Abstract
Viruses are ubiquitous components of marine ecosystems and are known to infect unicellular phycoerythrin-containing cyanobacteria belonging to the genus Synechococcus. A conserved region from the cyanophage genome was identified in three genetically distinct cyanomyoviruses, and a sequence analysis revealed that this region exhibited significant similarity to a gene encoding a capsid assembly protein (gp20) from the enteric coliphage T4. The results of a comparison of gene 20 sequences from three cyanomyoviruses and T4 allowed us to design two degenerate PCR primers, CPS1 and CPS2, which specifically amplified a 165-bp region from the majority of cyanomyoviruses tested. A competitive PCR (cPCR) analysis revealed that cyanomyovirus strains could be accurately enumerated, and it was demonstrated that quantification was log-linear over ca. 3 orders of magnitude. Different calibration curves were obtained for each of the three cyanomyovirus strains tested; consequently, cPCR performed with primers CPS1 and CPS2 could lead to substantial inaccuracies in estimates of phage abundance in natural assemblages. Further sequence analysis of cyanomyovirus gene 20 homologs would be necessary in order to design primers which do not exhibit phage-to-phage variability in priming efficiency. It was demonstrated that PCR products of the correct size could be amplified from seawater samples following 100× concentration and even directly without any prior concentration. Hence, the use of degenerate primers in PCR analyses of cyanophage populations should provide valuable data on the diversity of cyanophages in natural assemblages. Further optimization of procedures may ultimately lead to a sensitive assay which can be used to analyze natural cyanophage populations both quantitatively (by cPCR) and qualitatively following phylogenetic analysis of amplified products.
It is now well-established that viruses are ubiquitous in marine ecosystems, and viruses are increasingly recognized and accepted as important contributors to element cycling in the microbial loop (for reviews see references 6 and 47). Unicellular phycoeryrthrin-containing cyanobacteria belonging to the genus Synechococcus are abundant in the photic zone of the world’s oceans, and it has been thought that these organisms are responsible for up to 25% of the total oceanic primary productivity (55), although this figure is likely to be reduced following the discovery of the very closely related and abundant marine prochlorophytes (13, 39, 50). Cyanophages which infect Synechococcus strains are abundant in the marine environment and are thought to be a significant factor in determining the dynamics of Synechococcus spp. populations (48, 49, 54). However, some of the key questions regarding the ecological significance of cyanophages in the regulation of Synechococcus abundance and community structure remain unanswered.
The major problem in establishing the role of cyanophages in determining the dynamics of Synechococcus populations is the lack of suitable techniques for studying cyanophage communities. Traditional methods for estimating virus abundance and productivity have utilized techniques such as transmission electron microscopy (4, 40, 60), most-probable-number assays (54), radiotracer analysis (18, 44–46), inhibition experiments (5, 22), and measurements of dissolved esterase activity (8, 51). However, the main disadvantage of each of these methods is its lack of specificity. It is impossible to attribute specific roles to individual viruses if viruses are always studied as a diverse, mixed population in which different host-virus systems may interact completely differently within a given environment. In an attempt to study specific virus populations, Hennes et al. (24) successfully used fluorescently stained viruses as probes to detect specific bacteria and cyanobacteria in mixed microbial communities. These workers used the brightly fluorescent cyanine-based dyes YOYO-1 and POPO-1, which have very high binding coefficients for nucleic acids (25) and can be used to stain and visualize individual viruses by epifluorescence microscopy (23). Such techniques, however, are very time-consuming.
It is only recently that molecular techniques have been employed to investigate alga-virus populations (for a review see reference 59). Cottrell and Suttle (15) used DNA hybridization studies to establish the existence of genetically distinct viruses (MpV) which infect Micromonas pusilla and discovered a very diverse community of MpV. An alternative approach is to develop algal virus-specific PCR primers which recognize viral DNA sequences in order to investigate diversity. This approach has been successfully utilized to study marine microbial diversity (for a review see reference 20), primarily by using rRNAs as markers for species diversity. The virus genome does not contain rRNA sequences; therefore, it is necessary to identify other suitable sequences for this purpose. Chen and Suttle (10, 11) identified PCR primers based on B-family (α-like) DNA polymerase genes which could detect microalgal viruses. Algal virus-specific PCR primers were subsequently used to amplify DNA polymerase gene fragments from concentrated seawater obtained from the Gulf of Mexico (12), and again it was demonstrated that there was a diverse community of both MpV-like viruses and other unknown members of the Phycodnaviridae. In addition, Chen and Suttle (9) constructed a phylogenetic tree based on DNA polymerase gene fragments and determined that microalgal viruses are more closely related to each other than to other double-stranded DNA viruses and form a distinct phyletic group termed the Phycodnaviridae.
Using an alternative approach, Brautigam et al. (7) designed PCR primers based on a coat protein gene (30) of a virus (EsV) which infects the marine brown alga Ectocarpus siliculosus (Phaeophyceae). This set of primers was used to detect viral DNA in Ectocarpus siliculosus and investigate the life cycle of EsV during its propagation in the host. In a subsequent study Sengco et al. (43) used the same primers to detect the presence of viral DNA in extracts of unialgal Ectocarpus cultures obtained from the coasts of oceans around the world and found that at least 50% contained viral DNA.
In this study we used a different approach to design a set of PCR primers which specifically recognized sequences in DNA from cyanophages infecting phycoerythrin-containing marine Synechococcus strains. We used Southern hybridization analysis to attempt to identify a region of DNA which was common to all such cyanophages and which would provide the basis for the design of specific primers. It was hoped that such primers might permit the development of a quantitative assay for specific cyanophage DNA, which would facilitate analysis of the dynamics and diversity of natural cyanophage assemblages.
MATERIALS AND METHODS
Viruses, host strains, and media.
The three genetically distinct marine cyanophage isolates which lysed Synechococcus sp. strain WH7803 and were used for full sequence analysis of the bacteriophage T4 gene 20 homolog were isolated previously by plaque assay from coastal water collected off Plymouth, United Kingdom (strain S-PM2) (58), in Woods Hole harbor in Massachusetts (strain S-WHM1) (58), and in a bay adjacent to Raunefjorden, Norway, which is 20 km south of Bergen, Norway (strain S-BnM1) (56). The viruses used for the PCR specificity study are shown in Table 1. The marine cyanophage strains isolated during this study were isolated by plaque assay, as previously described (58), from water obtained from Hydrostation S (30°10′N, 64°30′W) in the Sargasso Sea (strains S-BM3 and S-BM6) and from water obtained off the west coast of Bermuda (strains S-BM4, S-BM5, S-BP1, S-BP2, and S-BP3). Different virus strains were kindly donated to us; marine cyanophage strains φ2, φ9, φ12, φ14, φ33, and φ34 were donated by John Waterbury, and strains S-PWM1 and S-PWM3 were donated by Curtis Suttle. Other bacteriophage and virus host systems used for PCR specificity studies included marine bacteriophage strains H2/1, H4/4, and H54/1 (from Karlheinz Moebus); marine algal virus strain PpV-01, which infected the haptophyte Phaeocystis pouchetii (from Anita Jacobsen); four uncharacterized P. pouchetii-specific viruses tentatively classified as strains PpV-02, PpV-04, PpV-05, and PpV-06 (from Kiezo Nagasaki); and freshwater cyanophage strain AN-15, which infected Anabaena sp. strain PCC7120 (from Dave Adams). The bacteriophage and virus lysates used for specificity studies included lysates of bacteriophage strain PW3a-P1, which infected Vibrio natriegens, and algal virus strain MpV-Sp1, which infected the photosynthetic flagellate M. pusilla (from Curtis Suttle). DNA extracted from coliphage strain T4 was purchased from Sigma.
