A 31-year-old man with a history of primary sclerosing cholangitis (PSC) and ulcerative colitis (UC) presented to the outpatient hepatology clinic for evaluation of a dominant common bile duct (CBD) stricture in a background of PSC. He had been diagnosed with PSC 6 months prior based on elevated serum liver biochemistries, alkaline phosphatase and aminotransferases, and magnetic resonance cholangiopancreatography (MRCP) that showed multifocal biliary strictures, with a high-grade distal CBD stricture consistent with sclerosing cholangitis. He was asymptomatic at the time of diagnosis. An endoscopic retrograde cholangiopancreatography (ERCP) showed a dominant distal CBD stricture with marked proximal dilation. Biliary brushings sent for cytology showed “atypical cells suspicious for adenocarcinoma.” An endoscopic ultrasound (EUS) described a possible distal CBD lesion measuring 12×5 mm concerning cholangiocarcinoma. A pancreaticoduodenectomy was recommended. Before proceeding, he sought a second opinion.
He described feeling well without weight loss, abdominal pain, or pruritus. He had been diagnosed with UC 8 years prior. He was previously prescribed oral mesalamine, but he no longer required medical therapy. He had no family history of liver disease, inflammatory bowel disease, or colorectal cancer. He denied alcohol and drug use. His medications included rabeprazole 20 mg daily and ursodiol 300 mg twice daily.
On physical examination, he was well-appearing with anicteric sclerae. His abdomen was soft without hepatosplenomegaly. There was no evidence of jaundice, muscle wasting, or asterixis.
Laboratory data included aspartate aminotransaminase 54 U/L, alanine aminotransferase 88 U/L, total bilirubin 0.5 mg/dL, alkaline phosphatase 119 U/L (reference range 45–115 U/L), international normalized ratio 1.0, albumin 4.9 g/dL, carbohydrate antigen 19-9 (CA 19-9) 8 U/mL (reference range <35 U/mL), and IgG4 50.2 mg/dL.
Repeat MRCP again demonstrated the high-grade distal CBD stricture with proximal ductal dilation (Fig. 1A). There was a mild mural enhancement of the CBD in the area of the stricture but no distinct mass. Repeat EUS also failed to show evidence of a distinct mass. In addition to the distal CBD stricture and proximal CBD dilation, ERCP demonstrated a diffuse beaded appearance of intrahepatic bile ducts consistent with PSC (Fig. 1). Cholangioscopy showed mild mucosal irregularity in the distal CBD. Biliary brushings were obtained for cytology and fluorescent in situ hybridization (FISH). Directed biopsies were obtained for pathology. The biliary brushings showed ductal epithelial cells with mild atypia. The FISH was negative for aneuploidy. The biopsies showed epithelial cells without evidence of malignancy.
FIGURE 1.
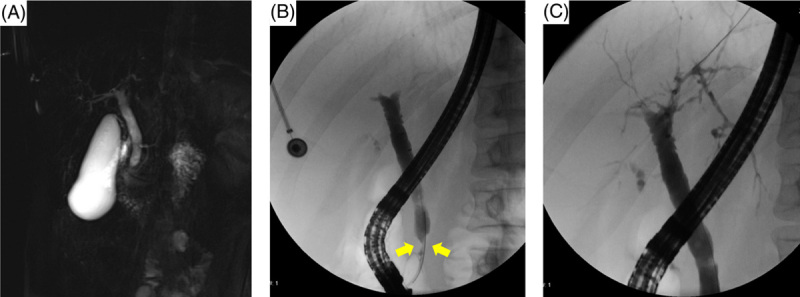
MRCP (A) and ERCP with extrahepatic (B) and intrahepatic (C) cholangiogram for evaluation of primary sclerosing cholangitis with a dominant stricture. There is irregular narrowing of intrahepatic bile ducts and dominant stricture of the distal CBD (yellow arrows). Abbreviation: CBD, common bile duct; ERCP, endoscopic retrograde cholangiopancreatography; MRCP, magnetic resonance cholangiopancreatography.
Three months later, he underwent repeat MRCP and EUS/ERCP with repeat biliary sampling. MRCP showed a stable high-grade distal CBD stricture with mild mural enhancement without discrete mass lesions. EUS demonstrated stable dilation of the CBD without evidence of a distinct mass. Periportal lymph nodes measuring up to 2.8 cm in maximum diameter sampled by fine needle aspiration showed benign findings of reactive hyperplasia. ERCP showed stable cholangiographic findings. Directed biopsies of the dominant distal CBD stricture showed no evidence of malignancy. Brush cytology showed mild atypia, and FISH was again negative for aneuploidy.
