Abstract
PURPOSE
Alcohol-related myopathy is one of the earliest alcohol-associated pathological tissue changes that is progressively exacerbated by cumulative long-term alcohol misuse. Acute and chronic alcohol use leads to changes in skeletal muscle mass and function. As discussed in this evidence-based review, alcohol-mediated mechanisms are multifactorial with effects on anabolic and catabolic signaling, mitochondrial bioenergetics, extracellular matrix remodeling, and epigenomic alterations. However, systematic studies are limited, especially regarding the acute effects of alcohol on skeletal muscle.
SEARCH METHODS
This review focuses on peer-reviewed manuscripts published between January 2012 and November 2022 using the search terms “alcohol” or “ethanol” and “skeletal muscle” in MEDLINE, PubMed, and Web of Science using EndNote reference management software.
SEARCH RESULTS
Eligible manuscripts included full-length research papers that discussed acute and chronic effects of alcohol on skeletal muscle mass and function in both clinical and preclinical studies. The review also includes alcohol-mediated skeletal muscle effects in the context of comorbidities. The three databases together yielded 708 manuscripts. Of these, the authors excluded from this review 548 papers that did not have “alcohol” or “muscle” in the title and 64 papers that were duplicates or did not discuss skeletal muscle. Thus, of all the manuscripts considered for this review, 96 are included and 612 are excluded. Additionally, relevant papers published earlier than 2012 are included to provide context to the review.
DISCUSSION AND CONCLUSIONS
Both acute and chronic alcohol use decrease protein synthesis and increase protein degradation. Alcohol also impairs mitochondrial function and extracellular matrix remodeling. However, there is a gap in the literature on the known alcohol-mediated mechanisms, including senescence, role of immune activation, and interorgan communication, on the development of alcohol-related myopathy. With increased life expectancy, changing alcohol use patterns, and increasing frequency of alcohol use among females, current observational studies are needed on the prevalence of alcohol-related myopathy. Additionally, the compounding effects of acute and chronic alcohol use on skeletal muscle with aging or exercise, in response to injury or disuse, and in the context of comorbidities including diabetes and human immunodeficiency virus (HIV), call for further investigation. Though evidence suggests that abstinence or reducing alcohol use can improve muscle mass and function, they are not restored to normal levels. Hence, understanding the pathophysiological mechanisms can help in the design of therapeutic strategies to improve skeletal muscle health.
Keywords: alcohol, muscles, skeletal, comorbidity, protein synthesis, proteolysis, metabolism, mitochondria
Alcohol misuse is the most common form of substance misuse and is associated with liver, cardiovascular, and metabolic diseases as well as with infections and cancers.1 Although an estimated 20% to 25% of people who drink heavily develop alcohol-related liver disease,2 40% to 60% of people with alcohol misuse have alcohol-related myopathy.3 Evidence that alcohol use leads to skeletal muscle (SKM) weakness, even in the absence of neuropathology, was independently documented in the 1800s by James Jackson4 and Magnus Huss.5 More empirical reports that alcohol or its metabolites could directly or indirectly lead to adaptations of SKM mass and function and that there are differences with acute and chronic alcohol misuse were formulated in the 1950s and 1960s.6–8
SKM mass is maintained by the balance of anabolic (protein synthesis) and catabolic (protein breakdown) signaling. Major anabolic stimuli—including amino acids, insulin, insulin-like growth factor 1 (IGF-1)—and mechanical loading promote protein synthesis by converging on the mechanistic/mammalian target of rapamycin (mTOR) signaling pathway (reviewed by Bourgeois et al.9 and Steiner et al.10). SKM protein breakdown occurs through activation of the ubiquitin proteasome pathway (UPP) and SKM-specific ubiquitin ligases or atrogenes; atrogin-1 (also known as muscle atrophy F-box, or MAFbx) and muscle RING-finger protein-1 (MuRF-1) are often used as markers of UPP activation. The second major protein breakdown pathway is activation of the autophagic-lysosomal system that degrades misfolded proteins by formation of a phagophore followed by engulfing of degraded proteins.9–12 Myofibers, their structural components, and the extracellular matrix intricately communicate to maintain SKM structure. Though most adult muscle growth is driven by hypertrophy of existing myofibers, muscle stem cells (satellite cells) contribute to myofiber regeneration, especially in response to injury or atrophy.13,14 Finally, being highly dynamic and with high energy demands, SKM relies heavily on mitochondria for bioenergetic demands, redox balance, and programmed death signaling (reviewed by Bourgeois et al.9). Evidence suggests that alcohol significantly affects all these major attributes of SKM mass and function, as discussed in this review.
Most current knowledge of the systematic cellular and molecular alterations seen with alcohol-related myopathy is from preclinical rodent studies.15 However, the pathophysiological mechanisms of alcohol misuse are complex and are influenced by genetics, sex, lifestyle factors, psychosocial determinants, health comorbidities, and patterns of alcohol use.16 Published literature in the 1990s and early 2000s provided epidemiological evidence for the prevalence of alcohol-related myopathy.17,18 With the changing patterns of alcohol use,19,20 changes in dietary and lifestyle choices,21 increase in life expectancy,22 and increasing frequency of alcohol misuse among females,19 there is a need for recent studies on the prevalence and the disease course of alcohol-related myopathy. Moreover, despite evidence for the high prevalence of alcohol-related myopathy, there is limited literature on its effects on aging, whole body metabolism, response to injury or atrophy, and exercise.
One of the challenges in assessing the effects of alcohol consumption on skeletal muscle and other systems is the sometimes-inconsistent definition of drink sizes and drinking levels, particularly when comparing studies conducted in different countries (see Table 1). The National Institute on Alcohol Abuse and Alcoholism provides information about drinking patterns for adults and defines a standard drink in the United States,23 although standard drink definitions sometimes differ in other countries.24 The World Health Organization maintains a global database that provides information on several alcohol-related topics, including levels and patterns of alcohol use.16
Table 1.
Alcohol Consumption: Drink Sizes and Drinking Levels in Select Countries
| Defining Drinking Levels | ||||
|---|---|---|---|---|
| United States | Iceland and United Kingdom | China, France, Ireland, and Spain | Austria | |
| Standard drink23 | 0.6 fluid oz or 14 g pure alcohol (12 oz regular beer, 5 oz wine, or 1.5 oz distilled spirits) | 8 g pure alcohol | 10 g pure alcohol | 20 g pure alcohol |
| Binge drinking23 | Pattern of drinking that brings blood alcohol concentration to 0.08% or higher in 2 hours (four or more U.S. standard drinks in women; five or more U.S. standard drinks in men) | |||
| Heavy drinking23 | Women: Consuming more than three drinks per day or more than seven drinks per week Men: Consuming more than four drinks per day or more than 14 drinks per week | |||
| World Health Organization: Alcohol Consumption Categories 16 | ||||
| Abstainer | Consumed no alcohol in lifetime or in past 12 months | |||
| Former drinker | Previously drank alcohol but no consumption in the past 12 months | |||
| Consumer | Consumed alcohol in the past 12 months | |||
| Heavy episodic drinker | Consumed 60 g or more alcohol on at least one occasion in the past 30 days | |||
Search Methods
This review is based on a literature search of three databases—PubMed, MEDLINE (OvidSP), and Web of Science’s Core Collection (Thomas Reuters)—using the EndNote program. The literature search included articles published between January 2012 and November 2022. The search terms used were “ethanol” and “skeletal muscle” as MeSH terms in PubMed and title, abstract, and keywords for MEDLINE and Web of Science.
Results of the Literature Search
The three databases yielded 708 papers, and titles were screened to include only papers that had the terms alcohol or ethanol, and muscle in the title. With this, 548 manuscripts were excluded. Also excluded were 64 manuscripts that were either duplicates across the three databases or manuscripts that did not discuss SKM. Thus, 96 manuscripts are included in the discussion. Eligible studies were those that included acute or chronic effects of alcohol on SKM mass and function in both clinical and preclinical studies.
This review briefly discusses the salient literature related to alcohol effects on SKM prior to 2012 to provide context. Following this, database search results are organized based on acute and chronic effects of alcohol on SKM metabolic signaling pathways as well as structural and functional adaptations. The review also discusses the effects of alcohol on SKM in the context of some comorbidities, including HIV, pain, cancer, and disuse. Finally, the review discusses gaps in literature and identifies some of the salient future directions that can be pursued.
Results of the Reviewed Studies
Overview
The effect of alcohol misuse on SKM mass and function is referred to as acute and chronic alcohol-related myopathy. Acute alcohol-related myopathy presents clinically as breakdown of damaged muscle tissue (rhabdomyolysis)25 and is the most frequent cause of nontraumatic rhabdomyolysis.26 This can occur even with a single binge-drinking session (blood alcohol concentration 0.08 g/dL), and symptoms generally resolve after 1 to 2 weeks of abstinence.27 Alcohol-related rhabdomyolysis predominantly affects muscles of the pelvic and shoulder girdles and is associated with increased circulating levels of creatinine kinase and myoglobin, compartment syndrome particularly of the lower extremities, and, in severe cases, acute renal failure.25,28 Evidence also indicates that people who have chronic alcohol-related myopathy can be prone to rhabdomyolysis following an alcohol binge and can show signs of episodic myalgia, muscle weakness, and dark-colored urine.26 An estimated 0.5% to 2.0% of people with alcohol misuse present with acute alcohol-related myopathy.26 Apart from rhabdomyolysis, acute alcohol affects SKM anabolic protein synthesis pathways29–34 and catabolic pathways.35
Chronic alcohol-related myopathy (CAM) is the most frequent form of alcohol-related myopathy with an overall prevalence of 2,000 per 100,000 people with alcohol misuse.17 Clinical signs associated with CAM are progressive proximal muscle weakness, type II fiber (fast twitch glycolytic fibers) atrophy, pain, and myotonia.26,36 Onset of CAM is associated with cumulative lifetime or long-term high-dose alcohol consumption.17,26 Thus, clinical manifestations of CAM are seen in older individuals (ages 40 to 60) and are more common in people with other comorbidities, with ~ 50% of people with alcohol-related liver cirrhosis and ~ 80% of people with alcohol-related cardiomyopathy presenting with CAM.26,37,38 CAM is characteristically marked by decreased protein synthesis, dysregulation of proteins in the insulin signaling pathway and the mTOR complex 1 (mTORC1) pathway, and dysregulation of myofibrillar and sarcoplasmic proteins.39–41 In addition, chronic alcohol intake increases SKM catabolic signaling.39,42,43 The effects of chronic alcohol use on SKM mitochondrial function are not clear. Early studies in people with CAM showed a lack of association with mitochondrial energy supply.44 However, other studies indicate that chronic alcohol consumption increases SKM glycogen and lipid storage, with megamitochondria and dilated sarcoplasmic reticulum.40
These alcohol-associated molecular changes could potentially affect muscle strength. In people with a history of alcohol misuse, mean strength increased from baseline over a 5-year abstinence period, but remained significantly weaker than age-matched controls, with more than half of them still showing histological signs of myopathy.3 People who consume a single drink (1 g ethanol/kg body weight) showed a significant decrease in peak strength even 36 or 60 hours post-exercise, indicating that alcohol use accentuates the loss of both dynamic and static strength seen with eccentric exercise.45 However, consumption of low-dose alcohol (0.5 g ethanol/kg body weight) after eccentric muscle exercise does not affect muscle force.46
Thus, epidemiological and molecular data from seminal work provide evidence that both acute and chronic alcohol use adversely affect SKM and clinically manifest as rhabdomyolysis or weakness of the proximal muscles, respectively. Building on this clinical and preclinical research, and with advances in cellular and molecular assays over the past decade, significant strides have been achieved on elucidating the pathophysiological adaptations that lead to acute and chronic alcohol-related myopathy.