TABLE 1.
Viruses used in the PCR specificity study, including cyanophages whose DNAs were amplified by cyanophage-specific PCR primers CPS1 and CPS2 and viruses whose DNAs were not amplified by CPS1 and CPS2
| Virus
|
Host(s) | Area of isolation | Reference or source | |
|---|---|---|---|---|
| Strain | Familya | |||
| Cyanophages amplified by primers CPS1 and CPS2 | ||||
| S-PM2 | M | WH7803, WH8012, WH8018 | Plymouth, United Kingdom | 58 |
| S-BnM1 | M | WH7803 | Bergen, Norway | 56 |
| S-WHM1 | M | WH7803, WH8012 | Woods Hole, Mass. | 58 |
| S-RSM1 | M | WH7803 | Red Sea, Eilat, Israel | 56 |
| S-RSM2 | M | WH7803 | Red Sea, Eilat, Israel | 56 |
| S-BM1 | M | WH7803, WH8012, WH8018 | Bermuda | 58 |
| S-BM3 | M | WH7803, WH8103 | Sargasso Sea | This study |
| S-BM4b | M | WH7803, WH8018 | Bermuda | This study |
| S-BM5b | M | WH8018 | Bermuda | This study |
| S-BM6 | M | WH7803 | Sargasso Sea | This study |
| S-MM1 | M | WH7803 | Miami, Fla. | 56 |
| S-MM2 | M | WH7803 | Miami, Fla. | 56 |
| S-MM3 | M | WH7803 | Miami, Fla. | 56 |
| S-MM4 | M | WH7803 | Miami, Fla. | 56 |
| S-MM5 | M | WH7803 | Miami, Fla. | 56 |
| S-MM7 | M | WH7803 | Miami, Fla. | 56 |
| φ2 | M | WH7803, WH8012, WH8018 | Sargasso Sea | 54 |
| φ9 | M | WH7803, WH8012, WH8018 | Woods Hole, Mass. | 54 |
| WH8103 | ||||
| φ14 | M | WH8103 | Gulf Stream | 54 |
| φ33 | M | WH7803 | Gulf Stream | 54 |
| φ34 | M | WH8103 | Gulf Stream | 54 |
| S-PWM1 | M | WH7803 | Gulf of Mexico | 49 |
| S-PWM3 | M | WH7803, SYN48, SNC2, SNC1c | Gulf of Mexico | 49 |
| Viruses not amplified by primers CPS1 and CPS2 | ||||
| S-BP1 | P | WH8018 | Bermuda | This study |
| S-BP2 | P | WH8018 | Bermuda | This study |
| S-BP3 | P | WH7803 | Bermuda | This study |
| φ12 | P | WH8018 | Gulf Stream | 54 |
| AN-15 | M | Anabaena sp. strain PCC7120 | Freshwater | 27 |
| PW3a-P1 | M | Vibrio natriegens | Gulf of Mexico | 49 |
| H2/1 | M | H2 | North Sea | 17 |
| H4/4 | P | H4 | North Sea | 17 |
| H54/1 | S | H54 | North Sea | 17 |
| PpV-01 | Ph | Phaeocystis pouchetii | Raunefjorden, Norway | 28 |
| PpV-02d | Ph | Phaeocystis pouchetii | Raunefjorden, Norway | |
| PpV-04d | Ph | Phaeocystis pouchetii | Raunefjorden, Norway | |
| PpV-05d | Ph | Phaeocystis pouchetii | Raunefjorden, Norway | |
| PpV-06d | Ph | Phaeocystis pouchetii | Raunefjorden, Norway | |
| MpV-Sp1 | Ph | Micromonas pusilla | Southern California | 16 |
| T4 | M | Escherichia coli | Sigma | |
M, Myoviridae; P, Podoviridae; S, Siphoviridae; Ph, Phycodnaviridae.
Larger products (length, ca. 800 bp) were amplified.
SYN48 and SNC2 are red phycoerythrin-containing strains of a marine Synechococcus species, and SNC1 is a green phycocyanin-containing strain of a marine Synechococcus species.
Unpublished results.
The main strain of Synechococcus sp. used to propagate cyanophages throughout this study, representing marine cluster A (53), was WH7803 (formerly designated DC2), which originated from the North Atlantic (33°45′N, 67°30′W) (55). The other Synechococcus sp. strains used for cyanophage isolation included WH8018, WH8012, and WH8103, which are also representatives of marine cluster A. Synechococcus sp. strains were routinely grown in 100-ml batch cultures in 250-ml conical flasks under constant illumination (5 to 36 microeinsteins m−2 s−1) at 25°C in artificial seawater (ASW) medium (57). Larger volumes were grown in 1-liter culture vessels to which 0.5 g of NaHCO3 liter−1 was added; these larger volumes were aerated with filtered air, stirred continuously, and maintained at 25°C in a water bath. The vessels were illuminated from above by warm-white fluorescent strip lights. P. pouchetii AJ01 was obtained from the culture collection of the University of Bergen, Bergen, Norway, and was maintained in 50-ml batch cultures at 5°C in f/2 medium (21) by using a cycle consisting of 16 h of light (ca. 36 microeinsteins of warm-white light m−2 s−1) and 8 h of darkness. Marine bacterial strains H2, H4, and H54 were kindly provided by Karlheinz Moebus, and each strain was grown in 75% (vol/vol) ASW medium enriched with 0.05% (wt/vol) Casamino Acids, 0.5% (wt/vol) peptone, and 0.1% (wt/vol) yeast extract (ESWIII); all three strains were grown at 25°C with vigorous stirring. Each strain was maintained on agar plates prepared by supplementing ESWIII with 1.5% (wt/vol) Bacto Agar (Difco).
Marine cyanophages isolated from water collected from Hydrostation S (Sargasso Sea) and from coastal water collected off Bermuda were assigned to a cyanophage family based on their morphological appearance following a transmission electron microscope analysis, as previously described (58). In addition, host ranges of newly isolated cyanophages were determined by a plaque assay and by lysis of liquid cultures in microtiter plates by using Synechococcus sp. strains WH7803, WH8012, WH8018, and WH8103 as host strains, as previously described (58).
Cloning and sequencing of cyanophage DNA.
Cyanophage DNA was extracted and purified from 50-ml liquid lysates by phenol extraction and alcohol precipitation, as previously described (58). The DNA was then subjected to restriction endonuclease digestion carried out under the conditions recommended by the manufacturers. DNA fragments were cloned into various restriction sites in the plasmid vector pUC19 (61), and plasmid DNA was prepared by alkaline lysis by using standard procedures, as described previously (33). A Southern analysis was conducted at different degrees of stringency; cross-hybridization to target DNA was detected and DNA probes were prepared by using a digoxigenin DNA labelling and detection kit (Boehringer Mannheim). Cloned cyanophage DNA fragments were excised and manually sequenced by the dideoxy chain terminator-M13 vector method (35, 42) by using a Sequenase version 2.0 DNA sequencing kit (United States Biochemical Corp.) and α-35S-labelled dATP (Amersham International plc). Potential open reading frames (ORFs) were identified from the resulting sequence data by using MicroGenie sequence analysis software (41) and were subsequently aligned with known genes obtained from the GenBank database by using Genetics Computer Group sequence analysis software (19).
