Abstract
Objective
This study aimed to validate the surgical and oncologic outcomes of robotic surgery with sentinel node navigation surgery (SNNS) in endometrial cancer.
Methods
This study included 130 patients with endometrial cancer, who underwent robotic surgery, including hysterectomy, bilateral salpingo-oophorectomy, and pelvic SNNS at the Department of Obstetrics and Gynecology of Kagoshima University Hospital. Pelvic sentinel lymph nodes (SLNs) were identified using the uterine cervix 99m Technetium-labeled phytate and indocyanine green injections. Surgery-related and survival outcomes were also evaluated.
Results
The median operative and console times and volume of blood loss were 204 (range: 101–555) minutes, 152 (range: 70–453) minutes, and 20 (range: 2–620) mL, respectively. The bilateral and unilateral pelvic SLN detection rates were 90.0% (117/130) and 5.4% (7/130), respectively, and the identification rate (the rate at which at least one SLN could be identified on either side) was 95% (124/130). Lower extremity lymphedema occurred in only 1 patient (0.8%), and no pelvic lymphocele occurred. Recurrence occurred in 3 patients (2.3%), and the recurrence site was the abdominal cavity, with dissemination in 2 patients and vaginal stump in one. The 3-year recurrence-free survival and 3-year overall survival rates were 97.1% and 98.9%, respectively.
Conclusion
Robotic surgery with SNNS for endometrial cancer showed a high SLN identification rate, low occurrence rates of lower extremity lymphedema and pelvic lymphocele, and excellent oncologic outcomes.
Keywords: Endometrial Cancer, Prognosis, Surgery, Sentinel Lymph Node, Lymph Nodes
Synopsis
Robotic surgery with sentinel node navigation surgery (SNNS) showed high identification rate of sentinel lymph node in endometrial cancer. The occurrence rate of lower extremity lymphedema and pelvic lymphocele was low. The present study demonstrated excellent oncologic outcome of robotic surgery with SNNS in patient with endometrial cancer.
Graphical Abstract
INTRODUCTION
Endometrial cancer is a common gynecological cancer, and surgical treatments include hysterectomy, bilateral salpingo-oophorectomy, and lymph node (LN) dissection. With technological advancement in recent years, minimally invasive surgery (MIS), especially robotic surgery for endometrial cancer, has become mainstream; some reports have shown that it is more useful than conventional open or laparoscopic surgery [1,2,3,4]. In contrast, comprehensive lymphadenectomy is an essential procedure for endometrial cancer staging because lymph node metastasis (LNM) is an important poor prognostic factor. However, its therapeutic significance is still unknown [5,6,7]. Moreover, comprehensive lymphadenectomy has been associated with bleeding, nerve damage, bowel obstruction, lower-extremity lymphedema, and pelvic lymphocele [8]. Consequently, the usefulness of sentinel lymph node (SLN) mapping in early-stage endometrial cancer has been widely reported in recent years [9,10,11,12] and is recommended in various guidelines [13,14,15]. In prostate cancer, previous reports described that SLN mapping improves detection of positive nodes and potentially lowers recurrence rates with subsequent optimization of patient management, without compromising patient safety [16]. However, most SLN mapping studies followed SLN mapping with systematic pelvic lymphadenectomy.
Sentinel node navigation surgery (SNNS), which omits comprehensive LN dissection if there is no metastasis in the SLN, is useful for improving patient quality of life (QOL) and diagnosing micro-metastasis; however, it is important to determine if it does not adversely affect prognosis. Few studies have described the prognosis of SNNS with robotic surgery in large cohorts of patients with endometrial cancer. This study aimed to validate robotic surgery with SNNS in endometrial cancer with respect to surgical outcome and prognosis.
