ABSTRACT
Aims
Ticagrelor is associated with a lower risk of ischemic events than clopidogrel. However, it is uncertain whether the benefits of more intensive anti-ischemic therapy outweigh the risks of major bleeding in patients who have a high bleeding risk (HBR). Therefore, this study compared ticagrelor and clopidogrel in myocardial infarction (MI) patients with HBR.
Methods and results
This study included all patients enrolled in the SWEDEHEART registry who were discharged with dual antiplatelet therapy using ticagrelor or clopidogrel following MI between 2010 and 2017. High bleeding risk was defined as a PRECISE-DAPT score ≥25. Information on ischemic events, major bleeding, and mortality was obtained from national registries, with 365 days of follow-up. Additional outcomes include major adverse cardiovascular events (MACE), a composite of MI, stroke and all-cause mortality, and net adverse clinical events (NACE), a composite of MACE and bleeding. This study included 25 042 HBR patients, of whom 11 848 were treated with ticagrelor. Ticagrelor was associated with a lower risk of MI, stroke, and MACE, but a higher risk of bleeding compared to clopidogrel. There were no significant differences in mortality and NACE. Additionally, when examining the relationship between antiplatelet therapy and bleeding risk in 69 040 MI patients, we found no statistically significant interactions between the PRECISE-DAPT score and treatment effect.
Conclusions
We observed no difference in NACE when comparing ticagrelor and clopidogrel in HBR patients. Moreover, we found no statistically significant interactions between bleeding risk and the comparative effectiveness of clopidogrel and ticagrelor in a larger population of MI patients.
Keywords: Myocardial infarction, Major bleeding, Risk score, Dual antiplatelet therapy
Introduction
Dual antiplatelet therapy (DAPT) using a combination of aspirin and oral P2Y12-receptor inhibitors is the cornerstone of antithrombotic pharmacological therapy in patients with myocardial infarction (MI).1,2 Guidelines recommend that DAPT should include potent P2Y12-receptor inhibitors, such as ticagrelor, as they are associated with a lower risk of ischemic events than clopidogrel.2–5 However, studies show that while more intensive antiplatelet regimens decrease the risk of ischemic events, they also increase the risk of bleeding,5–10 which is also associated with a worse prognosis in MI patients.11,12 Therefore, it is uncertain whether the benefits of more intensive anti-ischemic therapy outweigh the risks of major bleeding in individuals who have a high bleeding risk (HBR). In fact, contrary to the current guidelines, real-world data demonstrate that clopidogrel is commonly used in MI patients with HBR.13–16 Thus, there is an ongoing debate as to what may be the optimal strategy for antiplatelet therapy following MI in patients who are at an elevated risk of major bleeding.9,16,17
In a previous study by our group, we compared ticagrelor and clopidogrel in a cohort of MI patients who were >80 years old. Although we observed a reduced risk of ischemic events in elderly patients treated with ticagrelor, we also observed that the ticagrelor group experienced a greater number of bleeding events and had a higher mortality rate.18 Several studies have shown that older patients are more susceptible to bleeding, which we believe to be a possible explanation for why ticagrelor was associated with a higher mortality rate than clopidogrel in our previous study of elderly MI patients.19–22 However, age is but one of several factors that are known to be associated with HBR.23,24 This study aimed to assess treatment outcomes following DAPT using either clopidogrel or ticagrelor in a general population of MI patients who are at an elevated risk of bleeding. Based on our previous findings, we hypothesized that ticagrelor would be associated with a lower risk of ischemic events, but a higher risk of both major bleeding and all-cause mortality in MI patients who have a high risk of bleeding.