Surveillance for cholangiocarcinoma with CA 19-9 and MRCP was performed every 6 months in addition to serum liver biochemistries and assessment for new symptoms such as pruritus.
Two years after his initial consultation visit, he had an asymptomatic rise in his serum liver biochemistries prompting a repeat ERCP with biliary brushings again sent for cytology and FISH. Due to the challenge of diagnosing early cholangiocarcinoma and expanding the clinical accessibility of genetic testing for early cancer detection, a next-generation sequencing (NGS) assay called the SNaPshot was also performed. SNaPshot is an NGS assay developed at Massachusetts General Hospital, which assesses single-nucleotide variants and insertions/deletions in 104 known cancer genes. The brush cytology and FISH remained negative. However, NGS demonstrated genetic variants in KRAS and TP53 on 2 consecutive samples obtained 3 months apart. His case was reviewed in a multidisciplinary hepatopancreaticobiliary conference, and after discussions with the patient, the decision was made to proceed with surgical resection. The patient underwent an uncomplicated modified pancreaticoduodenectomy with cholecystectomy and periportal and retroperitoneal lymph node dissection. Surgical pathology showed multifocal low-grade dysplasia and one focus of high-grade dysplasia in the distal CBD stricture with superimposed inflammatory changes. No malignancy was found in the resected bile duct or in the 27 sampled lymph nodes. His postoperative recovery was unremarkable.
He continued surveillance for cholangiocarcinoma with CA 19-9 and MRCP every 6 months along with annual surveillance colonoscopy for colorectal cancer. He is doing well five years after surgery, with stable and mild elevation in liver biochemical tests and no evidence of malignancy.
DISCUSSION
Screening for cholangiocarcinoma is an essential part of the care of patients with PSC; patients with PSC are at an increased risk for the development of cholangiocarcinoma, with an annual risk of 2% and a 30-year cumulative incidence of ~20%.1 In the clinical spectrum of PSC, it is important to understand that some patients have a higher risk for cholangiocarcinoma. The presence of a dominant stricture (stenosis of the CBD to ≤1.5 mm or hepatic duct to ≤1 mm) and comorbid UC are associated with an increased risk of cholangiocarcinoma in patients with PSC.2,3 In contrast, patients with small duct PSC and young patients (<20 y) with PSC have been shown to be at a decreased risk for cholangiocarcinoma, and surveillance in this population may not be necessary.3–5
The development of cholangiocarcinoma in patients with PSC is associated with poor prognosis. However, surveillance for cholangiocarcinoma in patients with PSC has been associated with improved survival.6,7 In a group of highly selected candidates with early diagnosis undergoing liver transplantation, the 5-year survival was >70%.8 Nevertheless, the diagnosis of cholangiocarcinoma in patients with PSC, especially discerning benign from malignant strictures, remains a challenge due to the lack of sufficiently sensitive surveillance methods (Table 1).
TABLE 1.
Sensitivity and specificity of surveillance and diagnostic tools for cholangiocarcinoma in patients with PSC
| Test modality | Sensitivity, % | Specificity, % |
|---|---|---|
| Surveillance | ||
| CA 19-9 | Varies widely based on the cutoff value used | |
| MRCP | 89 | 75 |
| Diagnosis | ||
| Brush cytology | 18–40 | ~100 |
| FISH | 41 | 98 |
| NGS | Unknown | |
Abbreviations: FISH, fluorescent in situ hybridization; NGS, next-generation sequencing; MRCP, magnetic resonance cholangiopancreatography.
