Abstract
Aims: Serum phosphate control is crucial for the progression of vascular and valvular calcifications. Strict phosphate control is recently suggested; however, there is a lack of convincing evidence. Therefore, we explored the effects of strict phosphate control on vascular and valvular calcifications in incident patients undergoing hemodialysis.
Methods: A total of 64 patients undergoing hemodialysis from our previous randomized controlled trial were included in this study. Coronary artery calcification score (CACS) and cardiac valvular calcification score (CVCS) were evaluated using computed tomography and ultrasound cardiography at baseline and 18 months after the initiation of hemodialysis. The absolute changes in CACS (ΔCACS) and CVCS (ΔCVCS) and the percent change in CACS (%ΔCACS) and CVCS (%ΔCVCS) were calculated. Serum phosphate level was measured at 6, 12, and 18 months after the initiation of hemodialysis. Moreover, phosphate control status was evaluated using the area under the curve (AUC) by the amount of time spent with a serum phosphate level of ≥ 4.5 mg/dL and the extent to which this threshold exceeded over the observation period.
Results: ΔCACS, %ΔCACS, ΔCVCS, and %ΔCVCS were significantly lower in the low AUC group than in the high AUC group. ΔCACS and %ΔCACS were also significantly lower. ΔCVCS and %ΔCVCS tended to be lower in patients whose serum phosphate level never exceeded 4.5 mg/dL than in those whose serum phosphate level continuously exceeded 4.5 mg/dL. AUC significantly correlated with ΔCACS and ΔCVCS.
Conclusion: Consistently strict phosphate control may slow the progression of coronary and valvular calcifications in incident patients undergoing hemodialysis.
Keywords: Cardiac valvular calcification, Coronary artery calcification, Hemodialysis, Strict phosphate control
See editorial vol. 30: 1549-1551
Introduction
Cardiovascular disease (CVD) is a leading cause of death in patients undergoing hemodialysis. It is well known that cardiac calcification is an important risk factor for CVD events and mortality 1 , 2) . Cardiac calcification primarily consists of coronary artery calcification (CAC) and cardiac valvular calcification (CVC). Several patients with advanced-stage chronic kidney disease (CKD) already have CAC and CVC at the initiation of hemodialysis 3) . Moreover, these abnormalities gradually or sometimes rapidly progress as the patients undergo hemodialysis for a long time. The most important problem is that cardiac calcification is closely related to the pathophysiology that results in the occurrence of various CVDs such as coronary artery disease, aortic disease, and stroke 4 , 5) . Therefore, it is believed that halting the progression of cardiac calcification after the initiation of hemodialysis is crucial.
There are several risk factors for cardiac calcification. The classic risk factors commonly observed in patients undergoing hemodialysis are aging, diabetes mellitus, smoking, dyslipidemia, and hypertension 6) . The non-classic risk factors are anemia, uremic toxins, mineral disorders, oxidative stress, inflammation, activation of the renin-angiotensin-aldosterone and sympathetic nervous system, and volume overload. They are associated with the progression of cardiac calcification in this population 7 - 9) . Hyperphosphatemia is the most important risk factor for cardiac calcification 10 , 11) and most closely related to worse clinical outcomes 12 - 14) . The KDIGO guideline recommends lowering serum phosphate levels to the normal range in patients with CKD stage 3a-5D 15) . However, as this recommendation is primarily based on the results of observational studies, there is a lack of firm evidence regarding the target level of serum phosphorus control in patients with CKD, including patients undergoing hemodialysis.
This study aimed to investigate the relationship between consistently strict phosphate control status and CAC and CVC progression after the initiation of hemodialysis.
Methods
Study Design and Population
This study was a post hoc analysis of our previous randomized controlled trial (RCT) wherein we compared the effect of lanthanum carbonate (LC) with that of calcium carbonate (CC) on CAC and cardiac abnormalities after the initiation of hemodialysis 16) . Patients with contraindications to LC and CC and those with a history of parathyroidectomy were excluded from the previous study. Furthermore, those with insufficient data were excluded from this study. Among participants of the previous study, 64 patients whose CAC score (CACS) was ≥ 30 at baseline were included in this study. Moreover, we evaluated the CVC score (CVCS) for 34 patients who had available data. Serum phosphate levels were controlled between 3.5 and 6.0 mg/dL using appropriate phosphate binders according to the Japanese Society of Dialysis Therapy guidelines for managing chronic kidney disease-mineral and bone disorder (CKD-MBD) 17) . Our previous study was conducted according to the principles of the Declaration of Helsinki. The study protocols were approved by the Institutional Review Board of Kobe University Graduate School of Medicine (approval No. 230019). Written informed consent was obtained from all individual participants included in the study.
Evaluation of Serum Phosphate Control Status
Serum phosphate levels were evaluated at 6, 12, and 18 months after the initiation of hemodialysis. Blood samples were collected from patients before dialysis on the first day of a weekly dialysis session. To evaluate the burden of serum phosphate excursions during the observation periods, we calculated the total area under the curve (AUC) for serum phosphate levels according to the previous study 18) : total AUC=sum of the surface areas of two trapezoids created by the amount of time spent with a serum phosphate level of ≥ 4.5 mg/dL and the extent to which this threshold exceeded over the 6-month period. Subsequently, the mean monthly AUC was calculated by dividing the total AUC by 12 ( Fig.1A ) . The median AUC for all patients was 0.443. The patients were classified into two groups based on the value. Moreover, to evaluate the excursions in serum phosphate control, we counted the number of times wherein the serum phosphate level exceeded 4.5 mg/dL among three observational time points (6, 12, and 18 months) in each patient ( Fig.1B ) .
Fig.1. Evaluation of serum phosphate control.
