Abstract
Objective
The efficacy of intra-abdominal cytoreductive surgery in patients with endometrial cancer and distant metastasis is equivocal. We investigated the effectiveness of such surgical treatment and whether it should be performed before or after chemotherapy (CT).
Methods
This study included patients with an International Federation of Gynecology and Obstetrics stage IVB endometrial cancer who received initial treatment at our hospital between January 2006 and December 2017.
Results
We retrospectively reviewed 67 patients with stage IVB endometrial cancer with distant metastases and classified them into preceding surgery (PS, n=23), chemotherapy followed by a surgery (CS, n=27), and CT (n=17) groups. We examined the achievement of resection with [R (1)] or without [R (0)] intra-abdominal macroscopic residue and survival. The median survival time for R (0) was 44 (95% confidence interval [CI]=9–not available [NA]) months in the PS group and 27 (95% CI=11–NA) months in the CS group. The median survival time for R (1) was 9 (95% CI=0–24) months in the PS group and 12 (95% CI=7–19) months in the CS group. The similar prognosis in both groups was worse with R (1) than with R (0). The survival curve for R (1) in the resection groups was similar to that of the CT group.
Conclusion
Achieving resection without intra-abdominal macroscopic residue for endometrial cancer with distant metastases, whether before or after CT, could extend patients’ survival.
Keywords: Endometrial Cancer, Stage IVB, Distant Metastasis, Cytoreductive Surgery, Intra-abdominal Resection
Synopsis
The benefit of intra-abdominal cytoreductive surgery for endometrial cancer with distant metastases is not clear. Even if distant metastases remain, intra-abdominal no residual resection could extent patient’s survival. Intra-abdominal no residual resection has the same prognosis whether performed before or after chemotherapy.
INTRODUCTION
Endometrial cancer is the most frequent gynecological cancer in developed countries; its prevalence has been increasing continuously [1]. According to the International Federation of Gynecology and Obstetrics (FIGO) and the Japan Society of Obstetrics and Gynecology (JSOG), approximately 80% of patients with endometrial cancer are diagnosed at an early stage with a relatively favorable prognosis [2,3]. Reports of the JSOG indicated that stage IV endometrial cancer was infrequent in 2017, accounting for 7.5% of all endometrial cancers (0.2% with IVA, 7.1% with IVB, 0.2% with unknown), with a 5-year survival rate of 21%–26% [2,3]. The prognosis was poorer for patients with stage IVB and distant metastasis than those with stage IVB and intraperitoneal dissemination. Therefore, treatments to improve the prognosis of patients with stage IVB disease and distant metastasis are needed.
Chemotherapy (CT) is the initial treatment for patients with stage IVB disease and distant metastases. In some cases, a multidisciplinary treatment that combines CT with surgery could be effective. Several retrospective studies have suggested that cytoreductive surgery for stage IV endometrial cancer improves overall survival (OS) [4,5,6]. There are few reports on intra-abdominal cytoreductive surgery for patients with stage IVB and distant metastases [7,8], none of which are prospective studies. Some patients have intra-abdominal metastases, while others have extra-abdominal metastases. The treatment strategies are often determined individually, due to differences in tumor spread and the patient’s general condition [3].
This study aimed to investigate the effectiveness of intra-abdominal cytoreductive surgery in treating endometrial cancer with distant metastasis and whether surgery should be performed before or after CT.
MATERIALS AND METHODS
1. Patient population
Patients with endometrial cancer (FIGO stage IVB) who received their first treatment at our hospital between January 2006 and December 2017 were retrospectively assessed. We reviewed medical records, including physical examination notes, CT records, operative records, pathology reports, laboratory results, and prognosis information. We excluded from the study patients with non-epithelial malignancies, other active cancers, those who received radiotherapy as the initial treatment, and those who received only palliative care due to poor general condition.
2. Study definitions
The staging was based on FIGO 2009 guidelines, and the tumor grade and histology used the World Health Organization 2014 classification [9,10]. The staging was determined before treatment by histological biopsy analysis and imaging studies. Stage IVB was divided into those with distant metastases and those with only intraperitoneal metastases. Intraperitoneal metastases: excluding metastasis vagina, pelvic serosa or adnexa. Distant metastasis: including metastasis to inguinal lymph nodes other than para-aortic or pelvic nodes [10]. Histological type I includes Endometrial Grades 1 or 2, and mucinous carcinoma. Histological type II includes Endometrial Grade 3, serous, clear, and carcinosarcoma. OS was estimated from initial treatment for metastatic endometrial cancer to the date of death or last contact.
