Abstract
Cancer and its treatment produce deleterious symptoms across the phases of care. Poorly controlled symptoms negatively affect quality of life and result in increased health-care needs and hospitalization. The Improving the Management of symPtoms during And following Cancer Treatment (IMPACT) Consortium was created to develop 3 large-scale, systematic symptom management systems, deployed through electronic health record platforms, and to test them in pragmatic, randomized, hybrid effectiveness and implementation trials. Here, we describe the IMPACT Consortium’s conceptual framework, its organizational components, and plans for evaluation. The study designs and lessons learned are highlighted in the context of disruptions related to the COVID-19 pandemic.
The symptom burden that people with cancer experience is considerable and persistent, and those symptoms can lead to hospitalization, emergency department (ED) visits, or discontinuation of treatment (1-5). Patient-reported outcome assessment is increasingly used by cancer centers to improve the screening and management of symptoms and functioning (6), but evidence for the optimal approach to implementation of electronic patient-reported outcomes systems is limited (7-9). Unlike manual collection, electronic patient-reported outcomes systems automate assessment administration, scoring, and reporting, which can reduce burden on patients and health systems. When electronic patient-reported outcomes systems are well integrated into the electronic health record (EHR) system, information is available to clinicians so that they can conveniently coordinate responses such as education, referral, and prescriptions. Many electronic patient-reported outcomes systems, however, do not interface well with EHR systems, do not follow evidence-based guidance, and do not integrate tailored responses into clinical workflows. We formed a research consortium, Improving the Management of symPtoms during And following Cancer Treatment (IMPACT), to address these challenges by developing, implementing, and testing the effectiveness of systematic electronic symptom management systems in cancer care delivery.
The impetus for IMPACT came from the Cancer Moonshot (10), a federal effort to accelerate cancer research and catalyze improved outcomes. Cancer Moonshot convened scientific experts who identified the need for approaches to monitor and manage the debilitating side effects of cancer and its treatment. In response, the National Cancer Institute (NCI) funded cooperative agreements to support IMPACT and deploy and evaluate electronic symptom management systems in diverse cancer care settings across the United States.
The IMPACT Consortium supports 3 large, pragmatic clinical trials using electronic patient-reported outcomes to prompt clinician responses consistent with evidence-based guidelines. The trials use hybrid effectiveness-implementation designs (11) and implementation science approaches (12) to promote integration of research findings into policy and practice. Consortium-wide intervention effects on symptoms and health services will be evaluated across the cancer continuum and among typically underrepresented and medically underserved populations.
This article describes the conceptual, methodologic, and organizational components of IMPACT, including study designs, intervention components, and endpoints. It highlights challenges and lessons learned in deploying and scaling electronic patient-reported outcomes surveillance and management systems, integrating clinical decision support–based interventions and the benefits of implementation science to address intervention fidelity and adaptations, including complications imposed by the COVID-19 pandemic.
IMPACT conceptual framework
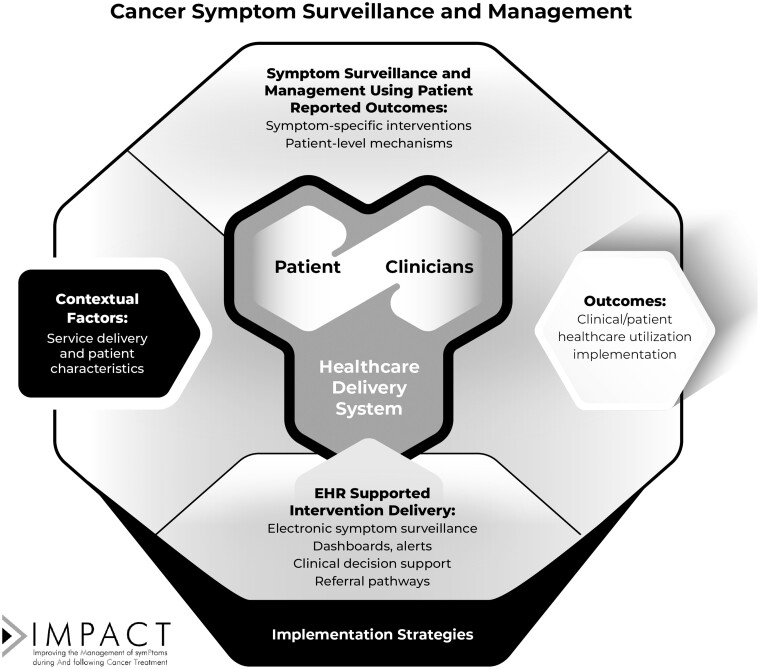
The IMPACT conceptual framework (Figure 1) highlights patient-reported outcomes symptom surveillance, patient-clinician dyads, and intervention deployment through EHR systems in health-care systems using implementation science approaches. The framework includes the following key elements:
Figure 1.
IMPACT conceptual framework. IMPACT = Improving the Management of symPtoms during And following Cancer Treatment.
Monitoring and management of cancer patient symptoms and functional limitations. Symptom surveillance and management couple regular patient reporting of symptoms with clinical responses. Patient-level mechanisms include promoting patient knowledge, self-efficacy for self-management, and behavior change.
EHR-based symptom surveillance and decision support. Interventions deploy electronic patient-reported outcomes systems in EHR systems through integrated patient portals. Informatics strategies include automated patient-reported outcomes assessment, patient reminders, dashboards on which to view trends, clinical decision support, and materials to support patient-self management tailored to specific patient-reported outcome responses.
Contextual factors: service delivery context and patient characteristics. Factors expected to influence implementation (13) include characteristics of the electronic system (eg, EHR integration, ease of patient-reported outcomes administration); extent of portal adoption, intervention integration into clinical workflow, and support from clinicians and administrators; and patient characteristics (eg, demographics, type of cancer, phase of treatment, digital literacy and access).
Implementation strategies. IMPACT trial designs support best practices for intervention development, adaptation, and scaling across diverse care settings. Strategies include education and training, workflow modification, task shifting, audit and feedback, practice champions, and clinician reminders. Implementation strategies are tailored to evaluate intervention acceptability, feasibility, scalability, and sustainability.
Outcomes. IMPACT evaluates symptom relief and functional improvement (through patient-reported outcomes), health-care utilization (eg, unplanned hospitalizations, ED visits), and implementation (eg, adoption, scalability, sustainability). The overall effects of the Consortium will be based on demonstration of clinically meaningful outcomes.