MATERIALS AND METHODS
From July 2018 to May 2022, 130 patients with endometrial cancer underwent robotic surgery using the da Vinci Xi® surgical system (Intuitive Surgical, Sunnyvale, CA, USA), including hysterectomy, bilateral salpingo-oophorectomy, and pelvic SNNS, at the Department of Obstetrics and Gynecology of Kagoshima University Hospital. The inclusion criteria were endometrial cancer with endometrioid adenocarcinoma grade 1/2 confirmed by histological biopsy, age >18 years, and patients with suspected preoperative International Federation of Gynecology and Obstetrics (FIGO) stage IA. Patients with suspected pelvic LNM on preoperative computed tomography (CT) or those who received neoadjuvant chemotherapy were excluded. This study was approved by the Institutional Ethics Committee of Kagoshima University Hospital (approval No. 20-K04), and informed consent was obtained from all the patients.
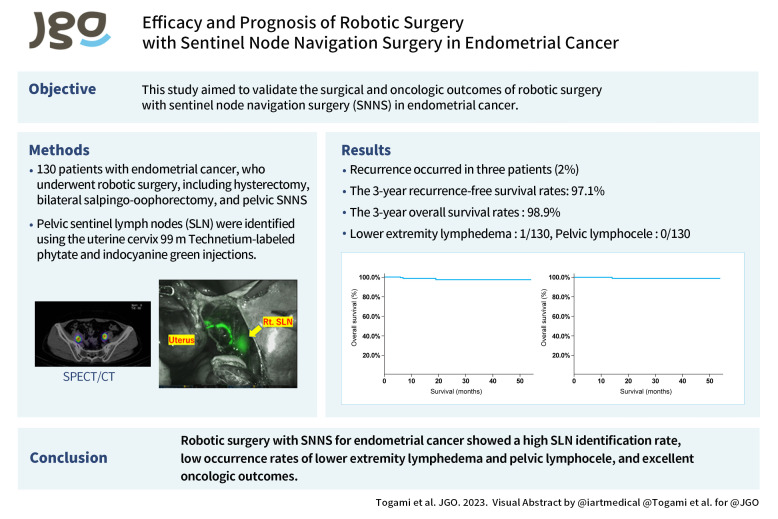
To detect the pelvic SLN, 99m Technetium-labeled phytate (148 MBq [4.0 mCi] per patient) was injected into 4 quadrants (0, 3, 6, and 9 o’clock positions) of the cervix on the day before surgery. Lymphoscintigraphy and single-photon emission CT with CT were performed to determine SLN localization. Indocyanine green (ICG) was injected into the cervix immediately before surgery, and the SLN was identified intraoperatively using a hybrid method (radioisotope and ICG). Intraoperatively, pelvic SLNs were first identified as bright LNs by near-infrared fluorescence imaging (SPIES; Karl Storz Endoscopy, Tokyo, Japan) using ICG. Thereafter, pelvic SLNs were identified using a gamma probe (Neo2000; Neoprobe Corporation, Dublin, OH, USA) as radioactive LNs. These 2 identification methods identified the same SLNs in most cases, but the number and sites of SLNs did not always match. All removed SLNs were incised at 2-mm intervals and subjected to rapid intraoperative pathological diagnosis. SLN ultrastaging was not performed in this study. Pelvic LN dissection (PLD) was omitted if no LNM was found in bilateral SLNs. If LNM was found in both SLNs, a bilateral PLD was performed; if the SLN could not be identified, an ipsilateral PLD was performed.
Postoperative chemotherapy was administered in cases with intermediate- and high-risk of recurrence, such as those with deep myometrial invasion, lymphovascular space invasion, histologic types other than G1/2 endometrial carcinoma, and positive LNs at the final histopathological examination. Patient information such as information on lymphedema and prognosis were collected by gynecologic oncologists during every follow-up visit to an outpatient clinic (the median follow-up period was 18 months [range: 2–47]).
We evaluated the clinicopathological results of robotic surgery with SNNS for endometrial cancer and analyzed the prognostic value of these results. The JMP software (version 14; SAS Institute Inc., Cary, NC, USA) was used for statistical analysis. A p-value of <0.05 was considered statistically significant.