Acute Effects of Alcohol on SKM
Acute alcohol effects on SKM anabolic signaling
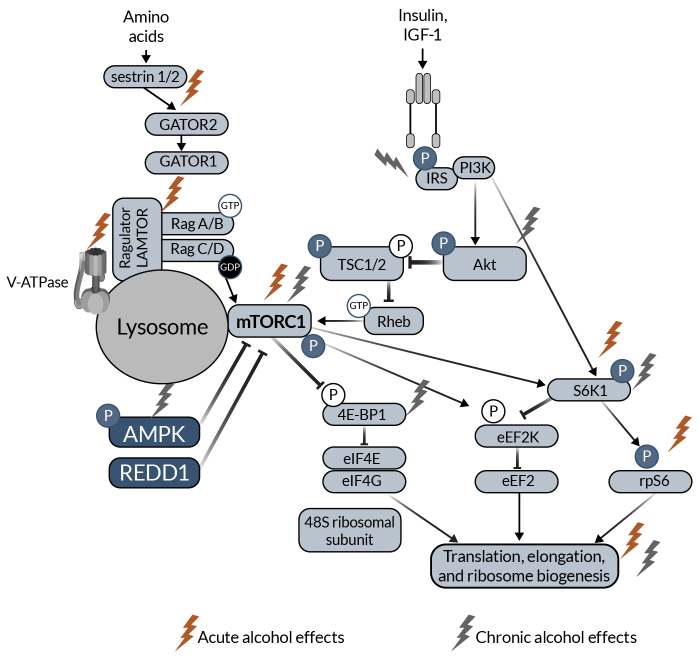
The major SKM anabolic pathway is the mTORC1 pathway leading to muscle protein synthesis (Figure 1). Anabolic stimuli (e.g., insulin, IGF-1) activate phosphoinositide 3 kinase, which phosphorylates and activates protein kinase B (Akt). Akt phosphorylates and inactivates tuberous sclerosis complex 1 and 2 (TSC1, TSC2) through inhibition of TSC2 guanosine-triphosphate hydrolase (GTPase) activity. TSC2 GTPase removes GTP from Ras homolog enriched in brain (Rheb). TSC2 inactivity allows GTP-bound Rheb to accumulate, which stimulates mTORC1 activity. Downstream of mTORC1, S6 kinase 1 (S6K1) is activated, allowing for the activation of ribosomal protein S6. Additionally, eukaryotic initiation factor 4E-binding protein (4E-BP1) is inactivated downstream of mTORC1. Both activation of S6K1 and inactivation of 4E-BP1 increase translational machinery, allowing protein synthesis to increase. Amino acids also regulate the mTORC1 pathway. When amino acids (e.g., leucine) bind to sestrin 1/2, sestrin dissociates from GTPase activating proteins (GAP) toward Rags complex 2 (GATOR2). This decreases the inhibitory effect of GATOR2 on GATOR1. Together, there is a conformational change to Ragulator, which has late endosomal/lysosomal adaptor and MAPK and mTORC1 activators (LAMTOR) increasing GTP-bound RagA and guanosine diphosphate-bound RagC that ultimately activates mTORC1. mTORC1 is also regulated by several other upstream proteins, including AMP-activated protein kinase (AMPK) and regulated in development and DNA damage response 1 (REDD1).10,47
Figure 1. Schematic representation of mTOR anabolic signaling in skeletal muscle.
The schematic highlights proteins that have been shown to be affected by acute (red lightning bolt icon) and chronic (light gray lightning bolt icon) alcohol intake in the skeletal muscle. Note: 4E-BP1, eukaryotic initiation factor 4E-binding protein; Akt, protein kinase B; AMPK, AMP-activated protein kinase; eEF2k, eukaryotic elongation factor 2 kinase; GAP, GTPase activating proteins; GATOR2, toward Rags complex 2; IGF-1, insulin-like growth factor 1; IRS, insulin receptor substrate; LAMTOR, late endosomal/lysosomal adaptor and MAPK and mTORC1 activators; mTORC1, mechanistic/mammalian target of rapamycin complex 1; PI3K, phosphoinositide 3 kinase; REDD1, regulated in development and DNA damage response 1; Rheb, Ras homolog enriched in brain; S6K1, S6 kinase 1; TSC1, tuberous sclerosis complex 1; TSC2, tuberous sclerosis complex 2.
In vitro studies in C2C12 myotubes showed that 100 mM ethanol modulates Rag and AMPK/TSC2/Rheb signaling, decreasing the anabolic effects of leucine.48 A single intraperitoneal alcohol injection (3 g/kg) in either fasted or fed male mice prevented the increase in fed-state protein synthesis and phosphorylation of ribosomal protein S6 kinase at threonine 389 (S6K1Thr389).49,50 In the fed state, alcohol administration decreased the association of Raptor and RagC with immunoprecipitated LAMTOR1 and increased sestrin1–GATOR2 and vacuolar-type ATPase V1 association with LAMTOR1 within 1 hour of administration, dysregulating protein–protein interactions of the Rag-Ragulator complex.50 However, a study using the same alcohol administration paradigm in REDD1 knockout mice indicated that REDD1 may not play a role in alcohol-mediated decreased protein synthesis, but may be involved in UPP-mediated protein breakdown.51
Male mice that were injected with 3 g/kg alcohol intraperitoneally and administered electrically stimulated muscle contractions decreased stimulation-induced total rate of protein synthesis and blunted the phosphorylation of S6K1 (Thr421/Ser424 and Thr389) and its substrate rpS6 (Ser240/244), indicating that acute alcohol administration dysregulates stimulation-induced changes in protein synthesis and mTORC1 signaling.52 A small clinical study among trained male and female participants who were administered alcohol (1.09 g/kg fat-free body mass) 10 to 20 minutes after an acute heavy resistance exercise trial found decreased exercise-induced phosphorylation of the mTOR(Ser2448) and S6K1 (Thr389) in males, with no changes in females.53 Other studies also showed that alcohol (1.5 g/kg body mass) consumed after exercise decreased mTORC1 signaling and protein synthesis.54
Acute alcohol-mediated alterations on the endocrine profile are thought to contribute to impaired SKM anabolic signaling. An alcohol binge post-exercise decreased testosterone to cortisol ratio55 or maintained increased testosterone levels post-exercise in healthy young males.56 These changes in the hormonal profile can potentially lead to decreased anabolic and increased catabolic signaling, thus affecting SKM protein balance; however, definitive studies are needed to prove this. Moreover, the effects of alcohol on estrogen signaling and their impact on SKM mass and function in both males and females remain largely unknown.
Acute alcohol effects on SKM catabolic signaling
A single intraperitoneal injection of alcohol (3 g/kg) blunted the expected fed-state decrease in autophagy in mice, with no significant effects on UPP.49 A single intraperitoneal injection of alcohol (5 g/kg) in female mice demonstrated altered expression of genes implicated in fatty acid oxidation, including peroxisome proliferator-activated receptor (PPAR) alpha and PPAR-beta, AMPK, and cluster of differentiation 36 (CD36), as well as of genes involved in protein breakdown, such as MuRF1, Krüppel-like factor 15 (Klf15), and branched chain amino acid transaminase 2 (Bcat2). These changes were associated with increased circulating corticosterone levels and dysregulation of energy substrate metabolism.57 C2C12 myoblasts treated with 100 mM of alcohol showed increased proteolysis. An inhibitor of autophagy (3-methyladenine) prevented the increase in proteolysis while a proteasome inhibitor (MG132) did not affect proteolysis, highlighting the relevance of activation of autophagic-lysosomal pathway to alcohol-induced catabolic responses in SKM.37
An acute intraperitoneal alcohol injection (5 g/kg) to female mice significantly disrupted mRNA expression of gastrocnemius clock genes as well as clock-controlled genes implicated in SKM function. Alcohol also increased circulating corticosterone levels and one of its target genes, REDD1, in SKM.58 The disruption of circadian clocks in different tissues is a possible mechanism of alcohol-induced tissue injury.59–61 It remains to be determined whether disruption of the clock genes and circadian rhythm is a mechanism of alcohol-mediated SKM dysfunction and whether there are sex- or age-specific effects.
Effects of acute alcohol exposure on SKM function
There are limited studies on the effects of acute alcohol exposure on SKM function. Studies in both male and female mice show that a single intraperitoneal injection of 5 g/kg alcohol decreased absolute and normalized peak-isometric (no change in length) tetanic (continuous muscle contraction) force generated in the triceps surae (gastrocnemius, soleus, and plantaris) muscles and increased fatigue within 1 hour of alcohol administration. These deficits were still present at 24 hours post-alcohol administration in male mice but not in female mice.62 In male mice administered 3 g/kg ethanol, there were no differences in twitch or tetanic force in the extensor digitorum longus muscle.63 Whether differential kinetics of alcohol clearance or sex hormone differences contribute to the sex-dependent effects warrants further investigation.
In a clinical study from the United States, individuals who consumed alcohol (1.09 g/kg lean mass) had decreased time to exhaustion when using a cycle ergometer 18 to 24 hours post-alcohol consumption, suggesting an alcohol-mediated detrimental impact on severe-intensity exercise performance. However, the single alcohol dose did not affect muscle power, strength, or fatigability (decrease in maximal force in response to contractile activity).64 Similarly, heavy episodic drinking (six to 20 or more standard drinks [defined as 8 g of alcohol]) on the previous day in male rugby players in New Zealand decreased lower body performance with no effect on isometric strength and sprint performance.65 In Australia, rugby players given 1 g ethanol/kg body weight 4 hours after a rugby match did not have statistically significant differences in maximal voluntary contraction, voluntary activation, or changes in creatine kinase, testosterone, or cortisol the morning after the match.66 In the United States, among females who performed two bouts of maximal single leg eccentric extension followed by a single drink (1.09 g/kg fat-free body mass), isometric torque was fully recovered and eccentric torque partially recovered after 48 hours. Using the same exercise regimen, females who consumed a single drink (0.88 g/kg body weight) had no effect on strength recovery.67,68 Though the exact mechanisms are not known, it is possible that estrogens play a protective role in muscle recovery in females.69,70
One possible explanation for the impaired strength with acute alcohol use is impaired glycolytic function, as severe intensity exercise relies heavily on anaerobic ATP production. In vitro exposure of primary male and female myoblasts to alcohol decreased glycolytic function.71 Alternatively, it is possible that the effects of alcohol are indirectly mediated by alterations in endocrine mediators. Acute alcohol binge drinking after resistance exercise increases cortisol levels, a known SKM catabolic mediator.55,72 Moreover, alcohol can interfere with SKM regeneration. Normally, muscle-damaging exercises activate regenerative processes with an initial inflammatory response to activate satellite cells.73 Acute binge alcohol intake during resistance exercise decreases the early SKM inflammatory response in both trained males and females,67,74 providing evidence that it can adversely affect SKM regenerative processes. Moreover, these studies and others have shown marked alcohol-induced decreased myoblast differentiation, suggesting impaired recovery from exercise could be due to decreased regeneration capacity.75,76
It should be noted that these effects of single, predetermined alcohol bouts on SKM function were examined in healthy and young males or females; however, whether this holds true in the general population, where the pattern and amount of alcohol consumed is variable, must be considered. Moreover, nutritional state and type of exercise can be confounding factors. In addition, the effect of acute alcohol binges on SKM function during exercise training in people with chronic alcohol misuse also necessitates investigation. In the BEER-High-Intensity Interval Training (BEER-HIIT) study conducted in Spain, females who consumed 12–24 g alcohol per day and males who consumed 24–36 g alcohol per day throughout the 10-week training period did not exhibit lower exercise-mediated increases in lean mass, aerobic fitness, and muscle strength.77,78 However, people in treatment for alcohol misuse have decreased isokinetic torque (strength), work, power, and isometric and isotonic muscle loading even after detoxification.79 This is compounded by the fact that ~ 15% of people with alcohol misuse have significant mobility impairment.80–82
In summary, acute alcohol exposure dysregulates multiple proteins in the mTORC1 pathway and decreases muscle protein synthesis. There is also evidence that acute alcohol use increases catabolic signaling by activating both UPP and autophagy. Acute alcohol intake before or after exercise affects SKM function and is influenced by gender, type of exercise strength, and amount of alcohol. However, the role of other confounders (e.g., age, chronic alcohol misuse) warrants further investigation.