Testing IMPACT: structural components and research interventions
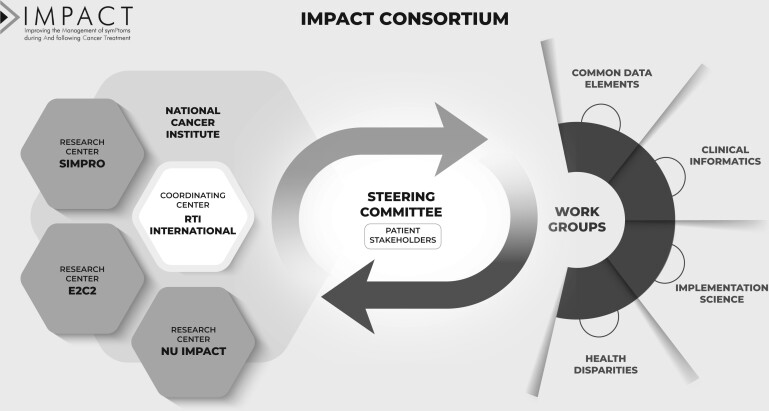
IMPACT consists of 3 research centers and a coordinating center, with NCI science officers as collaborators and advisors (Figure 2). IMPACT includes 2 patient representatives who provide feedback on study and Consortium activities. Trial characteristics for each research center are described in Table 1. All trials have institutional review board (IRB) approval and are registered on ClinicalTrials.gov. Research centers evaluate intervention implementation using the Reach, Effectiveness, Adoption, Implementation, and Maintenance (RE-AIM) framework (14) and the Consolidated Framework for Implementation Research (15-18). Each research center uses a mixed-methods approach to evaluate barriers and facilitators to study implementation effectiveness. Qualitative research methods assess the dynamic nature of health-care systems and the complex interplay of contextual factors as interventions are deployed. During the interventions, each research center used prespecified, iteratively refined, and explicit implementation strategies to support patient-reported outcome capture and clinician responses. All implementation strategies were tracked over time, and the relationship between implementation strategies, implementation outcomes, and clinical outcomes will be evaluated. Additionally, research centers are working on sustainability and dissemination planning, including sharing EHR-based decision support coding, such as clinician alerts, care pathways, order sets, smart phrases, and patient education materials. Intervention components will also be disseminated through channels such as searchable databases of evidence-based programs (eg, the NCI’s Evidence-Based Cancer Control Programs website [https://ebccp.cancercontrol.cancer.gov]).
Figure 2.
IMPACT Consortium conceptual framework. E2C2 = EHR-facilitated cancer symptom control; EHR = electronic health record; IMPACT = Improving the Management of symPtoms during And following Cancer Treatment; NU IMPACT = Northwestern University IMPACT; SIMPRO = Symptom Management IMplementation of Patient Reported Outcomes in Oncology.
Table 1.
Overview of the IMPACT research centersa
| Domain | SIMPRO | NU IMPACT | E2C2 | Commonalities |
|---|---|---|---|---|
| Population targets and settings | ||||
| Cancer site | Gastrointestinal, gynecological, thoracic cancers | Hematologic malignancies and all solid tumor cancers | All solid tumor cancers | Solid tumor cancers |
| Event triggering enrollment | Receipt of cancer surgery or initiation of new systemic therapy regimen via medical oncology | Medical oncology visit to establish care | Second medical oncology visit | 3 Research centers: Medical oncology visit |
| Treatment types | Surgery, systemic therapy |
|
Systemic, radiation, surgical therapy (including multimodal or no therapy) | Systemic therapy |
| Phases of care |
|
|
|
|
| Underserved populations of special interest | Black, rural, low socioeconomic status | Hispanic, Spanish speaking | Older adult, rural | All research centers doing outreach to underserved populations |
| Accrual target | ∼6000 | ∼12 000 | ∼40 000 | — |
| Catchment area | Maine, Massachusetts, Missouri, New Hampshire, Rhode Island, Tennessee, Vermont, West Virginia | Metropolitan Chicago area and surrounding suburbs | Minnesota, Iowa, Wisconsin | 2 Research centers: Midwest geographic region |
| Practice settings | 6 health systems (community-based hospitals and clinics, academic) | 1 health system (Northwestern Medicine); 32 outpatient medical oncology clinics | 1 health system (Mayo Clinic Health System); Mayo Clinic Rochester plus 4 regional health system clinics |
|
| Patient-reported outcomes surveillance | ||||
| Symptom domains targeted for routine monitoring |
|
PROMIS (pain, fatigue, depression, anxiety, physical function), PRO-CTCAE symptom terms (insomnia, nausea, vomiting, shortness of breath, constipation, diarrhea), Practical & Psychosocial Needs Checklist, Nutritional Needs Checklist | SPADE symptoms (insomnia, pain, anxiety, depression, and fatigue), and physical function. |
|
| Symptom monitoring tools | Modified from PRO-CTCAE (27) (items tailored to specific treatment regimens), PROMIS (33) | PROMIS computer-adaptive testing, problem checklists (investigator developed), PRO-CTCAE, toxicity grade (investigator developed) | 11-point numeric rating scale for SPADE symptoms (34), PROMIS |
|
| Symptom monitoring frequency |
|
|
Symptom surveys completed by patients before clinic appointment for a minimum of 6 mo during the preintervention phase. During the intervention phase, patients complete the same symptom survey before clinic appointment (at point of care and remotely) as well as monthly. | 2 research centers: 3, 6, 9, 12 mo |
| Mode of administration for symptom monitoring | Smartphone, tablet, laptop or desktop computer | Smartphone, tablet, laptop or desktop computer | Smartphone, tablet, laptop or desktop computer, interactive voice response | All research centers: smartphone, tablet, laptop or desktop computer |
| Patient-reported outcomes assessment setting | Outside clinic between visits | In clinic and outside clinic between visits | In clinic and outside clinic between visits |
|
| Intervention triggered by symptoms | ||||
| Symptom management |
|
|
|
All research centers: self-management educational materials; clinician alerts |
| Outcomes | ||||
| Patient-reported outcome measures |
|
|
|
3 research centers: pain, fatigue, depression, anxiety, physical function |
| Health-care utilization |
|
|
|
3 research centers: ED visits, hospitalizations, clinic visits, urgent care visits, supportive care visits, communication encounters |
| Cancer treatment |
|
|
|
3 research centers: duration of treatment, breaks, regimen changes, referrals, chemotherapy drug names |
| Patient experiences of care |
|
Consumer Assessment of Healthcare Providers and Systems Cancer Care (drug therapy) | N/A | 2 research centers: Consumer Assessment of Healthcare Providers and Systems Cancer Care drug therapy |
| Implementation evaluation |
|
|
|
3 research centers: RE-AIM evaluation framework, Consolidated Framework for Implementation Research, Normalisation Measure Development Questionnaire, Clinical Sustainability Assessment Tool |
| Measurement of usual care | Stepped-wedge, pre-post study design where health services outcomes for patients treated as part of usual care will be gathered before the intervention goes live at each site (preimplementation vs postimplementation evaluation of data in an automated patient registry); additional analysis of data on nonadopters and low adopters in the intervention condition | To address possible contamination effects within each clinical unit and allow for unit-level data collection for preimplementation and postimplementation comparison within and across clinical units, we will enroll, assess, and follow a cohort of participants before implementation of the program. Participants will receive the equivalent of the usual care condition, which does not involve the self-management intervention, for 6 mo. | Interviews and/or focus groups (care teams and patients): Clinician interviews guided by survey constructions (eg, acceptability, normalization) and Consolidated Framework for Implementation Research; patient interviews guided by RE-AIM framework | — |
| Special emphasis | Urban, rural; community, academic; limited-resource health settings; racially and socioeconomically diverse populations | Expanding access for Spanish speakers; urban and suburban | Suburban and rural; decision support; hybrid iteration of the collaborative care model | Focus on diverse geographic areas (high- and limited-resource settings), underserved populations |
E2C2 = EHR-facilitated cancer symptom control; ED = emergency department; EHR = electronic health record; N/A = not applicable; NU IMPACT = Northwestern University Improving the Management of symPtoms during And following Cancer Treatment; PROMIS = Patient Reported Outcomes Measurement Information System; PRO-CTCAE = Patient Reported Outcomes version of the Common Terminology Criteria for Adverse Events; RE-AIM = Reach, Effectiveness, Adoption, Implementation, and Maintenance; SIMPRO = Symptom Management IMplementation of Patient Reported Outcomes in Oncology; SPADE = sleep disturbance, pain, anxiety, depression, and low energy/fatigue.