RESULTS
Table 1 shows the clinicopathological characteristics of the 130 patients with EC. Median age and body mass index were 58 (range: 28–81) years and 27.4 (range: 16.7–53.1) kg/m2, respectively. The final pathology was endometrioid carcinoma in 128 (98.5%) patients, serous carcinoma in 1 (0.8%) patient, and clear cell carcinoma in 1 (0.8%) patient. The number of patients according to the FIGO staging (2009) was stage IA, 117 (90.0%) patients; IB, 4 (3.1%) patients; II, 1 (0.8%) patient; IIIA, 1 (0.8%) patient, and IIIC1, and 7 (5.4%) patients. Sixteen patients (12%) had lymphovascular space involvement, while the remaining 114 (87.7%) did not.
Table 1. Clinicopathological characteristics.
| Characteristics | Patients (n=130) | ||
|---|---|---|---|
| Age (yr) | 58 (28–81) | ||
| BMI (kg/m2) | 27.4 (16.7–53.1) | ||
| Final pathology | |||
| Endometrioid | 128 (98.5) | ||
| Grade 1 | 116 | ||
| Grade 2 | 10 | ||
| Grade 3 | 2 | ||
| Serous | 1 (0.8) | ||
| Clear cell | 1 (0.8) | ||
| FIGO staging (2009) | |||
| IA | 117 (90.0) | ||
| IB | 4 (3.1) | ||
| II | 1 (0.8) | ||
| IIIA | 1 (0.8) | ||
| IIIC1 | 7 (5.4) | ||
| LVSI | |||
| No | 114 (87.7) | ||
| Yes | 16 (12.3) | ||
Values are presented as median (range) or number (%).
BMI, body mass index; FIGO, International Federation of Gynecology and Obstetrics; LVSI, lymph-vascular space involvement.
Table 2 shows the surgery-related outcomes of the 130 patients with endometrial cancer. The median operation and console times were 204 (range: 101–555) and 152 (range: 70–453) minutes, respectively. The median volume of blood loss was 20 mL (range: 2–620), and conversion to laparotomy was not permitted. Regarding complications, only 1 patient (0.8%) had bladder injury during surgery, and 1 patient (0.8%) each had bowel obstruction, ureteral obstruction, and pelvic space infection postoperatively. Regarding adjuvant therapy, 17 patients (13.1%) received chemotherapy and 3 (2.3%) received radiotherapy.
Table 2. Surgery-related outcomes.
| Characteristics | Patients (n=130) | |
|---|---|---|
| Operation time (min) | 204 (101–555) | |
| Console time (min) | 152 (70–453) | |
| Blood loss (mL) | 20 (2–620) | |
| Conversion to laparotomy | 0 (0.0) | |
| Intra-operation complications | ||
| Bladder injury | 1 (0.8) | |
| Post-operation complications | ||
| Ileus | 1 (0.8) | |
| Ureteral stenosis | 1 (0.8) | |
| Pelvic space infection | 1 (0.8) | |
| Length of hospital stay (day) | 6 (4–14) | |
| Adjuvant therapy | ||
| None | 110 (84.6) | |
| Chemotherapy | 17 (13.1) | |
| Radiotherapy | 3 (2.3) | |
| Recurrence | ||
| No | 127 (97.7) | |
| Yes | 3 (2.3) | |
Values are presented as median (range) or number (%).
Table 3 shows the SNNS-related outcomes in the 130 patients. The bilateral and unilateral pelvic SLN detection rates were 90.0% (117/130) and 5.4% (7/130), respectively, and the identification rate (the rate at which at least one SLN could be identified on either side) was 95.4% (124/130). Only 6 (4.6%) patients had no SLNs. SLNs were detected in the obturator (52.7%), external iliac (38.6%), internal iliac (4.9%), common iliac (3.4%), and parametrial (0.4%) arteries. The median number of SLNs removed was 2; metastatic SLNs were found in 7 (5.4%) patients. Lower extremity lymphedema occurred in only 1 patient (0.8%), and no pelvic lymphocele occurred.
