Abstract
Oral pre-exposure prophylaxis (PrEP) is recommended for persons at substantial risk for HIV, including female sex workers (FSW), men who have sex with men (MSM), people who inject drugs (PWID), and transgender women (TGW). We report on a PrEP demonstration project at seven clinics in the Democratic Republic of the Congo. Routinely collected data were abstracted to assess PrEP uptake, scheduled visit attendance, and self-reported adherence. Between February and May 2018, 469 eligible clients were offered daily oral PrEP; 75.1% accepted: 78.7% FSW, 20.5% MSM, and 0.9% TGW. Two percent also identified as PWID. Attendance was 64.5% at one-month visits; 82.1% at three-month visits; and among 47.7% of clients who initiated PrEP at least six months before data abstraction, 85.8% at six-month visits. Among 66.3% of clients with at least one adherence assessment, 39% self-reported low adherence. Results demonstrate the acceptability of PrEP delivered in healthcare settings serving FSW, MSM, PWID, and TGW.
Keywords: Pre-exposure prophylaxis, HIV prevention, Democratic Republic of Congo, key populations, female sex workers, men who have sex with men, people who inject drugs
Introduction
Daily oral pre-exposure prophylaxis (PrEP) reduces HIV acquisition (Riddell et al., 2018). Yet globally PrEP use is low among groups at elevated risk for HIV (Djomand et al., 2020) including female sex workers (FSW) (Eakle et al., 2017; Kyongo et al., 2018), men who have sex with men (MSM) (Coulaud et al., 2018; Ogunbajo et al., 2019), transgender women (TGW) (Baral et al., 2013), and people who inject drugs (PWID) (Deryabina & El-Sadr, 2019; Escudero et al., 2014). This is a critical gap, as key populations (KP) have higher HIV prevalence and incidence compared to general populations, due to behavioral risk and high levels of poverty, criminalization, and pervasive stigma and discrimination, including in healthcare settings, that limit access to prevention resources (Brown & Peerapatanapokin, 2019). Evaluations of PrEP services can inform policies and programs to make effective prevention interventions accessible to KP most impacted by HIV (Case et al., 2019).
The Democratic Republic of Congo (DRC) has a low prevalence of HIV (0.8%) in the general population, with a much higher prevalence among FSW (7.5%), MSM (7.5%), and PWID (3.9%) (UNAIDS). To improve KP engagement in HIV services, the DRC Ministry of Public Health (MOH) designates several health facilities throughout the country as “KP-friendly” centers providing outreach, peer education, and testing, prevention, and treatment for HIV and other sexually transmitted infections (STI) (Programme National de Lutte Contre le VIH/SIDA et IST, 2017). To inform incorporation of PrEP into DRC’s national HIV guidelines, the MOH, ICAP at Columbia University, and the U.S. Centers for Disease Control and Prevention collaborated in a demonstration project of the first facility-based PrEP services for adults aged 18 years and older identifying as KP members. We report on the implementation and six-month outcomes at seven facilities.
Methods
The demonstration project introduced PrEP as a daily fixed-dose combination tablet of emtricitabine 200 mg-tenofovir disoproxil fumarate 300 mg and related services at four KP-friendly clinics in Kinshasa and three in Lubumbashi, for approximately 350 KP clients across all sites. The target number of PrEP clients was determined by a forecast of available PrEP medication supply. PrEP was offered in combination with other HIV prevention services, such as condoms and lubricant. PrEP-specific standard operating procedures, monitoring tools, and comprehensive training materials were developed (ICAP at Columbia University, 2017), and facility staff were trained on PrEP eligibility, initiation and follow-up, adherence counseling, and monitoring. Tools and training materials built on lessons learned in HIV care and treatment programs, such as the need to monitor visit attendance, address side effects, support adherence, and deliver KP-tailored HIV risk-reduction counseling.
Clinic peer workers were trained to educate KP on PrEP during outreach; healthcare workers also educated individuals testing HIV-negative about PrEP. PrEP eligibility assessment included: current behavioral risk for HIV acquisition, signs of acute HIV infection (AHI), willingness to take PrEP daily as prescribed, and allergies or contraindications to the regimen (WHO, 2017). Eligible clients were immediately initiated on PrEP, underwent screening and treatment for STIs, and received PrEP adherence and HIV risk-reduction counseling and condoms and lubricant.