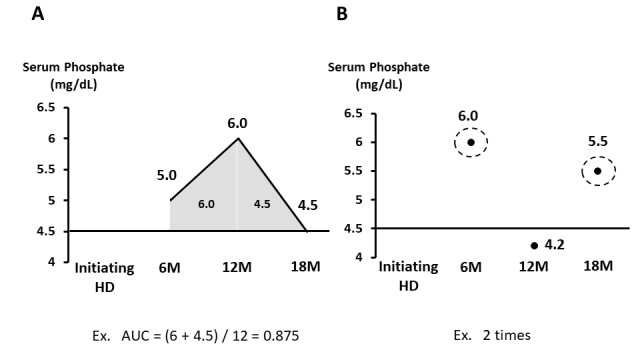
A. AUC for serum phosphate levels
B. Number of times wherein the serum phosphate level exceeded 4.5 mg/dL
AUC, area under the curve
Evaluation of Changes in CAC and CVC
CAC was evaluated using a multidetector-row computed tomography scanner (Optima CT660; GE Healthcare, Aquilion ONE; TOSHIBA), and CACS was calculated at baseline and 18 months after the initiation of hemodialysis as the Agatston score according to a previous study 19) .
CVC was evaluated by determining the calcification of the aortic valve, mitral valve, and mitral annulus by transthoracic echocardiography at baseline and 18 months after the initiation of hemodialysis, and CVCS was calculated according to the evaluation method described in a previous study 20) .
Based on the calculated CACS and CVCS, we evaluated the absolute change in CACS (ΔCACS) and CVCS (ΔCVCS) and the percentage change in CACS (%ΔCACS) and CVCS (%ΔCVCS).
Statistical Analysis
Statistical analyses were conducted using IBM SPSS Statistics version 27.0 (SPSS Inc., Illinois, USA). Continuous variables were expressed as mean±standard deviation or median (interquartile range). Student’s t-tests, χ2 test, and Wilcoxon signed-rank test were used to compare patient characteristics at baseline and changes in CACS and CVCS. Spearman’s correlation analysis was performed to investigate the correlation of AUC with ΔCACS, ΔCVCS, %ΔCACS, and %ΔCVCS. A multiple regression analysis was conducted to determine the independent factors that correlated with CACS and CVCS. P-values of <0.05 were considered statistically significant.
Results
Patient Characteristics
Table 1 shows the characteristics of study patients at baseline. A total of 64 patients were divided into two groups according to the median of AUC: low AUC group (n=32) and high AUC group (n=32). The conventional risk factors for CVD were comparable between the two groups. Regarding the CKD-MBD parameters, serum phosphate levels were significantly lower in the low AUC group than in the high AUC group (4.4±1.1 vs. 5.6±1.0 mg/dL, p<0.001). Serum calcium levels, serum intact PTH levels, and the use of phosphate binders and vitamin D agents were similar between the two groups. Moreover, baseline CACS showed no difference between the two groups. When they were divided into two groups based on the number of times wherein the serum phosphate level exceeded 4.5 mg/dL among three observational time points (0-time group or 3-times group), there were no significant differences in the clinical characteristics except for age and serum phosphate levels ( Supplementary Table 1 ) .
Table 1. Clinical characteristics between the low and high AUC groups at baseline.
| All (n= 64) | Low AUC (n= 32) | High AUC (n= 32) | P | |
|---|---|---|---|---|
| Age (year) | 67±10 | 69±10 | 65±11 | 0.088 |
| Male (%) | 50 (78.1) | 26 (81.3) | 24 (75.0) | 0.545 |
| Smoking (%) | 28 (43.8) | 13 (40.6) | 15 (46.9) | 0.614 |
| HT (%) | 63 (98.4) | 31 (96.9) | 32 (100) | 0.500 |
| DM (%) | 33 (51.6) | 14 (43.8) | 19 (59.4) | 0.211 |
| DLp (%) | 21 (32.8) | 9 (28.1) | 12 (37.5) | 0.424 |
| SBP (mmHg) | 146.2±20.8 | 145.8±17.3 | 146.4±23.8 | 0.910 |
| DBP (mmHg) | 72.7±13.5 | 73.1±11.3 | 72.3±15.3 | 0.807 |
| ACE-I/ARB (%) | 29 (45.3) | 15 (46.9) | 14 (43.8) | 0.896 |
| Statin (%) | 23 (35.9) | 12 (37.5) | 11 (34.4) | 0.872 |
| LC (%) | 29 (45.3) | 12 (37.5) | 17 (53.1) | 0.209 |
| CC (%) | 35 (54.7) | 20 (62.5) | 15 (46.9) | 0.209 |
| Sevelamer (%) | 4 (6.3) | 2 (6.3) | 2 (6.3) | 0.650 |
| Bixalomer (%) | 5 (7.8) | 2 (6.3) | 3 (9.4) | 0.555 |
| Vitamin D (%) | 31 (48.4) | 15 (46.9) | 16 (50.0) | 0.487 |
| Warfarin (%) | 3 (4.7) | 2 (6.3) | 1 (3.1) | 0.500 |
| Hb (g/dL) | 10.8±0.9 | 10.8±0.9 | 10.7±0.8 | 0.785 |
| cCa (mg/dL) | 8.9±0.6 | 8.9±0.6 | 8.8±0.6 | 0.553 |
| P (mg/dL) | 5.0±1.2 | 4.4±1.1 | 5.6±1.0 | <0.001 |
| Albumin (g/dL) | 3.8±0.3 | 3.8±0.3 | 3.8±0.3 | 0.900 |
| Intact PTH (pg/mL) | 152.8 (63.4–265.5) | 138.8 (57.6–226.1) | 173.0 (79.2–436.9) | 0.398 |
| CACS | 540.3 (149.7–1119.1) | 441.0 (109.3–1234.7) | 625.6 (185.1–1077.9) | 0.909 |
| CVCS | 2.0 (0.0–3.0) | 2.0 (0.5–4.0) | 2.0 (0.0–2.5) | 0.231 |
AUC, area under the curve; HT, hypertension; DM, diabetes mellitus; DLp, dyslipidemia; SBP, systolic blood pressure; DBP, diastolic blood pressure; ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin II type 1 receptor blocker; Hb, hemoglobin; cCa, corrected calcium; P, phosphate; PTH, parathyroid hormone; CACS, coronary artery calcium score; CVCS, cardiac valvular calcification score; CC, calcium carbonate; LC, lanthanum carbonate
Values are presented as mean±SD or median (interquartile range).