Methods
Study population
The Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies (SWEDEHEART) registry provides data on all patients admitted to coronary care units in Sweden with symptoms suggestive of MI.25 Currently, all Swedish hospitals that provide care for acute cardiac diseases (n = 74) report to the SWEDEHEART registry, with data for over 100 variables on baseline characteristics, medication on admission, in-hospital therapies, complications, and discharge medication being recorded for all patients (http://www.ucr.uu.se/swedeheart/). The accuracy of data entered into the SWEDEHEART registry is regularly evaluated against medical health records by independent reviewers, with a reported agreement of approximately 96%.25 Patients whose clinical data is recorded in the SWEDEHEART registry are not asked to provide informed consent, but they are informed about their participation in the SWEDEHEART-registry and that they may withdraw consent at any time should they wish to do so. The study protocol was approved by the regional ethics committee in Stockholm, Sweden (D-nr: 2012/60-31/2, 2014/1484-32, 2015/332-32).
The study population consisted of MI patients who had received DAPT using a combination of aspirin and either clopidogrel or ticagrelor following a diagnosis of acute MI. The study participants were consecutively enrolled in the SWEDEHEART registry between 1 January 2010, and 31 December 2017. Bleeding risk was assessed using the PRECISE-DAPT score. High bleeding risk was defined as a PRECISE-DAPT score of 25 or greater, moderate bleeding risk was defined as a PRECISE-DAPT score of 18–24, low bleeding risk was defined as a PRECISE-DAPT score of 11–17, and very low bleeding risk was defined as a PRECISE-DAPT score of 10 or less.23 We used the alternative 4-item version of the PRECISE-DAPT score, consisting of age, haemoglobin concentration, prior bleeding, and creatinine clearance, because data on the white blood cell count was unavailable in the SWEDEHEART registry. Creatinine clearance was estimated using the Cockcroft–Gault equation.23,26
To emulate the design of a putative clinical trial, we excluded patients who presented with comorbidities that would make them unlikely to be selected for randomization in a clinical trial of antiplatelet agents, such as dementia or ongoing dialysis treatment. Additional exclusion criteria include treatment with direct oral anticoagulants, vitamin K antagonists or P2Y12-receptor inhibitors on admission, age <18 years, in-hospital mortality, serious in-hospital bleeding (requiring blood transfusion or surgery), and hospitalization exceeding 60 days. Moreover, we also excluded patients who had incomplete data concerning any of the individual components of the 4-item PRECISE-DAPT score (i.e. age, haemoglobin concentration, prior bleeding, or creatinine clearance).
Exposure
Patients were considered exposed to either ticagrelor or clopidogrel if there was a corresponding drug dispensation recorded in the Swedish Prescribed Drug register within two weeks of hospital discharge. In cases where data on drug dispensation was unavailable (n = 1009), we instead determined the exposure for clopidogrel or ticagrelor based on data recorded in the SWEDEHEART registry concerning DAPT at discharge.
Outcomes
The endpoints of this study consist of all-cause mortality, recurrent MI, ischemic stroke, and major bleeding, as well as the composite outcomes of major adverse cardiovascular events (MACE) and net adverse clinical events (NACE). Major adverse cardiovascular events was defined as a composite of all-cause mortality, MI and ischemic stroke, while NACE was defined as a composite of MACE and major bleeding. The outcomes of recurrent MI, ischemic stroke, and major bleeding were defined using a set of International Classification of Diseases (ICD) codes, as detailed in the supplementary materials (see Supplementary material online, Table SI). All outcomes were assessed as time to the first event within 365 days of the discharge date or until the end of the follow-up period on the 31 December 2017, whichever occurred first.
Data on readmission due to adverse cardiovascular events and major bleeding was obtained from the Swedish national patient registry, which is based on reported ICD codes from all hospital admissions in Sweden. The registration of recurrent MI within the first month after hospital discharge required concomitant entries in both the Swedish national patient registry and the SWEDEHEART registry. Data on vital status was acquired from the Swedish population registry.
Statistical analysis
Continuous variables are presented either using means and standard deviations (SDs) or using medians and interquartile ranges (IQRs), as appropriate. Categorical variables are presented using counts and percentages.