Response to preoperative CT was evaluated using the Response Evaluation Criteria in Solid Tumors (RECIST) classification (version 1.1) [11]. Age (<65 vs. ≥65 years) was defined as previously reported [12].
We retrospectively classified the patients into 3 groups according to their initial treatment. The preceding surgery (PS) group included patients who underwent cytoreductive surgery as their initial treatment and any postoperative therapy. The chemotherapy followed by surgery (CS) group included patients who underwent cytoreductive surgery after CT. The CT group included patients who did not undergo cytoreductive surgery after CT.
3. Surgery and CT
The patient’s treatment plans were discussed with the Cancer Board based on the extent of the disease and the patient’s general condition. Those determined to be able to undergo intra-abdominal lesion resection underwent surgery and were administered CT as adjuvant therapy. CT was administered to patients whose condition precluded undergoing complete intra-abdominal lesion resection. Surgery was performed once the CT made it possible. The basic surgical procedures used for endometrial cancer were total hysterectomy, bilateral adnexectomy, omentectomy, lymph node dissection, and cytoreduction. Cytoreductive surgery included diaphragmatic, spleen, partial liver, and bowel resection. Surgery for distant metastases included partial lung resection for resectable lung metastases and femoral head replacement for femoral head metastases. Total hysterectomy, bilateral adnexectomy, and tumor reduction were performed in all patients who underwent surgery. Intra-abdominal residual disease after surgery was defined as R (0): no macroscopic residual tumor or R (1): with macroscopic residual tumor [13]. Common Terminology Criteria for Adverse Events version 5.0 was used to assess perioperative complications [14]. We performed 2–6 courses of preoperative CT. The standard treatments comprised doxorubicin/cisplatin (doxorubicin 60/m2, cisplatin 50/m2, day 1, q 21 days), docetaxel/cisplatin (docetaxel 70/m2, cisplatin 60/m2, day 1, q 21 days), or paclitaxel/cisplatin (paclitaxel 175/m2, carboplatin area under the curve of 6 mg/mL/min, day 1, q 21 days) [15].
4. Statistical analysis
Cox regression model was used, and univariate and multivariate analyses were performed to identify prognostic factors for points of initial therapy. The endpoint for efficacy of surgery was OS and was compared between the PS and CS groups (n=50) and CT group (n=17). The endpoint of prognosis after surgery was compared between PS (n=23) vs. CS (n=27) groups. Median OS was estimated using the Kaplan-Meier method, and the confidence interval (CI) was calculated using the variance obtained by the Greenwood formula. Hazard ratios (HRs) were estimated using Cox regression model, with point estimates and 2-sided 95% CIs. The Easy R, version 1.55 was used for statistical analyses [1,16].
5. Ethics statement
All patients gave informed consent before participating in this study. We conducted this study in accordance with the Declaration of Helsinki, and the protocol was accepted by the Ethics Committee of the Cancer Institute Ariake Hospital, Japanese Foundation for Cancer Research (approval number, No. 2018-1245).