PCR amplification and determination of primer specificity.
Either 1 μl of filtered (pore size, 0.2 μm) test virus lysate or 1 μl of a 1:10 dilution of test virus DNA was added to 49 μl of a PCR mixture (in a 0.65-ml silicon-treated test tube) which contained Taq DNA polymerase buffer (20 mM Tris-HCl [pH 8.4], 50 mM KCl), 10 mM MgCl2, each deoxynucleoside triphosphate at a concentration of 0.2 mM, 2 pmol of cyanophage-specific primer CPS1, and 2 pmol of cyanophage-specific primer CPS2 (Fig. 1); then the mixture was overlaid with 50 μl of paraffin oil. The sequences of the cyanophage-specific primers were based on sequence data obtained from cyanophage genes sequenced in this study (Fig. 1). The calculated melting temperatures for CPS1 (5′-GTAG[T/A]ATTTTCTACATTGA[C/T]GTTGG-3′) and CPS2 (5′-GGTA[G/A]CCAGAAATC[C/T]TC[C/A]AGCAT-3′) were 66 to 68 and 64 to 70°C, respectively. Negative controls contained all of the reagents except either virus lysate or virus DNA. PCR amplification was carried out with a DNA thermal cycler (Perkin-Elmer), and reactions were conducted by using the following conditions: there was a hot start at 94°C (5 min), and then the heating block was kept at 80°C until 1.25 U of Taq DNA polymerase had been added to each reaction mixture; this was followed by denaturation at 94°C (1 min), annealing at 55°C (1 min), and extension at 72°C (1 min) for 35 cycles of amplification. The PCR products (15 μl) were electrophoresed on a 1.2% (wt/vol) agarose gel in 1× TBE (89 mM Tris-HCl, 89 mM boric acid, 2 mM EDTA; pH 8.0) and were visualized by ethidium bromide staining.
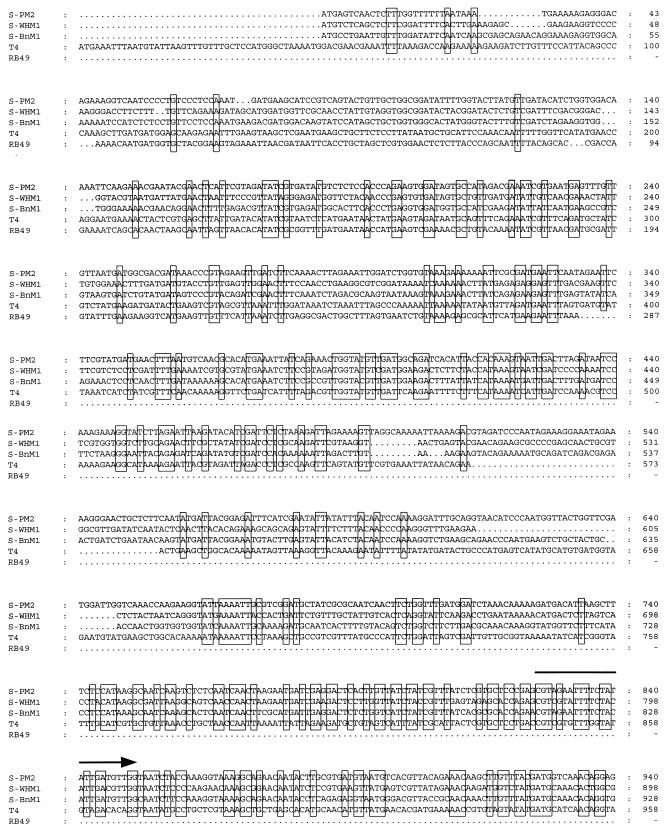
FIG. 1.
Alignment of the nucleic acid sequences of ORFs of three marine cyanomyovirus strains (S-PM2, S-WHM1, and S-BnM1; GenBank accession no. AFO16384, AFO16385, and AFO16386, respectively) with the sequence of coliphage T4 gene 20, which encodes a capsid assembly protein (34), and with partial sequence data for the same region of coliphage RB49 (37). Conserved bases are enclosed in boxes. The 25-mer forward primer CPS1 (5′-GTAG[T/A]ATTTTCTACATTGA[C/T]GTTGG-3′) and the 23-mer reverse primer CPS2 (5′-GGTA[G/A]CCAGAAATC[C/T]TC[C/A]AGCAT-3′) cyanophage-specific sequences are indicated by arrows.
Competitive PCR (cPCR).
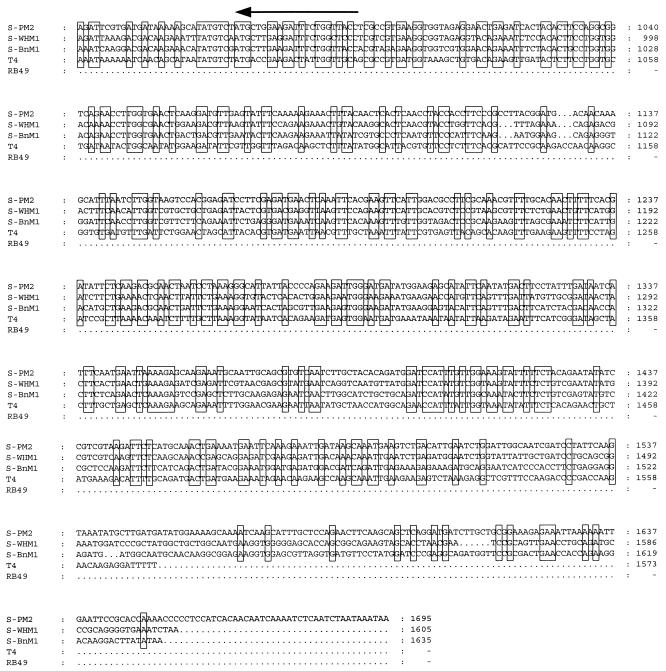
Competitor DNA was constructed by cloning a 193-bp SphI-PvuII fragment excised from the plasmid vector pUC19 (61) into the EcoNI site of a 1.5-kb PstI-EcoRI fragment isolated from cyanophage strain S-BnM1, which interrupted the region amplified by CPS1 and CPS2 (Fig. 2). The resulting 1.7-kb fragment was cloned into pUC19 to generate pNJF3. Following restriction by PstI and EcoRI, the 1.7-kb fragment was purified with a GeneClean II kit (Bio 101 Inc.) according to the manufacturer’s instructions, and this preparation was used as the competitor molecule, which gave a PCR amplification product of 358 bp.
FIG. 2.
Design of the internal standard for cPCR in which the cyanophage-specific primers CPS1 and CPS2 are used. A 1.5-kb PstI-EcoRI fragment, isolated from cyanophage S-BnM1, was cloned into pUC19 (pWHW04). A 193-bp SphI-PvuII fragment from pUC19 was subsequently cloned into an EcoNI site in pWHW04 to enlarge the region amplified by the primers.