To account for confounding by indication, we used inverse probability treatment weighting (IPTW) to achieve a similar distribution of observed baseline covariates for the ticagrelor- and clopidogrel groups.27 A multivariable logistic regression model was used to estimate propensity scores, with the assigned treatment as the dependent variable. The model consisted of 37 covariates: cardiovascular risk factors (age, sex, smoking, and hypertension), the PRECISE-DAPT score and its various components, calendar year, prior cardiovascular events (MI, stroke, and major bleeding), prior revascularization therapy (coronary artery bypass grafting, and percutaneous coronary intervention [PCI]), comorbidities (congestive heart failure, diabetes mellitus, renal failure, chronic obstructive pulmonary disease, peripheral arterial disease, and cancer), medications at hospital admission (aspirin, beta-blockers, calcium channel antagonists, digoxin, angiotensin-converting enzyme [ACE] inhibitors or angiotensin receptor blockers [ARB], diuretics, statins, and inotropes), the presence of ST-segment elevation, Killip class, coronary angiography during the hospital stay, PCI during the hospital stay, medical therapy at discharge (diuretics, beta-blockers, calcium channel antagonists, digoxin, ACE-inhibitor or ARB, statins, and medication for diabetes mellitus), creatinine clearance, haemoglobin concentration, and hospital length of stay. The computed propensity scores were used to calculate weights for each study participant. The weights were subsequently stabilized by multiplying the weight by the probability of being exposed to ticagrelor treatment for those exposed and the probability of being unexposed to ticagrelor treatment for those unexposed, in order to decrease the variance of the effect estimates.28 Weights were truncated at the 99th percentile, to limit the potential influence of outliers. We calculated standardized mean differences (SMD) between the two treatment groups for all parameters, to examine variable balance before and after IPTW. Weighting was considered successful if the SMD was between −0.1 and 0.1.
In addition to IPTW, we also included the above-mentioned 39 covariates in IPTW adjusted Cox proportional hazards models, to obtain a doubly robust estimate of causal effect.29 The proportional hazards assumption was evaluated by examining the Schoenfeld residuals. Additionally, weighted Kaplan–Meier curves were plotted to compare treatment groups concerning the cumulative incidences of the outcomes.
All statistical analyses were performed using R Statistical Software (version 4.1.1; R Foundation for Statistical Computing, Vienna, Austria).
Sensitivity analyses
To further explore the relationship between the choice of antiplatelet therapy and bleeding risk in MI patients who are treated with DAPT, we performed several sensitivity analyses to examine the interaction between treatment effect and bleeding risk following antiplatelet therapy using either clopidogrel or ticagrelor. First, we examined the change in the hazard ratio for several treatment outcomes following DAPT across different values of the PRECISE-DAPT score. These results were derived from a proportional hazards model, which included the PRECISE-DAPT score as a continuous variable modelled using restricted cubic splines (with 3 knots) and an interaction term between the PRECISE-DAPT score and antiplatelet therapy. Next, we also examined differences in the absolute risk of adverse events across several pre-specified subgroups of MI patients who had different degrees of bleeding risk.
Results
Study population
A total of 25 042 MI patients with HBR were included in this study (Supplemental materials, Supplemental Figure I). Of the included study participants, 11 848 (47.3%) were treated with ticagrelor and the remainder received clopidogrel, with the proportion of patients being treated with ticagrelor increasing over time from 0% in 2010 to 80% in 2017 (see Supplementaty material online, Figure II). High bleeding risk patients who were treated with ticagrelor were, on average, younger and more likely to present with ST-segment elevation and undergo invasive therapy than HBR patients who were treated with clopidogrel (Table 1). Despite these differences between the two treatment groups, we observed similar distributions of baseline covariates after covariate adjustment using IPTW (see Table 1; and Supplementary material online, Figure III).
Table 1.