PrEP clients were instructed to return for a one-month follow-up visit, including HIV testing, evaluation for AHI, STI screening and treatment, HIV risk assessment for continued PrEP eligibility, and risk reduction counseling and provision of condoms and lubricant. They were asked to report missed doses (fewer than two, or two or more pills over the past seven days), counseled on adherence, given a 60-day refill of PrEP, and instructed to return in 60 days. At that visit, clients again received HIV testing and eligibility assessment, risk reduction and adherence services, and a 90-day refill of PrEP. Thereafter follow-up visits were scheduled at 90-day intervals. A dedicated PrEP coordinator worked closely with the clinics in each city to build capacity and address challenges. Enhanced outreach including calls, texts, and home visits to remind clients of upcoming visits and reschedule missed appointments was initiated after attendance at one-month visits was reviewed and gaps were noted.
To evaluate the demonstration project, trained data clerks abstracted routinely-collected data from clinic registers and client visit forms. Analyses were conducted using SAS version 9.4. We report demographic and KP sub-population characteristics; proportion of eligible clients accepting PrEP; and proportions attending scheduled follow-up visits at one month, three months and six months post-initiation. We also examined the completeness of clinical HIV testing and self-reported adherence assessments at each follow-up visit. Visit attendance at one month was defined as a documented clinic visit at one-month post-initiation within a window of 14 days before or after, and within 30 days before or after at the scheduled three- and six-month visits post-initiation. Those who were re-engaged through enhanced outreach were re-scheduled for a follow-up visit, which was recorded as one-, three-, or six-months post-initiation, according to the schedule window.
All clients who started PrEP at least three months prior to data abstraction were included in estimates of attendance at one- and three-month visits. To analyze attendance at the six-month visit, only clients who had started PrEP at least six months prior to data abstraction were included. Self-reported adherence was defined as low if the client reported two or more pills missed over the seven days preceding a study visit; high adherence was defined as fewer than two pills missed. The project was not designed to compare outcomes by KP group; thus, results are presented using descriptive statistics without tests of statistical significance. The DRC MOH National Ethics Committee for Health and the Columbia University Institutional Review Board provided ethical review and approval for the demonstration project. A waiver of consent was granted for retrospective review of existing client-level data.
Results
Between February and May 2018, 469 eligible KP clients were offered oral daily PrEP. Among these clients, 352 (75.1%) accepted, 201 (57.1%) in Kinshasa and 151 (42.9%) in Lubumbashi. Among the 117 declining PrEP, 58 (49.6%) indicated that they were not interested in daily medication, and 35 (29.9%) did not believe they needed PrEP. Clients initiating PrEP included 277 (78.7%) FSW, 72 (20.5%) MSM, and three (0.9%) TGW. Six FSW and 1 MSM (2.0%) were also categorized as PWID. Nearly all (346, 98.3%) clients reported sex work as the main income source and all reported having sex with men, including 17.0% who were men reporting sex with men and women. The median age was 30 years (interquartile range [IQR] 24–35) (Table 1).
Table 1.
Characteristics of PrEP clients (N = 352).
| n | % | |
|---|---|---|
| Gender | ||
| Female | 277 | 78.7 |
| Male | 72 | 20.5 |
| Transgender woman | 3 | 0.9 |
| Age (years) | ||
| Median (IQR) | 30 (24–35) | |
| 18–19 | 19 | 5.4 |
| 20–24 | 68 | 19.3 |
| 25–29 | 86 | 24.4 |
| 30–34 | 79 | 22.4 |
| 35–39 | 55 | 15.6 |
| 40–44 | 23 | 6.5 |
| 45+ | 22 | 6.3 |
| Have sex with | ||
| Men only | 292 | 83.0 |
| Men and women | 60 | 17.0 |
| Selling sex is main incomea | ||
| Yes | 346 | 98.3 |
| No | 5 | 1.4 |
| Refused to reply | 1 | 0.3 |
| Injected drugsa | ||
| Yes | 7 | 2.0 |
| No | 336 | 95.5 |
| Refused to reply | 9 | 2.6 |
| Marital status | ||
| Single | 278 | 79.0 |
| Married or cohabitating | 24 | 6.8 |
| Divorced/separated/widowed | 32 | 9.1 |
| Missing | 18 | 5.1 |
Past six months.
Overall, 64.5% of clients who started PrEP attended the one-month visit: 62.1% of FSW, 73.6% of MSM, and 66.7% of TGW. The three-month visit was attended by 289 (82.1%) of 352 clients initiated, including 225 (81.2%) FSW and 64 (88.9%) MSM. Among clients attending the three-month visit, 91 (31.5%) had missed the one-month visit (data not shown) and were reengaged through outreach. Among 168 (47.7%) clients who initiated PrEP at least six months before data abstraction began, 144 (85.8%) attended the six-month visit, including 116 (86.6%) FSW and 28 (84.8%) MSM (Table 2). Among them, nine (6.3%) had missed their three-month visit and five had missed both one-month and three-month visits (data not shown).