Table 3. SLN-related outcomes.
| Characteristics | Patients (n=130) | |
|---|---|---|
| SLN mapping | ||
| Bilateral | 117 (90.0) | |
| Unilateral | 7 (5.4) | |
| None | 6 (4.6) | |
| SLN locations (n=264) | ||
| Obturator | 139 (52.7) | |
| External iliac | 102 (38.6) | |
| Internal iliac | 13 (4.9) | |
| Common iliac | 9 (3.4) | |
| Parametrial | 1 (0.4) | |
| No. of SLN removed | 2 (0–6) | |
| SLN metastasis | 7 (5.4) | |
| Lower extremity lymphedema | 1 (0.8) | |
| Pelvic lymphocele | 0 (0.0) | |
Values are presented as median (range) or number (%).
SLN, sentinel-lymph node.
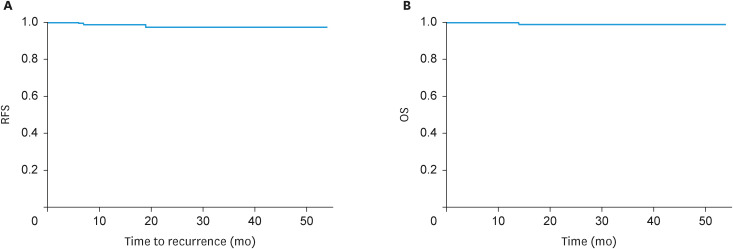
Recurrence occurred in 3 (2.3%) patients, and the recurrence site was the abdominal cavity, with dissemination, in 2 patients, and vaginal stump in one. One patient had deep myometrial and cervical stromal invasion and received postoperative chemotherapy, while the other 2 were in the low-risk group for recurrence and did not receive chemotherapy. The 3-year recurrence-free survival (RFS) and 3-year overall survival (OS) rates were 97.1% and 98.9%, respectively (Fig. 1).
Fig. 1. (A) Three-year RFS rate. (B) Three-year OS rate.
OS, overall survival; RFS, recurrence-free survival.
DISCUSSION
This study investigated the surgical and oncological outcomes of robotic surgery with SNNS in patients with endometrial cancer. Many studies have reported that SLN mapping for endometrial cancer is very useful in the diagnosis of micro-metastasis of SLN, as well as for avoiding lower extremity lymphedema and lymphocele [12,17,18,19]. The results showed that SLN mapping with robotic surgery for endometrial cancer had a high identification rate and that prognosis was also feasible.
Robotic surgery, an MIS, is rapidly gaining popularity for endometrial cancer treatment. A previous study showed that robotic surgery is associated with reduced blood loss and less conversion to open surgery than conventional laparoscopic surgery [3]. In addition, a randomized controlled trial of robotic versus conventional laparoscopic surgery concluded that robotic surgery offers an effective and safe alternative for the surgical treatment of endometrial cancer [1]. In our study, the median blood loss was also very low (20 mL), and no conversion to laparotomy was required., supporting the findings of previous studies.
SNNS, which can omit systematic lymphadenectomy if there are no SLN metastases, is a good match for MIS, and the combination can greatly improve patient QOL. The SENTI-ENDO trial, a multicenter prospective study, reported an 89% SLN identification rate in the pelvic region (at least one SLN could be identified), a 69% bilateral SLN identification rate, 84% sensitivity, a 97% negative predictive value, and recommended SLN biopsy for endometrial cancer in low- and intermediate-risk recurrence groups [9]. In the FIRES trial, a multicenter prospective cohort, the SLN identification rate (at least one SLN could be identified) was 86%, the bilateral SLN identification rate was 52%, the sensitivity and negative predictive values were 97.2% and 99.6%, respectively, and it concluded that pelvic SLN biopsy with ICG administration into the cervix is a useful alternative to lymphadenectomy as a staging method [17]. Our previous study also reported a good SLN identification rate of 96%, sensitivity of 91%, and negative predictive value of 99%, making it a useful and safe alternative to PLD, especially for low-risk recurrent endometrial cancers [11]. In the present study, the bilateral and unilateral pelvic SLN detection rates were 90% (117/130) and 5% (7/130), respectively, and the identification rate (the rate at which at least one SLN could be identified on either side) was 95% (124/130). In this study, if LNM was found within a SLN on either side, a bilateral PLD was performed. Although the significance of PLD in endometrial cancer is controversial, several reports have shown the prognostic benefit of PLD [20,21,22]. We believe that bilateral PLD can improve the prognosis in such cases, since there is a possibility of metastasis to other LNs than the SLN in patients with positive SLN metastasis.