Patient demographics before and after inverse probability of treatment weighting
| Unweighted | Weighted | |||||
|---|---|---|---|---|---|---|
| Clopidogrel | Ticagrelor | SMD | Clopidogrel | Ticagrelor | SMD | |
| Demographics | ||||||
| n | 13 0194 | 11 0848 | 12 627.03 | 9051.07 | ||
| Age, mean (SD) | 80.01 (8.09) | 77.07 (7.51) | 0.299 | 79.01 (8.61) | 79.11 (7.66) | 0.012 |
| Sex, female | 6318 (47.9) | 5162 (43.6) | 0.087 | 5844.3 (46.3) | 4129.2 (45.6) | 0.013 |
| PRECISE-DAPT score, median [Q1, Q3] | 35.0 [30.00, 42.00] | 32.0 [28.00, 38.00] | 0.282 | 34.00 [29.00, 41.00] | 33.00 [29.00, 40.00] | 0.033 |
| Creatinine clearance (mL/min), median [Q1, Q3] | 45.5 [35.09, 54.80] | 48.9 [40.02, 57.20] | 0.228 | 46.63 [36.21, 56.06] | 47.04 [37.61, 56.12] | 0.022 |
| Prior bleeding | 1580.0 (12.0) | 1328.0 (11.2) | 0.024 | 1556.8 (12.3) | 1086.0 (12.0) | 0.010 |
| Hemoglobin (g/dL), mean (SD) | 130.7 (16.99) | 133.6 (16.68) | 0.172 | 131.48 (17.24) | 132.01 (16.98) | 0.031 |
| Length of stay (days), median [Q1, Q3] | 4.00 [3.00, 7.00] | 4.00 [3.00, 6.00] | 0.153 | 4.00 [3.00, 6.00] | 4.00 [3.00, 6.00] | 0.040 |
| Calender year | 1.607 | 0.777 | ||||
| 2010 | 3237.0 (24.5) | 0.0 (0.0) | 1705.5 (13.5) | 0.0 (0.0) | ||
| 2011 | 3207.0 (24.3) | 36.0 (0.3) | 1708.7 (13.5) | 168.5 (1.9) | ||
| 2012 | 2239.0 (17.0) | 1136.0 (9.6) | 1801.5 (14.3) | 1614.6 (17.8) | ||
| 2013 | 1365.0 (10.3) | 1813.0 (15.3) | 1707.7 (13.5) | 1596.5 (17.6) | ||
| 2014 | 1056.0 (8.0) | 1988.0 (16.8) | 1589.8 (12.6) | 1463.4 (16.2) | ||
| 2015 | 827.0 (6.3) | 2169.0 (18.3) | 1476.6 (11.7) | 1406.4 (15.5) | ||
| 2016 | 695.0 (5.3) | 2372.0 (20.0) | 1399.7 (11.1) | 1438.8 (15.9) | ||
| 2017 | 568.0 (4.3) | 2334.0 (19.7) | 1237.5 (9.8) | 1363.0 (15.1) | ||
| Current smokers | 1364.0 (10.3) | 1504.0 (12.7) | 0.074 | 1443.6 (11.4) | 1032.8 (11.4) | 0.001 |
| Comorbidities | ||||||
| MI | 3962.0 (30.0) | 2525.0 (21.3) | 0.201 | 3317.6 (26.3) | 2198.0 (24.3) | 0.046 |