Although there is no universally agreed upon cholangiocarcinoma surveillance strategy in patients with PSC, multiple societies suggest CA 19-9 with MRCP every 6–12 months for patients with large duct PSC. Routine screening is not recommended for patients with small duct PSC, although occasional MRCP to assess for transition to large duct disease is indicated. ERCP to obtain biliary brushings for brush cytology and FISH is reserved for the evaluation of a dominant stricture, particularly if the dominant stricture is accompanied by symptoms and/or changes in serum liver biochemistries.5,9,10 CA 19-9 can be helpful as a biomarker but is of limited predictive value.10 Brush cytology has been shown to have limited sensitivity (18–40%) but excellent specificity (approaching 100%) for the diagnosis of cholangiocarcinoma.9,11 The addition of FISH improves diagnostic yield over conventional cytology and the presence of serial polysomy increases the positive predictive value.12–14 A positive FISH test is one of the criteria that qualifies patients for cholangiocarcinoma exception points per UNOS Guidelines. Despite the addition of FISH to the routine workup of a dominant stricture, current detection methods for cholangiocarcinoma have suboptimal sensitivity. A recent single-center study of multimodal testing in patients with PSC undergoing ERCP for the evaluation of biliary strictures suggests that applying NGS with assessment for relevant variants to biliary brushing samples may add sensitivity to brush cytology and FISH in cholangiocarcinoma surveillance as highlighted by this case.15 Our ability to detect early-stage cholangiocarcinoma or high-grade biliary dysplasia remains limited, and our need for better tools remains acute.
In conclusion, surveillance for cholangiocarcinoma in patients with large duct PSC with periodic MRCP and CA 19-9 is considered the standard of care. Patients with PSC who develop a dominant stricture should undergo ERCP with a sampling of the stricture utilizing cytology/pathology and FISH (Fig. 2). NGS may increase the sensitivity of biliary brushings for the diagnosis of cholangiocarcinoma and high-grade biliary dysplasia but requires additional study before it can be recommended for routine use alongside cytology and FISH. Early diagnosis of cholangiocarcinoma in these patients is critical as surgical resection and liver transplantation have the potential to improve prognosis.
FIGURE 2.
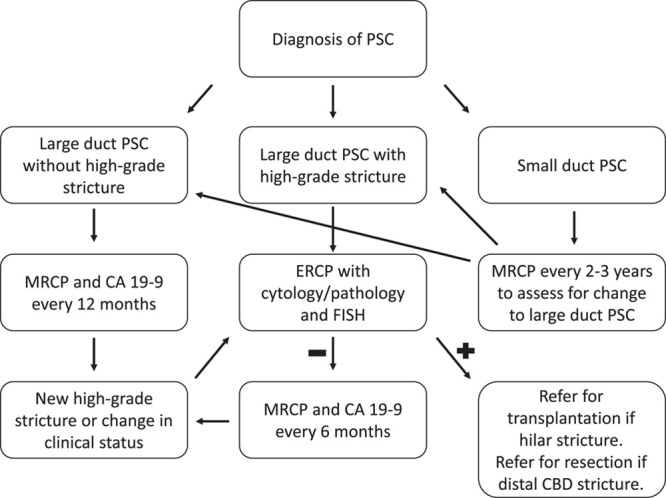
Cholangiocarcinoma surveillance in primary sclerosing cholangitis. Abbreviation: CBD, common bile duct; ERCP, endoscopic retrograde cholangiopancreatography; FISH, fluorescent in situ hybridization; MRCP, magnetic resonance cholangiopancreatography; PSC, primary sclerosing cholangitis.
KEY POINTS
Surveillance for cholangiocarcinoma in adult patients with large duct PSC should include imaging with MRCP and serum CA 19-9 every 6–12 months.
Patients with large duct PSC and a dominant stricture and those with co-existing UC are at the highest risk for cholangiocarcinoma.
Patients with small duct PSC and younger patients (<20 y) are at low risk for cholangiocarcinoma and may not warrant surveillance.
ERCP with tissue sampling for cytology/pathology and FISH should be used in the event of a new dominant stricture or change in clinical status.
The current diagnostic tools for cholangiocarcinoma in patients with PSC lack adequate sensitivity. NGS holds promise as a test that may improve the sensitivity of biliary brushings to diagnose cholangiocarcinoma and high-grade biliary dysplasia, but further testing is required.
Footnotes
Abbreviations: CBD, common bile duct; ERCP, endoscopic retrograde cholangiopancreatography; EUS, endoscopic ultrasound; FISH, fluorescent in situ hybridization; NGS, next-generation sequencing; MRCP, magnetic resonance cholangiopancreatography; PSC, primary sclerosing cholangitis; UC, ulcerative colitis.
Contributor Information
Eric M. Przybyszewski, Email: eprzybyszewski@mgh.harvard.edu.
Daniel S. Pratt, Email: DSPRATT@mgh.harvard.edu.
CONFLICTS OF INTEREST
The authors have no conflicts to report.