Combining robotic surgery (MIS) with SNNS can considerably improve patient QOL; however, there are limited data on the safety of omitting lymphadenectomy in SLN metastasis-negative cases.
Recurrence occurred in 3 (2%) patients, and the 3-year RFS and 3-year OS rates were 97.1% and 98.9%, respectively. In our previous study, we reported no significant between-group difference in 5-year RFS (laparoscopic surgery with lymphadenectomy group, 96.3%; open surgery with lymphadenectomy group, 92.6%) in 155 patients with low-risk endometrial cancer [23]. Buda et al. [24] compared the oncologic outcomes between SLN mapping and lymphadenectomy in patients with preoperative stage I endometrial cancer. They found that the 3-year disease-free survival was 90.4% and 89.6% (p=0.433) in the SLN mapping and lymphadenectomy groups, respectively. Although these studies were performed laparoscopically and laparotomically, we suggest that SLN mapping is equivalent to oncologic outcomes comparable to lymphadenectomy. Some reports have examined the oncological outcomes of SLN mapping and lymphadenectomy with robotic surgery. Jebens Nordskar et al. [25] investigated the long-term outcomes of 108 endometrial cancer patients who underwent robot-assisted surgery and SLN mapping. LNM was found in 17 (16%) patients, and the 5-year RFS and OS rates were 95.4% and 92.6%, respectively. Our 3-year RFS and 3-year OS rates can be considered favorable.
In our previous study [26], the incidence of lower extremity lymphedema and pelvic lymphocele in patients with endometrial cancer who underwent pelvic lymphadenectomy was 14.9% and 11.4%, respectively. However, a previous study [27] showed that the occurrence rate of lower extremity lymphedema in patients with endometrial cancer who underwent SNNS was 2.3%. In this study, lower extremity lymphedema occurred in only one (1%) patient, and no pelvic lymphocele occurred. Lower extremity lymphedema and pelvic lymphocele rarely occur with SNNS, suggesting that SNNS contributes to improved QOL.
This study has several limitations. First, this was a retrospective study and inherently carries the risk of selection bias. Second, although, to our knowledge, this is the largest case series of robotic surgery with SNNS in patients with endometrial cancer, the number of cases remains relatively low. Future studies with larger study populations are required to confirm our findings.
In conclusion, our data demonstrated that robotic surgery with SNNS for endometrial cancer showed a high SLN identification rate and a low occurrence rate of lower extremity lymphedema and pelvic lymphocele. The 3-year RFS and 3-year OS rates were 97.1% and 98.9%, respectively, and the oncologic outcomes were favorable. Prospective studies are needed to validate the safety and oncological outcomes of robotic surgery with SNNS for endometrial cancer.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Investigation: T.S., F.M., M.M.
- Supervision: K.H.
- Writing - original draft: T.S.
- Writing - review & editing: Y.S., K.H.