Supplementary Table 1. Clinical characteristics depending on the serum phosphate excursions.
| All (n= 33) | 0 times (n= 8) | 3 times (n= 25) | p | |
|---|---|---|---|---|
| Age (year) | 67.9±9.9 | 74.1±10.2 | 65.9±9.1 | 0.038 |
| Male (%) | 27 (81.8) | 8 (100) | 19 (76.0) | 0.160 |
| Smoking (%) | 14 (42.4) | 3 (37.5) | 11 (44.0) | 0.539 |
| HT (%) | 33 (100) | 8 (100) | 25 (100) | - |
| DM (%) | 17 (51.5) | 3 (37.5) | 14 (56.0) | 0.307 |
| DLp (%) | 9 (27.3) | 0 (0.0) | 9 (36.0) | 0.053 |
| SBP (mmHg) | 150.1±23.1 | 149.7±20.2 | 150.2±24.3 | 0.959 |
| DBP (mmHg) | 74.9±14.9 | 76.9±14.4 | 74.4±15.2 | 0.706 |
| ACE-I/ARB (%) | 19 (57.6) | 4 (50.0) | 15 (60.0) | 0.461 |
| Statin (%) | 12 (36.4) | 2 (25.0) | 10 (40.0) | 0.373 |
| Lanthanum carbonate (%) | 18 (54.5) | 4 (50.0) | 14 (56.0) | 0.541 |
| Calcium carbonate (%) | 15 (45.5) | 4 (50.0) | 11 (44.0) | 0.541 |
| Sevelamer (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
| Bixalomer (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
| Vitamin D (%) | 10 (30.3) | 2 (25.0) | 8 (32.0) | 0.539 |
| Warfarin (%) | 2 (6.1) | 1 (12.5) | 1 (4.0) | 0.432 |
| Hb (g/dL) | 8.8±1.3 | 9.2±0.8 | 8.7±1.4 | 0.074 |
| cCa (mg/dL) | 8.7±0.6 | 8.5±0.4 | 8.8±0.6 | 0.279 |
| P (mg/dL) | 5.5±1.3 | 4.7±1.6 | 5.8±1.0 | 0.018 |
| Albumin (g/dL) | 3.4±0.5 | 3.5±0.6 | 3.3±0.4 | 0.444 |
| Intact PTH (pg/mL) | 239.0 (134.7 – 397.2) | 252.6 (95.9 – 500.5) | 239.0 (140.2 – 397.2) | 0.848 |
| CACS | 632.4 (131.8 – 1144.5) | 864.8 (219.1 – 1927.3) | 632.4 (108.4 – 1098.5) | 0.352 |
| CVCS | 2.0 (0.0 – 2.25) | 1.0 (0.0 – 2.75) | 2.0 (0.0 – 2.25) | 0.721 |
AUC, area under the curve; HT, hypertension; DM, diabetes mellitus; DLp, dyslipidemia; SBP, systolic blood pressure; DBP, diastolic blood pressure; ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin II type 1 receptor blocker; Hb, hemoglobin; cCa, corrected calcium; P, phosphate; PTH, parathyroid hormone; CACS, coronary artery calcium score; CVCS, cardiac valvular calcification score.
Values are presented as mean±SD or median (interquartile range).
Effects of Serum Phosphate Control on CAC Progression
We evaluated the serum phosphate control status in two aspects: the burden and times of deviations from the strict target for phosphate control (target range of serum phosphate levels<4.5 mg/dL). To evaluate the effects of strict control of serum phosphate on CAC progression, we compared the ΔCACS and %ΔCACS between the low and high AUC groups. ΔCACS and %ΔCACS were significantly lower in the low AUC group than in the high AUC group ( Fig.2A and B ) . Furthermore, ΔCACS and %ΔCACS significantly and positively correlated with AUC ( Fig.3 ) . After adjustment by classic risk factors such as age, smoking, hyperlipidemia, and diabetes mellitus, AUC significantly correlated with ΔCACS (r=0.269, p<0.05). Even adjusted by age, sex, and baseline CACS, AUC significantly correlated with ΔCACS ( Supplementary Table 2 ) . Regarding the number of times wherein the serum phosphate level exceeded 4.5 mg/dL among the three observational time points, ΔCACS and %ΔCACS were significantly lower in patients with zero excursions than in those with three excursions ( Fig.2C and D ) .
Fig.2. Changes in CAC.
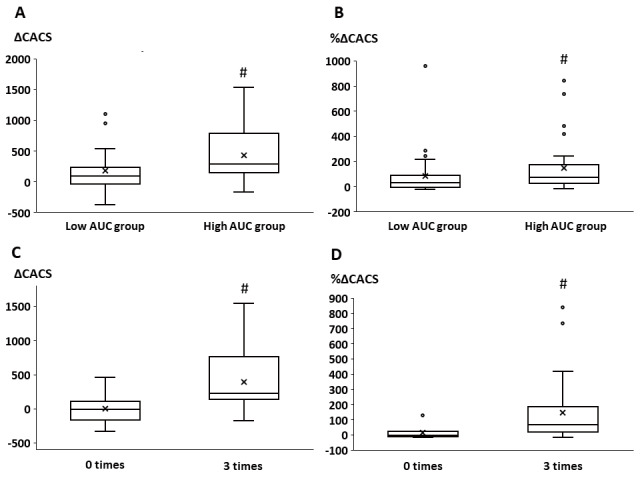
A. Changes in CACS between the low and high AUC groups
B. Percentage changes in CACS between the low and high AUC groups
C. Changes in CACS between patients with zero excursions from the target serum phosphate range and those with three excursions
D. Percentage changes in CACS between patients with zero excursions from the target serum phosphate range and those with three excursions
CAC, coronary artery calcification; CACS, coronary artery calcification score; AUC, area under the curve
#; p<0.05
Fig.3. Correlation between changes in CAC and AUC for serum phosphate levels.
