Google Scholar]
- McTeague LM, Lang PJ, Laplante MC, Cuthbert BN, Shumen JR, Bradley MM (2010) Aversive imagery in posttraumatic stress disorder: Trauma recurrence, comorbidity, and physiological reactivity. Biol Psychiatry. 67:346–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CA III, Grillon C, Southwick SM, Davis M, Charney DS (1995) Fearpotentiated startle in posttraumatic stress disorder. Biol Psychiatry. 38:378–385. [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T (2018) Fear processing, psychophysiology, and PTSD. Harv Rev Psychiatry. 26:129–141. [DOI] [PubMed] [Google Scholar]
- Nunnally JC (1978) Psychometric theory (2nd ed). New York: McGraw-Hill. [Google Scholar]
- Panagioti M, Gooding P, Tarrier N (2009) Post-traumatic stress disorder and suicidal behavior: A narrative review. Clin Psychol Rev. 29:471–482. [DOI] [PubMed] [Google Scholar]
- Peres JF, Newberg AB, Mercante JP, Simao M, Albuquerque VE, Peres MJ, Nasello AG (2007) Cerebral blood flow changes during retrieval of traumatic memories before and after psychotherapy: A SPECT study. Psychol Med. 37:1481–1491. [DOI] [PubMed] [Google Scholar]
- Rapaport MH, Clary C, Fayyad R, Endicott J (2005) Quality-of-life impairment in depressive and anxiety disorders. Am J Psychiatry. 162:1171–1178. [DOI] [PubMed] [Google Scholar]
- Rollman GB, Harris G (1987) The detectability, discriminability, and perceived magnitude of painful electrical shock. Percept Psychophys. 42:257–268. [DOI] [PubMed] [Google Scholar]
- Roy MJ, Costanzo ME, Leaman S (2012) Psychophysiologic identification of subthreshold PTSD in combat veterans. Annu Rev Cyberther Telemed. 181:149–155. [PubMed] [Google Scholar]
- Schmitz A, Grillon C (2012) Assessing fear and anxiety in humans using the threat of predictable and unpredictable aversive events (the NPU-threat test). Nat Protoc. 7: 527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Fox AS (2016) Contributions of the central extended amygdala to fear and anxiety. J Neurosci. 36:8050–8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankman SA, Funkhouser CJ, Klein DN, Davila J, Lerner D, Hee D (2018) Reliability and validity of severity dimensions of psychopathology assessed using the Structured Clinical Interview for DSM-5 (SCID). Int J Methods Psychiatr Res. 27:e1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankman SA, Gorka SM (2015) Psychopathology research in the RDoC era: Unanswered questions and the importance of the psychophysiological unit of analysis. Int J Psychophysiol. 98:330–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankman SA, Nelson BD, Sarapas C, Robison-Andrew EJ, Campbell ML, Altman SE, Gorka SM (2013) A psychophysiological investigation of threat and reward sensitivity in individuals with panic disorder and/or major depressive disorder. J Abnorm Psychol. 122:322–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, O’Hara MW, Naragon-Gainey K, Koffel E, Chmielewski M, Kotov R, Ruggero CJ (2012) Development and validation of new anxiety and bipolar symptom scales for an expanded version of the IDAS (the IDAS-II). Assessment. 19: 399–420. [DOI] [PubMed] [Google Scholar]
- Weathers FW, Bovin MJ, Lee DJ, Sloan DM, Schnurr PP, Kaloupek DG, Keana TM, Marx BP (2018) The Clinician-Administered PTSD Scale for DSM-5 (CAPS-5): Development and initial psychometric evaluation in military veterans. Psychol Assess. 30:383–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaba M, Kirmeier T, Ionescu IA, Wollweber B, Buell DR, Gall-Kleebach DJ, Schmidt U (2015) Identification and characterization of HPA-axis reactivity endophenotypes in a cohort of female PTSD patients. Psychoneuroendocrinology. 55:102–115. [DOI] [PubMed] [Google Scholar]