Table 2.
Attendance at 1, 3, and 6 months after PrEP initiation, by KP group (n = 352).
| Percent attended visit | ||||
|---|---|---|---|---|
| Number enrolleda |
1 month (%) |
3 months (%) |
6 monthsb (%) |
|
| Female sex worker | 277 | 62.1 | 81.2 | 86.6 |
| Men who have sex with men | 72 | 73.6 | 88.9 | 84.8 |
| People who inject drugs | 7 | 71.4 | 100.0 | 100.0 |
| Transgender woman | 3 | 66.7 | 0.0 | 0.0 |
| Total | 352a | 64.5 | 82.1 | 85.8 |
7 (2.0%) clients were classified into more than one KP group, including six FSW-PWID and 1 MSM-PWID.
134 FSW, 33 MSM, 5 PWID, and 1 TGW (n = 168 unique clients) had six months of observation time and were included.
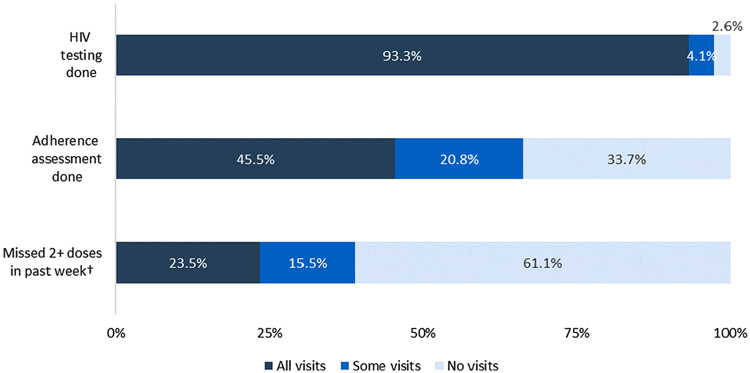
Among 341 clients with at least one follow-up visit after starting PrEP, HIV testing was performed at every follow-up for 318 (93.3%); at some visits for 14 (4.1%) others; and nine (2.6%) had no testing at any follow-up visits (Figure 1). No client who initiated PrEP and had subsequent HIV testing was diagnosed with HIV infection. Adherence assessments were completed at every visit for 155 (45.5%) clients; at some visits for 71 (20.8%) clients; and no adherence assessments were completed for 115 (33.7%). Among 226 clients with at least one visit in which adherence assessment was documented, 53 (23.5%) reported low adherence at all follow-up visits; 35 (15.5%) reported low adherence at some visits (Figure 1).
Figure 1.
Frequency of HIV testing, PrEP adherence assessments, and client-reported low adherence (defined as two or more doses missed in past seven days) at follow-up visits (n = 352).
†Among 226 clients with at least one adherence assessment completed
Conclusions
The real-world setting of this demonstration project is a strength that sheds light on the feasibility of facility-based PrEP for KP in resource-limited settings. Existing facility staff provided PrEP services with support from the PrEP coordinator in each city. Our dependence on routinely-collected clinic data to evaluate outcomes has limitations, as these sources may have gaps and offer limited insight into PrEP adherence over time. In addition, we cannot distinguish between clients who chose to discontinue PrEP because they felt they were no longer at risk for HIV and did not need it, and clients who missed appointments for other reasons. Finally, the clients constituted a convenience sample not representative of all at-risk KP in Kinshasa and Lubumbashi, or in other settings.
In the first demonstration project of PrEP for KP in routine care settings in DRC, we found high uptake and visit attendance among eligible clients, likely due to outreach supporting visit attendance. However, visit attendance was uneven and self-reported adherence was frequently low. Findings highlight the need to strengthen the implementation of PrEP services, including HIV testing and adherence assessment so that clients receive the full benefit of combination HIV prevention. These results demonstrate the demand for PrEP among KP and that this critical intervention can be effectively delivered in existing HIV care settings.
Acknowledgements
The authors gratefully acknowledge the collaboration of staff members at the KP-friendly clinics in Kinshasa and Lubumbashi whose dedication made this demonstration project possible, as well as the participation of the clinic clients.