RESULTS
1. Patient characteristics
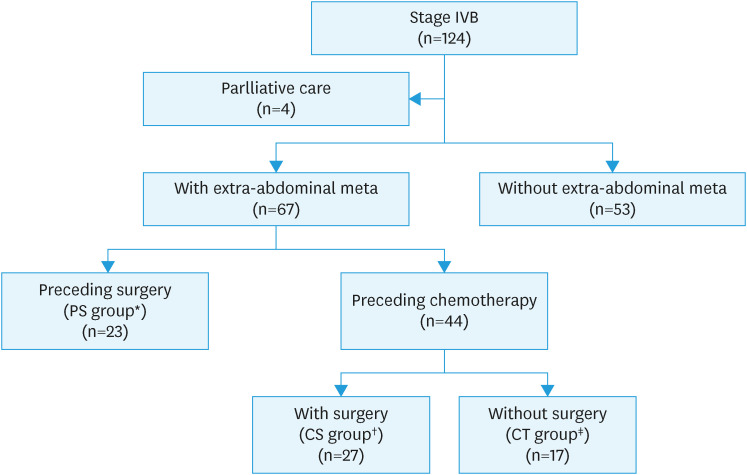
A total of 124 patients with stage IVB endometrial cancer were diagnosed at our hospital during the study period. After excluding 4 who received only palliative treatment, we examined the data of 120 patients (Fig. 1). The median age was 60.5 (range 30–85) years, and the median follow-up was 17 (range 1–131) months (Table 1). Sixty-seven patients had distant metastases, 23 in the PS group, 27 in the CS group, and 17 in the CT group (Fig. 1). In all groups, histology was more frequent in Type II than in Type I (75.6%–85.2%). The distant metastasis sites varied among groups and included the lung, liver, and bone; the rate of distant metastasis to 2 or more organs was higher in the CT group (64.7%) than in the other 2 groups (34.8% and 40.7%) (Table 1).
Fig. 1. Patient recruitment flow chart.
CS, chemotherapy followed by surgery; CT, chemotherapy; PS, preceding surgery.
*Patients who underwent surgery as the initial treatment, regardless of postoperative treatment.
†Patients who underwent cytoreductive surgery after CT.
‡Patients who did not undergo cytoreductive surgery before or after CT.
Table 1. Characteristics of patients with stage IVB endometrial cancer and extra-abdominal metastasis.
| Factor | Group | PS* | CS† | CT‡ |
|---|---|---|---|---|
| Follow-up (mo) | 17 (1–131) | |||
| Age (yr) | 60.5 (30–85) | |||
| Total | 23 | 27 | 17 | |
| Age | <65 | 13 (56.5) | 22 (81.5) | 13 (76.5) |
| ≥65 | 10 (43.5) | 5 (18.5) | 4 (23.5) | |
| Performance status | 0, 1 | 23 (100.0) | 26 (96.3) | 15 (88.2) |
| 2, 3 | 0 (0.0) | 1 (3.7) | 2 (11.8) | |
| Body mass index | <25 | 17 (73.9) | 23 (85.2) | 14 (82.4) |
| ≥25 | 6 (26.1) | 4 (14.8) | 3 (17.6) | |
| Hypertension | 7 (30.4) | 4 (14.8) | 2 (11.8) | |
| Diabetes mellitus | 2 (8.7) | 1 (3.7) | 0 (0.0) | |
| Histological type | I | 5 (21.7) | 4 (14.8) | 4 (23.5) |
| II | 18 (78.3) | 23 (85.2) | 13 (76.5) | |
| Abdominal dissemination | Without | 18 (78.3) | 10 (37.0) | 5 (29.4) |
| With | 5 (21.7) | 17 (63.0) | 12 (70.6) | |
| Distant lymph meta | Without | 16 (69.6) | 13 (48.1) | 7 (41.2) |
| With | 7 (30.4) | 14 (51.9) | 10 (58.8) | |
| Lung metastasis | Without | 9 (39.1) | 16 (59.3) | 10 (58.8) |
| With | 14 (60.9) | 11 (40.7) | 7 (41.2) | |
| Liver metastasis | Without | 21 (91.3) | 22 (81.5) | 12 (70.6) |
| With | 2 (8.7) | 5 (18.5) | 5 (29.4) | |
| Bone metastasis | Without | 15 (65.2) | 16 (59.3) | 10 (58.8) |
| With | 8 (34.8) | 11 (40.7) | 7 (41.2) | |
| No. of extra-abdominal metastasis organs | <2 | 15 (65.2) | 16 (59.3) | 6 (35.3) |
| ≥2 | 8 (34.8) | 11 (40.7) | 11 (64.7) | |
Values are presented as median (range) or number (%).
CS, chemotherapy followed by surgery; CT, chemotherapy; PS, preceding surgery.
*Patients treated by surgery as the initial treatment, regardless of postoperative treatment.
†Patients who underwent cytoreductive surgery after CT.
‡Patients who did not undergo cytoreductive surgery before or after CT.
2. Prognostic factors and survival analysis
The median OS for the entire cohort was 19 (95% CI=14–24) months, and the 5-year OS proportion was 25.3%. The respective values for the 67 patients with distant metastasis were 14 (95% CI=11–21) months and 21.6%.