REFERENCES
- 1. Boonstra K, Weersma RK, van Erpecum KJ, Rauws EA, Spanier BWM, Poen AC, et al. Population-based epidemiology, malignancy risk, and outcome of primary sclerosing cholangitis. Hepatology. 2013;58:2045–2055. [DOI] [PubMed] [Google Scholar]
- 2. Chapman MH, Webster GJ, Bannoo S, Johnson GJ, Wittmann J, Pereira SP. Cholangiocarcinoma and dominant strictures in patients with primary sclerosing cholangitis: a 25-year single-centre experience. Eur J Gastroenterol Hepatol. 2012;24:1051–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weismüller TJ, Trivedi PJ, Bergquist A, Imam M, Lenzen H, Ponsioen CY, et al. Patient age, sex, and inflammatory bowel disease phenotype associate with course of primary sclerosing cholangitis. Gastroenterology. 2017;152:1975–1984.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Deneau MR, El‐Matary W, Valentino PL, Abdou R, Alqoaer K, Amin M, et al. The natural history of primary sclerosing cholangitis in 781 children: a multicenter, international collaboration. Hepatology. 2017;66:518–527. [DOI] [PubMed] [Google Scholar]
- 5. Bowlus CL, Lim JK, Lindor KD. AGA clinical practice update on surveillance for hepatobiliary cancers in patients with primary sclerosing cholangitis: Expert Review. Clin Gastroenterol Hepatol. 2019;17:2416–2422. [DOI] [PubMed] [Google Scholar]
- 6. Kaya M, de Groen PC, Angulo P, Nagorney DM, Gunderson LL, Gores GJ, et al. Lindor Treatment of cholangiocarcinoma complicating primary sclerosing cholangitis: the Mayo Clinic experience. Am J Gastroenterol. 2001;96:1164–1169. [DOI] [PubMed] [Google Scholar]
- 7. Ali AH, Tabibian JH, Nasser‐Ghodsi N, Lennon RJ, DeLeon T, Borad MJ, et al. Surveillance for hepatobiliary cancers in patients with primary sclerosing cholangitis. Hepatology. 2018;67:2338–2351. [DOI] [PubMed] [Google Scholar]
- 8. Rea DJ, Heimbach JK, Rosen CB, Haddock MG, Alberts SR, Kremers WK, et al. Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Ann Surg. 2005;242:451–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chapman R, Fevery J, Kalloo A, Nagorney DM, Boberg KM, Shneider B, et al. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51:660–678. [DOI] [PubMed] [Google Scholar]
- 10. Lindor KD, Kowdley KV, Harrison EM. ACG Clinical Guideline: Primary Sclerosing Cholangitis. Am J Gastroenterol. 2015;110:646–659. [DOI] [PubMed] [Google Scholar]
- 11. Charatcharoenwitthaya P, Enders FB, Halling KC, Lindor KD. Utility of serum tumor markers, imaging, and biliary cytology for detecting cholangiocarcinoma in primary sclerosing cholangitis. Hepatology. 2008;48:1106–1117. [DOI] [PubMed] [Google Scholar]
- 12. Bangarulingam SY, Bjornsson E, Enders F, Barr Fritcher EG, Gores G, Halling KC, et al. Long‐term outcomes of positive fluorescence in situ hybridization tests in primary sclerosing cholangitis. Hepatology. 2010;51:174–180. [DOI] [PubMed] [Google Scholar]
- 13. Fritcher EBG, Kipp BR, Voss JS, Clayton AC, Lindor KD, Halling KC, et al. Primary sclerosing cholangitis patients with serial polysomy fluorescence in situ hybridization results are at increased risk of cholangiocarcinoma. Am J Gastroenterol. 2011;106:2023–2028. [DOI] [PubMed] [Google Scholar]
- 14. Moreno Luna LE, Gores GJ. Advances in the diagnosis of cholangiocarcinoma in patients with primary sclerosing cholangitis. Liver Transpl. 2006;12(S2):S15–S19. [DOI] [PubMed] [Google Scholar]
- 15. Scheid JF, Rosenbaum MW, Przybyszewski EM, Krishnan K, Forcione DG, Iafrate AJ, et al. Next‐generation sequencing in the evaluation of biliary strictures in patients with primary sclerosing cholangitis. Cancer Cytopathol. 2022;130:215–230. [DOI] [PubMed] [Google Scholar]