Footnotes
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Baral SD, Poteat T, Stromdahl S, Wirtz AL, Guadamuz TE, & Beyrer C (2013). Worldwide burden of HIV in transgender women: A systematic review and meta-analysis. The Lancet Infectious Diseases, 13(3), 214–222. 10.1016/S1473-3099(12)70315-8 [DOI] [PubMed] [Google Scholar]
- Brown T, & Peerapatanapokin W (2019). Evolving HIV epidemics: The urgent need to refocus on populations with risk. Current Opinion in HIV and AIDS, 14(5), 337–353. 10.1097/COH.0000000000000571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case KK, Gomez GB, & Hallett TB (2019). The impact, cost and cost-effectiveness of oral pre-exposure prophylaxis in sub-Saharan Africa: A scoping review of modelling contributions and way forward. Journal of the International AIDS Society, 22(9), Article e25390. 10.1002/jia2.25390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulaud PJ, Sagaon-Teyssier L, M’Madi Mrenda B, Maradan G, Mora M, Bourrelly M, Dembele Keita B, Keita AA, Anoma C, Babo Yoro SA, Dah TTE, Coulibaly C, Mensah E, Agbomadji S, Bernier A, Couderc C, Laurent C, Spire B, & The CohMSM Study Group. (2018). Interest in HIV pre-exposure prophylaxis in men who have sex with men in West Africa (CohMSM ANRS 12324 – Expertise France). Tropical Medicine & International Health, 23(10), 1084–1091. 10.1111/tmi.13129 [DOI] [PubMed] [Google Scholar]
- Deryabina AP, & El-Sadr WM (2019). Optimizing HIV prevention and treatment outcomes for persons with substance use in Central Asia: What will it take? Current Opinion in HIV and AIDS, 14(5), 374–380. 10.1097/COH.0000000000000565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djomand G, Bingham T, Benech I, Muthui M, Savva H, Alamo S, Manopaiboon C, Wheeler T, & Mital S (2020). Expansion of HIV Preexposure Prophylaxis to 35 PEPFAR-supported early program adopters, October 2016–September 2018. MMWR. Morbidity and Mortality Weekly Report, 69(8), 212–215. 10.15585/mmwr.mm6908a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eakle R, Gomez GB, Naicker N, Bothma R, Mbogua J, Cabrera Escobar MA, Saayman E, Moorhouse M, Venter WDF, & Rees H, On behalf of the TAPS Demonstration Project Team (2017). HIV pre-exposure prophylaxis and early antiretroviral treatment among female sex workers in South Africa: Results from a prospective observational demonstration project. PLOS Medicine, 14(11), Article e1002444. 10.1371/journal.pmed.1002444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escudero DJ, Lurie MN, Kerr T, Howe CJ, & Marshall BD (2014). HIV pre-exposure prophylaxis for people who inject drugs: A review of current results and an agenda for future research. Journal of the International AIDS Society, 17(1), 18899. 10.7448/IAS.17.1.18899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICAP at Columbia University. (2017). ICAP Pre-exposure Prophylaxis (PrEP) package (Includes training material in French). Retrieved January 29, 2021, from https://icap.columbia.edu/tools_resources/icap-pre-exposure-prophylaxis-prep-package-2/
- Kyongo JK, Kiragu M, Karuga R, Ochieng C, Ngunjiri A, Wachihi C, Musyoki H, Digolo L, Otiso L, Gelmon L, Kilonzo N, & Mukoma W (2018, July 23–27). How long will they take it? Oral pre-exposure prophylaxis (PrEP) retention for female sex workers, men who have sex with men and young women in a demonstration project in Kenya. In Proceedings of the 22nd International AIDS Conference, Amsterdam. [Google Scholar]
- Ogunbajo A, Leblanc NM, Kushwaha S, Boakye F, Hanson S, Smith MDR, & Nelson LE (2019). Knowledge and acceptability of HIV pre-exposure prophylaxis (PrEP) among men who have sex with men (MSM) in Ghana. AIDS Care, 1–7. 10.1080/09540121.2019.1675858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Programme National de Lutte Contre le VIH/SIDA et IST. (2017). Rapport annuel 2017 Ministere de la Sante Publique. Republique Democratique du Congo. [Google Scholar]
- Riddell JT, Amico KR, & Mayer KH (2018). HIV pre-exposure prophylaxis: A review. JAMA, 319(12), 1261–1268. 10.1001/jama.2018.1917 [DOI] [PubMed] [Google Scholar]
- UNAIDS. (2020). AIDSinfo. Retrieved May 28, 2021, from https://aidsinfo.unaids.org/
- WHO. (2017). WHO implementation tool for pre-exposure prophylaxis (PrEP) of HIV infection. Retrieved May 28, 2021, from https://www.who.int/hiv/pub/prep/prep-implementation-tool/en/