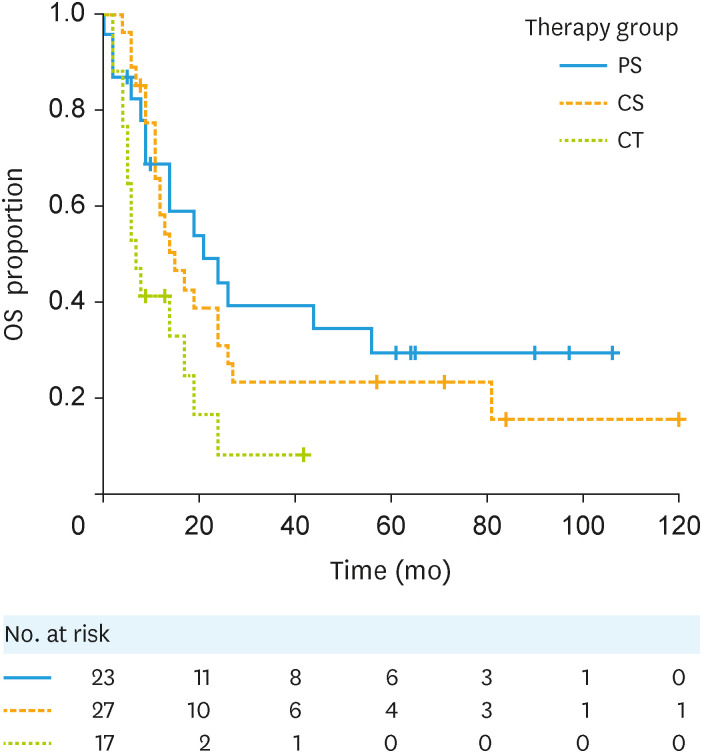
Among the 67 patients with distant metastases, the median OS in the PS group was 21 (95% CI=9–56) months, and their 5-year OS proportion was 29.4%. The respective values among the preceding CT group; the CS group was 15 (95% CI=11–24) months and 23.2%, the CT group was 7 (95% CI=4–17) months and not available (NA) (Fig. 2). Performance status of 2 or 3 was the only independent predictor of poor prognosis (HR=4.50; 95% CI=1.22–16.58). Inclusion in the PS group was not an independent prognostic factor (HR=0.83; 95% CI=0.41–1.69; Table 2).
Fig. 2. Kaplan-Meier curves for the OS of patients with stage IVB endometrial cancer with by treatment group. PS vs. CS: HR (95% CI)=1.26 (0.65–2.44). PS vs. CT: HR (95% CI)=2.54 (1.20–5.37).
CI, confidence interval; CS, chemotherapy followed by surgery; CT, chemotherapy; HR, hazard ratio; OS, overall survival; PS, preceding surgery.
Table 2. Cox multivariate analysis for prognostic factors in patients with stage IVB endometrial cancer and distant metastasis.
| Factor | HR (95% CI) |
|---|---|
| Age <65 yr | - |
| Age ≥65 yr | 1.02 (0.51–2.06) |
| Type I* | - |
| Type II† | 2.01 (0.89–4.53) |
| Performance atatus 0, 2 | - |
| Performance atatus 2, 3 | 4.50 (1.22–16.58) |
| With abdominal dissemination | 1.51 (0.82–2.79) |
| Without abdominal dissemination | - |
| Preceding chemotherapy | - |
| Preceding surgery | 0.83 (0.41–1.69) |
CI, confidence interval; HR, hazard ratio.
*Includes Endometrial Grades 1 and 2, mucinous carcinoma.
†Includes Endometrial Grade 3, serous, clear, and carcinosarcoma.
3. Treatment efficacy
The complete and partial response rate (RECIST version 1.1) was 66.7% in the CS group and 0% in the CT group (Table 3).
