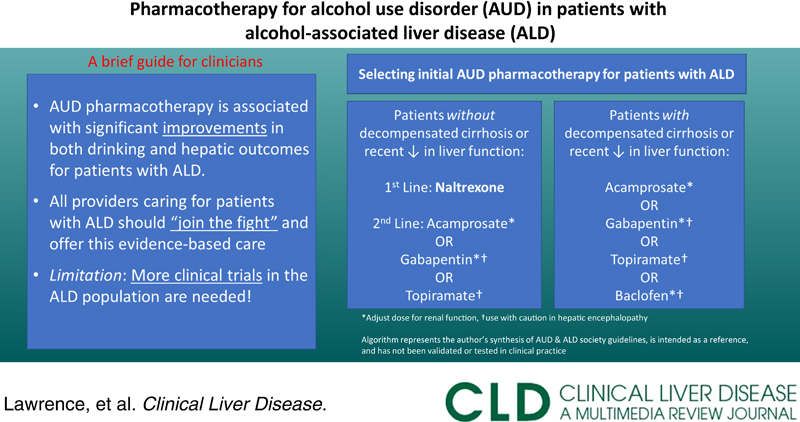
Quality care for patients living with alcohol-associated liver disease (ALD) includes identification and treatment of comorbid alcohol use disorder (AUD).1 In addition to recommending behavioral interventions, providers caring for this population should feel comfortable prescribing AUD pharmacotherapy, which is safe, effective, and associated with reduced hepatic decompensation and mortality.2–4 Many gastroenterologists, however, report discomfort prescribing these medications due to limited exposure in training as well as concerns about safety and efficacy.5 Although a multidisciplinary approach involving an addiction specialist is ideal, a timely referral is not always feasible. For clinicians seeking to incorporate this evidence-based care into their practice, we herein provide a brief review of the pharmacology, evidence, and key prescribing considerations for AUD pharmacotherapy in patients with ALD. Table 1 provides a summary of key prescribing considerations, and Figure 1 offers an approach for selecting an initial agent.
TABLE 1.
Summary of key prescribing considerations for alcohol use disorder pharmacotherapy
| Agent | Dosage | Efficacya | Considerations in liver disease | Special considerations, adverse effects (AE), and monitoring | FDA approved for AUD? |
|---|---|---|---|---|---|
| Acamprosate | Start at 666 mg TID; 999 mg BID is bioequivalent and simpler 6 | Significantly increased rates of abstinence vs. placebo 7–11
NNT to achieve additional cases of abstinence has been estimated between 7.511 and 9.097 |
No hepatic metabolism and considered safe in liver disease, although it has not been studied in CP C cirrhosis6
No known interaction with common antirejection medicines used after transplant12 |
Use 50% recommended dose in impaired renal function, avoid it completely if CrCl < 30 mL/min 6,13
GI side effects and anxiety are the most common AE7,9 Monitor renal function in elderly patients |
Yes |
| Naltrexone | Naltrexone hydrochloride: dose at 50 mg PO daily Naltrexone long-acting injection: dose at 380 mg IM q30 days |
Significant improvement in abstinence and reduction in heavy drinking, when combined with counseling 9,14,15
NNT to prevent a return to heavy drinking has been estimated at 8.611 |
Metabolism is altered in severe liver disease. Avoid in CP C cirrhosis16
Historic concern for hepatoxicity, although experience and data suggest this is rare17–19 No known interaction with common antirejection medicines used after transplant12 |
Caution with severe renal impairment. avoid initiating in patients who recently used opioids. Nausea, dizziness, vomiting, diarrhea, somnolence most common AEs15,17 IM only: injection site reactions, hematoma20 Check liver enzymes after 1 month of treatment and every 3 months thereafter. |
Yes |
| Baclofen | Titrate from 5 mg TID to 10 mg TID21 over 3 days | Improved abstinence rates among patients with cirrhosis22
Studies in the general population are less promising23,24 |
Minimal hepatic metabolism. Only AUD medication studied in patients with cirrhosis, although those with pre-existing HE were excluded.22 Avoid in HE. No known interaction with common antirejection medicines used after transplant12 |
Dose reduction by 33%, 50%, and 66% if CrCl 50–80 mL/min, 30–50 mL/min, and <30 mL/min, respectively25
Sedation, vertigo, and paresthesias are known adverse effects23 Routine monitoring of renal function |
No |
| Gabapentin | Start at 300 mg daily, increase based on response and tolerability by 300 mg every 1–2 days to 600 mg TID target dose 26,27 | Increases abstinence and reduces heavy drinking, particularly among patients with higher self-reported withdrawal symptoms. NNT to achieve a case of abstinence estimated to be 826,27 | No hepatic metabolism. Avoid in HE. No known interaction with common antirejection medicines used after transplant12 |
Dose reduction required in renal dysfunction: manufacturer recommends daily dose not exceed 1400 mg if CrCl <59, 700 if CrCl <29, and 300 if CrCl< 15. Somnolence, headache, muscle aches, and GI upset are the most common AEs28 Routine monitoring of renal function. |