Chronic Effects of Alcohol on SKM
Studies to identify when CAM develops are difficult and confounded by underreporting of its overall prevalence. A clinical observational study among males from Russia reports that CAM takes about 10 years to develop, with proximal paresis occurring only in people who have muscle atrophy.83
Some of the common causes that lead to dysfunctional SKM mass, especially with chronic alcohol use, are increased inflammation and oxidative stress. Zebrafish exposed to 0.5% alcohol for 8 weeks had decreased body weight and muscle fiber cross-sectional area with increased expression of the pro-inflammatory cytokines interleukin 1-beta (IL-1-beta) and tumor necrosis factor alpha (TNF-alpha) and increased expression of high-mobility cassette-1/toll-like receptor 4/nuclear factor-kappa B (HMGB1/TLR4/NF-kappa B) signaling proteins.84 Similarly, chronic alcohol-fed rats showed increased SKM TNF-alpha and IL-6 expression and activation of the Janus kinase (JNK) pathway.85 Clinical studies also confirmed that TNF-alpha expression was negatively associated with lean muscle mass in people with chronic alcohol misuse.86 The increase in inflammation has also been linked to SKM oxidative stress and tissue dysfunction.87 Rats with chronic exposure to alcohol had decreased antioxidant enzyme activity and increased malondialdehyde content.88 The contribution of oxidative stress to increased protein degradation and SKM dysfunction was demonstrated in C2C12 myoblasts and mitochondria-targeted Mito-TEMPO attenuated alcohol-mediated increase in autophagy.89
Chronic alcohol effects on SKM anabolic signaling
Chronic alcohol intake upregulates IGF binding protein-1 and myostatin, leading to decreased SKM protein synthesis.11 Chronic alcohol feeding of rats for 14 weeks decreased SKM protein synthesis and prevented the anabolic effects of leucine administration irrespective of sex, indicating that there are no sex-specific effects of alcohol on SKM protein synthesis. However, the study also showed that at 6 weeks, males had decreased SKM protein synthesis, but there were no changes in females.90 Alcohol administration for 4 weeks in female mice found dephosphorylation of mTORC1 and AMPK, which was mechanistically linked to an increase in protein phosphatase 2A.91 Additionally, chronic alcohol administration in mice, ethanol treatment of C2C12 myoblasts, and analyses in people with alcohol-related cirrhosis all demonstrated that alcohol had synergistic effects with increased ammonia to impair SKM protein synthesis and increase protein breakdown.92 Multiomics analyses of alcohol-treated C2C12 cells and SKM from ethanol-fed mice identified several beta-hydroxymethyl-butyrate–responsive targets. Moreover, beta-hydroxymethyl-butyrate restored ethanol-induced decreased mTORC1 signaling, protein synthesis, and mitochondrial respiration as well as decreased sarcopenic phenotype.93 In a study in Russia, females who reported consuming about 11 units of ethanol per day (1 unit is 10 ml of pure [96%] ethanol) for an average of 5.6 ± 0.6 years had decreased plasma IGF1 levels and decreased SKM expression of insulin receptor substrate (IRS-1), p-AktB, and p-4E-BP1. This was also associated with decrease in cross-sectional fiber area of both type I (slow oxidative) and II (fast glycolytic) fibers.94 Similarly, middle-aged males with chronic alcohol misuse had decreased circulating IGF-1 levels and reduced SKM expression of IRS-1 and p-4E-BP1. This was also associated with increased mRNA expression of heat shock proteins and atrogenes and a relative increase in the proportion of fast glycolytic muscle fibers,95,96 indicating both a decrease in anabolic signaling and an increase in catabolic signaling.
Studies suggest that chronic alcohol consumption does not affect SKM glucose uptake,97 despite the fact that chronic ethanol administration in rats reduced gastrocnemius expression of IRS-1, Akt, and p70S6K.98 Chronic alcohol intake increased triglyceride deposition and decreased glucose uptake in SKM, which can lead to metabolic dysregulation.99 Additionally, pigs fed a hypercaloric high-fat diet and alcohol diet for 7 weeks showed increased expression of proteins in the insulin signaling pathway and hyperglycemia.100 In addition, PPAR-delta activation protected against alcohol-induced decreased Akt phosphorylation and increased mitochondrial uncoupling, indicating that PPAR-delta activation can protect against alcohol-induced SKM lipotoxicity and insulin resistance.99
Physical activity or structured exercise generally is beneficial to anabolic signaling. However, female mice that were provided access to running wheels for 5 weeks and access to 20% ethanol in water during the last 5 days had decreased exercise-induced SKM p70S6K phosphorylation and increased MAFbx expression, suggesting that perhaps physical activity alone might not be sufficient to counteract the effects of alcohol-mediated SKM changes.101
Chronic alcohol effects on SKM catabolic signaling
Zebrafish exposed to 0.5% alcohol for 8 weeks had increased expression of markers of SKM atrophy and autophagy. This was associated with concomitant increases in reactive oxygen species content, decreased mRNA expression of antioxidant enzymes, and protein expression of Nox2,84 linking the catabolic changes to increased oxidative stress. Similarly, rats fed 3 g/kg body weight of alcohol for 4 weeks showed increased SKM MuRF1 expression, decreased pAkt/Akt ratio, and increased p-FoxO/FoxO ratio102 indicating UPP activation. In contrast, chronic alcohol feeding did not result in increased SKM expression of atrogenes in older rats.103 However, there are some discrepancies regarding the activation of SKM autophagy with chronic alcohol misuse. For example, some research showed increased expression of autophagy markers in people with alcohol-related cirrhosis and chronic alcohol-fed mice.37 However, this was not observed in other studies of chronic alcohol-fed mice11 or in primary myoblasts isolated from in vivo chronic binge alcohol administered to macaques.76
Chronic alcohol effects on SKM structural characteristics
A clinical study from Brazil showed that people who consumed more than 80 g alcohol per day and followed a sedentary lifestyle had reduced SKM index and phase angle (SKM specific indicator of cellular membrane integrity).104 Similarly, in the United States, people with chronic alcohol misuse had significantly lower whole SKM area37 and lower femoral and gluteal muscle areas.105 However, in a large 12-year observational study among Korean people, high protein intake compared to low protein intake was protective against the development of low SKM mass index. Alcohol consumption in females but not males reduced the protective effect of high protein intake. Among the total participant population who consumed diets with high protein content, heavy drinking was not associated with development of low SKM mass index,106 suggesting that dietary and lifestyle modifications potentially can prevent the imbalance in protein turnover with alcohol use.107 In an observational study from Russia, females who reported consuming 11 ± 1 units of ethanol/day for an average of 5.6 ± 0.6 years had decreased cross-sectional area of both type I and type II fibers; decreased expression of titin and nebulin, two large proteins involved in maintaining the sarcomere structure; and increased expression of the protease calpain-1 and ubiquitinated proteins.94 These findings are consistent with those from preclinical studies showing that chronic alcohol administration for 6 months in rats increased autolysis of mu-calpain; decreased titin, nebulin, and titin hyperphosphorylation; and led to the development of hindlimb muscle atrophy.108 Chronic alcohol also decreased expression of myosin heavy chain (MHC), a major motor protein in the thick filament, and troponin-T, which is necessary for myosin and actin positioning.63 Adult fish maintained at 0.5% ethanol for 8 weeks showed decreased SKM cross-sectional area, and this was associated with decreased SKM miR-140 expression and increased miR-146a expression. miR-140 targets the Notch signaling pathway, whereas miR-146a targets the Notchantagonist Numb, and these changes in miRNAs are implicated as mechanisms for the observed decreased SKM cross-sectional area.109
In addition to the contractile and structural SKM proteins, extracellular matrix (ECM) remodeling plays a critical role in regeneration, anabolic signaling, and mitochondrial function. Adult male rats on an alcohol-containing liquid diet for 24 weeks increased expression of collagens, hydroxyproline, and alpha-smooth muscle actin (marker of myofibroblast activation). This was associated with an increase in other matrisome proteins, including integrin-alpha-5, L-selectin, platelet endothelial cell adhesion molecule (PECAM), secreted protein acidic and rich in cysteine (SPARC), and ADAM metallopeptidase with thrombospondin type 1 motif 2 (ADAMTS2). Alcohol also increased the inflammatory cytokines TNF-alpha, IL-12, and IL-6, and decreased IL-10 mRNA expression,110 likely contributing to the observed increase in ECM deposition. Finally, chronic alcohol feeding in rats increased expression of transforming growth factor-beta 1 (TGF-beta) and associated receptors along with downstream signaling components,111 as well as expression of matrix metalloproteinase 9,112 providing evidence for ECM remodeling and promotion of an SKM profibrotic phenotype.
Chronic alcohol effects on SKM functional characteristics
In a longitudinal study among Japanese men and women, alcohol use was positively associated with decreased grip strength, and this association did not change over a 2-year period.113 Similarly, in a cross-sectional study among people living in China, men who consumed more than 25 g of alcohol per day had increased risk of low muscle mass and grip strength.114 In animal studies, female mice consuming 20% alcohol in water for 40 weeks had decreased grip strength and decreased lean muscle mass, without major neuromuscular junction changes, suggesting that muscle weakness is potentially driven by muscle atrophy.115 In other studies, fatigability and alterations in twitch and tetanic tension were seen with chronic alcohol intake.63 Evidence from studies using alpha-E83K mutant mice suggested that acute alcohol increased force and that this was due to direct actions of alcohol on the extracellular region of the neuromuscular nicotinic acetylcholine receptor (nAChR).116
Chronic alcohol effects on mitochondrial function
Caenorhabditis elegans exposed to ethanol had decreased expression of mitochondrial fission factor dynamin-related protein 1 (DRP-1), and mitochondrial network fragmentation leading to mitochondrial unfolded protein response, which was mechanistically linked to SKM weakness.117 Similarly, rats fed a chronic alcohol diet had decreased levels of mitofusin-1, dysregulation of mitochondrial topoisomerase, and decreased mitochondrial membrane integrity.118 Chronic alcohol administration also increased SKM 4-hydroxy-2-nonenal, decreased mitochondrial Complex IV and V activity, and decreased acetylcholinesterase expression, potentially indicating that mitochondrial dyshomeostasis was associated with inhibition of acetylcholinesterase, leading to myofiber atrophy.98 In addition, chronic alcohol feeding in the context of high-fat diet for 6 weeks decreased SKM Complex I and III activity, antioxidant activity, as well as increased lipid peroxidation in both male and female mice.119 A study using parkin-knockout mice fed an alcohol diet for 12 weeks showed that parkin was critical for alcohol-mediated disruption of mitochondrial complex activity, autophagy/mitophagy balance, and apoptosis.120 Finally, ethanol treatment of primary myoblasts isolated from male and female macaques during 5 days of differentiation decreased extracellular acidification rate, an indicator of glycolysis, and increased maximal oxygen consumption rate. These changes were associated with decreased differentiation, suggesting that bioenergetic alterations regulate alcohol-mediated impaired myogenesis.71 Despite these indications of alcohol-mediated mitochondrial changes, few systematic studies have focused on mitochondrial bioenergetics and function with acute and chronic alcohol intake.
