HISTORY OF IMMUNOSUPPRESSION FOR LIVER TRANSPLANT
The first liver transplant was performed by Dr. Thomas E. Starzl in 1963, and the first patient to survive more than a year after the transplant received a combination of azathioprine, steroids, and antilymphocyte globulin. Infectious complications and rejections were common, and 1-year survival was <30%. Calcineurin inhibitors (CNIs), including cyclosporine and tacrolimus, were introduced in the 1980s and changed the landscape of liver transplant, with less rejection and a more favorable side effect profile, resulting in better overall outcomes.1
Management of immunosuppression (IS) is a balancing act between achieving tolerance and minimizing side effects and should be tailored to each patient’s risk of rejection and comorbidities. The goal of this review is to provide a summary of the most frequently used IS regimens, their mechanisms, and common side effects.
IS REGIMENS
Post-transplant IS can be looked at in 2 phases: induction and maintenance. The induction phase can further be subdivided into the acute perioperative management period (days 0–4) and the initial 3 months after transplant (Figure 1). The perioperative induction management varies based on transplant center practices. Some centers use IL-2 receptor antibodies (basiliximab) in the perioperative induction phase as part of a renal-sparing strategy to delay the initiation of CNI. Pulse dose corticosteroids are used in the perioperative induction phase and then tapered and typically weaned by 3 months post-transplant in the absence of rejection or a history of autoimmune hepatitis (Table 1).
FIGURE 1.
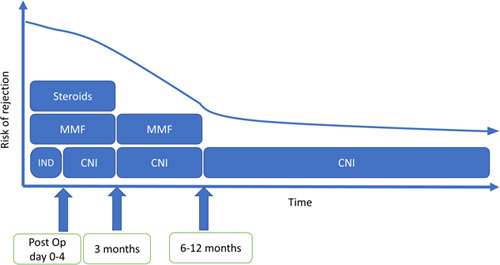
A sample renal-sparing medication regimen after liver transplant.
TABLE 1.
Most common immunosuppression medications, mechanism of action, and timing of use after liver transplant
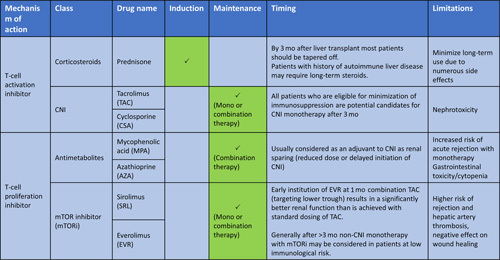
Abbreviations: AZA, azathioprine; CNI, calcineurin inhibitor; MPA, mycophenolic acid; mTORi, mammalian target of rapamycin inhibitors.
The maintenance phase can be subdivided into 3–6, 6–12, and >12 months post-transplant. As time from transplant increases, the risk of acute rejection decreases, and less IS is required to prevent acute cellular rejection.2 There are 3 main classes of medications that are most commonly used in this phase: (1) CNIs (such as tacrolimus and cyclosporine), (2) mammalian target of rapamycin inhibitors (mTORi) (such as sirolimus and everolimus), and (3) antimetabolites such as mycophenolic acid and azathioprine.
CNIs are the mainstay of maintenance phase, with tacrolimus being preferred over cyclosporine, as it is more effective in prevention of acute rejection and has superior patient and graft survival.2,3 During the first year post-transplant, the use of combination therapy is common, most often mycophenolic acid and CNI, to target lower trough levels and minimize renal toxicity caused by CNI. Generally, after 6–12 months, the majority of patients will transition to monotherapy (either CNI or less commonly mTORi). Tacrolimus trough level is followed closely to adjust the dose (with a higher trough goal in the first 3 months which is then decreased over the remainder of the year). In the absence of autoimmune hepatitis or a history of rejection, tacrolimus can be adjusted to achieve a trough level no >5 ng/mL after 12 months post-transplant.2 After 5 years, if there is good graft function (normal liver chemistries), trough levels just above the lower limit of detection are acceptable.2
Non-CNI monotherapy with mTORi can be considered in patients at low immunological risk and may be of interest in patients with a history of malignancy or those experiencing significant CNI-related side effects. Although used at many centers, sirolimus is not Food and Drug Administration (FDA) approved for liver transplant. Everolimus, which is a synthetic derivative of sirolimus, is FDA approved. In general, mTORi should be avoided in the first 30 days after transplant because of the increased risk for hepatic artery thrombosis and impaired wound healing. It has been proposed that in patients with HCC (within Milan criteria), mTORi-based regimens can achieve higher recurrence-free and overall survival, and can also reduce the risk of nonmelanoma skin cancer recurrence, renal cell cancer, and neuroendocrine tumors2; however, more data are needed to confirm this hypothesis.