| CHF | 2304.0 (17.5) | 1173.0 (9.9) | 0.221 | 1853.2 (14.7) | 1212.8 (13.4) | 0.037 |
| Diabetes mellitus | 3526.0 (26.7) | 3133.0 (26.4) | 0.006 | 3413.1 (27.0) | 2431.0 (26.9) | 0.004 |
| Stroke | 1663.0 (12.6) | 1056.0 (8.9) | 0.119 | 1503.3 (11.9) | 990.6 (10.9) | 0.030 |
| Renal failure | 910.0 (6.9) | 572.0 (4.8) | 0.088 | 807.5 (6.4) | 592.1 (6.5) | 0.006 |
| COPD | 1266.0 (9.6) | 1024.0 (8.6) | 0.033 | 1200.4 (9.5) | 854.9 (9.4) | 0.002 |
| Peripheral arterial disease | 1055.0 (8.0) | 690.0 (5.8) | 0.086 | 937.9 (7.4) | 650.6 (7.2) | 0.009 |
| Hypertension | 9202.0 (69.7) | 8244.0 (69.6) | 0.004 | 8894.7 (70.4) | 6458.0 (71.4) | 0.020 |
| Cancer | 635.0 (4.8) | 471.0 (4.0) | 0.041 | 608.7 (4.8) | 437.3 (4.8) | 0.001 |
| Prior medical interventions | ||||||
| CABG | 1537.0 (11.6) | 969.0 (8.2) | 0.116 | 1292.3 (10.2) | 841.2 (9.3) | 0.032 |
| PCI | 2118.0 (16.1) | 1744.0 (14.7) | 0.037 | 1975.8 (15.6) | 1392.9 (15.4) | 0.007 |
| Type of myocardial infarction | ||||||
| STEMI | 3653.0 (27.7) | 4514.0 (38.1) | 0.223 | 4006.4 (31.7) | 2986.1 (33.0) | 0.027 |
| Killip class > 1 | 448.0 (3.4) | 267.0 (2.3) | 0.069 | 374.6 (3.0) | 246.3 (2.7) | 0.015 |
| Medications on admission | ||||||
| Aspirin | 6755.0 (51.2) | 4778.0 (40.3) | 0.219 | 5932.5 (47.0) | 4037.6 (44.6) | 0.048 |
| Beta-blockers | 5759.0 (43.6) | 4290.0 (36.2) | 0.152 | 5210.6 (41.3) | 3578.2 (39.5) | 0.035 |
| Calcium channel antagonists | 3295.0 (25.0) | 3033.0 (25.6) | 0.014 | 3232.1 (25.6) | 2302.6 (25.4) | 0.004 |
| Digoxin | 122.0 (0.9) | 37.0 (0.3) | 0.078 | 87.7 (0.7) | 48.3 (0.5) | 0.021 |
| ACE-inhibitor or ARB | 5517.0 (41.8) | 4900.0 (41.4) | 0.009 | 5347.6 (42.4) | 3783.7 (41.8) | 0.011 |
| Diuretics | 4284.0 (32.5) | 2876.0 (24.3) | 0.183 | 3732.9 (29.6) | 2516.9 (27.8) | 0.039 |
| Statins | 4352.0 (33.0) | 3459.0 (29.2) | 0.082 | 3995.0 (31.6) | 2706.3 (29.9) | 0.038 |
| In-hospital treatments | ||||||