Triplicate decimal dilutions of filtered (pore size, 0.2 μm) cyanophage lysates (diluted in ASW) were coamplified with 2 pg of competitor DNA under the reaction conditions described above. The resulting amplification products (target and competitor) were separated by electrophoresis on a 1.2% (wt/vol) agarose gel in 1× TBE. The products were then quantified by comparing the relative intensities of PCR products (visualized by ethidium bromide staining) obtained from the negative image of a Polaroid photograph by using a computing densitometer (Molecular Dynamics Ltd.) and Image Quant (version 3.3) software. Calibration curves were drawn by plotting log10 target product/competitor product relative intensities on the y axis against log10 total number of cyanophage particles on the x axis. The cyanophage numbers in each decimal dilution were determined by using the nucleic acid dye TOTO-1 iodide (Molecular Probes, Inc.); a 10-μl aliquot from each cyanophage decimal dilution was added to 1 ml of 50% (vol/vol) autoclaved ASW containing 0.5 μM TOTO-1 iodide and left overnight at room temperature in the dark. The stained cyanophages were filtered, with gentle suction, onto a 0.02-μm-pore-size Anodisc 25 filter (Whatman) with a 0.45-μm-pore-size cellulose nitrate backing membrane (Whatman). The cyanophages were visualized and enumerated at a magnification of ×1,000 under oil by using a Nikon Labophot-2A fluorescence microscope and filter block B-2A (excitation wavelength, 450 to 490 nm; 510-nm dichromic mirror; 520-nm barrier filter); cyanophages appeared as small, bright yellow dots against a black background.
To eliminate the possibility that unpackaged DNA was amplified during PCR (released following cyanophage lysis), cyanophage dilutions were pretreated with a solution containing 1 μg of DNase I (Sigma) per ml at room temperature for 30 min. Following the DNase treatment 1 μl of each DNase-treated lysate dilution was amplified as described above, except that the cyanophage-specific primers and competitor template DNA were not added until after the hot start; this step was necessary to denature any remaining DNase in the PCR mixture.
PCR amplification of natural samples.
Seawater was collected in 1-liter polyethylene bottles from coastal waters off Trinidad and Barbados in the Caribbean Sea during January 1997. Seawater samples were passed through 0.2-μm-pore-size filters to remove cellular material, and 10-ml aliquots of the filtrate were concentrated by ultracentrifugation at 114,000 × g for 1.5 h at 4°C with a type SW40Ti rotor in a Beckman preparative ultracentrifuge. The supernatant was carefully removed, and the cyanophage pellet was resuspended in 100 μl of ASW and stored at 4°C in the dark. A 1-μl aliquot of the virus concentrate was then amplified with primers CPS1 and CPS2 by using the conditions described above (no competitor DNA or DNase was used in the reaction mixture). In addition, 1- and 10-μl aliquots of unconcentrated filtered (pore size, 0.2 μm) seawater were amplified in 50- and 100-μl reaction mixtures (to dilute excess salts), respectively; the PCR components were altered accordingly in the 100-μl reaction mixtures to maintain the same overall reaction concentrations.
Nucleotide sequence accession numbers.
The GenBank nucleotide sequence accession numbers for the ORFs of cyanophage strains S-PM2, S-WHM1, and S-BnM1 sequenced are AFO16384, AFO16385, and AFO16386, respectively.
RESULTS
DNA sequencing and design of PCR primers.
In a previous study (58), it was established by Southern hybridization that there were a limited number of restriction fragments which cross-hybridized in DNA extracted from five cyanophages which infected the phycoerythrin-containing marine organism Synechococcus sp. strain WH7803. This analysis was extended by using eight marine cyanophage isolates (results not shown), and cross-hybridizing fragments were cloned from cyanophage strains S-PM2, S-WHM1, and S-BnM1. Complete double-stranded DNA sequence data were obtained for each of the fragments, which revealed that they all contained ORFs which encoded polypeptides with high degrees of similarity (Fig. 1). Database searches revealed that these conserved ORFs showed significant similarity to gene 20 from coliphage T4, which encodes a capsid assembly protein (T4 gp20) (34) (Fig. 1).
An alignment of the DNA sequences of T4 gene 20 and the putative cyanophage homologs revealed conserved regions in each of the cyanophage ORFs that were not conserved in the T4 gene (Fig. 1), and two degenerate cyanophage-specific primers were designed based on these regions; the forward 25-mer primer was designated CPS1 (5′-GTAG[T/A]ATTTTCTACATTGA[C/T]GTTGG-3′), and the reverse 23-mer primer was designated CPS2 (5′-GGTA[G/A]CCAGAAATC[C/T]TC[C/A]AGCAT-3′).
Specificity of PCR primers.
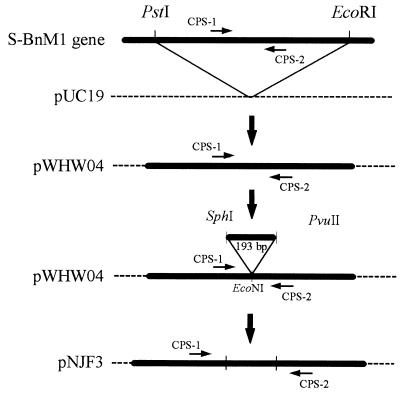
During this study, two cyanophage strains were isolated from water collected from Hydrostation S (Sargasso Sea); following a transmission electron microscopy analysis these strains were assigned to the family Myoviridae and were designated S-BM3 and S-BM6. Five cyanophages were isolated from coastal water collected off Bermuda; two of these cyanophages (S-BM4 and S-BM5) were assigned to the family Myoviridae, and three (S-BP1, S-BP2, and S-BP3) were assigned to the family Podoviridae (Table 1). To determine the specificity of the cyanophage-specific primers CPS1 and CPS2, PCR amplification was performed with a wide range of cyanophages isolated both during this study and from other sources, as well as with a range of other cyanophages, bacteriophages, and algal viruses (Table 1). It was established that CPS1 and CPS2 amplified only DNA from marine cyanophages belonging to the family Myoviridae (Cyanomyoviridae) (Table 1), and in most instances products that were 165 bp long were obtained (Fig. 3). Products that were ca. 800 bp long were obtained from cyanomyovirus strains S-BM4 and S-BM5. When the PCR annealing temperature was decreased to 50°C, more intense products that were ca. 800 bp long were amplified from S-BM4 and S-BM5, but a larger product that was ca. 2 kb long was obtained with coliphage T4 DNA when the same PCR conditions were used (results not shown).
FIG. 3.
Determination of the sensitivity of cyanophage-specific PCR primers CPS1 and CPS2. Serial dilutions of a filtered (pore size, 0.2 μm) cyanophage strain S-BnM1 lysate were enumerated by TOTO-1 iodide staining and were amplified in the absence of competitor DNA. Lanes 1 to 6, preparations containing ca. 1.9 × 106, 1.9 × 105, 1.9 × 104, 1.9 × 103, 190, and 19 cyanophage strain S-BnM1 particles per reaction mixture, respectively. A 15-μl aliquot of each PCR mixture was electrophoresed on a 1.2% agarose gel in 1× TBE buffer.
cPCR.
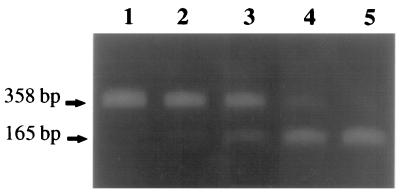
Coamplification of cyanophage strain S-BnM1 lysate and competitor DNA resulted in 165- and 358-bp amplification products, respectively (Fig. 4). The product intensities in the tracks varied depending on the number of cyanophages added to each PCR mixture. Cyanophage numbers as low as ca. 103 cyanophage particles per PCR mixture were detected in the presence of competitor (Fig. 4, lane 2); cyanophage numbers lower than 103 cyanophage particles per PCR mixture were not detected, and competitor DNA was preferentially amplified (Fig. 4, lane 1). cPCR was performed with cyanophage strains S-BnM1, S-MM5, and S-BM3 individually following DNase treatment to remove unpackaged virus DNA. The calibration curves for each cyanophage target DNA and competitor amplification products were subsequently plotted against total cyanophage numbers, and a different plot was obtained for each strain (Fig. 5). In the absence of competitor DNA, PCR products were obtained down to a concentration of ca. 190 cyanophage particles (as enumerated with TOTO-1 iodide) per reaction mixture following amplification of decimal dilutions of a filtered (pore size, 0.2 μm) cyanophage strain S-BnM1 lysate (the cyanophage titer prior to decimal dilution was 1.9 × 109 cyanophage particles ml−1) (Fig. 3).