No |
| Topiramate | Begin at 25 mg per day, increase in 25–50 mg increments to 150 mg BID over 8 weeks29 | Decreases drinks per day, decreases heavy drinking days, and increases percent days abstinent compared with placebo.29–31 | Partial hepatic metabolism. Consider 30% dose reduction in severe hepatic disease32
Avoid in HE. No known interaction with common antirejection medicines used after transplant12 |
Dose reduction of 50% recommended in renal impairment (CrCl <70) Cognitive impairment, paresthesia, nephrolithiasis, and anorexia are known AEs 33 Routine monitoring of renal function |
No |
Common clinically relevant efficacy metrics include rates of abstinence and heavy drinking. Study definitions for abstinence duration are provided when available in the text. Heavy drinking is typically defined as 4 or more drinks per day for women and 5 or more drinks per day for men.
Abbreviations: AUD, alcohol use disorder; CP C, Child-Pugh-C; CrCl, creatinine clearance; FDA, Food and Drug Administration; GI, gastrointestinal; IM, intramuscular; NNT, number needed to treat.
FIGURE 1.
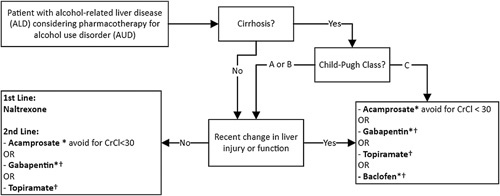
Approach to selecting initial alcohol use disorder pharmacotherapy option in alcohol-associated liver disease. This algorithm represents the authors best synthesis of AUD-focused and ALD-focused society guideline recommendations for AUD pharmacotherapy in the ALD population, it is intended as a reference only and has not been validated or tested in clinical practice. *Adjust dose for renal function. †Use with caution in HE. Abbreviation: CrCl, creatinine clearance.
FOOD AND DRUG ADMINISTRATION–APPROVED PHARMACOTHERAPY OPTIONS
Acamprosate
Acamprosate attenuates alcohol-associated elevations in glutaminergic activity through action on N-methyl-d-aspartate receptors.34 Its effectiveness in maintaining abstinence is well established.7–11 It is well tolerated, with gastrointestinal symptoms and anxiety being the most common side effects, but rare.7,9 Typically dosed at 666 mg 3 times a day, acamprosate dosed at 999 mg twice daily is bioequivalent and may improve adherence.6 The American Psychiatric Association (APA) and UK National Institute for Health and Care Excellence recommend it as a first-line option for AUD.13,35 The Veterans Health Administration (VA) recommends it as first line but qualifies the supporting evidence as weak.36 The World Federation of Societies of Biologic Psychiatry cites high-quality evidence regarding its use, although it cautions that some trials do not show the effect on relapse prevention.37 Acamprosate does not undergo hepatic metabolism and is likely safe in Child-Pugh A or B cirrhosis, although data regarding safety in liver disease is limited.34,38 The dose should be reduced for renal impairment and avoided if creatinine clearance (CrCl) is <30 mL/min.13 The American Association for the Study of Liver Diseases recommends consideration of acamprosate to treat AUD in ALD.1
Disulfiram
Although Food and Drug Administration has approved AUD since the 1950s, disulfiram is hepatically metabolized and associated with hepatotoxicity and liver failure.39 Given these risks and the availability of safer treatments for this population, disulfiram is not recommended for AUD in patients with ALD.1
Naltrexone
Naltrexone is an opioid receptor antagonist that decreases cravings through additional effects on the hypothalamic-pituitary axis.40 It improves rates of abstinence and reduces the risk of return to heavy drinking.9,14,15 In addition to gastrointestinal side effects, dizziness and somnolence have also been reported.15,17 Naltrexone has simple dosing, available as a 50-mg tablet taken once daily or a monthly 380 mg intramuscular injection. The APA, National Institute for Health and Care Excellence, and VA recommend naltrexone as the first line for AUD, and the WFSB characterizes evidence supporting its use as “abundant.”13,35–37 The US Substance Abuse and Mental Health Services Administration guidelines recommend several days of abstinence before initiating naltrexone.41 There are historical concerns for naltrexone-related hepatoxicity, and although liver function should be monitored frequently while on therapy, experience and data suggest that this is rare.17–19 Nonetheless, the metabolism of naltrexone is altered in liver disease, and it should be avoided in patients with recent changes in liver function and Child-Pugh-C cirrhosis, pending additional safety data.16 Naltrexone should not be initiated in patients recently taking opioids.