| PCI | 7335.0 (55.6) | 9976.0 (84.2) | 0.656 | 8528.9 (67.5) | 6454.0 (71.3) | 0.082 |
| Coronary angiography | 9204.0 (69.8) | 10 945.0 (92.4) | 0.603 | 10 026.8 (79.4) | 7412.3 (81.9) | 0.063 |
| Inotropes | 217.0 (1.6) | 275.0 (2.3) | 0.049 | 249.5 (2.0) | 195.2 (2.2) | 0.013 |
| Diuretics | 2879.0 (21.8) | 2111.0 (17.8) | 0.101 | 2662.2 (21.1) | 1821.2 (20.1) | 0.024 |
| Medications on discharge | ||||||
| Beta-blockers | 11 542.0 (87.5) | 10 464.0 (88.3) | 0.026 | 11 049.0 (87.5) | 7925.6 (87.6) | 0.002 |
| Calcium channel antagonists | 2943.0 (22.3) | 2570.0 (21.7) | 0.015 | 2844.4 (22.5) | 2035.7 (22.5) | 0.001 |
| Digoxin | 111.0 (0.8) | 28.0 (0.2) | 0.083 | 74.2 (0.6) | 35.6 (0.4) | 0.028 |
| ACE-inhibitor or ARB | 9989.0 (75.7) | 9909.0 (83.6) | 0.198 | 9923.9 (78.6) | 7224.0 (79.8) | 0.030 |
| Diabetes medications | 2646.0 (20.1) | 2407.0 (20.3) | 0.007 | 2557.1 (20.3) | 1838.7 (20.3) | 0.002 |
| Statins | 11 118.0 (84.3) | 11 131.0 (93.9) | 0.315 | 11 092.3 (87.8) | 8015.7 (88.6) | 0.022 |
Outcomes in patients with a high bleeding risk
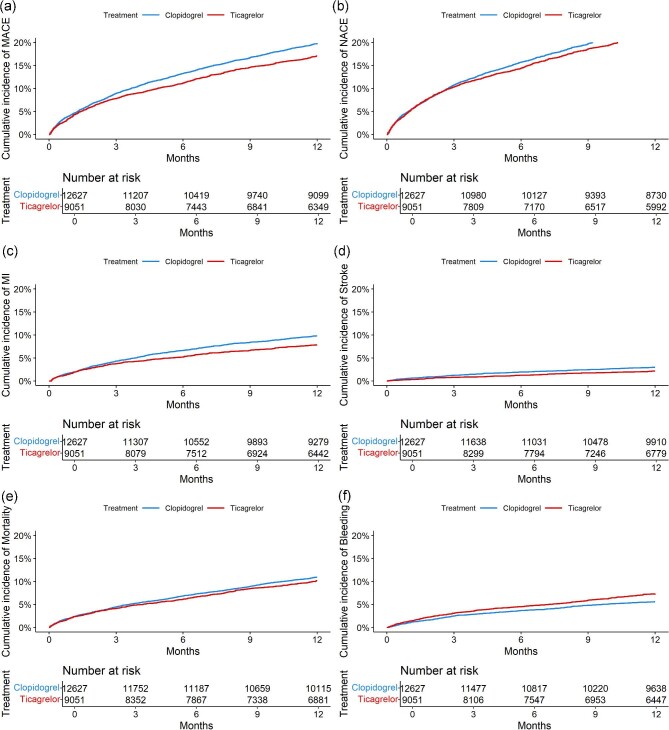
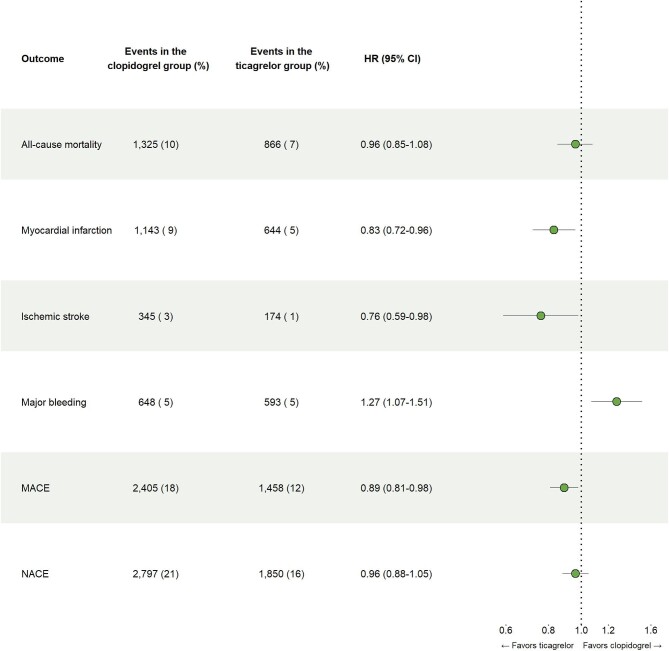
Ticagrelor was associated with a lower risk of MI (hazard ratio [HR], 0.83 [95% CI 0.72–0.96]), stroke (HR, 0.76 [95% CI 0.59–0.98]) and MACE (HR, 0.89 [95% CI 0.81–0.98]), and a higher risk of bleeding compared to clopidogrel (HR, 1.27 [95% CI 1.07–1.51]) in MI patients with HBR (Figures 1 and 2). There were no significant differences in mortality (HR 0.96 [95% CI 0.85–1.08]) and NACE (HR 0.96 [95% CI 0.88–1.05]).
Figure 1.
Weighted cumulative incidence of treatment outcomes in HBR patients. (a), Major adverse cardiovascular event. (b), Net adverse clinical event. (c), Myocardial infarction. (d), Ischemic stroke. (e), All-cause mortality. (f), Major bleeding.
Abbreviations: MACE = Major adverse cardiovascular event, NACE = Net adverse clinical event.
Figure 2.
Treatment outcomes in high bleeding risk patients.