IMPACT research centers
Enhanced EHR-facilitated cancer symptom control
The EHR-facilitated cancer symptom control (E2C2) (ClinicalTrials.gov ID NCT03892967) research center is conducted by the Mayo Clinic and tests the effectiveness of routine, periodic, EHR-delivered symptom surveillance using web-based patient-reported outcomes captured through the patient portal from tablets in clinic waiting rooms or by interactive voice response. Study details have been published previously (19). In brief, monitoring is paired with symptom care manager–led collaborative care and multicomponent cancer symptom management to improve symptom burden, physical function, and health-care utilization. Secondary objectives are to identify implementation strategies that enhance feasibility, adoption, and reach and to design and adapt tools (eg, clinical decision support, educational materials, and EHR-based clinical workflows) to support scaling of evidence-based interventions for symptoms and impaired physical function in diverse populations with cancer.
EHR-facilitated cancer symptom control is implemented and evaluated in Mayo Clinic Rochester and 4 regional Mayo Clinic Health System clinic groups using a hybrid effectiveness-implementation, type II, stepped-wedge trial design. Patients are randomly assigned in a cluster fashion to 1 of 5 intervention start dates every 8 months during a 46-month intervention period in 5 steps (each made up of several clusters). Clusters are defined by clinic site within the Mayo Clinic Health System and by cancer type at Mayo Clinic Rochester. In accordance with the stepped-wedge design (20), the ratio of preintervention to intervention data collection varies across steps; patients in the step 5 cluster have intervention data collected for only 8 months at most (less for those who initiate medical oncology care after the step 5 intervention start date).
During the preintervention period, patients complete surveys (including numeric rating scales for sleep disturbance, pain, anxiety, depression, and low energy (or fatigue) [SPADE] symptoms and physical dysfunction) from home through the patient portal approximately 4 days before medical oncology clinic appointments. Those who do not complete an assessment at home are asked to complete it on a tablet while waiting for their clinic appointment. Those who do not respond at home or in the clinic can subsequently complete their survey over the phone using an interactive voice response system. After intervention initiation, patients receive surveys before clinic visits and monthly to capture symptoms they experience between visits. During the intervention, patients complete surveys after the second medical oncology visit (to avoid patients with single consultations only).
The E2C2 deploys a bundled, stepped-care intervention based on the collaborative care model. Patients with at least 1 moderate or severe symptom receive additional EHR-delivered educational materials to support self-management. Patients with at least 1 severe symptom are called by a symptom care manager (a nurse, social worker, or physical therapist). The symptom care manager uses evidence-based algorithms to assess and develop individualized symptom management plans emphasizing self-management. The intervention and implementation strategies include EHR-based clinical decision support, sharing symptom scores with oncology clinicians, and facilitating implementation of evidence-based approaches (including referrals and orders for pharmacologic and nonpharmacologic symptom management). Eligible participants are 18 years of age or older, speak English, and are receiving medical oncology care for a solid tumor at Mayo Clinic in Rochester or for a solid tumor or a hematologic malignancy in Mayo Clinic Health System community clinics. The IRB waived individual consent, deeming E2C2 a standard-of-care study. Planned accrual is approximately 40 000 participants.
Primary effectiveness outcomes include SPADE symptoms and selected Patient-Reported Outcomes Measurement Information System (PROMIS) measures. Secondary effectiveness outcomes include receipt of guideline-concordant symptom management and rates of ED visits and hospitalizations. Implementation outcomes include acceptability, feasibility, clinician adoption, and patient receptivity and engagement, captured through semistructured patient and clinician interviews and focus groups, along with EHR-derived metrics of clinician engagement with the intervention (eg, orders for supportive care interventions and referrals). Generalized linear maximum likelihood mixed modelling will be used to estimate intervention effects. Qualitative analysis will be theoretically guided by constructs from normalization process theory (21), Consolidated Framework for Implementation Research, and RE-AIM models. Special emphasis is placed on understanding the experiences of and intervention adoption among older adults (greater than or equal to 65 years of age) and those living in rural communities.
Northwestern University IMPACT
The Northwestern University (NU) IMPACT (ClinicalTrials.gov ID NCT03988543) research center is testing the effectiveness and implementation of a systemwide symptom monitoring and management program in Northwestern Medicine outpatient medical oncology clinics. NU IMPACT provides tools in English and Spanish and will examine patient clinical outcomes, health-care utilization, cancer treatment delivery, and implementation outcomes. Study details have been published previously (22). In brief, NU IMPACT uses a type II hybrid effectiveness-implementation design that includes a cluster-randomized, modified, stepped-wedge trial and an embedded patient-randomized study to address implementation and effectiveness research questions independently and simultaneously.
NU IMPACT deploys and evaluates an EHR-integrated cancer patient-reported outcomes symptom monitoring program that includes symptom measures (using PROMIS) and 2 supportive oncology care needs checklist items. All cancer patient-reported outcomes scores are captured in the EHR. Elevated scores and patient-endorsed care needs prompt clinical alerts through EHR in-basket messages to designated clinicians. The cluster-randomized trial includes 7 clusters of 32 clinical units (range = 1-9 clinical units per cluster). The prospective, observational implementation group of the stepped wedge began in September 2020, with the remaining 6 clusters randomly assigned to begin quarterly. Each cluster has a 6-month preimplementation phase for consent-based enrollment into the study before transitioning to the postimplementation phase. Implementation strategies to enhance engagement with cancer patient-reported outcomes are introduced at the system level at the start of the study, then at the cluster level just before the start of the postimplementation phase in each cluster, with the aim of increasing adoption, reach, and sustainment of cancer patient-reported outcome symptom screening.
In postimplementation phases, through an embedded patient-level randomized trial, cancer patient-reported outcomes constitutes a usual care condition that is compared with an enhanced care condition. Enhanced care builds on the cancer patient-reported outcomes symptom screening and aims to increase patient self-efficacy regarding symptom management. Intervention components and implementation strategies include tabular and graphic feedback on reported symptoms in the EHR patient portal and use of a targeted, web-based tool called My NM (Northwestern Medicine) Care Corner. This tool includes patient-centered information about cancer-related concerns and self-management materials, including communication skills; it is provided to postimplementation participants randomly assigned to enhanced care. Based on cancer patient-reported outcomes assessments, enhanced care participants receive automated reinforcement emails, calls, or text messages encouraging them to access My NM Care Corner. Mild, moderate, or severe symptoms trigger information about a tailored, secure link to My NM Care Corner, with written materials and audio options. Participants with normal-range symptoms receive a link to My NM Care Corner and a message that their symptoms are in the normal range (and they may continue to access the website and patient resources pages). All enhanced care participants can review their cancer patient-reported outcomes assessment results. When severe symptoms are reported, clinical alerts are sent to oncology clinicians for both the usual care and the enhanced care conditions.