OFF-LABEL PHARMACOTHERAPY OPTIONS
Baclofen
Baclofen is a γ-aminobutyric acid (GABA)B agonist that suppresses dopamine release and attenuates alcohol-associated reward pathways.42 It is the only medication discussed that has been studied in cirrhosis. In a randomized controlled trial (RCT), 10 mg administered 3 times daily improved 12-week abstinence rates compared with placebo.22 Higher doses have been used for AUD in the general population, although lower doses (5–10 mg 3 times a day) may be more effective and better tolerated.21,43 Sedation is common, particularly if abstinence is not achieved before use. Up and down titration over several days is needed to ensure tolerability and prevent discontinuation reactions. It is not recommended in patients with HE, and dose reduction should be considered in patients with reduced renal function.21,43 Despite the favorable findings in cirrhosis, studies in the general population are less promising.23,24 Thus, the American Association for the Study of Liver Diseases recommends baclofen for the treatment of AUD in ALD, but it is not included in guidelines for the general population.1
Gabapentin
Gabapentin is a GABA analog that reduces excitatory neurotransmission and affects the downstream mediation of neurologic changes associated with withdrawal and abstinence in heavy drinkers.28 Compared with a placebo, gabapentin significantly increases 12-week abstinence rates and reduces heavy drinking.26 These effects are greatest among patients with alcohol withdrawal symptoms.27 Somnolence, dizziness, and headache are the known side effects.28 For AUD, gabapentin is initiated at 300 mg daily and increased in 300 mg increments, based on response and tolerability, every 1–2 days to a target dose of 600 mg 3 times daily.26,27 In cirrhosis, lower initial doses may be considered. The APA and VA recommend gabapentin as second line in AUD.13,36 Gabapentin can precipitate HE, and extra caution is advised for patients with decompensated cirrhosis.44 It also must be dose-reduced in kidney disease.
Topiramate
Topiramate likely improves AUD by dampening excitatory activity through the potentiation of GABA and inhibition of glutamate.29 Topiramate reduces heavy drinking days and increases percent days abstinent when compared with a placebo.29–31 Dosing requires slow escalation; trials typically start with 25 mg daily, increasing to a maximum of 300 mg per day in 2 divided doses over 8 weeks.29,31 Confusion, somnolence, paresthesias, and nephrolithiasis have been reported.29,31,33 Cognitive effects may limit tolerability and confound the clinical picture in patients with HE.12 There is minimal hepatic metabolism, but a dose reduction of 30% has been recommended for severe hepatic impairment.32 The manufacturer suggests halving the dose for CrCl <70. The VA and APA recommend it as a first-line and second-line option in the general population, respectively.13,36
CONCLUSIONS
Pharmacotherapy for AUD is safe and effective in improving drinking outcomes in the general population. For patients with concurrent ALD, however, evidence is mostly limited to retrospective data. More prospective studies involving chronic liver disease, including decompensated cirrhosis, are needed. Despite this, the ongoing risk alcohol use poses for negative outcomes should make barriers for accessing AUD pharmacotherapy low. Armed with the essential knowledge of how to prescribe these medications, those caring for patients with ALD should feel confident offering this evidence-based care.