References
- 1.Mäenpää MM, Nieminen K, Tomás EI, Laurila M, Luukkaala TH, Mäenpää JU. Robotic-assisted vs traditional laparoscopic surgery for endometrial cancer: a randomized controlled trial. Am J Obstet Gynecol. 2016;215:588.e1–588.e7. doi: 10.1016/j.ajog.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Argenta PA, Mattson J, Rivard CL, Luther E, Schefter A, Vogel RI. Robot-assisted versus laparoscopic minimally invasive surgery for the treatment of stage I endometrial cancer. Gynecol Oncol. 2022;165:347–352. doi: 10.1016/j.ygyno.2022.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xie W, Cao D, Yang J, Shen K, Zhao L. Robot-assisted surgery versus conventional laparoscopic surgery for endometrial cancer: a systematic review and meta-analysis. J Cancer Res Clin Oncol. 2016;142:2173–2183. doi: 10.1007/s00432-016-2180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gota T, Tomio K, Kurose T, Saito R, Nara R, Kin S, et al. The current status of robotic surgery for endometrial cancer in Japan. Glob Health Med. 2022;4:21–25. doi: 10.35772/ghm.2021.01077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benedetti Panici P, Basile S, Maneschi F, Alberto Lissoni A, Signorelli M, Scambia G, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100:1707–1716. doi: 10.1093/jnci/djn397. [DOI] [PubMed] [Google Scholar]
- 6.ASTEC study group. Kitchener H, Swart AMC, Qian Q, Amos C, Parmar MK, et al. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009;373:125–136. doi: 10.1016/S0140-6736(08)61766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Todo Y, Kato H, Kaneuchi M, Watari H, Takeda M, Sakuragi N. Survival effect of para-aortic lymphadenectomy in endometrial cancer (SEPAL study): a retrospective cohort analysis. Lancet. 2010;375:1165–1172. doi: 10.1016/S0140-6736(09)62002-X. [DOI] [PubMed] [Google Scholar]
- 8.Hareyama H, Hada K, Goto K, Watanabe S, Hakoyama M, Oku K, et al. Prevalence, classification, and risk factors for postoperative lower extremity lymphedema in women with gynecologic malignancies: a retrospective study. Int J Gynecol Cancer. 2015;25:751–757. doi: 10.1097/IGC.0000000000000405. [DOI] [PubMed] [Google Scholar]
- 9.Ballester M, Dubernard G, Lécuru F, Heitz D, Mathevet P, Marret H, et al. Detection rate and diagnostic accuracy of sentinel-node biopsy in early stage endometrial cancer: a prospective multicentre study (SENTI-ENDO) Lancet Oncol. 2011;12:469–476. doi: 10.1016/S1470-2045(11)70070-5. [DOI] [PubMed] [Google Scholar]
- 10.Soliman PT, Westin SN, Dioun S, Sun CC, Euscher E, Munsell MF, et al. A prospective validation study of sentinel lymph node mapping for high-risk endometrial cancer. Gynecol Oncol. 2017;146:234–239. doi: 10.1016/j.ygyno.2017.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Togami S, Kawamura T, Fukuda M, Yanazume S, Kamio M, Kobayashi H. Prospective study of sentinel lymph node mapping for endometrial cancer. Int J Gynaecol Obstet. 2018;143:313–318. doi: 10.1002/ijgo.12651. [DOI] [PubMed] [Google Scholar]
- 12.Touhami O, Grégoire J, Renaud MC, Sebastianelli A, Plante M. Performance of sentinel lymph node (SLN) mapping in high-risk endometrial cancer. Gynecol Oncol. 2017;147:549–553. doi: 10.1016/j.ygyno.2017.09.014. [DOI] [PubMed] [Google Scholar]
- 13.Concin N, Matias-Guiu X, Vergote I, Cibula D, Mirza MR, Marnitz S, et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int J Gynecol Cancer. 2021;31:12–39. doi: 10.1136/ijgc-2020-002230. [DOI] [PubMed] [Google Scholar]
- 14.Koh WJ, Abu-Rustum NR, Bean S, Bradley K, Campos SM, Cho KR, et al. Uterine neoplasms, version 1.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16:170–199. doi: 10.6004/jnccn.2018.0006. [DOI] [PubMed] [Google Scholar]
- 15.Nagase S, Katabuchi H, Hiura M, Sakuragi N, Aoki Y, Kigawa J, et al. Evidence-based guidelines for treatment of uterine body neoplasm in Japan: Japan Society of Gynecologic Oncology (JSGO) 2009 edition. Int J Clin Oncol. 2010;15:531–542. doi: 10.1007/s10147-010-0138-6. [DOI] [PubMed] [Google Scholar]