Table 3. Characteristics of patients after initial surgical treatment or CT.
| Factor | Group | PS* | CS† | CT‡ |
|---|---|---|---|---|
| Total | 23 | 27 | 17 | |
| Effect of preceding CT | CR/PR | NA | 18 (66.7) | 0 (0.0) |
| SD/PD | NA | 9 (33.3) | 17 (100.0) | |
| Intra-abdominal residual disease | R (0)§ | 16 (69.6) | 11 (40.7) | NA |
| R (1)∥ | 7 (30.4) | 16 (59.3) | NA | |
| Presence of distant metastases at the time of surgical treatment | Absent | 3 (13.0) | 8 (29.6) | NA |
| Present | 20 (87.0) | 19 (70.4) | NA | |
| CTCAE version 5.0 Grades 3 or 4 | With | 1 (4.3) | 0 (0.0) | NA |
| Without | 22 (95.7) | 27 (100) | NA |
Values are presented as number (%).
CR, complete response; CS, chemotherapy followed by surgery; CT, chemotherapy; CTCAE, Common Terminology Criteria for Adverse Events; PR, partial response; NA, not applicable; PD, progressive disease; SD, stable disease.
*Patients who underwent surgery as the initial treatment, regardless of postoperative treatment.
†Patients who underwent cytoreductive surgery after initial CT.
‡Patients who did not undergo cytoreductive surgery before or after CT.
§No macroscopic residual tumor.
∥With macroscopic residual tumor.
The median OS of patients who underwent cytoreductive surgery (PS + CS) was 17 (95% CI=12–26) months, significantly longer than the 7 (95% CI=4–17) months in the CT group (HR [95% CI]=2.86 [1.18–4.21]; Fig. S1) despite the high residual rate of distant metastases after intra-abdominal cytoreductive surgery (87% in the PS group and 70.4% in the CS group; Table 3).
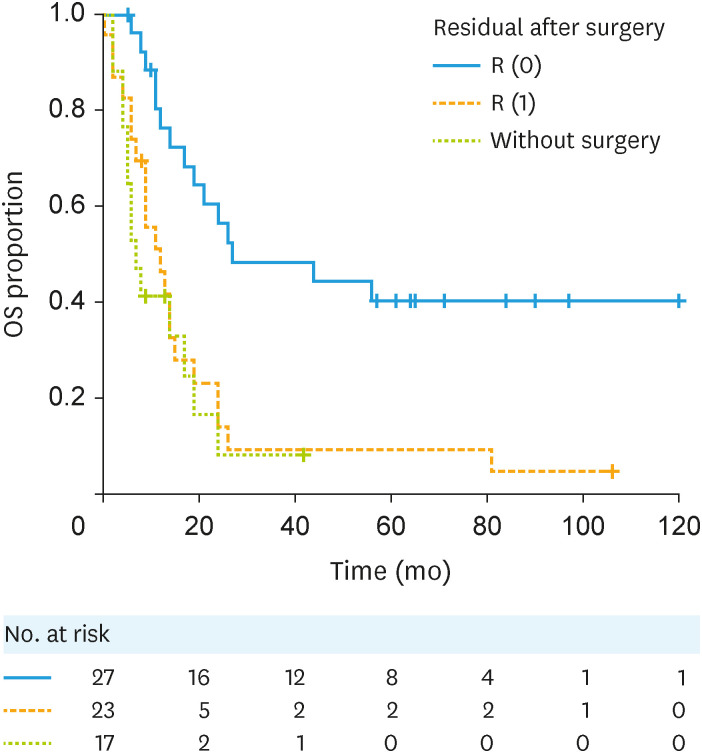
The median OS of patients (PS + CS groups) with R (0) and R (1) and those without surgical resection (CT group) were 27 (95% CI=17–NA), 12 (95% CI=7–15), and 7 (95% CI=4–17) months, respectively. The survival time of the R (0) group was significantly longer than that of the R (1) and CT groups. R (0) vs. R (1): HR (95% CI)=3.02 (1.53–5.4), R (0) vs. Without surgery: HR (95% CI)=3.83 (1.79–8.17) (Fig. 3).
Fig. 3. Kaplan-Meier curves for the OS of patients with stage IVB endometrial cancer with or without intra-abdominal residual disease after surgery. R (0): no macroscopic residual tumor; R (1): with macroscopic residual tumor. R (0) vs. R (1): HR (95% CI)=3.02 (1.53–5.94). R (0) vs. Without surgery: HR (95% CI)=3.83 (1.79–8.17).