In summary, chronic alcohol-mediated increases in inflammation and oxidative stress contribute to CAM. Chronic alcohol exposure dysregulates multiple proteins in the mTORC1 signaling pathway and decreases SKM protein synthesis. As seen with acute alcohol exposure, chronic alcohol exposure increases protein breakdown by impacting autophagy and UPP. Preclinical and clinical studies provide evidence for decreased SKM mass with chronic alcohol exposure. Though the exact mechanisms of alcohol’s effect on SKM structural and functional characteristics are not known, dysregulation of contractile proteins, muscle regulatory factors, and ion channels may be implicated.121 Evidence also suggests that physical exercise and protein-rich diets can potentially reduce the adverse effects of chronic alcohol intake on SKM.
Alcohol Effects on SKM in the Context of Comorbidities
SIV/HIV
With effective antiretroviral therapy (ART), people with HIV (PWH) have a near-normal life expectancy that has increased the earlier occurrence of age-associated comorbidities, including impaired SKM mass and function as well as frailty. Alcohol misuse is a maladaptive coping behavior among PWH122 that can exacerbate HIV-specific effects on SKM. Significant knowledge of the effects of chronic alcohol intake on SKM in the context of HIV has been derived from the rhesus macaque model. Chronic binge alcohol (CBA) administration in ART-naïve macaques infected with simian immunodeficiency virus (SIV), a clinically relevant preclinical model of HIV, increased SKM expression of pro-inflammatory cytokines and decreased antioxidant capacity.123,124 CBA produced dysregulation of epigenomic networks, including those of transcriptomic, DNA methylation, and miRNA implicated in ECM remodeling, pro-inflammatory milieu, protein homeostasis, calcium and ion homeostasis, neuromuscular junction signaling, and satellite cell growth and survival, providing evidence for mechanisms leading to CBA-mediated SKM loss at end-stage SIV infection.125 ECM remodeling was confirmed by increases in SKM hydroxy proline content and collagen expression, as well as by upregulation of expression of TGF-beta, tissue inhibitor of metalloproteinase (TIMP-1), and matrix metallopeptidase 2 and 9 (MMP2 and 9).126 CBA also activated UPP, increasing catabolic signaling.123
With effective ART regimens and controlled infection, the prevalence of overt SKM wasting in PWH has significantly decreased, especially during the asymptomatic stage of HIV disease. However, there are cellular and molecular changes that contribute to dysfunctional SKM mass. CBA administration decreased myoblast differentiation, myogenic gene expression, and SKM enriched miRs (myomiR) expression.127 Data also suggested that miR-206 targeted Class IIA histone deacetylase (HDAC4), and that an HDAC inhibitor could partially ameliorate CBA-mediated decrease in myoblast differentiation.128 Ongoing studies have explored the possibility of extracellular vesicles as mediators of intercellular communication contributing to decreased myoblast differentiation. Results showed significant alterations in expression of myomiRs in extracellular vesicles derived from myotubes formed from myoblasts isolated from CBA animals; however, no significant differences existed in extracellular vesicle size or concentration.129 CBA also dysregulated expression of genes implicated in mitochondrial homeostasis (e.g., peroxisome proliferator-activated receptor gamma, coactivator 1 beta [PGC-1b]; PPAR-alpha; estrogen-related receptor alpha; and superoxide dismutase) in SKM of ART-naïve SIV-infected male macaques at end-stage disease.130 In animals that were treated with ART and with controlled infection, CBA decreased succinate dehydrogenase activity (complex II of the electron transport chain) in type 1 and type 2 fibers, as well as myoblast maximal oxygen consumption rate. Results also indicated that formoterol, a beta-adrenergic agonist, increased myoblast PGC-1b expression and mitochondrial DNA, and improved maximal oxygen consumption rate,131 suggesting that exercise training or increased physical activity may help alleviate alcohol-related myopathy.
In a study among PWH with increased fasting plasma glucose but no diagnosed diabetes, negative indicators of myoblast bioenergetic health (proton leak, nonmitochondrial oxygen consumption rate, and bioenergetic health index) were higher among people with higher scores on the Alcohol Use Disorders Identification Test (AUDIT). This was also associated with increased mitochondrial volume and decreased expression of genes implicated in mitochondrial health.132 In the same cohort of PWH, circulating miR-206 was decreased in people with recent alcohol use as indicated by positive phosphatidylethanol (PEth).133 In another PWH cohort, body composition had significant modulatory effects on frailty, with higher fat-free mass index, body fat, and body mass index associated with decreased frailty risk. The study also indicated a negative association of frailty with fat-free mass index among people with detectable PEth, indicating that increased SKM mass is protective in PWH with alcohol use.134
SKM pain
SKM pain is associated with several comorbid conditions, including diabetes and HIV. A study in the United States that examined whether people wanted to drink more alcohol when they experienced SKM pain observed that men had a higher risk than women to self-medicate with alcohol.135 Preclinical evidence suggests that chronic alcohol administration in mice promotes SKM mechanical hyperalgesia, and that probiotics can significantly reduce this alcohol-induced SKM mechanical hyperalgesia.136 Moreover, in an SKM disuse atrophy model, chronic alcohol feeding of rats for 10 weeks produced mechanical hyperalgesia and associated pain-related neuroadaptations, indicating that chronic alcohol misuse could exacerbate complex pain regional syndrome.137
Cancer cachexia
The association among alcohol, muscle pain, and muscle functional mass also extends to other comorbidities. In mice injected with melanoma cells, those fed 20% alcohol (weight/volume) in water for 3 months had increased SKM inflammation, apoptosis, and protein degradation. There was also a significant decrease in satellite cell numbers and impaired myogenesis, indicating that alcohol exacerbated cancer-associated cachexia.138 In a Lewis lung carcinoma mouse model, chronic alcohol administration decreased expression of proteins in the mTORC1 pathway and increased expression of proteins of both UPP and autophagy, indicating a shift to increased SKM catabolism. Moreover, there was increased phosphorylation of Smad and extracellular signal-regulated kinase signaling proteins as well as increased expression of SKM and circulating myostatin, negative regulators of SKM mass.139 These studies suggest that alcohol use can exacerbate cancer-associated cachexia.
Disuse Atrophy and Injury
Another condition where alcohol can produce detrimental SKM changes is in response to disuse or injury. Alcohol misuse puts people at increased risk for injury and falls due to motor incoordination and peripheral neuropathies.140,141 In a model of barium chloride-induced injury of the tibialis anterior muscle, chronic alcohol exposure increased inflammation and fibrosis and decreased the cross-sectional area of regenerated muscle fibers.103 In a model of cryoinjury of tibialis anterior muscle, chronic alcohol administration increased inflammation, and low-level laser therapy decreased inflammation and improved recovery.142 In a model of SKM disuse, chronic alcohol feeding for 10 weeks in ovariectomized or intact female rats indicated that alcohol dysregulated genes implicated in regeneration and increased TGF-beta expression.134 In addition, primary macaque myoblasts isolated from in vivo CBA-administered macaques had decreased differentiation potential and concomitant decrease in myogenic gene expression,76 indicating that alcohol could affect SKM regenerative potential. In a model of disuse atrophy, repeated binge alcohol administration activated UPP and decreased protein synthesis.143 Based on evidence that alcohol can affect satellite cell regenerative function, exacerbate catabolic signaling, and curb protein synthesis, further studies on the effects of alcohol on muscle injury or disuse atrophy are warranted.
Alcohol, SKM, and Aging
Alcohol misuse is disproportionately on the rise among older individuals.20 Approximately 45% of current drinkers age 60 and older consume more than seven drinks per week, and 25% consume more than 14 drinks per week in the United States.144 Alcohol use among older individuals potentially decreases SKM mass and function,145 increasing the risk of morbidity.146–148 Thus, alcohol-related myopathy potentially can exacerbate age-related declines in SKM mass and function. However, there are few published studies on alcohol’s effects on SKM function in aging, and this warrants attention. Aged female F344 rats fed an alcohol diet for 20 weeks showed decreased lean mass and SKM protein synthesis with dysregulation of multiple proteins in the mTORC1 pathway but with no significant effects on catabolic pathways.149 In a large observational study of older males in Japan, alcohol misuse and liver fibrosis led to greater loss of SKM mass150 and increased intramuscular adipose tissue accumulation.151 A study among postmenopausal women found increased risk for sarcopenia among those with high-risk alcohol use.152 Similarly, in another large study among elderly Korean women, binge drinking once or more per week was associated with a higher risk for sarcopenia.152,153 However, there are some indications that alcohol consumption may not be associated with sarcopenia in older adults.154 The increased aging of the population and the rising frequency of alcohol use in both male and female aged individuals make this an important area in need of further investigation.
Summary and Recommendations for Future Work
As indicated in this review, although there is a large focus on the effects of both acute and chronic alcohol intake on the mTORC1 pathway and protein synthesis, gaps remain in the literature on alcohol’s effects on mitochondrial bioenergetics, protein degradation, and satellite cell function. Additionally, most of the published clinical and preclinical studies are either observational or descriptive, and future studies that are mechanistic and prove causality are highly warranted. This is particularly relevant with respect to acute effects of alcohol and in the context of exercise training, injuries, atrophy, and aging. There is also a gap in literature on additional alcohol-mediated mechanisms such as epigenomic alterations, role of senescence, effects of immune activation, and circadian signaling that can lead to impaired SKM mass and function. In addition, both clinical and preclinical studies on alcohol-mediated SKM effects in the presence of comorbidities are limited.