Footnotes
Abbreviations: ALD, alcohol-associated liver disease; APA, American Psychiatric Association; AUD, alcohol use disorder; CP C, Child-Pugh-C; CrCl, creatinine clearance; FDA, Food and Drug Administration; GABA, γ-aminobutyric acid; GI, gastrointestinal; IM, intramuscular; VA, Veterans Health Administration.
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of UCLA, the Department of Veterans Affairs, or the US government.
Contributor Information
Christopher Coe, Email: ccoe@mednet.ucla.edu.
Arpan Patel, Email: arpanpatel@mednet.ucla.edu.
David Lawrence, Email: David.Lawrence3@va.gov.
AUTHOR CONTRIBUTIONS
All: study concept and design; Christopher Coe: drafting of manuscript; all: critical revision of manuscript; Arpan Patel and David Lawrence: study supervision.
CONFLICTS OF INTEREST
The authors have no conflicts to report.
EARN MOC FOR THIS ARTICLE
REFERENCES
- 1. Crabb DW, Im GY, Szabo G, Mellinger JL, Lucey MR. Diagnosis and treatment of alcohol-associated liver diseases: 2019 Practice Guidance From the American Association for the Study of Liver Diseases. Hepatology. 2020;71:306–33. [DOI] [PubMed] [Google Scholar]
- 2. Rogal S, Youk A, Zhang H, Gellad WF, Fine MJ, Good CB, et al. Impact of alcohol use disorder treatment on clinical outcomes among patients with cirrhosis. 2080. Hepatology. 2020;71:2080–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mellinger JL, Fernandez A, Shedden K, Winder GS, Fontana RJ, Volk ML, et al. Gender disparities in alcohol use disorder treatment among privately insured patients with alcohol-associated cirrhosis. Alcohol Clin Exp Res. 2019;43:334–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vannier AGL, Shay JES, Fomin V, Patel SJ, Schaefer E, Goodman RP, et al. Incidence and progression of alcohol-associated liver disease after medical therapy for alcohol use disorder. JAMA Netw Open. 2022;5:E2213014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Im GY, Mellinger JL, Winters A, Aby ES, Lominadze Z, Rice J, et al. Provider attitudes and practices for alcohol screening, treatment, and education in patients with liver disease: a survey from the American Association for the Study of Liver Diseases Alcohol-Associated Liver Disease Special Interest Group. Clin Gastroenterol Hepatol. 2021;19:2407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saivin S, Hulot T, Chabac S, Potgieter A, Durbin P, Houin G. Clinical pharmacokinetics of acamprosate. Clinical Pharmacokinetics. 1998;35:5. [DOI] [PubMed] [Google Scholar]
- 7. Rösner S, Hackl-Herrwerth A, Leucht S, Lehert P, Vecchi S, Soyka M. Acamprosate for alcohol dependence. Cochrane Database Syst Rev. 2010;9:CD004332. 10.1002/14651858.CD004332.pub2 [DOI] [PubMed] [Google Scholar]
- 8. Donoghue K, Elzerbi C, Saunders R, Whittington C, Pilling S, Drummond C. The efficacy of acamprosate and naltrexone in the treatment of alcohol dependence, Europe versus the rest of the world: ameta-analysis. Addiction. 2015;110:920–30. [DOI] [PubMed] [Google Scholar]
- 9. Jonas DE, Amick HR, Feltner C, Bobashev G, Thomas K, Wines R, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014;311:1889–900. [DOI] [PubMed] [Google Scholar]
- 10. Cheng HY, McGuinness LA, Elbers RG, MacArthur GJ, Taylor A, McAleenan A, et al. Treatment interventions to maintain abstinence from alcohol in primary care: systematic review and network meta-analysis. The BMJ. 2020;371:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maisel NC, Blodgett JC, Wilbourne PL, Humphreys K, Finney JW. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013. Feb;108:275–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arab JP, Izzy M, Leggio L, Bataller R, Shah VH. Management of alcohol use disorder in patients with cirrhosis in the setting of liver transplantation. Nat Rev Gastroenterol Hepatol. 2022;19:45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reus VI, Fochtmann LJ, Bukstein O, Eyler AE, Hilty DM, Horvitz-Lennon M, et al. The American psychiatric association practice guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018;175:86–90. [DOI] [PubMed] [Google Scholar]