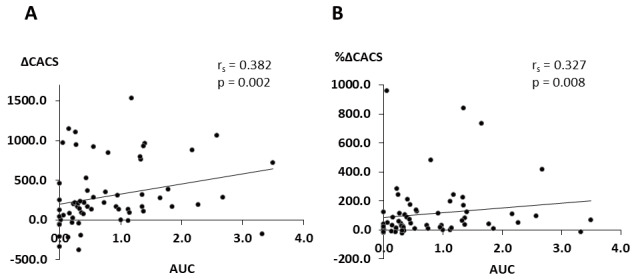
A. Relationship between changes in CACS and AUC
B. Relationship between percentage changes in CACS and AUC
CAC, coronary artery calcification; CACS, coronary artery calcification score; AUC, area under the curve
Supplementary Table 2. Correlation of vascular and valvular calcifications with clinical factors.
| ΔCACS | ΔCVCS | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| r | p | β | p | r | p | β | p | |
| Age | -0.189 | 0.135 | -0.159 | 0.263 | 0.156 | 0.377 | 0.237 | 0.217 |
| Gender | 0.013 | 0.922 | 0.087 | 0.510 | 0.148 | 0.405 | -0.005 | 0.979 |
| AUC | 0.299 | 0.016 | 0.271 | 0.035 | 0.394 | 0.021 | 0.401 | 0.025 |
| baseline CACS | -0.081 | 0.525 | -0.003 | 0.983 | -0.216 | 0.219 | -0.145 | 0.447 |
Effects of Serum Phosphate Control on CVC Progression
The low and high AUC groups showed no differences in CVCS at baseline. Other clinical characteristics except for serum phosphate levels were similar between the two groups ( Supplementary Tables 3 and 4 ) . ΔCVCS and %ΔCVCS were significantly higher in the high AUC group than in the low AUC group ( Fig.4A and B ) . ΔCVCS and %ΔCVCS were also significantly correlated with AUC ( Fig.5 ) . Even after adjustment by classic risk factors such as age, smoking, hyperlipidemia, and diabetes mellitus, AUC significantly correlated with ΔCVCS and %ΔCVCS (ΔCVCS: r=0.456, p<0.05; %ΔCVCS: r=0.492, p<0.05). Even adjusted by age, sex, and baseline CACS, AUC significantly correlated with ΔCVCS ( Supplementary Table 2 ) . Regarding the number of serum phosphate excursions, ΔCVCS and %ΔCVCS were lower in patients with zero excursions than in those with three excursions ( Fig.4C and D ) .
Supplementary Table 3. Clinical characteristics between the low AUC and high AUC groups among patients with CVCS.
| All (n= 34) | Low AUC (n= 17) | High AUC (n= 17) | p | |
|---|---|---|---|---|
| Age (year) | 69.4±9.2 | 70.9±10.0 | 67.8±8.4 | 0.342 |
| Male (%) | 26 (78.1) | 13 (76.5) | 13 (76.5) | 0.656 |
| Smoking (%) | 22 (64.7) | 12 (70.6) | 10 (58.8) | 0.473 |
| HT (%) | 34 (100.0) | 17 (100.0) | 17 (100.0) | - |
| DM (%) | 16 (47.1) | 7 (41.2) | 9 (52.9) | 0.492 |
| DLp (%) | 16 (47.1) | 6 (35.3) | 10 (58.8) | 0.169 |
| SBP (mmHg) | 145.2±19.3 | 144.5±16.9 | 145.9±22.0 | 0.828 |
| DBP (mmHg) | 68.4±11.1 | 69.7±10.8 | 67.1±11.5 | 0.503 |
| ACE-I/ARB (%) | 23 (45.3) | 11 (64.7) | 12 (70.6) | 0.714 |
| Statin (%) | 23 (67.6) | 10 (58.8) | 13 (76.5) | 0.271 |
| Lanthanum carbonate (%) | 16 (47.1) | 7 (41.2) | 9 (52.9) | 0.492 |
| Calcium carbonate (%) | 18 (52.9) | 10 (58.8) | 8 (47.1) | 0.492 |
| Sevelamer (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
| Bixalomer (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
| Vitamin D (%) | 11 (32.4) | 4 (23.5) | 7 (41.2) | 0.271 |
| Warfarin (%) | 3 (8.8) | 2 (11.8) | 1 (5.9) | 0.500 |
| Hb (g/dL) | 8.7±1.2 | 8.7±1.3 | 8.9±1.2 | 0.568 |
| cCa (mg/dL) | 8.8±0.5 | 8.8±0.7 | 8.7±0.4 | 0.469 |
| P (mg/dL) | 5.7±1.3 | 5.3±1.4 | 6.2±0.9 | 0.029 |
| Albumin (g/dL) | 3.3±0.4 | 3.2±0.4 | 3.4±0.5 | 0.425 |
| Intact PTH (pg/mL) | 235.0 (133.0 – 335.0) | 262.0 (109.0 – 389.0) | 231.0 (157.0 – 330.0) | 0.973 |
| CACS | 549.7 (141.8 – 1221.2) | 480.7 (142.5 – 1840.9) | 618.7 (131.8 – 1074.9) | 0.518 |
| CVCS | 2.0 (0.0 – 3.0) | 2.0 (0.5 – 4.0) | 2.0(0.0 – 2.5) | 0.231 |
AUC, area under the curve; HT, hypertension; DM, diabetes mellitus; DLp, dyslipidemia; SBP, systolic blood pressure; DBP, diastolic blood pressure; ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin II type 1 receptor blocker; Hb, hemoglobin; cCa, corrected calcium; P, phosphate; PTH, parathyroid hormone; CACS, coronary artery calcium score; CVCS, cardiac valvular calcification score.