FIG. 4.
cPCR of decimal dilutions of cyanophage strain S-BnM1 lysate, each coamplified with 2 pg of competitor DNA (Fig. 2) by using cyanophage-specific primers CPS1 and CPS2. Lanes 1 to 5, preparations containing ca. 190, 1.9 × 103, 1.9 × 104, 1.9 × 105, and 1.9 × 106 cyanophage strain S-BnM1 particles per reaction mixture, respectively. Cyanophage counts were determined by epifluorescence microscopy of lysate stained with the nucleic acid dye TOTO-1. A 15-μl aliquot of each PCR mixture was electrophoresed on a 1.2% agarose gel in 1× TBE buffer.
FIG. 5.
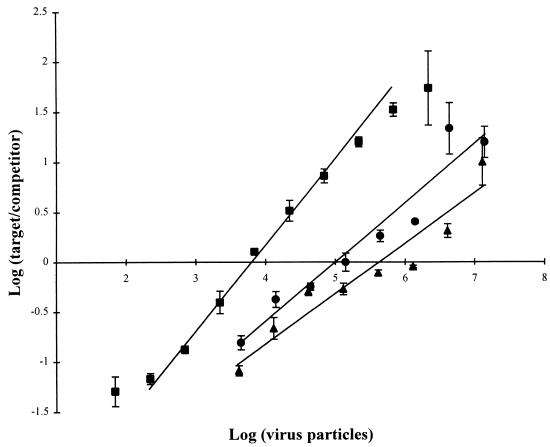
cPCR calibration curves for target cyanophage strains S-BnM1 (▪), S-MM5 (•), and S-BM3 (▴), obtained by using DNase-treated lysates. The data are plotted as log10 target product/competitor product relative intensities (estimated with a computer densitometer) (y axis) against log10 total number cyanophage particles enumerated by fluorescence microscopy with TOTO-1 iodide staining (x axis). Portions (2 pg) of competitor DNA (Fig. 2) were added to decimal dilutions of each cyanophage, and the DNAs were coamplified by using cyanophage-specific primers CPS1 and CPS2. Standard deviations of the means (n = 3) are indicated by error bars.
PCR amplification of natural samples.
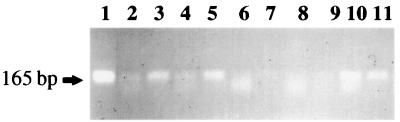
DNA from cyanophage communities collected from coastal seawater off Trinidad was amplified with primers CPS1 and CPS2 from 1- and 10-μl seawater aliquots added directly to a PCR mixture (Fig. 6, lanes 2 through 5). A clear band at 165 bp was observed in both filtered (pore size, 0.2 μm) and unfiltered seawater, although the yields of the products varied in the different seawater volumes amplified. No PCR products were obtained from coastal seawater collected off Barbados without prior cyanophage concentration (Fig. 6, lanes 6 through 9). Following ultracentrifugation of filtered (pore size, 0.2 μm) seawater collected off Barbados, clear bands at 165 bp were amplified from 1- and 10-μl aliquots of 100× concentrates added to reaction mixtures along with primers CPS1 and CPS2 (Fig. 6, lanes 10 and 11).
FIG. 6.
Analysis of fragments amplified by PCR from natural cyanophage communities collected from coastal seawater off Trinidad and Barbados by using primers CPS1 and CPS2. Lanes 2 through 5 contained amplified products from either 1-μl (lanes 2 and 4) or 10-μl (lanes 3 and 5) aliquots of unconcentrated Trinidad seawater added to PCR mixtures; seawater was both filtered (pore size, 0.2 μm) (lanes 2 and 3) and not filtered (lanes 4 and 5) prior to amplification. Lanes 6 through 9 contained amplified products from either 1-μl (lanes 6 and 8) or 10-μl (lanes 7 and 9) aliquots of unconcentrated Barbados seawater added to PCR mixtures; seawater was both filtered (pore size, 0.2 μm) (lanes 6 and 7) and not filtered (lanes 8 and 9) prior to amplification. Lanes 10 and 11 contained amplified products from 1- and 10-μl aliquots, respectively, of filtered (pore size, 0.2 μm) Barbados seawater concentrated 100× by ultracentrifugation. Lane 1 was a positive control lane containing amplified cyanophage strain S-BnM1 lysate added to filtered (pore size, 0.2 μm) Barbados water. A 15-μl aliquot of each PCR mixture was electrophoresed on a 1.2% agarose gel in 1× TBE buffer.
DISCUSSION
Strains belonging to the abundant picoplanktonic phycoerythrin-containing marine genus Synechococcus form a distinct group termed marine cluster A (53), which, on the basis of molecular evidence, appears to be a discrete phylogenetic group (38). Extensive morphological characterization of phages infecting this group of organisms identified them as members of the three families of tailed phages; however, the vast majority were contractile tailed phages belonging to the family Myoviridae (54). We report here the characterization of a set of homologous genes from myoviruses infecting members of the phycoerythrin-containing marine Synechococcus strains. Our results allowed us to develop PCR primers capable of specifically detecting these viruses in natural assemblages and should in the longer term facilitate analysis of the dynamics and diversity of viral populations in response to corresponding changes in host populations.
During a previous study it was demonstrated by Southern hybridization that when cyanomyovirus strain S-WHM1 DNA was used as a probe, at least two BamHI restriction fragments from other cyanomyoviruses contained homologous sequences (58). In the current study, this region was cloned from three cyanomyovirus strains isolated from different oceanographic provinces, the English Channel off Plymouth, United Kingdom (strain S-PM2; formerly misclassified as siphonovirus strain S-PS1), Woods Hole harbor in Massachusetts (strain S-WHM1), and Raunefjorden, Norway (strain S-BnM1). Sequence analysis of this region revealed that the DNA from the cyanophages encoded ORFs which had a high degree of homology to each other (Fig. 1) and encoded polypeptides that were approximately 550 amino acids long (Fig. 7). Database searches revealed that the polypeptides encoded by these ORFs exhibited ∼36% identity with gp20 of the well-characterized coliphage T4. T4 g20, an essential gene in head morphogenesis, encodes the minor capsid protein gp20 (34). The product of gene 20 in coliphage T4 is responsible for the initiation of head assembly by formation of the proximal vertex (52), for DNA packaging (26), and for binding with the head-tail junction (14). Thus, the cyanomyoviruses studied not only are similar morphologically to T4, but also contain a region of DNA encoding an apparently similar function.
FIG. 7.
Alignment of the predicted amino acid sequences encoded by ORFs of marine cyanomyovirus strains S-PM2, S-WHM1, and S-BnM1 (GenBank accession no. AFO16384, AFO16385, and AFO16386, respectively) with the sequence of the product of coliphage T4 gene 20, which encodes a capsid assembly protein (34), and with the predicted sequence of amino acids translated from partial sequence data for the same region of coliphage RB49 (37). Conserved residues are enclosed in boxes.