COMMON ISSUES WITH IS
Drug-drug interactions
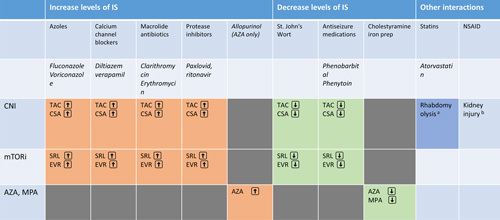
Both CNI and mTORi are metabolized by CYP-450 3A4 and therefore have interactions with many drugs. It is important to consider these interactions and adjust dosage accordingly, as completely avoiding drug-drug interaction is usually not possible. Common classes of drugs that inhibit CYP-450 3A4 include azoles, macrolides, protease inhibitors, and calcium-channel blockers (especially non-dihydropyridine) (Table 2). Of note, the recently approved antiviral Paxlovid for the treatment of COVID-19 contains protease inhibitor ritonavir, which is a potent CYP-450 3A4 inhibitor that leads to very high CNI levels and requires close monitoring.4
TABLE 2.
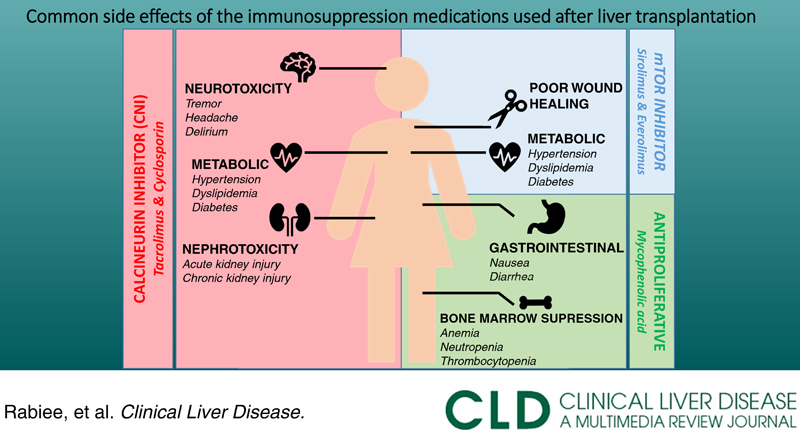
Most common side effects of immunosuppression medications used after liver transplant
Abbreviations: AKI, acute kidney injury; AZA, azathioprine; CKD, chronic kidney disease; CNI, calcineurin inhibitor; CSA, cyclosporine; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; MPA, mycophenolic acid; mTORi, mammalian target of rapamycin inhibitors; PRES, posterior reversible encephalopathy syndrome; TAC, tacrolimus.
Kidney injury
One of the major side effects of CNI is kidney injury. CNI can cause both acute and chronic nephrotoxicity (lasting >3 mo). Although acute nephrotoxicity is reversible and is caused by afferent arterial vasoconstriction, chronic nephrotoxicity, which is caused by interstitial fibrosis, is irreversible and can even lead to End Stage Renal Disease. The cumulative incidence of chronic stage 3 kidney disease ranges from 36% to 57% with CNI.5 The risk of kidney injury can be reduced with a lower dose and/or delayed initiation of CNI after transplant (use of renal-sparing regimens).