Values are presented as mean±SD or median (interquartile range).
Supplementary Table 4. Clinical characteristics depending on the serum phosphate excursions among patients with CVCS.
| All (n= 18) | 0 time (n= 4) | 3 times (n= 14) | p | |
|---|---|---|---|---|
| Age (year) | 70.0±9.2 | 78.3±6.7 | 67.6±8.5 | 0.018 |
| Male (%) | 15 (83.3) | 4 (100.0) | 11 (78.6) | 0.446 |
| Smoking (%) | 11 (61.1) | 3 (75.0) | 8 (57.1) | 0.485 |
| HT (%) | 18 (100.0) | 4 (100.0) | 14 (100.0) | - |
| DM (%) | 8 (44.4) | 2 (50.0) | 6 (42.9) | 0.618 |
| DLp (%) | 7 (38.9) | 0 (0.0) | 7 (50.0) | 0.108 |
| SBP (mmHg) | 145.7±20.3 | 144.3±20.9 | 146.1±20.9 | 0.875 |
| DBP (mmHg) | 67.6±10.2 | 66.8±6.7 | 67.8±11.2 | 0.864 |
| ACE-I/ARB (%) | 11 (61.1) | 2 (50.0) | 9 (64.3) | 0.515 |
| Statin (%) | 12 (66.7) | 2 (50.0) | 10 (71.4) | 0.407 |
| Lanthanum carbonate (%) | 10 (55.6) | 2 (50.0) | 8 (57.1) | 0.618 |
| Calcium carbonate (%) | 8 (44.4) | 2 (50.0) | 6 (42.9) | 0.618 |
| Sevelamer (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
| Bixalomer (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
| Vitamin D (%) | 7 (38.9) | 2 (50.0) | 5 (35.7) | 0.515 |
| Warfarin (%) | 2 (11.1) | 1 (25.0) | 1 (7.1) | 0.405 |
| Hb (g/dL) | 9.0±1.1 | 9.2±0.8 | 8.9±1.3 | 0.705 |
| cCa (mg/dL) | 8.6±0.4 | 8.3±0.2 | 8.7±0.4 | 0.053 |
| P (mg/dL) | 5.7±1.2 | 4.3±1.1 | 6.1±1.0 | 0.005 |
| Albumin (g/dL) | 3.4±0.5 | 3.4±0.4 | 3.4±0.5 | 0.979 |
| Intact PTH (pg/mL) | 235.0 (158.8 – 335.0) | 161.0 (36.8 – 484.8) | 239.0 (179.0 – 335.0) | 0.442 |
| CACS | 510.1 (139.4 – 1122.0) | 821.4 (219.1 – 1927.3) | 495.8 (104.5 – 1058.7) | 0.327 |
| CVCS | 2.0 (0.0 – 2.25) | 1.0 (0.0 – 2.75) | 2.0 (0.0 – 2.25) | 0.721 |
AUC, area under the curve; HT, hypertension; DM, diabetes mellitus; DLp, dyslipidemia; SBP, systolic blood pressure; DBP, diastolic blood pressure; ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin II type 1 receptor blocker; Hb, hemoglobin; cCa, corrected calcium; P, phosphate; PTH, parathyroid hormone; CACS, coronary artery calcium score; CVCS, cardiac valvular calcification score.
Values are presented as mean±SD or median (interquartile range).
Fig.4. Changes in CVC.
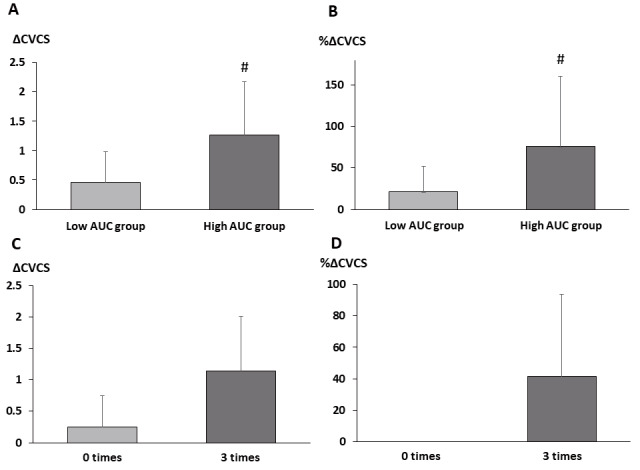
A. Changes in CVCS between the low and high AUC groups
B. Percentage changes in CVCS between the low and high AUC groups
C. Changes in CVCS between patients with zero excursions from the target serum phosphate range and those with three excursions
D. Percentage changes in CVCS between patients with zero excursions from the target serum phosphate range and those with three excursions
CVC, cardiac valvular calcification; CVCS, cardiac valvular calcification score; AUC, area under the curve
#; p<0.05
Fig.5. Correlation between changes in CVC and AUC for serum phosphate levels.