- 14. Streeton C, Whelan G. Naltrexone, a relapse prevention maintenance treatment of alcohol dependence: a meta-analysis of randomized controlled trials. Alcohol Alcohol. 2001;36:544–52. [DOI] [PubMed] [Google Scholar]
- 15. Anton RF, O’malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, et al. Combined pharmacotherapies and bBehavioral interventions for alcohol dependence the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003–17. [DOI] [PubMed] [Google Scholar]
- 16. Bertolotti M, Ferrari A, Vitale G, Stefani M, Trenti T, Loria P, et al. Effect of liver cirrhosis on the systemic availability of naltrexone in humans. J Hepatol. 1991;21:505–11. [DOI] [PubMed] [Google Scholar]
- 17. Carmen B, Angeles M, Ana M, María AJ. Efficacy and safety of naltrexone and acamprosate in the treatment of alcohol dependence: a systematic review. 99 Addiction. 2004:811–28. [DOI] [PubMed] [Google Scholar]
- 18. Yen MH, Ko HC, Tang FI, Lu RB, Hong JS. Study of hepatotoxicity of naltrexone in the treatment of alcoholism. Alcohol. 2006;38:117–20. [DOI] [PubMed] [Google Scholar]
- 19. Ayyala D, Bottyan T, Tien C, Pimienta M, Yoo J, Stager K, et al. Naltrexone for alcohol use disorder: hepatic safety in patients with and without liver disease. Hepatol Commun. 2022;00:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ahamad K, Korthuis PT, Lum PJ, Johnson C, Wood E. A delayed injection-site reaction in a patient receiving extended-release naltrexone. Subst Abus. 2016;37:278–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Addolorato G, Caputo F, Capristo E, Domenicali M, Bernardi M, Janiri L, et al. Baclofen efficacy in reducing alcohol craving and intake: a preliminary double-blind randomized controlled study. Alchol Alcohol. 2002;37:504–8. [DOI] [PubMed] [Google Scholar]
- 22. Addolorato G, Leggio L, Ferrulli A, Cardone S, Vonghia L, Mirijello A, et al. Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol-dependent patients with liver cirrhosis: randomised, double-blind controlled study. Lancet. 2007;370:1915–22. [DOI] [PubMed] [Google Scholar]
- 23. Minozzi S, Saulle R, Rösner S. Baclofen for alcohol use disorder. Cochrane Database Syst Rev. 2018. 2018 11:CD012557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bschor T, Henssler J, Müller M, Baethge C. Baclofen for alcohol use disorder—a systematic meta-analysis. Acta Psychiatr Scand. 2018;138:232–42. [DOI] [PubMed] [Google Scholar]
- 25. Vlavonou R, Perreault MM, Barrière O, Shink E, Tremblay PO, Larouche R, et al. Pharmacokinetic characterization of baclofen in patients with chronic kidney disease: dose adjustment recommendations.. The J Clin Pharmacol. 2014;54:584–92. [DOI] [PubMed] [Google Scholar]
- 26. Mason BJ, Quello S, Goodell V, Shadan F, Kyle M, Begovic A. Gabapentin treatment for alcohol dependence: a randomized controlled trial. JAMA Intern Med. 2014;174:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Anton RF, Latham P, Voronin K, Book S, Hoffman M, Prisciandaro J, et al. Efficacy of gabapentin for the treatment of alcohol use disorder in patients with alcohol withdrawal symptoms: a randomized clinical trial. JAMA Intern Med. 2020;180:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opinon on Investigations Drugs. 2017;27:113–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Johnson BA, Ait-Daoud N, Bowden CL, DiClemente CC, Roache JD, Lawson K, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361:1677–85. [DOI] [PubMed] [Google Scholar]