CI, confidence interval; HR, hazard ratio; OS, overall survival.
The intra-abdominal R (0) resection rates in the PS and CS groups were 69.6% and 40.7%, respectively (Table 3). The respective median survivals of patients with R (0) and R (1) were 44 (95% CI=9–NA) and 9 (95% CI=0–24) months in the PS group and 27 (95% CI=11–NA) and 12 (95% CI=7–19) months in the CS group, with no difference between the groups in those with either R (0) or R (1). The prognosis of patients with R (1) was worse than with R (0) in both groups. The OS curve for patients with R (1) in the resection group (PS + CS) was similar to that of the CT group (Fig. S2).
DISCUSSION
The present study showed that intra-abdominal cytoreductive surgery prolonged the OS of patients with endometrial cancer and distant metastasis, especially those who achieved R (0) resection. The 5-year OS proportion at our institution was 25.3%, similar to previous reports [2,3]. Patients in this study were evaluated for surgery by the Cancer Board; however, the efficacy of intra-abdominal cytoreductive surgery for patients with distant metastases has been unclear, and judgments varied. Since the present findings suggest that intra-abdominal resection may improve prognosis, even in patients with distant metastasis, a positive decision could be made in the future. It is hoped that this process will improve the prognosis of patients with stage IVB disease.
The rate of PS in previous reports of endometrial cancer with distant metastasis was 63.6%–83.3%, higher than at our hospital (34.8%) [8,9], possibly because patients at our hospital were referred to CT before surgery if it was assumed that R (0) could not be achieved. The complete intra-abdominal surgery rate at our hospital did not differ between the PS and CS groups, and similar OS was noted regardless of whether the initial therapy was surgery or CT. These results suggest that achieving R (0) could improve prognosis regardless of the therapy sequence.
The risk of perioperative complications in endometrial cancer is associated with comorbidities, such as diabetes and hypertension; additionally, comorbidities and complications have been reported to potentially affect oncologic outcomes [17,18]. In this study, no difference was observed in comorbidities, such as diabetes and hypertension, between groups (Table 1) and the incidence of major complications in Grades 3–4 did not change with the timing of surgical treatment (Table 3).
The optimal timing for surgery in patients with endometrial cancer and distant metastasis remains unclear. In our study, the PS and CS groups had similar survival proportions (Fig. 2). Furthermore, multivariate analysis showed that inclusion in the PS group was not an independent prognostic factor. Tobias et al. [19] reported that the use of neoadjuvant chemotherapy (NACT) increased from 106 of 661 patients (16.0%; 95% CI=13.2%–18.8%) in 2010 to 224 of 938 (23.9%; 95% CI=21.2%–26.6%) in 2015 (p<0.001). However, while patients with primary debulking surgery (PDS) are at an increased risk of early death by surgery, they have a more favorable long-term prognosis. Khouri et al. [20] reported that less than half of patients undergoing NACT for endometrial cancer underwent interval debulking surgery (IDS) because the response of endometrial cancer to NACT was less sensitive than that of ovarian cancer; however, if IDS could be performed, survival was improved. Our results show that in both resection groups, the prognosis of patients with R (1) was worse than in those with R (0), with no difference between PS and CS (Fig. S2). This outcome was observed, despite the high residual rate of distant metastases after intra-abdominal cytoreductive surgery (87% in the PS group and 70.4% in the CS group; Table 3). Furthermore, the OS curve for those with R (1) was similar to that of those who did not undergo surgery (Fig. 3). These results suggest that surgery that achieves R (0) could improve survival, even in patients with endometrial cancer and distant metastases. Regardless of whether a case is PDS or IDS, surgery should be performed when it can be undertaken with the aim of achieving intra-abdominal R (0).