NU IMPACT’s cluster-randomized trial, along with the embedded patient-randomized trial, allows for within-site and between-site analysis of the effects of system-level changes on an observational group of patients completing patient-reported outcomes under usual care before and after the health-care system begins offering enhanced care. Once each cluster begins the postimplementation phase, patients who have provided consent and enrolled during the preimplementation phase continue with usual care. Patients consenting to participate in the postimplementation phase are randomly assigned to either enhanced care or usual care over 6 months of rolling enrollment. The trial uses a “staircase” recruitment and intervention design, aligned with the postimplementation phase of the stepped-wedge design. Participants are followed for 12 months and complete monthly assessments. Planned accrual for evaluation of implementation outcomes is approximately 12 000 patients, with 6000 in the observational preimplementation group and 6000 in the postimplementation patient-level randomized trial. Of the 6000 participants observed during the postimplementation phase, it is expected that 1000 will consent to be randomly assigned to enhanced care vs usual care (500 to enhanced care; 500 to usual care). Randomization will be stratified by site, gender, language preference, and cancer treatment phase (curative intent; non–curative intent; post-treatment survivorship).
Eligible patients are 18 years of age or older, have a current confirmed diagnosis of cancer or a history of cancer in the past 10 years, received (in the past year) or plan to receive care in at least 1 Northwestern Medicine region—Central (city of Chicago), West (western Chicago suburbs), or North (northern Chicago suburbs)—and can read and understand English or Spanish. The study was IRB approved; eligible and interested patients provide consent electronically. Depending on the timing of enrollment, participants are placed into the pre- or postimplementation group.
Primary effectiveness outcomes include longitudinal symptoms and toxicities (patient-reported outcomes), health-care utilization, and cancer care delivery. Secondary effectiveness outcomes include clinical outcomes (cancer recurrence, progression, second cancers, cancer-specific and all-cause mortality). Implementation process data are collected at the clinical unit, clinic, and clinician levels using quantitative and qualitative methods in a sequential mixed-methods analysis. Implementation outcomes (adoption and reach) are collected within and between each cluster to evaluate the effect of the implementation strategies. Outcomes are captured through semistructured clinician interviews and focus groups as well as EHR-derived metrics of clinician engagement (receipt of alerts, orders for supportive care interventions, referrals). Intervention effects of the enhanced care vs usual care embedded trial will use longitudinal modeling of patient-reported outcomes. In generalized, linear mixed models, random effects will be included for individuals and fixed effects for intervention condition (enhanced care vs usual care), site, treatment phase, gender, language preference, and the baseline patient-reported outcomes value. Implementation outcomes will be analyzed by fitting a generalized linear mixed model or using generalized estimating equations, with time as a fixed effect for each step and a random effect for each cluster (23,24). Outcomes will be evaluated for equity and representativeness. Implementation process and sustainability, through clinician interviews and surveys, will be analyzed using mixed methods, with repeated-measures analysis of variance for quantitative metrics and thematic content analysis for qualitative data.
Symptom Management IMplementation of Patient Reported Outcomes in Oncology
A multidisciplinary team from 6 health systems form the Symptom Management IMplementation of Patient Reported Outcomes in Oncology (SIMPRO; ClinicalTrials.gov ID NCT03850912) research center. Study details have been published previously (25). The aim is to develop and evaluate the effects of an electronic symptom management system for routine patient-reported outcomes symptom surveillance and management in medical and surgical oncology treatment settings. Primary endpoints are rates of emergency care for symptom management and hospital readmission following postsurgical discharge. Secondary endpoints are changes in symptom burden over time and patient experiences. Exploratory endpoints include treatment duration and delays. The study includes patients recovering from cancer surgery or receiving chemotherapy for gastrointestinal, gynecologic, or thoracic cancers in academic and community-based hospitals or clinics in rural and suburban locations.
Multistakeholder panels that include 32 patients and 194 clinicians and staff stakeholders informed the development of the electronic symptom management system. A centralized IRB review deemed electronic symptom management system to be standard care and waived individual patient consent requirements. The intervention and implementation strategies include 1) prompting patients to report symptoms at predefined intervals, 2) tracking symptom profiles over time in an EHR-embedded flowsheet, 3) triggering use of self-management information using electronically delivered tip sheets in response to mild to moderate symptoms, 4) alerting patients to contact their clinicians in response to reports of severe symptoms, 5) alerting clinicians about patients with severe symptoms, 6) facilitating symptom burden monitoring of user-defined patient cohorts through dashboards in the EHR system, and 7) integrating secure smartphone or tablet apps for patient data reporting.
SIMPRO’s electronic symptom management system was developed in conjunction with research collaborators at Epic (Verona, WI) and is fully integrated into Epic EHR systems at the following health systems: Dana-Farber Cancer Institute (Massachusetts), Dartmouth-Hitchcock Medical Center (New Hampshire), Baptist Memorial Health Care (Tennessee and Mississippi), Lifespan Cancer Institute (Rhode Island), West Virginia University (West Virginia), and Maine Medical Center (Maine). Two tailored electronic symptom management system questionnaires were developed: 1 for surgical and 1 for medical oncology patients. Questionnaires include items modified from the Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) (26). PRO-CTCAE is designed to evaluate symptom reports based on a symptom’s frequency, severity, or interference with usual or daily activities (27). Nine symptom-based items from the PRO-CTCAE and 2 global items measuring overall well-being and functional status are included in both questionnaires. Global assessments are collected through 5-item pictogram choices. Patients can report additional symptoms, if relevant. Electronic symptom management system questionnaires are accessed through the Epic MyChart portal from internet-enabled devices. Patient registries identify eligible patients based on diagnosis or Common Procedural Terminology codes. Reminders are sent to patients when an electronic symptom management system questionnaire is unanswered after several days. MyChart displays previously reported symptoms and gives patients access to a library of tip sheets that provide support for self-management and indicate when to call for help. If a severe symptom is reported, automatic alerts are sent to clinicians through the Epic in-basket, and patients are prompted to contact their care team. When patients report only mild to moderate symptoms, they are prompted to view relevant self-management tip sheets. All patient symptom reports and trends are available to clinicians in the EHR (28).
For surgical patients, electronic symptom management system questionnaires are deployed on a tapering schedule from 3 times to once a week for the first 2 months postoperatively. For patients receiving chemotherapy, electronic symptom management system questionnaires are deployed twice a week on the fourth and seventh day from cycle initiation. Study outcomes are extracted from the EHR up to 12 months after enrollment.
SIMPRO uses a stepped-wedge, cluster-randomized design to determine the effectiveness of the electronic symptom management system through 7 steps in a wedge, with each SIMPRO health system randomized in 2 stages. The health systems are randomized to receive interventions based on the sequence of the treatment groups (ie, medical oncology first and surgery second at 3 health systems or surgery first and medical oncology second at the other 3 health systems). For each pairing, SIMPRO randomly assigns the sites to 1 of the steps. No 2 health systems are assigned to the same step or sequence. Targeted accrual is approximately 6000 patients. The first 2 health systems began using the electronic symptom management system in fall 2019, and the final 2 health systems deployed the electronic symptom management system in 2022.