Metabolic syndrome
Metabolic syndrome is a major concern post-transplant and is becoming more relevant as more patients with NASH cirrhosis undergo liver transplant. Corticosteroids, CNI, and mTORi are associated with the development of metabolic syndrome (Table 3). Metabolic syndrome has been reported in >50% of patients 6 months after liver transplant and is associated with an increased risk of cardiovascular events.6,7 Metabolic syndrome includes new-onset diabetes (30%–40%), hypertension (60%–70%), dyslipidemia (50%–70%), and obesity.8 First-line treatment for hypertension includes calcium-channel blockers. In the treatment of dyslipidemia, and as statins interact with cyclosporine, switching to tacrolimus should be considered. Treatment of diabetes is based on endocrine guidelines, but the modification of IS regimen can also be considered.9
TABLE 3.
Most common drug-drug interactions with immunosuppression medications used after liver transplant
| Category | Side effects | Culprit Agents | Approach |
|---|---|---|---|
| Nephrotoxicity | AKI CKD |
CNI | Use of renal sparing regimen at time of transplant Addition of a antimetabolites. to allow a lower trough target |
| Cardiovascular and metabolic syndrome | Diabetes | CNI |TAC > CSA), steroids | Treat based on endocrine diabetes management guidelines |
| Hypertension | CNI |CSA>TACj, steroids | Treatment with nifedipine or earvedilol | |
| Hyperlipidemia | mTORi> CSA> TAC, steroids | mTORi dose-red jction or switching to alternative medication Use caution in using statins with CSA Pravastatin may be preferred in CSA |
|
| Bone marrow suppression | Leukopenia Neutropenia Anemia Thrombocytopenia |
Antiproliferative (MPA, AZA), mTORi | Switch to a different agent or dose reduction G-CSF/G M-C5F to treat neutropenia |
| Gastrointestinal symptoms | Nausea and vomiting, Diarrhea | Antiproliferative (MPA. AZA) | Need to rule out other causes, can consider switching to Myfortic (enteric coated) |
| Neurotoxicity | Tremors, headache, seizure, delirium, mood changes, PRE5 | CNI | Addition of Magnesium Switching to Envaraus for tremors [peak related) Can consider switching to cyclosporine from tacrolimus for PRES |
Abbreviations: AKI: Acute Kidney Injury; CKD: Chronic Kidney Disease; CNI: Calcineurin Inhibitor; TAC: Tacrolimus; CSA: Cyclosporine; MMF: Mycophenolic acid; AZA: Azathioprine; PRES: posterior reversible encephalopathy syndrome; mTORi: Mammalian Target of Rapamycin Inhibitors; G-C5F: Granulocyte colony-stimulating factor; GM-C5F: Granulacyte-macrophage colony-stimulating factor.
Bone marrow suppression
Antiproliferative agents, including mycophenolate, azathioprine, and mTORi, can cause bone marrow suppression and cytopenia. Initial treatment involves dose reduction of culprit medications (commonly IS and/or valganciclovir) and evaluation for cytomegalovirus. In post-transplant patients with severe neutropenia (absolute neutrophil count <500), a granulocyte colony-stimulating factor may be required to improve neutrophil counts and reduce infection risk.
Gastrointestinal symptoms
Antiproliferative agents can cause nausea, vomiting, and diarrhea. Initial management includes dose reduction where possible. Switching to mycophenolate sodium may improve symptoms as it is enteric coated.
Neurotoxicity
CNI can cause tremors, seizures, headaches, mood changes, altered mental status, and posterior reversible encephalopathy syndrome. Major neurotoxicities such as posterior reversible encephalopathy syndrome should be managed by conversion to a different IS (either within the same class or a different class).10 Less severe forms of neurotoxicity can be managed symptomatically. Hypomagnesemia caused by CNIs is a risk factor for worsening neurotoxicity, so magnesium supplements can be helpful.10 The use of long-acting tacrolimus (Envarsus XR) may reduce tremors, which are peak-related11; however, it should be noted that Envarsus XR is not FDA approved for liver transplant (Table 4).
TABLE 4.