Few studies have identified whether the effects of alcohol are directly mediated or the result of alcohol metabolism and generation of metabolites such as acetaldehyde.37 Although there is evidence of alcohol directly affecting SKM function, especially in ex vivo systems, it is debatable whether significant alcohol metabolism occurs in SKM. It is possible that acetaldehyde can cause adverse effects in SKM. Similarly, the possibility that alcohol-associated SKM effects result from actions of soluble factors or extracellular vesicles released from distant organs, in a true interorgan communication, remains to be explored.155,156
Regarding published clinical studies on alcohol-related myopathy, the lack of objective measures of alcohol use, with few studies including biomarkers of alcohol use, confound the ability to draw conclusions and may explain the sometimes discrepant reports in the literature. Most clinical studies rely on self-report of alcohol use instead of validated questionnaires for assessing alcohol use, making it difficult to draw conclusions and comparisons across populations. With the mission of research and health care institutions, and the community at large, for rigor and reproducibility, it is recommended that peer-reviewed studies use standardized alcohol use questionnaires or biomarkers of alcohol use. For example, AUDIT, timeline follow-back, and lifetime drinking history are established self-report questionnaires that can be used, and PEth is a reliable biomarker of recent alcohol use.157,158
Abstinence is the most effective treatment for alcohol-related myopathy. Abstinence results in significant improvement in muscle strength,3,159 but fails to reach similar levels to those of age-matched controls. Reducing alcohol use is also a good strategy to improve functional SKM mass.3,159 An ideal strategy to improve SKM function in persons with alcohol misuse is to increase physical activity or structured physical exercise while reducing alcohol consumption. The efficacy of exercise-induced beneficial effects on SKM function in subjects with varying levels of alcohol use remains to be determined. Similarly, diet modifications also may help improve SKM function and remain to be systematically studied. Overall, elucidating specific mechanisms and increasing fundamental knowledge of alcohol-mediated effects on SKM can help design therapeutic targets to improve SKM health and overall quality of life.
Acknowledgments
The authors acknowledge research support provided by the National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health, under award numbers P60 AA009803 and F30 AA029358. All figures were generated using Biorender.com.
Footnotes
Disclosures
The authors declare no competing financial or nonfinancial interests.
Publisher’s Note
Opinions expressed in contributed articles do not necessarily reflect the views of the National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health. The U.S. government does not endorse or favor any specific commercial product or commodity. Any trade or proprietary names appearing in Alcohol Research: Current Reviews are used only because they are considered essential in the context of the studies reported herein.
References
- 1.GBD Alcohol and Drug Use Collaborators. The global burden of disease attributable to alcohol and drug use in 195 countries and territories, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Psychiatry. 2018;5(12):987–1012. doi: 10.1016/S2215-0366(18)30337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mellinger JL. Epidemiology of alcohol use and alcoholic liver disease. Clin Liver Dis (Hoboken) 2019;13(5):136–139. doi: 10.1002/cld.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Estruch R, Sacanella E, Fernández-Solá J, Nicolás JM, Rubin E, Urbano-Márquez A. Natural history of alcoholic myopathy: A 5-year study. Alcohol Clin Exp Res. 1998;22(9):2023–2028. doi: 10.1111/j.1530-0277.1998.tb05911.x. [DOI] [PubMed] [Google Scholar]
- 4.Jackson J. On a peculiar disease resulting from the use of ardent spirits. N Engl J Med. 1822;11:351–353. doi: 10.1056/NEJM182210010110402. [DOI] [Google Scholar]
- 5.Huss M. Alcoholismus Chronicus, or Chronic Alcohol Disease. Br Foreign Med Chir Rev. 1852;9(18):339–354. [PMC free article] [PubMed] [Google Scholar]
- 6.Hed R, Larsson H, Wahlgren F. Acute myoglobinuria; report of a case with fatal outcome. Acta Med Scand. 1955;152(6):459–463. doi: 10.1111/j.0954-6820.1955.tb03504.x. [DOI] [PubMed] [Google Scholar]
- 7.Hed R, Lundmark C, Fahlgren H, Orell S. Acute muscular syndrome in chronic alcoholism. Acta Med Scand. 1962;171(5):585–599. doi: 10.1111/j.0954-6820.1962.tb04224.x. [DOI] [PubMed] [Google Scholar]
- 8.Fahlgren H, Hed R, Lundmark C. Myonecrosis and myoglobinuria in alcohol and barbiturate intoxication. Acta Med Scand. 1957;158(6):405–412. doi: 10.1111/j.0954-6820.1957.tb15508.x. [DOI] [PubMed] [Google Scholar]
- 9.Bourgeois BL, Levitt DE, Molina PE, Simon L. Chronic alcohol and skeletal muscle. In: Patel VB, Preedy VR, editors. Handbook of Substance Misuse and Addictions. Springer; Cham: 2022. pp. 943–967. [Google Scholar]
- 10.Steiner JL, Lang CH. Dysregulation of skeletal muscle protein metabolism by alcohol. Am J Physiol Endocrinol Metab. 2015;308(9):E699–E712. doi: 10.1152/ajpendo.00006.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steiner JL, Gordon BS, Lang CH. Moderate alcohol consumption does not impair overload-induced muscle hypertrophy and protein synthesis. Physiol Rep. 2015;3(3) doi: 10.14814/phy2.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White PJ, St-Pierre P, Charbonneau A, et al. Protectin DX alleviates insulin resistance by activating a myokine-liver glucoregulatory axis. Nat Med. 2014;20(6):664–669. doi: 10.1038/nm.3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen W, Datzkiw D, Rudnicki MA. Satellite cells in ageing: Use it or lose it. Open Biol. 2020;10(5):200048. doi: 10.1098/rsob.200048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dumont NA, Bentzinger CF, Sincennes MC, Rudnicki MA. Satellite cells and skeletal muscle regeneration. Compr Physiol. 2015;5(3):1027–1059. doi: 10.1002/cphy.c140068. [DOI] [PubMed] [Google Scholar]
- 15.Levitt DE, Molina PE, Simon L. Pathophysiological mechanisms of alcoholic myopathy - Lessons from rodent models. J Vet Ani Sci. 2021;52(2):107–116. doi: 10.51966/jvas.2021.52.2.107-116. [DOI] [Google Scholar]
- 16.World Health Organization. Global Information System on Alcohol and Health. 2023. https://www.who.int/data/gho/data/themes/global-information-system-on-alcohol-and-health .
- 17.Preedy VR, Ohlendieck K, Adachi J, et al. The importance of alcohol-induced muscle disease. J Muscle Res Cell Motil. 2003;24(1):55–63. doi: 10.1023/a:1024842817060. [DOI] [PubMed] [Google Scholar]
- 18.Urbano-Márquez A, Estruch R, Fernandez-Solá J, Nicolás JM, Paré JC, Rubin E. The greater risk of alcoholic cardiomyopathy and myopathy in women compared with men. JAMA. 1995;274(2):149–154. doi: 10.1001/jama.1995.03530020067034. [DOI] [PubMed] [Google Scholar]
- 19.Keyes KM, Jager J, Mal-Sarkar T, Patrick ME, Rutherford C, Hasin D. Is there a recent epidemic of women’s drinking? A critical review of national studies. Alcohol Clin Exp Res. 2019;43(7):1344–1359. doi: 10.1111/acer.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keyes KM. Alcohol use in the older adult US population: Trends, causes, and consequences. Alcohol. 2023;107:28–31. doi: 10.1016/j.alcohol.2022.05.005. [DOI] [PubMed] [Google Scholar]
- 21.Baik D, Bird K. Dietary Lifestyle Changes. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK587401/ [PubMed] [Google Scholar]
- 22.World Health Organization. GHE: Life expectancy and healthy life expectancy. https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-life-expectancy-and-healthy-life-expectancy# .
- 23.National Institute on Alcohol Abuse and Alcoholism. Alcohol’s Effects on Health: Research-based information on drinking and its impact. No date. https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking .
- 24.Alcohol Research: Current Reviews Editorial Staff. Drinking Patterns and Their Definitions. Alcohol Res. 2018;39(1):17–18. [PMC free article] [PubMed] [Google Scholar]
- 25.Silberstein EB, Bove KE. Visualization of alcohol-induced rhabdomyolysis: A correlative radiotracer, histochemical, and electron-microscopic study. J Nucl Med. 1979;20(2):127–129. [PubMed] [Google Scholar]
- 26.Urbano-Márquez A, Fernandez-Solá J. Effects of alcohol on skeletal and cardiac muscle. Muscle Nerve. 2004;30(6):689–707. doi: 10.1002/mus.20168. [DOI] [PubMed] [Google Scholar]
- 27.Preedy VR, Adachi J, Ueno Y, et al. Alcoholic skeletal muscle myopathy: Definitions, features, contribution of neuropathy, impact and diagnosis. Eur J Neurol. 2001;8(6):677–687. doi: 10.1046/j.1468-1331.2001.00303.x. [DOI] [PubMed] [Google Scholar]
- 28.Ong JP, Thomas LA. Alcohol related non-traumatic rhabdomyolysis and compartment syndrome. Acute Med. 2007;6(1):33–34. [PubMed] [Google Scholar]
- 29.Kumar V, Frost RA, Lang CH. Alcohol impairs insulin and IGF-I stimulation of S6K1 but not 4E-BP1 in skeletal muscle. Am J Physiol Endocrinol Metab. 2002;283(5):E917–E928. doi: 10.1152/ajpendo.00181.2002. [DOI] [PubMed] [Google Scholar]
- 30.Koll M, Beeso JA, Kelly FJ, et al. Chronic alpha-tocopherol supplementation in rats does not ameliorate either chronic or acute alcohol-induced changes in muscle protein metabolism. Clin Sci (Lond) 2003;104(3):287–294. doi: 10.1042/CS20020312. [DOI] [PubMed] [Google Scholar]
- 31.Lang CH, Frost RA, Kumar V, Wu D, Vary TC. Impaired protein synthesis induced by acute alcohol intoxication is associated with changes in eIF4E in muscle and eIF2B in liver. Alcohol Clin Exp Res. 2000;24(3):322–331. doi: 10.1111/j.1530-0277.2000.tb04614.x. [DOI] [PubMed] [Google Scholar]
- 32.Lang CH, Frost RA, Vary TC. Acute alcohol intoxication increases REDD1 in skeletal muscle. Alcohol Clin Exp Res. 2008;32(5):796–805. doi: 10.1111/j.1530-0277.2008.00637.x. [DOI] [PubMed] [Google Scholar]
- 33.Sneddon AA, Koll M, Wallace MC, et al. Acute alcohol administration inhibits the refeeding response after starvation in rat skeletal muscle. Am J Physiol Endocrinol Metab. 2003;284(5):E874–E882. doi: 10.1152/ajpendo.00209.2002. [DOI] [PubMed] [Google Scholar]
- 34.Preedy VR, Peters TJ. Acute effects of ethanol on protein synthesis in different muscles and muscle protein fractions of the rat. Clin Sci (Lond) 1988;74(5):461–466. doi: 10.1042/cs0740461. [DOI] [PubMed] [Google Scholar]
- 35.Vary TC, Frost RA, Lang CH. Acute alcohol intoxication increases atrogin-1 and MuRF1 mRNA without increasing proteolysis in skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2008;294(6):R1777–R1789. doi: 10.1152/ajpregu.00056.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernández-Solá J, Sacanella E, Estruch R, Nicolás JM, Grau JM, Urbano-Márquez A. Significance of type II fiber atrophy in chronic alcoholic myopathy. J Neurol Sci. 1995;130(1):69–76. doi: 10.1016/0022-510x(95)00005-m. [DOI] [PubMed] [Google Scholar]