Primary effectiveness outcomes include ED visits, cancer care delivery (chemotherapy treatment duration and delays), patient outcomes (self-efficacy, symptom burden), and satisfaction with care. Patient-level clinical data will be analyzed, noting that the usual care vs control group includes patients not exposed to the electronic symptom management system intervention. A subset of patients at each health system both assigned (n = 1800) and not assigned (n = 1800) to the electronic symptom management system are asked to complete a 1-time, cross-sectional questionnaire examining key care delivery domains (Table 1).
Implementation outcomes include patient reach, clinician-level and institution-level adoption, electronic patient-reported outcomes scalability and sustainability for symptom management, and the extent of electronic patient-reported outcomes system adaptation. SIMPRO is collecting qualitative data from stakeholders 1 year after electronic symptom management system launch at each step to obtain descriptive information about factors that influenced implementation across multiple levels. Intervention data will be analyzed using generalized linear mixed models, with hospitals included as random effects. Implementation outcomes will be analyzed using a mixed-methods approach, integrating quantitative and qualitative data.
IMPACT coordinating center
RTI International serves as the coordinating center for IMPACT. RTI facilitates the organizational, administrative, and scientific activities necessary to meet the Consortium’s goals. These goals include 1) fostering collaborations among investigators through regular conference calls and meetings; 2) preparing policy and procedural manuals and governance plans; 3) overseeing data harmonization, developing analysis plans, performing pooled analyses, and synthesizing findings; 4) maintaining a website (www.impactconsortium.org) and a bimonthly newsletter; 5) developing procedures to share data and disseminate findings; and 6) providing subject matter expertise in informatics, clinical decision support, implementation science, and stepped-wedge trial design.
IMPACT Consortium structure and processes
To accomplish IMPACT’s ambitious aims, we developed cross-cutting and collaborative groups (Figure 2). A steering committee, a publications committee, and several working groups form the collaborative structure of IMPACT and include members from all participating organizations. The steering committee is the main governing body and serves as the communication hub (including sharing successes and challenges to foster co-learning), decision making, problem resolution, development of Consortium-wide research questions and activities, collaboration in pooled analyses, and interpretation and dissemination of findings across the Consortium. The steering committee is composed of 2 representatives from the coordinating center and each research center and 3 NCI science officers, for a total of 11 members. The 2 cancer survivor representatives serve in an advisory capacity.
Working groups focus on cross-cutting themes, including 1) common data elements, 2) clinical informatics, 3) implementation science, and 4) health disparities. The Common Data Elements and Implementation Science working groups include efforts to standardize data collection (quantitative and qualitative) across research centers and establish procedures for data synthesis and transfer to the coordinating center. The Clinical Informatics Working Group documents and identifies metrics to measure patient and clinician experiences of the symptom management system structures and clinical decision support tools. The Health Disparities Working Group focuses on issues related to achieving health equity related to symptom management and intervention access for medically underserved populations and those with known health disparities in cancer and symptom management.
Accomplishments, early challenges, and lessons learned
The IMPACT Consortium was initiated in November 2018. Early research center work focused on building EHR-based symptom management systems, determining unique and Consortium-wide common data elements, engaging with institutional and clinical stakeholders, and establishing intervention protocols. Studies were conducted across geographically diverse cancer centers, and the current sample exceeds 45 000 patients, with more expected at study completion. Almost half are older adults (≥65 years of age) and less than 10% are young adults (aged 18-40 years); almost 60% of the sample is female. Patients in rural settings made up about one-third of the early sample; one-third are employed, and more than half of the sample are retired. Although inclusion of patients from racial and ethnic minority groups in the early data was low (<10% of patients were Black, Asian, American Indian, or other non-White race, and <5% were Hispanic), outreach efforts were developed to identify and improve racial and ethnic diversity (29). Additional outreach to increase participation of Spanish-speaking cancer survivors included newsletters and technical support offered in Spanish and using Spanish-speaking oncology clinicians for recruitment. All places on the cancer control continuum are represented, including patients undergoing active treatment (chemotherapy, immunotherapy, and surgical treatment) and those in survivorship or at end of life. IMPACT includes a wide range of cancer types; early data show the highest percentages represented by breast, lung, gynecologic, and gastrointestinal cancers.
At the time of the initial COVID-19 surge in March 2020, the research centers were in different stages of intervention deployment, with NU IMPACT in beta-testing, SIMPRO deploying its intervention in 2 of 6 health-care systems, and E2C2 delivering the intervention to its first cluster. All research centers were dramatically affected by the COVID-19 pandemic, with clinical restrictions and staffing reprioritization, changes in service delivery patterns, and patient attitude and behavior. The pandemic affected processes foundational to each research center’s research efforts, including 1) data collection, curation, and exchange; 2) intervention development and delivery; and 3) practice integration and implementation.
COVID-19–related challenges took several forms. COVID-19 affected both research and clinical staff, including through work furloughs and redeployments from study responsibilities to cover other essential hospital needs. It also introduced practice changes, including cancellation of nonessential procedures, shifts from in-person to virtual visits, and changes in treatment paradigms. The increase in virtual visits undermined the reliability of EHR patient-reported outcomes assignment because of a lack of specificity in Epic logic developed for questionnaire assignment across digital platforms (eg, tablets, laptops, smartphones). For patients with cancer, COVID-19 affected their access and receptivity to symptom management and supportive care (resulting in interruptions in physical and occupational therapy and increases in psychological care needs). New ambulatory clinic precautions (eg, decreased ability to deploy patient-reported outcomes assessments on tablets out of concern for contamination) were barriers to electronic patient-reported outcomes collection. These challenges required substantial shifts in outreach and intervention deployment by research centers. Solutions included pausing until new clinical policies were enacted, extended data-collection periods, and increased use of telehealth. Analyses are also planned to examine pandemic effects, including sensitivity analyses to examine potential biases, examination of sample characteristics, clinical encounters, and missing data rates. Additionally, consistent with our early findings, data from other studies of patient-reported outcomes in health-care settings suggest that the pandemic disproportionately negatively affected EHR portal enrollment and patient-reported outcomes completion by Black and Hispanic patients (30). In addition to the strategies IMPACT employed to mitigate these effects (29), future efforts will need to test symptom surveillance interventions in samples that are adequately powered and in those with lower English proficiency, lower digital literacy, and greater racial/ethnic diversity.
The COVID-19 pandemic also introduced accelerators of intervention uptake. It catalyzed a telehealth-friendly environment, spurred by need and coverage expansions for remotely delivered services across institutions. Longstanding reimbursement restrictions were relaxed to encourage the expansion of telehealth. Patient portal enrollment escalated sharply, potentially supporting better adherence to routine electronic patient-reported outcomes surveillance and greater receptivity to electronically receiving self-management and other support resources. Challenges with digital access, technology familiarity, and receptivity to electronic patient-reported outcomes assessment contribute to a digital divide in electronic patient-reported outcomes adoption and sustainment in clinical practice, and these aspects of digital health equity (31,32) are areas of active study within IMPACT.