- 30. Blodgett JC, Re AC, del, Maisel NC, Finney JW. A meta-analysis of topiramate’s effects for individuals with alcohol use disorders. Alcohol Clin Exp Res. 2014;38 16 1481–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Batki SL, Pennington DL, Lasher B, Neylan TC, Metzler T, Waldrop A, et al. Topiramate Treatment of Alcohol Use Disorder in Veterans with Posttraumatic Stress Disorder: A Randomized Controlled Pilot Trial. Alcohol Clin Exp Res. 2014;38:2169–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vidaurre J, Gedela S, Yarosz S. Antiepileptic drugs and liver disease. Pediatr Neurol. 2017;77:23–36. [DOI] [PubMed] [Google Scholar]
- 33. Shank RP, Maryanoff BE. Molecular pharmacodynamics, clinical therapeutics, and pharmacokinetics of topiramate. CNS Neurosci Ther. 2008;14:120–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kalk NJ, Lingford-Hughes AR. The clinical pharmacology of acamprosate. Br J Clin Pharmacol. 2014;77:315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. NICE: National Institute for Health and Care Excellence. Alcohol-use disorders: diagnosis, assessment and management of harmful drinking (high-risk drinking) and alcohol dependence clinical guideline. 2011.
- 36. US Department of Veterans Affairs: Management of Substance Use Disorders Work Group. . Veterans Affairs/Department of Defense Clinical Practice Guideline for the management of Substance use disorders. [Internet]. 2021. Accessed September 11, 2022. https://www.healthquality.va.gov/guidelines/MH/sud/VADoDSUDCPG.pdf
- 37. Soyka M, Kranzler HR, Hesselbrock V, Kasper S, Mutschler J, Möller HJ. Guidelines for biological treatment of substance use and related disorders, part 1: alcoholism, first revision. World J Biol Psychiatry. 201717;18:86–119. [DOI] [PubMed] [Google Scholar]
- 38. Tyson LD, Cheng A, Kelleher C, Strathie K, Lovendoski J, Habtemariam Z, et al. Acamprosate may be safer than baclofen for the treatment of alcohol use disorder in patients with cirrhosis: a first description of use in real-world clinical practice. Eur J Gastroenterol Hepatol. 2022;34:567–75. [DOI] [PubMed] [Google Scholar]
- 39. Chick J. Safety issues concerning the use of disulfiram in treating alcohol dependence. Drug Saf. 1999;20:427–35. [DOI] [PubMed] [Google Scholar]
- 40. Williams KL, Broadbear JH, Woods JH. Noncontingent and response-contingent intravenous ethanol attenuates the effect of naltrexone on hypothalamic-pituitary-adrenal activity in rhesus monkeys. Alcohol Clin Exp Res. 2004;28:566–71. [DOI] [PubMed] [Google Scholar]
- 41. Substance Abuse and Mental Health Services Administration. Medication for the treatment of alcohol use disorder: a brief guide. HHS Publication. 2015. Accessed September 11, 2022. http://store.samhsa.gov
- 42. Addolorato G, Leggio L. Safety and efficacy of baclofen in the treatment of alcohol-dependent patients. Curr Pharm Des. 2010;16:2113–7. [DOI] [PubMed] [Google Scholar]
- 43. Pierce M, Sutterland A, Beraha EM, Morley K, van den Brink W. Efficacy, tolerability, and safety of low-dose and high-dose baclofen in the treatment of alcohol dependence: a systematic review and meta-analysis. Eur Neuropsychopharmacol. 2018;28:795–806. [DOI] [PubMed] [Google Scholar]
- 44. Tapper EB, Henderson JB, Parikh ND, Ioannou GN, Lok AS. Incidence of and risk factors for hepatic encephalopathy in a population-based cohort of Americans with cirrhosis. Hepatol Commun. 2019;3:1510. [DOI] [PMC free article] [PubMed] [Google Scholar]