- 16.Mazzone E, Dell’Oglio P, Grivas N, Wit E, Donswijk M, Briganti A, et al. Diagnostic value, oncologic outcomes, and safety profile of image-guided surgery technologies during robot-assisted lymph node dissection with sentinel node biopsy for prostate cancer. J Nucl Med. 2021;62:1363–1371. doi: 10.2967/jnumed.120.259788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rossi EC, Kowalski LD, Scalici J, Cantrell L, Schuler K, Hanna RK, et al. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study. Lancet Oncol. 2017;18:384–392. doi: 10.1016/S1470-2045(17)30068-2. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka T, Terai Y, Ashihara K, Tsunetoh S, Akagi H, Yamada T, et al. The detection of sentinel lymph nodes in laparoscopic surgery for uterine cervical cancer using 99m-technetium-tin colloid, indocyanine green, and blue dye. J Gynecol Oncol. 2017;28:e13. doi: 10.3802/jgo.2017.28.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanner E, Puechl A, Levinson K, Havrilesky LJ, Sinno A, Secord AA, et al. Use of a novel sentinel lymph node mapping algorithm reduces the need for pelvic lymphadenectomy in low-grade endometrial cancer. Gynecol Oncol. 2017;147:535–540. doi: 10.1016/j.ygyno.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 20.Chan JK, Urban R, Cheung MK, Shin JY, Husain A, Teng NN, et al. Lymphadenectomy in endometrioid uterine cancer staging: how many lymph nodes are enough? A study of 11,443 patients. Cancer. 2007;109:2454–2460. doi: 10.1002/cncr.22727. [DOI] [PubMed] [Google Scholar]
- 21.Kim TH, Kim HS, Kim TJ, Chang SJ, Kim DY, Ryu SY, et al. Survival impact based on the thoroughness of pelvic lymphadenectomy in intermediate- or high-risk groups of endometrioid-type endometrial cancer: a multi-center retrospective cohort analysis. Gynecol Oncol. 2016;141:440–446. doi: 10.1016/j.ygyno.2016.03.031. [DOI] [PubMed] [Google Scholar]
- 22.Polterauer S, Khalil S, Zivanovic O, Abu-Rustum NR, Hofstetter G, Concin N, et al. Prognostic value of lymph node ratio and clinicopathologic parameters in patients diagnosed with stage IIIC endometrial cancer. Obstet Gynecol. 2012;119:1210–1218. doi: 10.1097/AOG.0b013e318255060c. [DOI] [PubMed] [Google Scholar]
- 23.Togami S, Kawamura T, Yanazume S, Kamio M, Kobayashi H. Comparison of survival outcomes between laparoscopic and open surgery in patients with low-risk endometrial cancer. Jpn J Clin Oncol. 2020;50:1261–1264. doi: 10.1093/jjco/hyaa116. [DOI] [PubMed] [Google Scholar]
- 24.Buda A, Di Martino G, Restaino S, De Ponti E, Monterossi G, Giuliani D, et al. The impact on survival of two different staging strategies in apparent early stage endometrial cancer comparing sentinel lymph nodes mapping algorithm and selective lymphadenectomy: an Italian retrospective analysis of two reference centers. Gynecol Oncol. 2017;147:528–534. doi: 10.1016/j.ygyno.2017.09.033. [DOI] [PubMed] [Google Scholar]
- 25.Jebens Nordskar N, Hagen B, V Vesterfjell E, Salvesen Ø, Aune G. “Long-term outcome in endometrial cancer patients after robot-assisted laparoscopic surgery with sentinel lymph node mapping”. Eur J Obstet Gynecol Reprod Biol. 2022;271:77–82. doi: 10.1016/j.ejogrb.2022.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Togami S, Kubo R, Kawamura T, Yanazume S, Kamio M, Kobayashi H. Risk factors for lymphatic complications following lymphadenectomy in patients with endometrial cancer. Taiwan J Obstet Gynecol. 2020;59:420–424. doi: 10.1016/j.tjog.2020.03.015. [DOI] [PubMed] [Google Scholar]
- 27.Niikura H, Toki A, Nagai T, Okamoto S, Shigeta S, Tokunaga H, et al. Prospective evaluation of sentinel node navigation surgery in Japanese patients with low-risk endometrial cancer-safety and occurrence of lymphedema. Jpn J Clin Oncol. 2021;51:584–589. doi: 10.1093/jjco/hyaa252. [DOI] [PubMed] [Google Scholar]