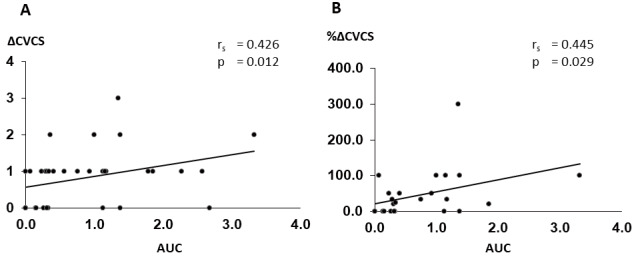
A. Relationship between changes in CVCS and AUC
B. Relationship between percentage changes in CVCS and AUC
CVC, cardiac valvular calcification; CVCS, cardiac valvular calcification score; AUC, area under the curve
Discussion
Our study demonstrated the following: (1) the progression of coronary and valvular calcifications was significantly slower in the low AUC group than in the high AUC group, (2) the changes in CACS were significantly lower and the changes in CVCS tended to be lower in patients whose serum phosphate level never exceeded 4.5 mg/dL than in those whose serum phosphate level continuously exceeded 4.5 mg/dL, and (3) AUC significantly correlated with the changes in CACS and CVCS.
In the clinical setting, we must focus on the serum phosphate level because it significantly contributes to CVD progression, such as vascular and valvular calcifications 11) . Phosphate is taken up into vascular smooth muscle cells (VSMCs) through the sodium-dependent phosphate cotransporter, PiT-1, on the surface of VSMCs and induces cellular apoptosis and the transformation of VSMCs into osteoblast-like cells 21 , 22) . These changes may be dependent on phosphate concentrations. These pathophysiological alterations in VSMCs lead to the progression of vascular calcification. Similar to that in VSMCs, phosphate also induces the transformation of interstitial valvular cells into osteoblast-like cells 23 , 24) . In addition to these changes, a decrease in the levels of inhibitors against vascular calcification also causes the progression of vascular and valvular calcifications 25) . Phosphate is also known to be related to both vascular intimal and medial calcifications 26 , 27) . Mild-to-moderate coronary intimal calcification is associated with coronary events, such as acute myocardial infarction and unstable angina, and severe intimal and medial calcifications are related to impaired coronary microvascular circulation 28) . Therefore, an appropriate control of serum phosphate levels may be essential in patients with CKD.
There is extensive research on the association between phosphate and vascular calcifications; however, several studies have reported that serum phosphate is an essential risk factor for valvular calcification. For instance, a large study on 6814 people without CVD demonstrated that higher serum phosphate levels were associated with a significantly greater prevalence of aortic valve calcification 29) . Moreover, another study reported that higher serum phosphate levels that were within the normal range were associated with valvular and annular calcifications in a community-based cohort of older adults 30) . Our recent study, which used the same original data as the present study, also indicated that changes in CVCS significantly correlated with average phosphate levels and were significantly higher in the high serum phosphate group than in the low serum phosphate group 20) . Altogether, phosphate control might be a crucial strategy to prevent vascular and valvular calcifications. The results of this study may support these findings.
There are several investigations regarding the target range of serum phosphate levels in patients undergoing hemodialysis. For example, data from the United States indicated that serum phosphorus concentrations of >5.0 mg/dL were associated with an increased relative risk of death 31) and those of >4.5 mg/dL with an increased relative risk of developing CVD 32) . A recent study based on data from European countries also reported that serum phosphate levels of 4.4 mg/dL were associated with the minimum relative risk of mortality 33) . Similarly, a Japanese study demonstrated that hazard ratios for mortality were significantly higher in patients with serum phosphate levels of >5.0 mg/dL than in those with serum phosphate levels of 4.0–4.9 mg/dL 34) . Furthermore, a recent RCT on patients undergoing hemodialysis demonstrated that the changes in CAC were significantly smaller in the strict phosphate control group (3.5–4.5 mg/dL) than in the standard phosphate control group (5.0–6.0 mg/dL) 35) . Based on these findings, we considered <4.5 mg/dL as the target serum phosphate level.
As mentioned earlier, although the control of serum phosphate levels is necessary to improve clinical outcomes in patients undergoing hemodialysis, they show frequent fluctuations in a time-dependent manner. Consequently, the evaluation of serum phosphate levels is a difficult and particularly important issue. There are several methods for evaluating the serum phosphate control status, but it is important to consider those that are more accurate and related to clinical outcomes 36) . A recent study reported the association between the calculated AUC by multiplying the time spent with serum phosphate levels of >4.5 mg/dL over a 6-month run-in period by the extent to which this threshold exceeded and CVD mortality 18) . The result of that study demonstrated that the adjusted hazard ratio of CVD mortality was significantly higher in patients whose monthly average AUC was >1 than in those whose monthly average AUC was 0. Similarly, we also evaluated the association between monthly average AUC and CAC and CVC progression. Consistent with the results of that study, our findings also indicated significant associations between these parameters. In addition, a recent review advocated that clinicians should take necessary action when a patient’s phosphate level exceeds the target range multiple times in a certain time period 36) . Therefore, it is also important to evaluate the amount of time spent within target serum phosphate levels in a certain time period. In fact, one study demonstrated a significant association between the time of achieving the guideline-recommended target phosphate range and all-cause death 12) . Even in this study, the progression of CAC and CVC was slower in patients whose serum phosphate level never exceeded 4.5 mg/dL than in those whose serum phosphate level continuously exceeded 4.5 mg/dL. These findings have drawn further attention to the importance of strict phosphate control in patients undergoing hemodialysis.
This study has some limitations. First, because the number of enrolled patients was relatively small, we could not perform statistically sufficient adjustments. However, as this study is a part of a previous RCT, the study patients were closely followed up and the quality of data was reliable. Second, this is not a prospective study but a post hoc analysis of our previous study. Therefore, to ascertain the clinical implication of consistently strict phosphate control for coronary and valvular calcifications, it is necessary to conduct a further prospective study in the near future. Third, we could not distinguish coronary intimal lesions from medial lesions. In this study, CACS was supposed to consist both intimal and medial calcifications because it is impossible to distinguish coronary intimal lesions from medial lesions using the currently available clinical imaging modalities. This remains a crucial issue in the clinical setting because both are important for the occurrence of CVD. Finally, since this study compared only baseline data and does not include all confounding factors, we cannot rule out the possibility that the serial changes in confounding factors and unknown confounding factors might influence the results.