There have been numerous reports of the isolation of phages with morphologies similar to T4 morphology, and although many of these phages were isolated with Escherichia coli or closely related hosts, some were isolated with more distantly related hosts, such as Pseudomonas, Vibrio, and Aeromonas strains (2, 32). A recent molecular study (37) of phages with T-even morphology revealed a group designated the pseudo T-even phages, whose genomes contained only a limited region with homology to the T4 genome. This region was characterized in coliphage RB49, and it contains genes whose products are virion structural components, such as components of the phage base plate. Roughly at the center of this region was the homolog of gp20. Although only partial sequence information is available for the RB49 gene 20 homolog (accession no. Z78091), the predicted sequence of the polypeptide is similar to the sequences of T4 and cyanomyovirus gp20 homologs.
It has been proposed (37) that this region of the viral genome, which encodes virion structural components, may be a mobile genetic module that can be exchanged as a unit between different viral genomes. The conservation of the genes encoded by the module could reflect the fact that alteration is constrained by the requirement for the gene products to undergo multiple protein-protein interactions during phage morphogenesis. Consequently, it may be appropriate to consider the marine cyanomyoviruses members of the group containing the pseudo T-even phages and to assume that this region of the genome may be exchanged between different viral strains during coinfection of susceptible Synechococcus hosts. Indeed, genetic exchange with viruses infecting more distantly related hosts must have occurred in the past and presumably continues to take place in the marine environment.
The results of the comparison of the gene 20 sequences of the three cyanomyoviruses and T4 allowed us to design two degenerate PCR primers which should specifically amplify a 165-bp region from the cyanomyoviruses. The results show that at an annealing temperature of 55°C the CPS1-CPS2 primer pair yielded a PCR product of the expected size from the majority of marine cyanomyovirus isolates but did not amplify DNA from marine cyanopodovirus isolates (Table 1). However, under the same PCR conditions larger products (length, ca. 800 bp) were obtained from cyanomyovirus strains S-BM4 and S-BM5. When the PCR annealing temperature was decreased to 50°C, more intense products that were ca. 800 bp long were amplified from these strains, but a larger ca. 2-kb product was obtained with coliphage T4 DNA (results not shown). Therefore, to maintain the ability of the PCR to specifically detect marine cyanomyoviruses, an annealing temperature of 55°C was adopted as the standard annealing temperature.
At an annealing temperature of 55°C, no products were amplified from morphologically similar phages belonging to the family Myoviridae infecting other hosts, including phages isolated from marine heterotrophic bacteria (strains H2/1 and PW3a-P1) and a freshwater cyanobacterium (strain AN-15) (Table 1); thus, primers CPS1 and CPS2 do appear to be specific for marine cyanomyoviruses. This is not surprising, since a recent survey of protein sequence identities revealed that the levels of sequence similarity between tailed phages are generally low, indicating that there has been extensive diversification (1). It was highly unlikely, therefore, that DNA from other unrelated phages and viruses would be amplified following PCR with primers CPS1 and CPS2.
Lysis of a phage-infected host leads to the release of mature phage particles. However, in addition, unpackaged phage DNA may be released, and this DNA can act as a target for PCR and is amplified just as efficiently as the DNA packaged within the phage capsid. In the marine environment dissolved DNA concentrations range from 1 to 15 μg liter−1 in coastal and open ocean oligotrophic surface waters (3). Approximately 50% of the DNA from the marine environment that can be filtered with a 0.2-μm-pore-size filter has been shown to be truly soluble or free (cf. bound DNA in viral capsids) (29). Although it is thought that viruses do not contribute a significant amount of the oceanic dissolved DNA (29), this has yet to be rigorously proven. Thus, in many circumstances it may be important to distinguish between unpackaged phage DNA and intact phage particles. It was demonstrated that unpackaged DNA in laboratory cyanophage lysates increased the PCR product yield compared with lysates which had been pretreated with DNase (results not shown). Consequently, in subsequent attempts to develop cPCR methods for enumeration of cyanomyoviruses DNase-treated samples were used.
Given the apparent specificity of the primer pair used, it was also necessary to obtain an estimate of the sensitivity of this approach for detecting cyanomyoviruses. Amplified products were obtained from laboratory lysates with as few as ca. 190 particles per PCR mixture (Fig. 3, lane 5), which is equivalent to ca. 104 to 105 cyanophage particles ml−1. Cyanophage concentrations greater than 105 cyanophage particles ml−1 have been observed in the Gulf of Mexico (49), and consequently, it should be possible to detect cyanophages directly in PCR mixtures containing some seawater samples without prior concentration. We demonstrated that PCR products of the correct size could be amplified from seawater samples following 100× concentration (Fig. 6, lanes 10 and 11) and even directly without prior concentration (Fig. 6, lanes 2 through 5).
An attempt to develop a method for reliable quantification of the cyanophage DNA template resulted in the introduction of an internal standard (Fig. 2) which acted as a competitor template DNA. The competitor DNA was constructed by cloning a 193-bp fragment from pUC19 into the region of cyanophage strain S-BnM1 DNA amplified by CPS1 and CPS2. It was demonstrated that a constant amount (2 pg per reaction mixture) could be coamplified with decimal dilutions of cyanophage DNA (Fig. 4). The difference in the sizes of the competitor product (358 bp) and the cyanophage product (165 bp) was similar to the difference in the sizes of the products previously used to quantify human immunodeficiency virus promoters (207-bp difference in size) by PCR (62).
Calibration curves for cPCR products for cyanophage strains S-BnM1, S-MM5, and S-BM3 were log-linear over ca. 3 orders of magnitude of cyanophage concentrations (Fig. 5). Each curve was limited at the upper end by the PCR plateau and at the lower end by the amount of DNA needed for the bands to be visible; thus, for each individual phage strain, cPCR provided an accurate method for enumerating phage particles. Similar results have been observed previously. Leser (31) obtained a log-linear curve over 4 orders of magnitude for a marine Pseudomonas sp. in seawater; and Moller and Jansson (36) observed linearity over 2 orders of magnitude for genetically tagged cyanobacteria following cPCR. However, cPCR of cyanophage strains S-BnM1, S-MM5, and S-BM3 resulted in three different calibration curves with different sensitivities of detection (Fig. 5). Thus, cPCR with primers CPS1 and CPS2 should lead to substantial inaccuracies in estimates of phage abundance in natural assemblages. Degeneracy in the primers presumably led to biases in the intensities of amplification products. The results of further sequence analysis of cyanomyovirus gene 20 homologs may allow workers to design primers which do not give this phage-to-phage variability in priming efficiency.
However, the use of degenerate primers in PCR analysis of cyanophage populations may provide valuable data on the diversity of cyanophages in natural assemblages. PCR have previously been used to amplify algal genes from seawater and to determine phylogenetic relationships between phycodnaviruses (12). Our data extends such results to show that cyanophages can be detected in natural virus communities directly from untreated seawater. Further optimization of protocols may ultimately lead to a sensitive assay which can be used to analyze natural cyanophage populations both quantitatively (by cPCR) and qualitatively following phylogenetic analysis of amplified products.
ACKNOWLEDGMENTS
This study was funded in part by a Natural Environmental Research Council CASE award in conjunction with Plymouth Marine Laboratory, United Kingdom, and in part by Plankton Reactivity in the Marine Environment (PRIME) grant GST/02/1053, both awarded to N.H.M. PRIME is a community program funded by the Natural Environmental Research Council of the United Kingdom.