The number of patients in this single-center report was large, and the treatment strategy was consistent. However, this was a retrospective study, limited by the non-randomized treatment methods. Recently, molecular analysis, as well as traditional prognostic factors, have been used to individualize clinical management and treatment options for endometrial cancer [21]. The introduction of molecular/genomic profiling can help to tailor the most appropriate treatment. In the progressive/metastatic setting, molecular characterization provides important information regarding the most appropriate therapy. Among them, microsatellite instability-high/mismatch repair deficient/tumor mutation burden-high is an important (agnostic) biomarker [22]. In addition, pembrolizumab for microsatellite instability-high/mismatch repair deficient/tumor mutation burden-high and lenvatinib and pembrolizumab have become available as second-line treatments for metastatic or recurrent endometrial cancer [23]. Long-term survival has become possible for some patients with metastatic endometrial cancer with drug therapy alone. With the advent of more intense drug therapy, distant metastases are expected to be controlled and intraperitoneal debulking surgery may become more effective. Future treatment strategies may need to consider the molecular biology characteristics of patients.
ACKNOWLEDGEMENTS
For consultation on statistical analysis, contact Naoki Ishizuka and Satomi Ando. We would like to thank Editage (www.editage.com) for English language editing.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: K.M., Y.M., K.H.
- Data curation: K.M., K.N., A.Y., O.M., T.T.
- Formal analysis: K.M., K.N.
- Investigation: K.M.
- Methodology: Y.M., K.N.
- Supervision: Y.M., K.H.
- Validation: K.M., K.N.
- Writing - original draft: K.M.
- Writing - review & editing: Y.M., K.N., A.Y., O.M., T.T., K.H.
SUPPLEMENTARY MATERIALS
Kaplan-Meier curves for the OS of patients with stage IVB endometrial cancer with and without surgery. With surgery: hazard ratio (95% confidence interval)=2.86 (1.18–4.21).
Kaplan-Meier curves for the OS of patients with stage IVB endometrial cancer Whether or Not Intra-abdominal controlled surgery was achieved. R (0): no macroscopic residual tumor; R (1): with macroscopic residual tumor.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Creasman WT, Odicino F, Maisonneuve P, Quinn MA, Beller U, Benedet JL, et al. Carcinoma of the corpus uteri. FIGO 26th annual report on the results of treatment in gynecological cancer. Int J Gynaecol Obstet. 2006;95(Suppl 1):S105–S143. doi: 10.1016/S0020-7292(06)60031-3. [DOI] [PubMed] [Google Scholar]
- 3.Nagase S, Ohta T, Takahashi F, Enomoto T 2017 Committee on Gynecologic Oncology of the Japan Society of Obstetrics and Gynecology. Annual report of the committee on gynecologic oncology, the Japan Society of Obstetrics and Gynecology: annual patients report for 2015 and annual treatment report for 2010. J Obstet Gynaecol Res. 2019;45:289–298. doi: 10.1111/jog.13863. [DOI] [PubMed] [Google Scholar]
- 4.Goff BA, Goodman A, Muntz HG, Fuller AF, Jr, Nikrui N, Rice LW. Surgical stage IV endometrial carcinoma: a study of 47 cases. Gynecol Oncol. 1994;52:237–240. doi: 10.1006/gyno.1994.1038. [DOI] [PubMed] [Google Scholar]
- 5.Shih KK, Yun E, Gardner GJ, Barakat RR, Chi DS, Leitao MM., Jr Surgical cytoreduction in stage IV endometrioid endometrial carcinoma. Gynecol Oncol. 2011;122:608–611. doi: 10.1016/j.ygyno.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 6.Eto T, Saito T, Kasamatsu T, Nakanishi T, Yokota H, Satoh T, et al. Clinicopathological prognostic factors and the role of cytoreduction in surgical stage IVb endometrial cancer: a retrospective multi-institutional analysis of 248 patients in Japan. Gynecol Oncol. 2012;127:338–344. doi: 10.1016/j.ygyno.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 7.Numazaki R, Miyagi E, Konnai K, Ikeda M, Yamamoto A, Onose R, et al. Analysis of stage IVB endometrial carcinoma patients with distant metastasis: a review of prognoses in 55 patients. Int J Clin Oncol. 2009;14:344–350. doi: 10.1007/s10147-009-0878-3. [DOI] [PubMed] [Google Scholar]