Conclusion
The findings of our study suggested that consistently strict phosphate control slows the progression of coronary and valvular calcifications in incident patients undergoing hemodialysis.
Competing Interests
H. F. received speaker fees as honoraria from Kyowa Kirin, Bayer, Kissei, and Astellas and we received scholarship grants from Kyowa Kirin, Bayer and Chugai.
Funding
This research was partly supported by Bayer.
Author Contributions
M.S. wrote the text. H.F. revised it critically for important intellectual content. S.G. and K.K. analyzed the data. K.W. and K.S. interpreted the data. S.N. drafted this study. All co-authors reviewed and approved this paper.
References
- 1).Rennenberg R, Kessels A, Schurgers, Van Engelshoven J, de Leeuw P, Kroon A. Vascular calcifications as a marker of increased cardiovascular risk: a meta-analysis. Vasc Health Risk Manag, 2009; 5: 185-197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Raggi P, Bellasi A, Gamboa C, Ferramosca E, Ratti C, Block GA, Muntner P. All-cause mortality in hemodialysis patients with heart valve calcification. Clin J Am Soc Nephrol, 2011; 6: 1990-1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Kitamura K, Fujii H, Nakai K, Kono K, Goto S, Nishii T, Kono A, Nishi S. Relationship between cardiac calcification and left ventricular hypertrophy in patients with chronic kidney disease at hemodialysis initiation. Heart Vessels, 2017; 32: 1109-1116 [DOI] [PubMed] [Google Scholar]
- 4).Mehta A, Pandey A, Ayers CR, Khera A, Sperling LS, Szklo MS, Gottesman RF, Budoff MJ, Blaha MJ, Blumenthal RS, Nasir K, Joshi PH. Predictive Value of Coronary Artery Calcium Score Categories for Coronary Events Versus Strokes: Impact of Sex and Race: MESA and DHS. Circ Cardiovasc Imaging, 2020; 13: e010153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Lei MH, Wu YL, Chung SL, Chen CC, Chen WC, Hsu YC. Coronary Artery Calcium Score Predicts Long-Term Cardiovascular Outcomes in Asymptomatic Patients with Type 2 Diabetes. J Atheroscler Thromb, 2021; 28: 1052-1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Drüeke TB, Massy ZA. Atherosclerosis in CKD: differences from the general population. Nat Rev Nephrol, 2010; 6: 723-735 [DOI] [PubMed] [Google Scholar]
- 7).Chade AR, Lerman A, Lerman LO. Kidney in early atherosclerosis. Hypertension, 2005; 45: 1042-1049 [DOI] [PubMed] [Google Scholar]
- 8).Kon V, Linton MF, Fazio S. Atherosclerosis in chronic kidney disease: the role of macrophages. Nat Rev Nephrol, 2011; 7: 45-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Fujii H, Goto S, Fukagawa M. Role of Uremic Toxins for Kidney, Cardiovascular, AND Bone Dysfunction. Toxins, 2018; 10: 202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Shang D, Xie Q, Ge X, Yan H, Tian J, Kuang D, Hao CM, Zhu T. Hyperphosphatemia as an independent risk factor for coronary artery calcification progression in peritoneal dialysis patients. BMC Nephrol, 2015; 16: 107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Adeney KL, Siscovick DS, Ix JH, et al. Association of serum phosphate with vascular and valvular calcification in moderate CKD. J Am Soc Nephrol, 2009; 20: 381-387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Taniguchi M, Fukagawa M,Fujii N, Hamano T, Shoji T, Yokoyama K, Nakai S, Shigematsu T, Iseki K, Tsubakihara Y. Serum phosphate and calcium should be primarily and consistently controlled in prevalent hemodialysis patients. Ther Apher Dial, 2013; 17: 221-228 [DOI] [PubMed] [Google Scholar]
- 13).Dhingra R, Sullivan LM, Fox CS, Wang TJ, D’Agostino RB Sr, Gaziano JM, Vasan RS. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med, 2007; 167: 879-885 [DOI] [PubMed] [Google Scholar]
- 14).Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation, 2005; 112: 2627-2633 [DOI] [PubMed] [Google Scholar]
- 15).KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl (2011), 2017: 7; 1-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Fujii H, Kono K, Nakai K, Goto S, Tatsuya Nishii T, Kono A, Nishi S. Effects of Lanthanum Carbonate on Coronary Artery Calcification and Cardiac Abnormalities After Initiating Hemodialysis. Calcif Tissue Int, 2018; 102: 310-320 [DOI] [PubMed] [Google Scholar]
- 17).Fukagawa M, Yokoyama K, Koiwa F, Taniguchi M, Shoji T, Kazama JJ, Komaba H, Ando R, Kakuta T, Fujii H, Nakayama M, Shibagaki Y, Fukumoto S, Fujii N, Hattori M, Ashida A, Iseki K, Shigematsu T, Tsukamoto Y, Tsubakihara Y, Tomo T, Hirakata H, Akizawa T. Clinical practice guideline for the management of chronic kidney disease-mineral and bone disorder. Ther Apher Dial, 2013; 17: 247-288 [DOI] [PubMed] [Google Scholar]
- 18).Lopes MB, Karaboyas A, Bieber B, Pisoni RL, Walpen S, Fukagawa M, Christensson A, Evenepoel P, Pegoraro M, Robinson BM, Pecoits-Filho R. Impact of longer term phosphorus control on cardiovascular mortality in hemodialysis patients using an area under the curve approach: results from the DOPPS. Nephrol Dial Transplant, 2020; 35: 1794-1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol, 1990; 15: 827-832 [DOI] [PubMed] [Google Scholar]