We thank the staff and crew of the RV Weatherbird (Bermuda Biological Station for Research) who assisted in the collection of seawater samples for this study. We also thank Lois Vincent-Sealy for technical assistance. Finally, we thank all of the people who kindly donated viruses for this study.
Footnotes
PRIME (Plankton Reactivity in the Marine Environment) contribution number 61.
REFERENCES
- 1.Ackermann H-W, Elzanowski A, Fobo G, Stewart G. Relationships of tailed phages: a survey of protein sequence identity. Arch Virol. 1995;140:1871–1884. doi: 10.1007/BF01384350. [DOI] [PubMed] [Google Scholar]
- 2.Ackermann H-W, Kasatiya S S, Kawata T, Koga T, Lee J V, Mbiguino A, Newman F S, Vieu J-F, Zachary A. Classification of Vibrio bacteriophages. Intervirology. 1984;22:61–71. doi: 10.1159/000149535. [DOI] [PubMed] [Google Scholar]
- 3.Boehme J, Frischer M E, Jiang S C, Kellogg C A, Pichard S, Rose J B, Steinway C, Paul J H. Viruses, bacterioplankton, and phytoplankton in the southeastern Gulf of Mexico: distribution and contribution to oceanic DNA pools. Mar Ecol Prog Ser. 1993;97:1–10. [Google Scholar]
- 4.Børsheim K Y, Bratbak G, Heldal M. Enumeration and biomass estimation of planktonic bacteria and viruses by transmission electron microscopy. Appl Environ Microbiol. 1990;56:352–356. doi: 10.1128/aem.56.2.352-356.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bratbak G, Heldal M, Thingstad T F, Riemann B, Haslund O H. Incorporation of viruses into the budget of microbial C-transfer. A first approach. Mar Ecol Prog Ser. 1992;83:273–280. [Google Scholar]
- 6.Bratbak G, Thingstad F, Heldal M. Viruses and the microbial loop. Microb Ecol. 1994;28:209–221. doi: 10.1007/BF00166811. [DOI] [PubMed] [Google Scholar]
- 7.Brautigam M, Klein M, Knippers R, Muller D G. Inheritance and meiotic elimination of a virus genome in the host Ectocarpus siliculosus (Phaeophyceae) J Phycol. 1995;31:823–827. [Google Scholar]
- 8.Brussaard C P D, Riegman R, Noordeloos A A M, Cadee G C, Witte H, Kop A J, Nieuwland G, van Duyl F C, Bak R P M. Effects of grazing, sedimentation and phytoplankton cell lysis on the structure of a coastal pelagic food web. Mar Ecol Prog Ser. 1995;123:259–271. [Google Scholar]
- 9.Chen F, Suttle C A. Evolutionary relationships among large double-stranded DNA viruses which infect microalgae and other organisms, as inferred from DNA polymerase genes. Virology. 1996;219:170–178. doi: 10.1006/viro.1996.0234. [DOI] [PubMed] [Google Scholar]
- 10.Chen F, Suttle C A. Amplification of DNA polymerase gene fragments from viruses infecting microalgae. Appl Environ Microbiol. 1995;61:1274–1278. doi: 10.1128/aem.61.4.1274-1278.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen F, Suttle C A. Nested PCR with three highly degenerate primers for amplification and identification of DNA from related organisms. BioTechniques. 1995;18:609–612. [PubMed] [Google Scholar]
- 12.Chen F, Suttle C A, Short S M. Genetic diversity in marine algal communities as revealed by sequence analysis of DNA polymerase genes. Appl Environ Microbiol. 1996;62:2869–2874. doi: 10.1128/aem.62.8.2869-2874.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chisholm S W, Olson R J, Rettler E R, Waterbury J, Goericke R, Welschmeyer N. A novel free-living prochlorophyte occurs at high cell concentrations in the oceanic euphotic zone. Nature (London) 1988;334:340–343. [Google Scholar]
- 14.Coombs D, Eiserling F A. Studies on the structure, protein composition and assembly of the neck of bacteriophage T4. J Mol Biol. 1977;116:375–407. doi: 10.1016/0022-2836(77)90076-6. [DOI] [PubMed] [Google Scholar]
- 15.Cottrell M T, Suttle C A. Genetic diversity of algal viruses which lyse the photosynthetic picoflagellate Micromonas pusilla (Prasinophyceae) Appl Environ Microbiol. 1995;61:3088–3091. doi: 10.1128/aem.61.8.3088-3091.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cottrell M T, Suttle C A. Wide-spread and clonal variation in viruses which cause lysis of a cosmopolitan, eukaryote marine phytoplankter, Micromonas pusilla. Mar Ecol Prog Ser. 1991;78:1–9. [Google Scholar]
- 17.Frank H, Moebus K. An electron microscopic study of bacteriophages from marine waters. Helgol Meersunters. 1987;41:385–414. [Google Scholar]
- 18.Fuhrman J A, Noble R T. Viruses and protists cause similar bacterial mortality in coastal seawater. Limnol Oceanogr. 1995;40:1236–1242. [Google Scholar]
- 19.Genetics Computer Group. Program manual for the Wisconsin Package, version 8.1. Madison, Wis: Genetics Computer Group; 1994. [Google Scholar]
- 20.Giovannoni S J, Mullins T D, Field K G. Microbial diversity in oceanic systems: rRNA approaches to the study of unculturable microbes. NATO Adv Study Inst Ser Ser G. 1995;38:217–248. [Google Scholar]
- 21.Guillard R R L. Culture of phytoplankton for feeding marine invertebrates. In: Smith W L, Chanley M H, editors. Culture of marine invertebrate animals. New York, N.Y: Plenum; 1975. pp. 29–60. [Google Scholar]
- 22.Heldal M, Bratbak G. Production and decay of viruses in aquatic environments. Mar Ecol Prog Ser. 1991;72:205–212. [Google Scholar]
- 23.Hennes K P, Suttle C A. Direct counts of viruses in natural seawater and laboratory cultures by epifluorescence microscopy. Limnol Oceanogr. 1995;40:1054–1059. [Google Scholar]
- 24.Hennes K P, Suttle C A, Chan A M. Fluorescently labelled virus probes show that natural virus populations can control the structure of marine microbial communities. Appl Environ Microbiol. 1995;61:3623–3627. doi: 10.1128/aem.61.10.3623-3627.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirons G T, Fawcett J J, Crissman H A. TOTO and YOYO: new very bright fluorochromes for DNA content analysis by flow cytometry. Cytometry. 1994;15:129–140. doi: 10.1002/cyto.990150206. [DOI] [PubMed] [Google Scholar]
- 26.Hsiao C L, Black L W. Head morphogenesis of bacteriophage T4. III. The role of gene 20 in DNA packaging. Virology. 1978;91:26–38. doi: 10.1016/0042-6822(78)90352-5. [DOI] [PubMed] [Google Scholar]
- 27.Hu N-T, Thiel T, Giddings T H, Jr, Wolk C P. New Anabaena and Nostoc cyanophages from sewage settling ponds. Virology. 1981;114:236–246. doi: 10.1016/0042-6822(81)90269-5. [DOI] [PubMed] [Google Scholar]
- 28.Jacobsen A, Bratbak G, Heldal M. Isolation and characterization of a virus infecting Phaeocystis pouchetii (Prymnesiophyceae) J Phycol. 1996;32:923–927. [Google Scholar]