- 37.Thapaliya S, Runkana A, McMullen MR, et al. Alcohol-induced autophagy contributes to loss in skeletal muscle mass. Autophagy. 2014;10(4):677–690. doi: 10.4161/auto.27918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vural A, Attaway A, Welch N, Zein J, Dasarathy S. Skeletal muscle loss phenotype in cirrhosis: A nationwide analysis of hospitalized patients. Clinical Nutrition. 2020;39(12):3711–3720. doi: 10.1016/j.clnu.2020.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lang CH, Frost RA, Vary TC. Skeletal muscle protein synthesis and degradation exhibit sexual dimorphism after chronic alcohol consumption but not acute intoxication. Am J Physiol Endocrinol Metab. 2007;292(6):E1497–E506. doi: 10.1152/ajpendo.00603.2006. [DOI] [PubMed] [Google Scholar]
- 40.Rubin E, Katz AM, Lieber CS, Stein EP, Puszkin S. Muscle damage produced by chronic alcohol consumption. Am J Pathol. 1976;83(3):499–516. [PMC free article] [PubMed] [Google Scholar]
- 41.Pacy PJ, Preedy VR, Peters TJ, Read M, Halliday D. The effect of chronic alcohol ingestion on whole body and muscle protein synthesis — A stable isotope study. Alcohol Alcohol. 1991;26(5–6):505–513. doi: 10.1093/oxfordjournals.alcalc.a045152. [DOI] [PubMed] [Google Scholar]
- 42.Clary CR, Guidot DM, Bratina MA, Otis JS. Chronic alcohol ingestion exacerbates skeletal muscle myopathy in HIV-1 transgenic rats. AIDS Res Ther. 2011;8:30. doi: 10.1186/1742-6405-8-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koll M, Ahmed S, Mantle D, et al. Effect of acute and chronic alcohol treatment and their superimposition on lysosomal, cytoplasmic, and proteosomal protease activities in rat skeletal muscle in vivo. Metabolism. 2002;51(1):97–104. doi: 10.1053/meta.2002.28967. [DOI] [PubMed] [Google Scholar]
- 44.Cardellach F, Galofré J, Grau JM, et al. Oxidative metabolism in muscle mitochondria from patients with chronic alcoholism. Ann Neurol. 1992;31(5):515–518. doi: 10.1002/ana.410310509. [DOI] [PubMed] [Google Scholar]
- 45.Barnes MJ, Mundel T, Stannard SR. Acute alcohol consumption aggravates the decline in muscle performance following strenuous eccentric exercise. J Sci Med Sport. 2010;13(1):189–193. doi: 10.1016/j.jsams.2008.12.627. [DOI] [PubMed] [Google Scholar]
- 46.Barnes MJ, Mundel T, Stannard SR. A low dose of alcohol does not impact skeletal muscle performance after exercise-induced muscle damage. Eur J Appl Physiol. 2011;111(4):725–729. doi: 10.1007/s00421-010-1655-8. [DOI] [PubMed] [Google Scholar]
- 47.Kimball SR, Lang CH. Mechanisms underlying muscle protein imbalance induced by alcohol. Annu Rev Nutr. 2018;38:197–217. doi: 10.1146/annurev-nutr-071816-064642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hong-Brown LQ, Brown CR, Kazi AA, Navaratnarajah M, Lang CH. Rag GTPases and AMPK/TSC2/Rheb mediate the differential regulation of mTORC1 signaling in response to alcohol and leucine. Am J Physiol Cell Physiol. 2012;302(10):C1557–C1565. doi: 10.1152/ajpcell.00407.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steiner JL, Lang CH. Ethanol acutely antagonizes the refeeding-induced increase in mTOR-dependent protein synthesis and decrease in autophagy in skeletal muscle. Mol Cell Biochem. 2019;456(1–2):41–51. doi: 10.1007/s11010-018-3488-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Laufenberg LJ, Crowell KT, Lang CH. Alcohol acutely antagonizes refeeding-induced alterations in the Rag GTPase-Ragulator complex in skeletal muscle. Nutrients. 2021;13(4):1236. doi: 10.3390/nu13041236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steiner JL, Kimball SR, Lang CH. Acute alcohol-induced decrease in muscle protein synthesis in female mice is REDD-1 and mTOR-independent. Alcohol Alcohol. 2016;51(3):242–250. doi: 10.1093/alcalc/agv105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Steiner JL, Lang CH. Alcohol impairs skeletal muscle protein synthesis and mTOR signaling in a time-dependent manner following electrically stimulated muscle contraction. J Appl Physiol. 2014;117(10):1170–1179. doi: 10.1152/japplphysiol.00180.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duplanty AA, Budnar RG, Luk HY, et al. Effect of acute alcoholingestion on resistance exercise-induced mTORC1 signaling in human muscle. J Strength Cond Res. 2017;31(1):54–61. doi: 10.1519/JSC.0000000000001468. [DOI] [PubMed] [Google Scholar]
- 54.Parr EB, Camera DM, Areta JL, et al. Alcohol ingestion impairs maximal post-exercise rates of myofibrillar protein synthesis following a single bout of concurrent training. PLoS One. 2014;9(2):e88384. doi: 10.1371/journal.pone.0088384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haugvad A, Haugvad L, Hamarsland H, Paulsen G. Ethanol does not delay muscle recovery but decreases testosterone/cortisol ratio. Med Sci Sports Exerc. 2014;46(11):2175–2183. doi: 10.1249/MSS.0000000000000339. [DOI] [PubMed] [Google Scholar]
- 56.Vingren JL, Hill DW, Buddhadev H, Duplanty A. Postresistance exercise ethanol ingestion and acute testosterone bioavailability. Med Sci Sports Exerc. 2013;45(9):1825–1832. doi: 10.1249/MSS.0b013e31828d3767. [DOI] [PubMed] [Google Scholar]
- 57.Tice AL, Laudato JA, Fadool DA, Gordon BS, Steiner JL. Acute binge alcohol alters whole body metabolism and the time-dependent expression of skeletal muscle-specific metabolic markers for multiple days in mice. Am J Physiol Endocrinol Metab. 2022;323(3):E215–E230. doi: 10.1152/ajpendo.00026.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tice AL, Laudato JA, Rossetti ML, et al. Binge alcohol disrupts skeletal muscle core molecular clock independent of glucocorticoids. Am J Physiol Endocrinol Metab. 2021;321(5):E606–E620. doi: 10.1152/ajpendo.00187.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bailey SM. Emerging role of circadian clock disruption in alcohol-induced liver disease. Am J Physiol Gastrointest Liver Physiol. 2018;315(3):G364–G373. doi: 10.1152/ajpgi.00010.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Udoh US, Valcin JA, Gamble KL, Bailey SM. The molecular circadian clock and alcohol-induced liver injury. Biomolecules. 2015;5(4):2504–2537. doi: 10.3390/biom5042504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Davis BT, Voigt RM, Shaikh M, Forsyth CB, Keshavarzian A. Circadian mechanisms in alcohol use disorder and tissue injury. Alcohol Clin Exp Res. 2018;42(4):668–677. doi: 10.1111/acer.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Laudato JA, Tice AL, Call JA, Gordon BS, Steiner JL. Effects of alcohol on skeletal muscle contractile performance in male and female mice. PLoS One. 2021;16(8):e0255946. doi: 10.1371/journal.pone.0255946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Crowell KT, Laufenberg LJ, Lang CH. Chronic alcohol consumption, but not acute intoxication, decreases in vitro skeletal muscle contractile function. Alcohol Clin Exp Res. 2019;43(10):2090–2099. doi: 10.1111/acer.14179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shaw AG, Chae S, Levitt DE, Nicholson JL, Vingren JL, Hill DW. Effect of previous-day alcohol ingestion on muscle function and performance of severe-intensity exercise. Int J Sports PhysiolPerform. 2022;17(1):44–49. doi: 10.1123/ijspp.2020-0790. [DOI] [PubMed] [Google Scholar]
- 65.Prentice C, Stannard SR, Barnes MJ. Effects of heavy episodic drinking on physical performance in club level rugby union players. J Sci Med Sport. 2015;18(3):268–271. doi: 10.1016/j.jsams.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 66.Murphy AP, Snape AE, Minett GM, Skein M, Duffield R. The effect of post-match alcohol ingestion on recovery from competitive rugby league matches. J Strength Cond Res. 2013;27(5):1304–1312. doi: 10.1519/JSC.0b013e318267a5e9. [DOI] [PubMed] [Google Scholar]
- 67.Levitt DE, Luk HY, Duplanty AA, McFarlin BK, Hill DW, Vingren JL. Effect of alcohol after muscle-damaging resistance exercise on muscular performance recovery and inflammatory capacity in women. Eur J Appl Physiol. 2017;117(6):1195–1206. doi: 10.1007/s00421-017-3606-0. [DOI] [PubMed] [Google Scholar]
- 68.McLeay Y, Stannard SR, Mundel T, Foskett A, Barnes M. Effect of alcohol consumption on recovery from eccentric exercise induced muscle damage in females. Int J Sport Nutr Exerc Metab. 2017;27(2):115–121. doi: 10.1123/ijsnem.2016-0171. [DOI] [PubMed] [Google Scholar]
- 69.Williams T, Walz E, Lane AR, Pebole M, Hackney AC. The effect of estrogen on muscle damage biomarkers following prolonged aerobic exercise in eumenorrheic women. Biol Sport. 2015;32(3):193–198. doi: 10.5604/20831862.1150300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Minahan C, Joyce S, Bulmer AC, Cronin N, Sabapathy S. The influence of estradiol on muscle damage and leg strength after intense eccentric exercise. Eur J Appl Physiol. 2015;115(7):1493–1500. doi: 10.1007/s00421-015-3133-9. [DOI] [PubMed] [Google Scholar]
- 71.Levitt DE, Chalapati N, Prendergast MJ, Simon L, Molina PE. Ethanol-impaired myogenic differentiation is associated with decreased myoblast glycolytic function. Alcohol Clin Exp Res. 2020;44(11):2166–2176. doi: 10.1111/acer.14453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Koziris LP, Kraemer WJ, Gordon SE, Incledon T, Knuttgen HG. Effect of acute postexercise ethanol intoxication on the neuroendocrine response to resistance exercise. J Appl Physiol. 2000;88(1):165–172. doi: 10.1152/jappl.2000.88.1.165. [DOI] [PubMed] [Google Scholar]
- 73.Dumont NA, Rudnicki MA. Characterizing satellite cells and myogenic progenitors during skeletal muscle regeneration. In: Pellicciarii C, Biggiogera M, editors. Histochemistry of Single Molecules: Methods in Molecular Biology (MIMB) Vol. 1560. New York, NY: Humana Press; 2017. pp. 179–188. [DOI] [PubMed] [Google Scholar]
- 74.Levitt DE, Duplanty AA, Budnar RG, Jr, et al. The effect of post-resistance exercise alcohol ingestion on lipopolysaccharide-stimulated cytokines. Eur J Appl Physiol. 2016;116(2):311–318. doi: 10.1007/s00421-015-3278-6. [DOI] [PubMed] [Google Scholar]
- 75.Arya MA, Tai AK, Wooten EC, Parkin CD, Kudryavtseva E, Huggins GS. Notch pathway activation contributes to inhibition of C2C12 myoblast differentiation by ethanol. PLoS One. 2013;8(8):e71632. doi: 10.1371/journal.pone.0071632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Simon L, LeCapitaine N, Berner P, et al. Chronic binge alcohol consumption alters myogenic gene expression and reduces in vitro myogenic differentiation potential of myoblasts from rhesus macaques. Am J Physiol Regul Integr Comp Physiol. 2014;306(11):R837–R844. doi: 10.1152/ajpregu.00502.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Molina-Hidalgo C, De-la-O A, Jurado-Fasoli L, Amaro-Gahete FJ, Castillo MJ. Beer or ethanol effects on the body composition response to high-intensity interval training. The BEER-HIIT Study. Nutrients. 2019;11(4):909. doi: 10.3390/nu11040909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Molina-Hidalgo C, De-la-O A, Dote-Montero M, Amaro-Gahete FJ, Castillo MJ. Influence of daily beer or ethanol consumption on physical fitness in response to a high-intensity interval training program. The BEER-HIIT study. J Int Soc Sports Nutr. 2020;17(1):29. doi: 10.1186/s12970-020-00356-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.York JL, Hirsch JA, Pendergast DR, Glavy JS. Muscle performancein detoxified alcoholics. J Stud Alcohol. 1999;60(3):413–421. doi: 10.15288/jsa.1999.60.413. [DOI] [PubMed] [Google Scholar]