Finally, the use of implementation science and adaptive approaches by IMPACT research centers supported nimble trial evolution that was needed in response to a pandemic that profoundly disrupted clinical research efforts. The implementation science approach promotes opportunities to examine and manage the effects of the COVID-19 pandemic over time. Thus, the pragmatic design of the IMPACT trials allowed the research centers to successfully continue this research, despite COVID-19–related disruptions.
The IMPACT Consortium is generating substantial new knowledge on the implementation and effectiveness of symptom assessment and management interventions that will inform the feasibility of deployment of electronic patient-reported outcomes in routine frontline health-care delivery. Together, the research centers are developing and evaluating tools and best practices for electronic patient-reported outcomes–based surveillance and guideline-based symptom management across diverse geographic settings, health systems, and patient populations. Data derived from IMPACT will generate evidence for barriers and facilitators to effective symptom management, with an emphasis on meeting the needs of medically underserved and hard-to-reach populations. Collectively, the studies are poised to identify multilevel barriers to intervention success as well as strategies to mitigate challenges; it will also augment opportunities for optimal symptom management.
Acknowledgements
The opinions and views expressed by the authors are their own, the content is solely the responsibility of the authors, and this material should not be interpreted as representing the official viewpoint of the US Department of Health and Human Services, the National Institutes of Health, or the National Cancer Institute.
IMPACT Consortium group authorship: David Cella, PhD (Northwestern University Feinberg School of Medicine, Chicago, IL); Andrea Cheville, MD, MSCE (Mayo Clinic, Rochester MN); Michael J. Hassett, MD, MPH (Dana-Farber Cancer Institute, Boston, MA); Raymond U. Osarogiagbon, MBBS, FACP (Baptist Memorial Hospital, Memphis, TN); Deborah Schrag, MD, MPH (Memorial Sloan Kettering Cancer Institute, New York, NY); Sandra L. Wong, MD (Dartmouth-Hitchcock Medical Center, Lebanon, NH); Barbara L. Kroner, PhD, MPH (RTI International, Research Triangle Park, NC); Ashley Wilder Smith, PhD, MPH (National Cancer Institute, Bethesda, MD); Lisa DiMartino, PhD (Peter O'Donnell Jr. School of Public Health, University of Texas Southwestern Medical Center, Austin, TX, USA); Sofia Garcia, PhD (Northwestern University Feinberg School of Medicine, Chicago, IL); Joan Griffin, PhD (Mayo Clinic, Rochester, MN); Roxanne Jensen, PhD (National Cancer Institute, Bethesda, MD); Sandra Mitchell, PhD, CRNP (National Cancer Institute, Bethesda, MD); Kathryn Ruddy, MD, MPH (Mayo Clinic, Rochester, MN); Justin D. Smith, PhD (University of Utah Spencer Fox Eccles School of Medicine, Salt Lake City, UT); Betina Yanez, PhD (Northwestern University Feinberg School of Medicine, Chicago, IL); Jessica J. Bian, MD (Maine Medical Center, Portland, ME); Don S. Dizon, MD, FACP (Lifespan Cancer Institute, Providence, RI); Hannah W. Hazard-Jenkins, MD, FACS (West Virginia University Cancer Institute, Morgantown, WV); Mary-Anne Ardini (RTI International, Research Triangle Park, NC); Paige Ahrens, MS (Maine Medical Center, Portland, ME); Jessica Austin, PhD (Mayo Clinic, Scottsdale, AZ); Fiona Barrett (Dana-Farber Cancer Institute, Boston, MA); Michael Bass, MS (Northwestern University Feinberg School of Medicine, Chicago, IL); Megan Begnoche, RN, MSN (Lifespan Cancer Institute, Providence, RI); September Cahue, MPH (Northwestern University Feinberg School of Medicine, Chicago, IL); Kimberly Caron, RN, BSN, CCRC (Maine Medical Center, Portland, ME); Linda Chlan, PhD, RN (Mayo Clinic, Rochester, MN); Ava Coughlin, MAEd (Northwestern University Feinberg School of Medicine, Chicago, IL); Christine Cronin (Dana-Farber Cancer Institute, Boston, MA); Samira Dias, MPH (Dana-Farber Cancer Institute, Boston, MA); Nicolas Faris, MDiv (Baptist Memorial Hospital, Memphis, TN); Anne Marie Flores, PhD, PT (Northwestern University Feinberg School of Medicine, Chicago, IL); Martha Garcia (Northwestern University Feinberg School of Medicine, Chicago, IL); Karla Hemming, PhD (University of Birmingham, Edgbaston, Birmingham, UK); Jeph Herrin, PhD, MS (Yale University School of Medicine, New Haven, CT); Christine Hodgdon, MS (Guiding Researchers and Advocates to Scientific Partnerships, Baltimore, MD); Sheetal Kircher, MD (Northwestern University Feinberg School of Medicine, Chicago, IL); Kurt Kroenke, MD, MAC (Indiana University, Indianapolis, IN); Veronica Lam (Mayo Clinic, Rochester, MN); Nicola Lancki, MPH (Northwestern University Feinberg School of Medicine, Chicago, IL); Quan H. Mai, MS (Northwestern University Feinberg School of Medicine, Chicago, IL); Jennifer Mallow, PhD, FNP-BC (West Virginia University Cancer Institute, Morgantown, WV); Nadine J. McCleary, MD, MPH (Dana-Farber Cancer Institute, Boston, MA); Wynne Norton, PhD (National Cancer Institute, Bethesda, MD); Mary O’Connor, MS (Northwestern University Feinberg School of Medicine, Chicago, IL); Deirdre Pachman, MD (Mayo Clinic, Rochester, MN); Loretta Pearson, MPhil, CCRC (Dartmouth-Hitchcock Medical Center, Lebanon, NH); Frank Penedo, PhD (University of Miami, Miami, FL); Jewel Podratz, MBA (Mayo Clinic, Rochester, MN); Jennifer Popovic, DVM, MA (RTI International, Research Triangle Park, NC); Liliana Preiss, MSE (RTI International, Research Triangle Park, NC); Parvez Rahman, MHI (Mayo Clinic, Rochester, MN); Sarah Redmond, PhD, MA (Mayo Clinic, Rochester, MN); James Reich, PMP (Maine Medical Center, Portland, ME); Joshua Richardson, PhD (RTI International, Research Triangle Park, NC); Kimberly Richardson, MA (Black Cancer Collaborative, Chicago, IL); Jennifer Ridgeway, PhD (Mayo Clinic, Rochester, MN); Lila Rutten, PhD (Mayo Clinic, Rochester, MN); Karen Schaepe, PhD (Mayo Clinic, Rochester, MN) Denise Scholtens, PhD (Northwestern University Feinberg School of Medicine, Chicago, IL); Tiana Poirier-Shelton, MPH (Baptist Memorial Hospital, Memphis, TN); Philip Silberman, MA (Northwestern University Feinberg School of Medicine, Chicago, IL); Jaclyn Simpson, MBA (Baptist Memorial Hospital, Memphis, TN); Laura Tasker, BS, RT(N) (West Virginia University Cancer Institute, Morgantown, WV); Nathan Tesch, MS (Mayo Clinic, Rochester, MN); Cindy Tofthagen, PhD (Mayo Clinic, Jacksonville, FL); Angela Tramontano, MPH (Dana-Farber Cancer Institute, Boston, MA); Benjamin D. Tyndall, PhD (RTI International, Research Triangle Park, NC); Hajime Uno, PhD (Dana-Farber Cancer Institute, Boston, MA); Firas Wehbe, MD, PhD (Northwestern University Feinberg School of Medicine, Chicago, IL); and Bryan Weiner, PhD, MA (University of Washington, Seattle, WA).