- 8.Ueda Y, Enomoto T, Miyatake T, Egawa-Takata T, Ugaki H, Yoshino K, et al. Endometrial carcinoma with extra-abdominal metastasis: improved prognosis following cytoreductive surgery. Ann Surg Oncol. 2010;17:1111–1117. doi: 10.1245/s10434-009-0892-8. [DOI] [PubMed] [Google Scholar]
- 9.Creasman W. Revised FIGO staging for carcinoma of the endometrium. Int J Gynaecol Obstet. 2009;105:109. doi: 10.1016/j.ijgo.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Kurman RJ, Carcangiu ML, Herrington CS, Young RH. WHO classification of tumors of female reproductive organs. Lyon: International Agency for Research on Cancer; 2014. [Google Scholar]
- 11.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 12.Rajkumar S, Nath R, Lane G, Mehra G, Begum S, Sayasneh A. Advanced stage (IIIC/IV) endometrial cancer: role of cytoreduction and determinants of survival. Eur J Obstet Gynecol Reprod Biol. 2019;234:26–31. doi: 10.1016/j.ejogrb.2018.11.029. [DOI] [PubMed] [Google Scholar]
- 13.Eto T, Saito T, Shimokawa M, Hatae M, Takeshima N, Kobayashi H, et al. Status of treatment for the overall population of patients with stage IVb endometrial cancer, and evaluation of the role of preoperative chemotherapy: a retrospective multi-institutional study of 426 patients in Japan. Gynecol Oncol. 2013;131:574–580. doi: 10.1016/j.ygyno.2013.08.036. [DOI] [PubMed] [Google Scholar]
- 14.National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 [Internet] Bethesda, MD: National Cancer Institute; 2017. [cited 2023 Apr 23]. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf. [Google Scholar]
- 15.Nomura H, Aoki D, Susumu N, Mizuno M, Nakai H, Arai M, et al. Analysis of the relapse patterns and risk factors of endometrial cancer following postoperative adjuvant chemotherapy in a phase III randomized clinical trial. Gynecol Oncol. 2019;155:413–419. doi: 10.1016/j.ygyno.2019.09.023. [DOI] [PubMed] [Google Scholar]
- 16.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Donato V, D’Oria O, Giannini A, Bogani G, Fischetti M, Santangelo G, et al. Age-adjusted Charlson comorbidity index predicts survival in endometrial cancer patients. Gynecol Obstet Invest. 2022;87:191–199. doi: 10.1159/000525405. [DOI] [PubMed] [Google Scholar]
- 18.Giannini A, Di Donato V, Schiavi MC, May J, Panici PB, Congiu MA. Predictors of postoperative overall and severe complications after surgical treatment for endometrial cancer: the role of the fragility index. Int J Gynaecol Obstet. 2020;148:174–180. doi: 10.1002/ijgo.13020. [DOI] [PubMed] [Google Scholar]
- 19.Tobias CJ, Chen L, Melamed A, St Clair C, Khoury-Collado F, Tergas AI, et al. Association of neoadjuvant chemotherapy with overall survival in women with metastatic endometrial cancer. JAMA Netw Open. 2020;3:e2028612. doi: 10.1001/jamanetworkopen.2020.28612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khouri OR, Frey MK, Musa F, Muggia F, Lee J, Boyd L, et al. Neoadjuvant chemotherapy in patients with advanced endometrial cancer. Cancer Chemother Pharmacol. 2019;84:281–285. doi: 10.1007/s00280-019-03838-x. [DOI] [PubMed] [Google Scholar]
- 21.Golia D’Augè T, Cuccu I, Santangelo G, Muzii L, Giannini A, Bogani G, et al. Novel insights into molecular mechanisms of endometrial diseases. Biomolecules. 2023;13:499. doi: 10.3390/biom13030499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Donato V, Giannini A, Bogani G. Recent advances in endometrial cancer management. J Clin Med. 2023;12:2241. doi: 10.3390/jcm12062241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Makker V, Colombo N, Casado Herráez A, Santin AD, Colomba E, Miller DS, et al. Lenvatinib plus pembrolizumab for advanced endometrial cancer. N Engl J Med. 2022;386:437–448. doi: 10.1056/NEJMoa2108330. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Kaplan-Meier curves for the OS of patients with stage IVB endometrial cancer with and without surgery. With surgery: hazard ratio (95% confidence interval)=2.86 (1.18–4.21).
Kaplan-Meier curves for the OS of patients with stage IVB endometrial cancer Whether or Not Intra-abdominal controlled surgery was achieved. R (0): no macroscopic residual tumor; R (1): with macroscopic residual tumor.