- 80.Gunther O, Roick C, Angermeyer MC, Konig HH. The EQ-5D in alcohol dependent patients: Relationships among health-related quality of life, psychopathology and social functioning. Drug Alcohol Depend. 2007;86(2–3):253–264. doi: 10.1016/j.drugalcdep.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 81.Gossop M, Neto D, Radovanovic M, et al. Physical health problemsamong patients seeking treatment for alcohol use disorders: A study in six European cities. Addict Biol. 2007;12(2):190–196. doi: 10.1111/j.1369-1600.2007.00066.x. [DOI] [PubMed] [Google Scholar]
- 82.Kim K, Kim JS. The association between alcohol consumption patterns and health-related quality of life in a nationally representative sample of South Korean adults. PLoS One. 2015;10(3):e0119245. doi: 10.1371/journal.pone.0119245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nemirovskaya TL, Shenkman BS, Zinovyeva OE, Kazantseva Iu V, Samkhaeva ND. [The development of clinical and morphological manifestations of chronic alcoholic myopathy in men with prolonged alcohol intoxication]. Fiziol Cheloveka. 2015;41(6):65–69. Russian. [PubMed] [Google Scholar]
- 84.Wen W, Sun C, Chen Z, et al. Alcohol induces zebrafish skeletal muscle atrophy through HMGB1/TLR4/NF-kappaB signaling. Life(Basel) 2022;12(8):1211. doi: 10.3390/life12081211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lang CH, Derdak Z, Wands JR. Strain-dependent differences for suppression of insulin-stimulated glucose uptake in skeletal and cardiac muscle by ethanol. Alcohol Clin Exp Res. 2014;38(4):897–910. doi: 10.1111/acer.12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.González-Reimers E, Fernández-Rodriguez CM, Santolaria-Fernández F, et al. Interleukin-15 and other myokines in chronic alcoholics. Alcohol Alcohol. 2011;46(5):529–533. doi: 10.1093/alcalc/agr064. [DOI] [PubMed] [Google Scholar]
- 87.Molina PE, Gardner JD, Souza-Smith FM, Whitaker AM. Alcohol abuse: Critical pathophysiological processes and contribution to disease burden. Physiology. 2014;29(3):203–215. doi: 10.1152/physiol.00055.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Durán Castellón MC, González-Reimers E, López-Lirola A, et al. Alcoholic myopathy: Lack of effect of zinc supplementation. Food Chem Toxicol. 2005;43(9):1333–1343. doi: 10.1016/j.fct.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 89.Kumar A, Davuluri G, Welch N, et al. Oxidative stress mediates ethanol-induced skeletal muscle mitochondrial dysfunction and dysregulated protein synthesis and autophagy. Free Radic Biol Med. 2019;145:284–299. doi: 10.1016/j.freeradbiomed.2019.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lang CH. Lack of sexual dimorphism on the inhibitory effect of alcohol on muscle protein synthesis in rats under basal conditions and after anabolic stimulation. Physiol Rep. 2018;6(23):e13929. doi: 10.14814/phy2.13929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Davuluri G, Welch N, Sekar J, et al. Activated protein phosphatase 2A disrupts nutrient sensing balance between mechanistic target of rapamycin complex 1 and adenosine monophosphate-activated protein kinase, causing sarcopenia in alcohol-associated liver disease. Hepatology. 2021;73(5):1892–1908. doi: 10.1002/hep.31524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kant S, Davuluri G, Alchirazi KA, et al. Ethanol sensitizes skeletal muscle to ammonia-induced molecular perturbations. J Biol Chem. 2019;294(18):7231–7244. doi: 10.1074/jbc.RA118.005411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Singh SS, Kumar A, Welch N, et al. Multiomics-identified intervention to restore ethanol-induced dysregulated proteostasis and secondary sarcopenia in alcoholic liver disease. Cell Physiol Biochem. 2021;55(1):91–116. doi: 10.33594/000000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shenkman BS, Zinovyeva OE, Belova SP, et al. Cellular and molecular signatures of alcohol-induced myopathy in women. Am J Physiol Endocrinol Metab. 2019;316(5):E967–E976. doi: 10.1152/ajpendo.00513.2018. [DOI] [PubMed] [Google Scholar]
- 95.Mirzoev TM, Lomonosova YN, Zinovyeva OE, Lysenko EA, Shenkman BS, Nemirovskaya TL. Signaling targets of alcoholic intoxication in human skeletal muscle. Dokl Biochem Biophys. 2016;470(1):329–331. doi: 10.1134/S1607672916050069. [DOI] [PubMed] [Google Scholar]
- 96.Shenkman BS, Belova SP, Zinovyeva OE, et al. Effect of chronic alcohol abuse on anabolic and catabolic signaling pathways in human skeletal muscle. Alcohol Clin Exp Res. 2018;42(1):41–52. doi: 10.1111/acer.13531. [DOI] [PubMed] [Google Scholar]
- 97.Steiner JL, Crowell KT, Lang CH. Impact of alcohol on glycemic control and insulin action. Biomolecules. 2015;5(4):2223–2246. doi: 10.3390/biom5042223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nguyen VA, Le T, Tong M, Silbermann E, Gundogan F, de la Monte SM. Impaired insulin/IGF signaling in experimental alcohol-related myopathy. Nutrients. 2012;4(8):1058–1075. doi: 10.3390/nu4081058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Koh JH, Kim KH, Park SY, Kim YW, Kim JY. PPARdelta attenuates alcohol-mediated insulin resistance by enhancing fatty acid-induced mitochondrial uncoupling and antioxidant defense in skeletal muscle. Front Physiol. 2020;11:749. doi: 10.3389/fphys.2020.00749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Elmadhun NY, Lassaletta AD, Chu LM, Bianchi C, Sellke FW. Vodka and wine consumption in a swine model of metabolic syndrome alters insulin signaling pathways in the liver and skeletal muscle. Surgery. 2012;152(3):414–422. doi: 10.1016/j.surg.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Reed CH, Buhr TJ, Tystahl AC, Bauer EE, Clark PJ, Valentine RJ. The effects of voluntary binge-patterned ethanol ingestion and daily wheel running on signaling of muscle protein synthesis and degradation in female mice. Alcohol. 2022;104:45–52. doi: 10.1016/j.alcohol.2022.06.004. [DOI] [PubMed] [Google Scholar]
- 102.No SH, Moon HW, Kim JS. Effect of chronic alcohol intake on the expression of muscle atrophy-related proteins in growing rats. J Exerc Rehabil. 2022;18(4):235–239. doi: 10.12965/jer.2244314.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dekeyser GJ, Clary CR, Otis JS. Chronic alcohol ingestion delays skeletal muscle regeneration following injury. Regen Med Res. 2013;1:2. doi: 10.1186/2050-490X-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Coelho MPP, Diniz KGD, Bering T, et al. Skeletal muscle massindex and phase angle are decreased in individuals with dependence on alcohol and other substances. Nutrition. 2020;71:110614. doi: 10.1016/j.nut.2019.110614. [DOI] [PubMed] [Google Scholar]
- 105.Kvist H, Hallgren P, Jönsson L, et al. Distribution of adipose tissue and muscle mass in alcoholic men. Metabolism. 1993;42(5):569–573. doi: 10.1016/0026-0495(93)90214-9. [DOI] [PubMed] [Google Scholar]
- 106.So E, Joung H. Alcohol consumption reduces the beneficial influence of protein intake on muscle mass in middle-aged Korean adults: A 12-year community-based prospective cohort study. Nutrients. 2019;11(9):2143. doi: 10.3390/nu11092143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Smiles WJ, Parr EB, Coffey VG, Lacham-Kaplan O, Hawley JA, Camera DM. Protein coingestion with alcohol following strenuous exercise attenuates alcohol-induced intramyocellular apoptosis and inhibition of autophagy. Am J Physiol Endocrinol Metab. 2016;311(5):E836–E849. doi: 10.1152/ajpendo.00303.2016. [DOI] [PubMed] [Google Scholar]
- 108.Gritsyna YV, Salmov NN, Bobylev AG, et al. Increased autolysis of μ-calpain in skeletal muscles of chronic alcohol-fed rats. Alcohol Clin Exp Res. 2017;41(10):1686–1694. doi: 10.1111/acer.13476. [DOI] [PubMed] [Google Scholar]
- 109.Khayrullin A, Smith L, Mistry D, Dukes A, Pan YA, Hamrick MW. Chronic alcohol exposure induces muscle atrophy (myopathy) in zebrafish and alters the expression of microRNAs targeting the Notch pathway in skeletal muscle. Biochem Biophys Res Commun. 2016;479(3):590–595. doi: 10.1016/j.bbrc.2016.09.117. [DOI] [PubMed] [Google Scholar]
- 110.Steiner JL, Pruznak AM, Navaratnarajah M, Lang CH. Alcohol differentially alters extracellular matrix and adhesion molecule expression in skeletal muscle and heart. Alcohol Clin Exp Res. 2015;39(8):1330–1340. doi: 10.1111/acer.12771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hong-Brown LQ, Brown CR, Navaratnarajah M, Lang CH. Adamts1 mediates ethanol-induced alterations in collagen and elastin via a FoxO1-sestrin3-AMPK signaling cascade in myocytes. J Cell Biochem. 2015;116(1):91–101. doi: 10.1002/jcb.24945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang J, Liu Y, Zhang L, et al. Effects of increased matrix metalloproteinase-9 expression on skeletal muscle fibrosis in prolonged alcoholic myopathies of rats. Mol Med Rep. 2012;5(1):60–65. doi: 10.3892/mmr.2011.592. [DOI] [PubMed] [Google Scholar]
- 113.Cui Y, Huang C, Momma H, Sugiyama S, Niu K, Nagatomi R. The longitudinal association between alcohol consumption and muscle strength: A population-based prospective study. J Musculoskelet Neuronal Interact. 2019;19(3):294–299. [PMC free article] [PubMed] [Google Scholar]
- 114.Zhai J, Ma B, Qin J, et al. Alcohol consumption patterns and the risk of sarcopenia: A population-based cross-sectional study among Chinese women and men from Henan province. BMC Public Health. 2022;22(1):1894. doi: 10.1186/s12889-022-14275-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Moser SE, Brown AM, Clark BC, Arnold WD, Baumann CW. Neuromuscular mechanisms of weakness in a mouse model of chronic alcoholic myopathy. Alcohol Clin Exp Res. 2022;46(9):1636–1647. doi: 10.1111/acer.14907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Noori HR, Mücksch C, Vengeliene V, et al. Alcohol reduces muscle fatigue through atomistic interactions with nicotinic receptors. Commun Biol. 2018;1:159. doi: 10.1038/s42003-018-0157-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Oh KH, Sheoran S, Richmond JE, Kim H. Alcohol induces mitochondrial fragmentation and stress responses to maintain normal muscle function in Caenorhabditis elegans. FASEB J. 2020;34(6):8204–8216. doi: 10.1096/fj.201903166R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Eisner V, Lenaers G, Hajnóczky G. Mitochondrial fusion is frequent in skeletal muscle and supports excitation-contraction coupling. J Cell Biol. 2014;205(2):179–195. doi: 10.1083/jcb.201312066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ismaeel A, Laudato JA, Fletcher E, et al. High-fat diet augments the effect of alcohol on skeletal muscle mitochondrial dysfunction in mice. Nutrients. 2022;14(5):1016. doi: 10.3390/nu14051016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Peng H, Qin X, Chen S, et al. Parkin deficiency accentuates chronic alcohol intake-induced tissue injury and autophagy defects in brain, liver and skeletal muscle. Acta Biochim Biophys Sin (Shanghai) 2020;52(6):665–674. doi: 10.1093/abbs/gmaa041. [DOI] [PubMed] [Google Scholar]