Contributor Information
Ashley Wilder Smith, Outcomes Research Branch, Healthcare Delivery Research Program, National Cancer Institute, Bethesda, MD, USA.
Lisa DiMartino, Peter O’Donnell Jr. School of Public Health, University of Texas Southwestern Medical Center, Austin, TX, USA; RTI International, Washington, DC, USA.
Sofia F Garcia, Department of Medical Social Sciences and the Robert H. Lurie Comprehensive Cancer Center, Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
Sandra A Mitchell, Outcomes Research Branch, Healthcare Delivery Research Program, National Cancer Institute, Bethesda, MD, USA.
Kathryn J Ruddy, Department of Oncology, Mayo Clinic, Rochester, MN, USA.
Justin D Smith, Division of Health Systems Innovation and Research, Department of Population Health Sciences, Spencer Fox Eccles School of Medicine at the University of Utah, Salt Lake City, UT, USA.
Sandra L Wong, Department of Surgery, Dartmouth-Hitchcock Medical Center, Lebanon, NH, USA.
September Cahue, American Academy of Allergy, Asthma and Immunology, Chicago, IL, USA.
David Cella, Department of Medical Social Sciences and the Robert H. Lurie Comprehensive Cancer Center, Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
Roxanne E Jensen, Outcomes Research Branch, Healthcare Delivery Research Program, National Cancer Institute, Bethesda, MD, USA.
Michael J Hassett, Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA.
Christine Hodgdon, Guiding Researchers and Advocates to Scientific Partnerships, Baltimore, MD, USA.
Barbara Kroner, RTI International, Washington, DC, USA.
Raymond U Osarogiagbon, Multidisciplinary Thoracic Oncology Program, Baptist Cancer Center, Memphis, TN, USA.
Jennifer Popovic, Value Evidence and Outcomes, GSK, Waltham, MA, USA.
Kimberly Richardson, Black Cancer Collaborative, Chicago, IL, USA.
Deborah Schrag, Department of Medical Oncology, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Andrea L Cheville, Department of Physical Medicine and Rehabilitation, Mayo Clinic, Rochester, MN, USA.
IMPACT Consortium:
David Cella, Andrea Cheville, Michael J Hassett, Raymond U Osarogiagbon, Deborah Schrag, Sandra L Wong, Barbara L Kroner, Ashley Wilder Smith, Lisa DiMartino, Sofia Garcia, Joan Griffin, Roxanne Jensen, Sandra Mitchell, Kathryn Ruddy, Justin D Smith, Betina Yanez, Jessica J Bian, Don S Dizon, Hannah W Hazard-Jenkins, Mary-Anne Ardini, Paige Ahrens, Jessica Austin, Fiona Barrett, Michael Bass, Megan Begnoche, September Cahue, Kimberly Caron, Linda Chlan, Ava Coughlin, Christine Cronin, Samira Dias, Nicolas Farisiv, Anne Marie Flores, Martha Garcia, Karla Hemming, Jeph Herrin, Christine Hodgdon, Sheetal Kircher, Kurt Kroenke, Veronica Lam, Nicola Lancki, Quan H Mai, Jennifer Mallow, Nadine J McCleary, Wynne Norton, Mary O'Connor, Deirdre Pachman, Loretta Pearsonil, Frank Penedo, Jewel Podratz, Jennifer Popovic, Liliana Preiss, Parvez Rahman, Sarah Redmond, James Reich, Joshua Richardson, Kimberly Richardson, Jennifer Ridgeway, Lila Rutten, Karen Schaepe, Denise Scholtens, Tiana Poirier-Shelton, Philip Silberman, Jaclyn Simpson, Laura Tasker, Nathan Tesch, Cindy Tofthagen, Angela Tramontano, Benjamin D Tyndall, Hajime Uno, Firas Wehbe, and Bryan Weiner
Data availability
Descriptive information is provided in this commentary; no specific data are associated with this manuscript.
Author contributions
Ashley Wilder Smith, PhD, MPH (Conceptualization; Methodology; Supervision; Visualization; Writing—original draft; Writing—review & editing), Deborah Schrag, MD, MPH (Conceptualization; Funding acquisition; Supervision; Writing—review & editing), Kimberly Richardson, MA (Writing—review & editing), Jennifer Popovic, DVM, MA (Writing—review & editing), Raymond U. Osarogiagbon, MBBS (Conceptualization; Funding acquisition; Supervision; Writing—review & editing), Barbara Kroner, PhD (Conceptualization; Funding acquisition; Supervision; Writing—review & editing), Christine Hodgdon, MS (Writing—review & editing), Michael J. Hassett, MD, MPH (Supervision; Writing—review & editing), Andrea L. Cheville, MD (Conceptualization; Funding acquisition; Methodology; Supervision; Visualization; Writing—original draft; Writing—review & editing), Roxanne E. Jensen, PhD (Conceptualization; Writing—review & editing), September Cahue, MPH (Project administration; Writing—review & editing), Sandra L. Wong, MD (Conceptualization; Funding acquisition; Methodology; Supervision; Visualization; Writing—original draft; Writing—review & editing), Justin D. Smith, PhD (Conceptualization; Methodology; Visualization; Writing—original draft; Writing—review & editing), Kathryn J. Ruddy, MD, MPH (Conceptualization; Methodology; Project administration; Visualization; Writing—original draft; Writing—review & editing), Sandra A. Mitchell, PhD, CRNP (Conceptualization; Methodology; Visualization; Writing—original draft; Writing—review & editing), Sofia F. Garcia, PhD (Conceptualization; Methodology; Project administration; Supervision; Visualization; Writing—original draft; Writing—review & editing), Lisa DiMartino, PhD (Conceptualization; Methodology; Project administration; Visualization; Writing—original draft; Writing—review & editing), David Cella, PhD (Conceptualization; Funding acquisition; Supervision; Writing—review & editing) IMPACT Consortium.
Funding
The IMPACT Consortium is a Cancer Moonshot Research Initiative under the authorization of the 2016 United States 21st Century Cures Act. Research reported in this publication was supported by the NCI of the National Institutes of Health under award No. UM1CA233033 (Mayo Clinic, Rochester, MN), UM1CA233035 (Northwestern University, Chicago, IL), UM1CA233080 (Baptist Health System, Memphis, TN; Dana-Farber Cancer Institute, Boston, MA; Dartmouth-Hitchcock Medical Center, Lebanon, NH; Lifespan Health System, Providence, RI; Maine Medical Center, Portland, ME; and West Virginia University, Morgantown, WV), and U24CA232980 (RTI International, Research Triangle Park, NC).
Conflicts of interest
The authors have nothing to disclose.