- 20).Watanabe K, Fujii H, Kono K, Goto S, Nishi S. Comparison of the effects of lanthanum carbonate and calcium carbonate on the progression of cardiac valvular calcification after initiation of hemodialysis. BMC Cardiovasc Disord, 2020; 20: 39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Jono S, McKee MD, Murry CE, Shioi A, Nishizawa Y, Mori K, Morii H, Giachelli CM. Phosphate regulation of vascular smooth muscle cell calcification. Circ Res, 2000; 87: E10-17 [DOI] [PubMed] [Google Scholar]
- 22).Jimbo R, Kawakami-Mori F, Mu S, Hirohama D, Majtan B, Shimizu Y, Yatomi Y, Fukumoto S, Fujita T, Shimosawa T. Fibroblast growth factor 23 accelerates phosphate-induced vascular calcification in the absence of Klotho deficiency. Kidney Int, 2014; 85: 1103-1111 [DOI] [PubMed] [Google Scholar]
- 23).Rajamannan NM, Evans FJ, Aikawa E, Grande-Allen KJ, Demer LL, Heistad DD, Simmons CA, Masters KS, Mathieu P, O’Brien KD, Schoen FJ, Towler DA, Yoganathan AP, Otto CM. Calcific aortic valve disease: not simply a degenerative process: A review and agenda for research from the National Heart and Lung and Blood Institute Aortic Stenosis Working Group. Executive summary: Calcific aortic valve disease-2011 update. Circularion, 2011; 124: 1783-1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Otto CM. Calcific aortic stenosis--time to look more closely at the valve. N Engl J Med, 2008; 359: 1395-1398 [DOI] [PubMed] [Google Scholar]
- 25).Reynolds JL, Joannides AJ, Skepper JN, McNair R, Schurgers LJ, Proudfoot D, Jahnen-Dechent W, Weissberg PL, Shanahan CM. Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: a potential mechanism for accelerated vascular calcification in ESRD. J Am Soc Nephrol, 2004; 15: 2857-2867 [DOI] [PubMed] [Google Scholar]
- 26).Phan O, Ivanovski O, Nguyen-Khoa T, Mothu N, Angulo J, Westenfeld R, Ketteler M, Meert N, Maizel J, Nikolov IG, Vanholder R, Lacour B, Drüeke TB, Massy ZA. Sevelamer prevents uremia-enhanced atherosclerosis progression in apolipoprotein E-deficient mice. Circulation, 2005; 112: 2875-2882 [DOI] [PubMed] [Google Scholar]
- 27).Lanzer P, Hannan FM, Lanzer JD, Janzen J, Raggi P, Furniss D, Schuchardt M, Thakker R, Fok PW, Saez-Rodriguez J, Millan A, Sato Y, Ferraresi R, Virmani R, St Hilaire C. Medial Arterial Calcification: JACC State-of-the-Art Review. J Am Coll Cardiol, 2021; 78: 1145-1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Fujii H, Kono K, Nishi S. Characteristics of coronary artery disease in chronic kidney disease. Clin Exp Nephrol, 2019; 23: 725-732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Linefsky JP, O’Brien KD, Sachs M, Katz R, Eng J, Michos ED, Budoff MJ, de Boer IH, Kestenbaum B. Serum phosphate is associated with aortic valve calcification in the Multi-ethnic Study of Atherosclerosis (MESA). Atherosclerosis, 2014; 233: 331-337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Linefsky JP, O’Brien KD, Katz R, de Boer IH, Barasch E, Jenny NS, Siscovick DS, Kestenbaum B. Association of serum phosphate levels with aortic valve sclerosis and annular calcification: the cardiovascular health study. J Am Coll Cardiol, 2011; 58: 291-297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31).Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol, 2004; 15: 2208-2218 [DOI] [PubMed] [Google Scholar]
- 32).Slinin Y, Foley RN, Collins AJ. Calcium, phosphorus, parathyroid hormone, and cardiovascular disease in hemodialysis patients: the USRDS waves 1, 3, and 4 study. J Am Soc Nephrol, 2005; 16: 1788-1793 [DOI] [PubMed] [Google Scholar]
- 33).Fernández-Martín JL, Martínez-Camblor P, Dionisi MP, Floege J, Ketteler M, London G, Locatelli F, Gorriz JL, Rutkowski B, Ferreira A, Bos WJ, Covic A, Rodríguez-García M, Sánchez JE, Rodríguez-Puyol D, Cannata-Andia JB. Fernández-Martín JL, et al. Improvement of mineral and bone metabolism markers is associated with better survival in haemodialysis patients: the COSMOS study. Nephrol Dial Transplant, 2015; 30: 1542-1551 [DOI] [PubMed] [Google Scholar]
- 34).Nakai S, Akiba T, Kazama J, Yokoyama K, Fukagawa M, Tominaga Y, Iseki K, Tsubakihara Y. Effects of serum calcium, phosphorous, and intact parathyroid hormone levels on survival in chronic hemodialysis patients in Japan. Ther Apher Dial, 2008; 12: 49-54 [DOI] [PubMed] [Google Scholar]
- 35).Isaka Y, Hamano T, Fujii H, Tsujimoto Y, Koiwa F, Sakaguchi Y, Tanaka R, Tomiyama N, Tatsugami F, Teramukai S. Optimal Phosphate Control Related to Coronary Artery Calcification in Dialysis Patients. J Am Soc Nephrol, 2021; 32: 723-735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Martin KJ. How to evaluate phosphate control in patients on dialysis. Nephrol Dial Transplant, 2022; 37: 1830-1832 [DOI] [PMC free article] [PubMed] [Google Scholar]