Pharmacokinetics of commonly used immunosuppression medications after liver transplant
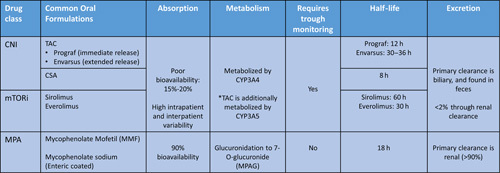
Abbreviations: CNI, calcineurin inhibitor; CSA, cyclosporine; MPA, mycophenolic acid; mTORi, mammalian target of rapamycin inhibitors; TAC, tacrolimus.
CONCLUSIONS
Innovation in IS over the past 80 years has led to a dramatic reduction in post-liver transplant mortality. This improved survival has shifted the focus of IS management away from rejection to minimization of IS-related morbidity and mortality. Modern IS management includes an individualized approach with the goal of exposing the patient to the minimum amount of IS required to maintain a healthy graft.
Footnotes
Abbreviations: AKI, acute kidney injury; AZA, azathioprine; CKD, chronic kidney disease; CNIs, calcineurin inhibitors; CSA, cyclosporine; FDA, Food and Drug Administration; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; MPA, mycophenolic acid; mTORi, mammalian target of rapamycin inhibitors; PRES, posterior reversible encephalopathy syndrome; TAC, tacrolimus.
Contributor Information
Anahita Rabiee, Email: anahita.rabiee@yale.edu.
Gianna Girone, Email: gianna.girone@ynhh.org.
Jessica P.E. Davis, Email: Jessica.Davis2@va.gov.
CONFLICTS OF INTEREST
The authors have no conflicts to report.
REFERENCES
- 1. Busuttil RW, Lake JR. Role of tacrolimus in the evolution of liver transplantation. Transplantation. 2004;77(suppl 9):S44–51. [DOI] [PubMed] [Google Scholar]
- 2. Charlton M, Levitsky J, Aqel B, O'Grady J, Hemibach J, Rinella M, et al. International Liver Transplantation Society Consensus Statement on immunosuppression in liver transplant recipients. Transplantation. 2018;102:727–43. [DOI] [PubMed] [Google Scholar]
- 3. Haddad EM, McAlister VC, Renouf E, Malthaner R, Kjaer MS, Gluud LL. Cyclosporin versus tacrolimus for liver transplanted patients. Cochrane Database Syst Rev. 2006;2006:Cd005161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lange NW, Salerno DM, Jennings DL, Choe J, Hedvat J, Kovac D, et al. Nirmatrelvir/ritonavir use: managing clinically significant drug-drug interactions with transplant immunosuppressants. Am J Transplant. 2022;22:1925–6. [DOI] [PubMed] [Google Scholar]
- 5. Moini M. Review on immunosuppression in liver transplantation. World J Hepatol. 2015;7:1355–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Watt KDS, Pedersen RA, Kremers WK, Heimbach JK, Charlton MR. Evolution of causes and risk factors for mortality post-liver transplant: results of the NIDDK long-term follow-up study. Am J Transplant. 2010;10:1420–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Laish I, Braun M, Mor E, Sulkes J, Harif Y, Ari ZB. Metabolic syndrome in liver transplant recipients: prevalence, risk factors, and association with cardiovascular events. Liver Transpl. 2011;17:15–22. [DOI] [PubMed] [Google Scholar]
- 8. Watt KDS, Charlton MR. Metabolic syndrome and liver transplantation: a review and guide to management. J Hepatol. 2010;53:199–206. [DOI] [PubMed] [Google Scholar]
- 9. Geissler EK, Schlitt HJ. Immunosuppression for liver transplantation. Gut. 2009;58:452–63. [DOI] [PubMed] [Google Scholar]
- 10. Anghel D, Tanasescu R, Campeanu A, Lupescu I, Podda G, Bajenaru O. Neurotoxicity of immunosuppressive therapies in organ transplantation. Maedica (Bucur). 2013;8:170–5. [PMC free article] [PubMed] [Google Scholar]
- 11. Langone A, Steinberg SM, Gedaly R, Chan LK, Shah T, Sethi KD, et al. Switching STudy of Kidney TRansplant PAtients with Tremor to LCP-TacrO (STRATO): an open-label, multicenter, prospective phase 3b study. Clin Transplant. 2015;29:796–805. [DOI] [PMC free article] [PubMed] [Google Scholar]