References
- 1. Xu H, Mohamed M, Flannery M, et al. An unsupervised machine learning approach to evaluating the association of symptom clusters with adverse outcomes among older adults with advanced cancer: a secondary analysis of a randomized clinical trial. JAMA Netw Open. 2023;6(3):e234198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Badger TA, Segrin C, Crane TE, et al. Social determinants of health and symptom burden during cancer treatment. Nurs Res. 2023;72(2):103-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sikorskii A, Given CW, Chang S, et al. Patient reported outcomes and unscheduled health services use during oral anti-cancer treatment. J Pain Symptom Manage. 2023;65(2):e115-e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Batra A, Yang L, Boyne DJ, et al. Associations between baseline symptom burden as assessed by patient-reported outcomes and overall survival of patients with metastatic cancer. Support Care Cancer. 2021;29(3):1423-1431. [DOI] [PubMed] [Google Scholar]
- 5. Cho B, Pérez M, Jeffe DB, et al. Factors associated with initiation and continuation of endocrine therapy in women with hormone receptor-positive breast cancer. BMC Cancer. 2022;22(1):837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jim HSL, Hoogland AI, Brownstein NC, et al. Innovations in research and clinical care using patient-generated health data. CA Cancer J Clin. 2020;70(3):182-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mooney K, Berry DL, Whisenant M, et al. Improving cancer care through the patient experience: how to use patient-reported outcomes in clinical practice. Am Soc Clin Oncol Educ Book. 2017;37:695-704. [DOI] [PubMed] [Google Scholar]
- 8. Rocque GB, Dent DN, Ingram SA, et al. Adaptation of remote symptom monitoring using electronic patient-reported outcomes for implementation in real-world settings. J Clin Oncol Oncol Pract. 2022;18(12):e1943-e1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Caissie A, Olson R, Barbera L, et al. Striving to fill in gaps between clinical practice and standards: the evolution of a pan-Canadian approach to patient-reported outcomes use. Curr Oncol. 2022;29(5):3698-3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Singer DS, Jacks T, Jaffee E.. A U.S. "Cancer Moonshot" to accelerate cancer research. Science. 2016;353(6304):1105-1106. [DOI] [PubMed] [Google Scholar]
- 11. Landes SJ, McBain SA, Curran GM.. An introduction to effectiveness-implementation hybrid designs. Psychiatry Res. 2019;280:112513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moullin JC, Dickson KS, Stadnick NA, et al. Ten recommendations for using implementation frameworks in research and practice. Implement Sci Commun. 2020;1:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nilsen P, Bernhardsson S.. Context matters in implementation science: a scoping review of determinant frameworks that describe contextual determinants for implementation outcomes. BMC Health Serv Res. 2019;19(1):189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Glasgow RE, Battaglia C, McCreight M, et al. Use of the reach, effectiveness, adoption, implementation, and maintenance (RE-AIM) framework to guide iterative adaptations: applications, lessons learned, and future directions. Front Health Serv. 2022;2:959565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Damschroder LJ, Aron DC, Keith RE, et al. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Damschroder LJ, Reardon CM, Widerquist MAO, et al. The updated Consolidated Framework for Implementation Research based on user feedback. Implement Sci. 2022;17(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kirk MA, Kelley C, Yankey N, et al. A systematic review of the use of the Consolidated Framework for Implementation Research. Implement Sci. 2016;11:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Waltz TJ, Powell BJ, Fernández ME, et al. Choosing implementation strategies to address contextual barriers: Diversity in recommendations and future directions. Implement Sci. 2019;14(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Finney Rutten LJ, Ruddy KJ, Chlan LL, et al. Pragmatic cluster randomized trial to evaluate effectiveness and implementation of enhanced EHR-facilitated cancer symptom control (E2C2). Trials. 2020;21(1):480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hemming K, Haines TP, Chilton PJ, et al. The stepped wedge cluster randomised trial: Rationale, design, analysis, and reporting. BMJ. 2015;350:h391. [DOI] [PubMed] [Google Scholar]
- 21. May CR, Cummings A, Girling M, et al. Using normalization process theory in feasibility studies and process evaluations of complex healthcare interventions: a systematic review. Implement Sci. 2018;13(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cella D, Garcia SF, Cahue S, et al. Implementation and evaluation of an expanded electronic health record-integrated bilingual electronic symptom management program across a multi-site Comprehensive Cancer Center: The NU IMPACT protocol. Contemp Clin Trials. 2023;128:107171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li F, Hughes JP, Hemming K, et al. Mixed-effects models for the design and analysis of stepped wedge cluster randomized trials: An overview. Stat Methods Med Res. 2021;30(2):612-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thompson JA, Hemming K, Forbes A, et al. Comparison of small-sample standard-error corrections for generalised estimating equations in stepped wedge cluster randomised trials with a binary outcome: a simulation study. Stat Methods Med Res. 2021;30(2):425-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hassett MJ, Wong S, Osarogiagbon RU, et al. ; SIMPRO Co-Investigators. Implementation of patient-reported outcomes for symptom management in oncology practice through the SIMPRO research consortium: a protocol for a pragmatic type II hybrid effectiveness-implementation multi-center cluster-randomized stepped wedge trial. Trials. 2022;23(1):506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Basch E, Reeve BB, Mitchell SA, et al. Development of the National Cancer Institute's patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J Natl Cancer Inst. 2014;106(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. NCI. Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE®).https://healthcaredelivery.cancer.gov/pro-ctcae/. Accessed October 18, 2023. [DOI] [PMC free article] [PubMed]
- 28. Hassett MJ, Cronin C, Tsou TC, et al. eSyM: an electronic health record-integrated patient-reported outcomes-based cancer symptom management program used by six diverse health systems. J Clin Oncol Clin Cancer Inform. 2022;6:e2100137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Davis KM, Wilbur KM, Metzger SB, et al. Symptom and needs assessment screening in oncology patients: alternate outreach methods during COVID-19. J Psychosoc Oncol. 2021;39(3):452-460. [DOI] [PubMed] [Google Scholar]
- 30. Sisodia RC, Rodriguez JA, Sequist TD.. Digital disparities: lessons learned from a patient reported outcomes program during the COVID-19 pandemic. J Am Med Inform Assoc. 2021;28(10):2265-2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pritchett JC, Patt D, Thanarajasingam G, et al. Patient-reported outcomes, digital health, and the quest to improve health equity. Am Soc Clin Oncol Educ Book. 2023;43:e390678. [DOI] [PubMed] [Google Scholar]
- 32. Patt D, Wilfong L, Hudson KE, et al. Implementation of electronic patient-reported outcomes for symptom monitoring in a large multisite community oncology practice: dancing the Texas two-step through a pandemic. J Clin Oncol Clin Cancer Inform. 2021;5:615-621. [DOI] [PubMed] [Google Scholar]
- 33. HealthMeasures. Patient-Reported Outcomes Measurement Information System®(PROMIS®). https://www.healthmeasures.net/explore-measurement-systems/promis. Accessed October 18, 2023.
- 34. Davis LL, Kroenke K, Monahan P, et al. The SPADE symptom cluster in primary care patients with chronic pain. Clin J Pain. 2016;32(5):388-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang R, Burgess E, Reddy M, et al. Provider perspectives on the integration of patient-reported outcomes in an electronic health record. JAMIA Open. 2019;2(1):73-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Descriptive information is provided in this commentary; no specific data are associated with this manuscript.