- 29.Jiang S C, Paul J H. Viral contribution to dissolved DNA in the marine environment as determined by differential centrifugation and kingdom probing. Appl Environ Microbiol. 1995;61:317–325. doi: 10.1128/aem.61.1.317-325.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klein M, Lanka S T J, Knippers R, Muller D G. Coat protein of the Ectocarpus siliculosus virus. Virology. 1995;206:520–526. doi: 10.1016/s0042-6822(95)80068-9. [DOI] [PubMed] [Google Scholar]
- 31.Leser T D. Quantitation of Pseudomonas sp. strain B13(FR1) in the marine environment by competitive polymerase chain reaction. J Microbiol Methods. 1995;22:249–262. [Google Scholar]
- 32.Liss A, Ackermann H-W, Mayer L W, Zierdt C H. Tailed phages of Pseudomonas and related bacteria. Intervirology. 1981;15:71–81. doi: 10.1159/000149216. [DOI] [PubMed] [Google Scholar]
- 33.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 34.Marusich E I, Mesyanzhinov V V. Nucleotide and deduced amino acid sequences of bacteriophage T4 gene 20. Nucleic Acids Res. 1989;17:7514. doi: 10.1093/nar/17.18.7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Messing J, Gronenborn B, Muller-Hill B, Hofschneider P H. Filamentous coliphage M13 as a cloning vehicle: insertion of a HindII fragment of the lac regulatory region in M13 replicative form in vitro. Proc Natl Acad Sci USA. 1977;74:3642–3646. doi: 10.1073/pnas.74.9.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moller A, Jansson J K. Quantification of genetically tagged cyanobacteria in Baltic Sea sediment by competitive PCR. BioTechniques. 1997;22:512–518. doi: 10.2144/97223rr02. [DOI] [PubMed] [Google Scholar]
- 37.Monod C, Repoila F, Kutateladze M, Tetart F, Krisch H M. The genome of the pseudo T-even bacteriophages, a diverse group that resembles T4. J Mol Biol. 1997;267:237–249. doi: 10.1006/jmbi.1996.0867. [DOI] [PubMed] [Google Scholar]
- 38.Palenik B. Cyanobacterial community structure as seen from RNA polymerase gene sequence analysis. Appl Environ Microbiol. 1994;60:3212–3219. doi: 10.1128/aem.60.9.3212-3219.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palenik B, Haselkorn R. Multiple evolutionary origins of prochlorophytes, the chlorophyll b-containing prokaryotes. Nature (London) 1992;355:265–267. doi: 10.1038/355265a0. [DOI] [PubMed] [Google Scholar]
- 40.Proctor L M, Fuhrman J A. Viral mortality of marine bacteria and cyanobacteria. Nature (London) 1990;343:60–62. [Google Scholar]
- 41.Queen C, Korn L J. A comprehensive sequence analysis program for the IBM PC. Nucleic Acids Res. 1984;12:581–599. doi: 10.1093/nar/12.1part2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sengco M R, Brautigam M, Kapp M, Muller D G. Detection of virus DNA in Ectocarpus siliculosus and E. fasciculatus (Phaeophyceae) from various geographic areas. Eur J Phycol. 1996;31:73–78. [Google Scholar]
- 44.Steward G F, Smith D C, Azam F. Abundance and production of bacteria and viruses in the Bering and Chukchi seas. Mar Ecol Prog Ser. 1996;131:287–300. [Google Scholar]
- 45.Steward G F, Wikner J, Cochlan W P, Smith D C, Azam F. Estimation of virus production in the sea. II. Field results. Mar Microbial Food Webs. 1992;6:79–90. [Google Scholar]
- 46.Steward G F, Wikner J, Smith D C, Cochlan W P, Azam F. Estimation of virus production in the sea. I. Method development. Mar Microbial Food Webs. 1992;6:57–78. [Google Scholar]
- 47.Suttle C A. The significance of viruses to mortality in aquatic microbial communities. Microb Ecol. 1994;28:237–243. doi: 10.1007/BF00166813. [DOI] [PubMed] [Google Scholar]
- 48.Suttle C A, Chan A M. Dynamics and distribution of cyanophages and their effect on marine Synechococcus spp. Appl Environ Microbiol. 1994;60:3167–3174. doi: 10.1128/aem.60.9.3167-3174.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suttle C A, Chan A M. Marine cyanophages infecting oceanic and coastal strains of Synechococcus: abundance, morphology, cross-infectivity and growth characteristics. Mar Ecol Prog Ser. 1993;92:99–109. [Google Scholar]
- 50.Urbach E, Robertson D L, Chisholm S W. Multiple evolutionary origins of prochlorophytes within the cyanobacterial radiation. Nature (London) 1992;355:267–270. doi: 10.1038/355267a0. [DOI] [PubMed] [Google Scholar]
- 51.van Boekel W H M, Hansen F C, Riegman R, Bak R P M. Lysis-induced decline of a Phaeocystis spring bloom and coupling with the microbial foodweb. Mar Ecol Prog Ser. 1992;81:269–276. [Google Scholar]
- 52.van Driel R, Couture E. Assembly of the scaffolding core of bacteriophage T4 preheads. J Mol Biol. 1978;123:713–719. doi: 10.1016/0022-2836(78)90217-6. [DOI] [PubMed] [Google Scholar]
- 53.Waterbury J B, Rippka R. The order Chroococcales. In: Staley J T, Bryant M P, Pfennig N, Holt J G, editors. Bergey’s manual of systematic bacteriology. Vol. 3. Baltimore, Md: Williams and Wilkins; 1989. pp. 1728–1746. [Google Scholar]
- 54.Waterbury J B, Valois F W. Resistance to co-occurring phages enables marine Synechococcus communities to coexist with cyanophages abundant in seawater. Appl Environ Microbiol. 1993;59:3393–3399. doi: 10.1128/aem.59.10.3393-3399.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Waterbury J B, Watson S W, Valois F W, Franks D G. Biological and ecological characterization of the marine unicellular cyanobacterium Synechococcus. Can Bull Fish Aquat Sci. 1986;214:71–120. [Google Scholar]
- 56.Wilson W H. Ph.D. thesis. Coventry, United Kingdom: University of Warwick; 1994. [Google Scholar]
- 57.Wilson W H, Carr N G, Mann N H. The effect of phosphate status on the kinetics of cyanophage infection in the oceanic cyanobacterium Synechococcus sp. WH7803. J Phycol. 1996;32:506–516. [Google Scholar]
- 58.Wilson W H, Joint I R, Carr N G, Mann N M. Isolation and molecular characterization of five marine cyanophages propagated on Synechococcus sp. WH7803. Appl Environ Microbiol. 1993;59:3736–3743. doi: 10.1128/aem.59.11.3736-3743.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilson W H, Mann N H. Lysogenic and lytic production in marine microbial communities. Aquat Microb Ecol. 1997;13:95–100. [Google Scholar]
- 60.Wilson, W. H., S. Turner, and N. H. Mann. 1998. Population dynamics of phytoplankton and viruses in a phosphate limited mesocosm and their effect on DMSP production. Estuarine Coastal Shelf Sci. 46(Suppl. A):49–59.
- 61.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:109–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 62.Zachar V, Thomas R A, Goustin A S. Absolute quantification of target DNA: a simple competitive PCR for efficient analysis of multiple samples. Nucleic Acids Res. 1993;21:2017–2018. doi: 10.1093/nar/21.8.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]