- 121.Alleyne J, Dopico AM. Alcohol use disorders and their harmful effects on the contractility of skeletal, cardiac and smooth muscles. Adv Drug Alcohol Res. 2021;1 doi: 10.3389/ADAR.2021.10011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wardell JD, Shuper PA, Rourke SB, Hendershot CS. Stigma, coping, and alcohol use severity among people living with HIV: A prospective analysis of bidirectional and mediated associations. Ann Behav Med. 2018;52(9):762–772. doi: 10.1093/abm/kax050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.LeCapitaine NJ, Wang ZQ, Dufour JP, et al. Disrupted anabolic and catabolic processes may contribute to alcohol-accentuated SAIDS-associated wasting. J Infect Dis. 2011;204(8):1246–1255. doi: 10.1093/infdis/jir508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Molina PE, Lang CH, McNurlan M, Bagby GJ, Nelson S. Chronic alcohol accentuates simian acquired immunodeficiency syndrome-associated wasting. Alcohol Clin Exp Res. 2008;32(1):138–147. doi: 10.1111/j.1530-0277.2007.00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Simon L, Hollenbach AD, Zabaleta J, Molina PE. Chronic binge alcohol administration dysregulates global regulatory gene networks associated with skeletal muscle wasting in simian immunodeficiency virus-infected macaques. BMC Genomics. 2015;16:1097. doi: 10.1186/s12864-015-2329-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dodd T, Simon L, LeCapitaine NJ, et al. Chronic binge alcohol administration accentuates expression of pro-fibrotic and inflammatory genes in the skeletal muscle of simian immunodeficiency virus-infected macaques. Alcohol Clin Exp Res. 2014;38(11):2697–2706. doi: 10.1111/acer.12545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Simon L, Ford SM, Jr, Song K, et al. Decreased myoblast differentiation in chronic binge alcohol-administered simian immunodeficiency virus-infected male macaques: Role of decreased miR-206. Am J Physiol Regul Integr Comp Physiol. 2017;313(3):R240–R250. doi: 10.1152/ajpregu.00146.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Adler K, Molina PE, Simon L. Epigenomic mechanisms of alcohol-induced impaired differentiation of skeletal muscle stem cells; role of Class IIA histone deacetylases. Physiol Genomics. 2019;51(9):471–479. doi: 10.1152/physiolgenomics.00043.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bourgeois BL, Levitt DE, Molina PE, Simon L. Differential expression of adipocyte and myotube extracellular vesicle miRNA cargo in chronic binge alcohol-administered SIV-infected male macaques. Alcohol. 2023;108:1–9. doi: 10.1016/j.alcohol.2022.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Duplanty AA, Simon L, Molina PE. Chronic binge alcohol-induced dysregulation of mitochondrial-related genes in skeletal muscle of simian immunodeficiency virus-infected rhesus macaques at end-stage disease. Alcohol Alcohol. 2017;52(3):298–304. doi: 10.1093/alcalc/agw107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Duplanty AA, Siggins RW, Allerton T, Simon L, Molina PE. Myoblast mitochondrial respiration is decreased in chronic binge alcohol administered simian immunodeficiency virus-infected antiretroviral-treated rhesus macaques. Physiol Rep. 2018;6(5):e13625. doi: 10.14814/phy2.13625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Levitt DE, Ferguson TF, Primeaux SD, et al. Skeletal muscle bioenergetic health and function in people living with HIV: Association with glucose tolerance and alcohol use. Am J PhysiolRegul Integr Comp Physiol. 2021;321(5):R781–R790. doi: 10.1152/ajpregu.00197.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bourgeois BL, Lin HY, Yeh AY, et al. Unique circulating microRNA associations with dysglycemia in people living with HIV and alcohol use. Physiol Genomics. 2022;54(1):36–44. doi: 10.1152/physiolgenomics.00085.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Levitt DE, Yeh AY, Prendergast MJ, et al. Chronic alcohol dysregulates skeletal muscle myogenic gene expression after hind limb immobilization in female rats. Biomolecules. 2020;10(3):441. doi: 10.3390/biom10030441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Stennett B, Anderson MB, Vitus D, et al. Sex moderates the effects of experimentally induced musculoskeletal pain on alcohol demand in healthy drinkers. Drug Alcohol Depend. 2021;219:108475. doi: 10.1016/j.drugalcdep.2020.108475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Green PG, Alvarez P, Levine JD. Probiotics attenuate alcohol-induced muscle mechanical hyperalgesia: Preliminary observations. Mol Pain. 2022;18:17448069221075345. doi: 10.1177/17448069221075345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cucinello-Ragland JA, Mitchell-Cleveland R, Bradley Trimble W, et al. Alcohol amplifies cingulate cortex signaling and facilitates immobilization-induced hyperalgesia in female rats. Neurosci Lett. 2021;761:136119. doi: 10.1016/j.neulet.2021.136119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang B, Zhang F, Zhang H, et al. Alcohol intake aggravates adipose browning and muscle atrophy in cancer-associated cachexia. Oncotarget. 2017;8(59):100411–100420. doi: 10.18632/oncotarget.22243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li Y, Zhang F, Modrak S, Little A, Zhang H. Chronic alcohol consumption enhances skeletal muscle wasting in mice bearing cachectic cancers: The role of TNFα/myostatin axis. Alcohol Clin Exp Res. 2020;44(1):66–77. doi: 10.1111/acer.14221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chen CM, Yoon Y-H. Usual alcohol consumption and risks for nonfatal fall injuries in the United States: Results from the 2004–2013 National Health Interview Survey. Subst Use Misuse. 2017;52(9):1120–1132. doi: 10.1080/10826084.2017.1293101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Julian T, Glascow N, Syeed R, Zis P. Alcohol-related peripheral neuropathy: A systematic review and meta-analysis. J Neurol. 2019;266(12):2907–2919. doi: 10.1007/s00415-018-9123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cespedes IC, Assis L, Thomaz RM, Panfilio CE, Goncalves L, Muniz ACR. Effects of low-level laser therapy on muscle repair in rats with chronic alcohol intake. Braz Arch Biol Techn. 2018:61. doi: 10.1590/1678-4324-2018161223. [DOI] [Google Scholar]
- 143.Vargas R, Lang CH. Alcohol accelerates loss of muscle and impairs recovery of muscle mass resulting from disuse atrophy. Alcohol Clin Exp Res. 2008;32(1):128–137. doi: 10.1111/j.1530-0277.2007.00548.x. [DOI] [PubMed] [Google Scholar]
- 144.Moos RH, Schutte KK, Brennan PL, Moos BS. Older adults’ alcohol consumption and late-life drinking problems: A 20-year perspective. Addiction. 2009;104(8):1293–1302. doi: 10.1111/j.1360-0443.2009.02604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Steffl M, Bohannon RW, Petr M, Kohlikova E, Holmerova I. Alcohol consumption as a risk factor for sarcopenia—a meta-analysis. BMC Geriatr. 2016;16:99. doi: 10.1186/s12877-016-0270-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li R, Xia J, Zhang XI, et al. Associations of muscle mass and strength with all-cause mortality among US older adults. Med Sci Sports Exerc. 2018;50(3):458–467. doi: 10.1249/MSS.0000000000001448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Volaklis KA, Halle M, Meisinger C. Muscular strength as a strong predictor of mortality: A narrative review. Eur J Intern Med. 2015;26(5):303–310. doi: 10.1016/j.ejim.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 148.Wang H, Hai S, Liu Y, Liu Y, Dong B. Skeletal muscle mass as a mortality predictor among nonagenarians and centenarians: A prospective cohort study. Sci Rep. 2019;9(1):2420. doi: 10.1038/s41598-019-38893-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Korzick DH, Sharda DR, Pruznak AM, Lang CH. Aging accentuates alcohol-induced decrease in protein synthesis in gastrocnemius. Am J Physiol Regul Integr Comp Physiol. 2013;304(10):R887–R898. doi: 10.1152/ajpregu.00083.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Onishi S, Miyai N, Zhang Y. Excessive alcohol intake and liver fibrosis are associated with skeletal muscle mass reduction in elderly men: The Wakayama study. Aging Clin Exp Res. 2022;34(1):185–192. doi: 10.1007/s40520-021-01902-2. [DOI] [PubMed] [Google Scholar]
- 151.Sjöholm K, Gripeteg L, Larsson I. Macronutrient and alcohol intake is associated with intermuscular adipose tissue in a randomly selected group of younger and older men and women. Clin Nutr ESPEN. 2016;13:e46–e51. doi: 10.1016/j.clnesp.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 152.Kwon YJ, Lim HJ, Lee YJ, et al. Associations between high-risk alcohol consumption and sarcopenia among postmenopausal women. Menopause. 2017;24(9):1022–1027. doi: 10.1097/GME.0000000000000879. [DOI] [PubMed] [Google Scholar]
- 153.Yoo JI, Ha YC, Lee YK, Hana C, Yoo MJ, Koo KH. High prevalence of sarcopenia among binge drinking elderly women: A nationwide population-based study. BMC Geriatr. 2017;17:114. doi: 10.1186/s12877-017-0507-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hong SH, Bae YJ. Association between alcohol consumption and the risk of sarcopenia: A systematic review and meta-analysis. Nutrients. 2022;14(16):3266. doi: 10.3390/nu14163266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dasarathy J, McCullough AJ, Dasarathy S. Sarcopenia in alcoholic liver disease: Clinical and molecular advances. Alcohol Clin Exp Res. 2017;41(8):1419–1431. doi: 10.1111/acer.13425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sato S, Namisaki T, Murata K, et al. The association between sarcopenia and endotoxin in patients with alcoholic cirrhosis. Medicine. 2021;100(36):e27212. doi: 10.1097/MD.0000000000027212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ferguson TF, Theall KP, Brashear M, et al. Comprehensive assessment of alcohol consumption in people living with HIV (PLWH): The New Orleans Alcohol Use in HIV Study. Alcohol Clin Exp Res. 2020;44(6):1261–1272. doi: 10.1111/acer.14336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring Alcohol Consumption. Totowa, NJ: Humana Press; 1992. pp. 41–72. [DOI] [Google Scholar]
- 159.Fernandez-Sola J, Nicolas JM, Sacanella E, et al. Low-dose ethanol consumption allows strength recovery in chronic alcoholic myopathy. QJM. 2000;93(1):35–40. doi: 10.1093/qjmed/93.1.35. [DOI] [PubMed] [Google Scholar]