Abbreviations: MACE = Major adverse cardiovascular event, NACE = Net adverse clinical event.
Association between bleeding risk and treatment effect
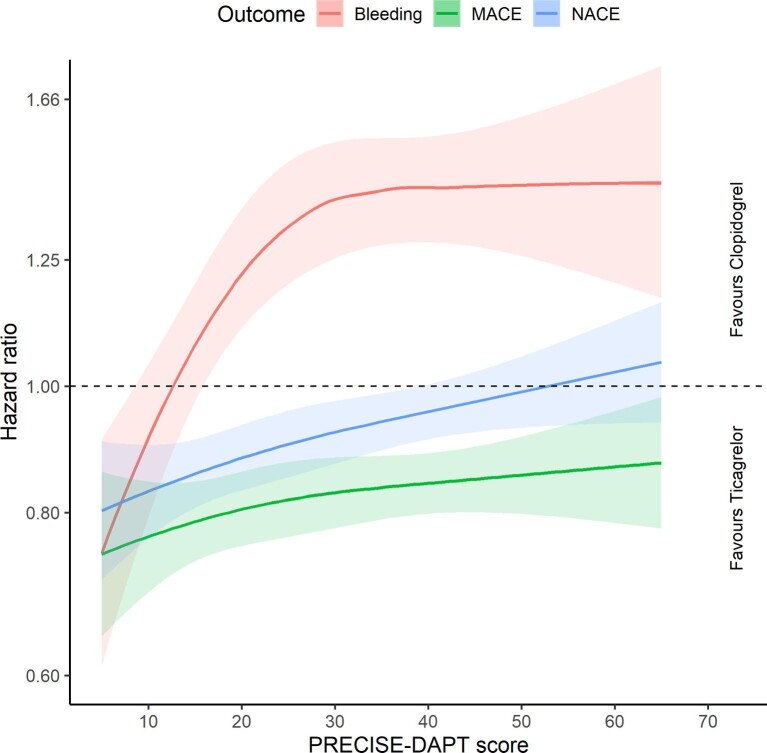
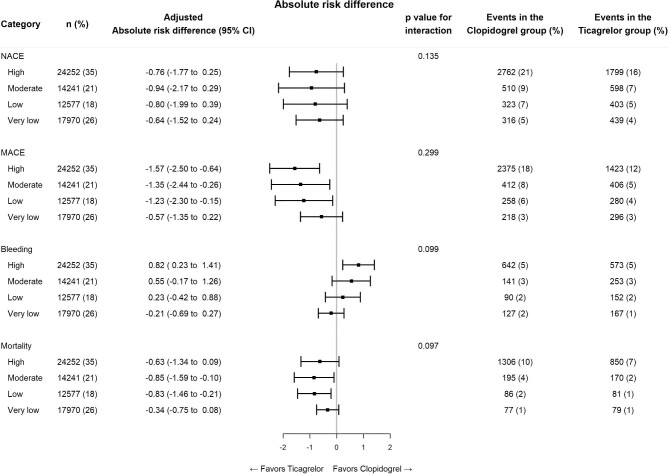
High bleeding risk patients were on average older and less likely to present with ST-segment elevation, undergo coronary angiography and receive ticagrelor than MI patients who had a lower bleeding risk (see Supplementary material online, Table S2). Ticagrelor therapy was generally more favourable than the use of clopidogrel in patients who had a lower bleeding risk than in patients who had a higher bleeding risk when examining the outcome of NACE, although this interaction was not statistically significant (Figures 3 and 4). While ticagrelor was associated with a higher absolute risk of major bleeding and a lower absolute risk of ischemic events in HBR patients than in patients who presented with a lower bleeding risk, we found that the relative difference between ticagrelor and clopidogrel remained virtually unchanged when comparing patients outcomes among individuals who presented with a moderate- or a high bleeding risk.
Figure 3.
Relationship between treatment effect and bleeding risk, assessed using the 4-item version of the PRECISE-DAPT score.
Abbreviations: MACE = Major adverse cardiovascular event, NACE = Net adverse clinical event.
Figure 4.
Comparison of clopidogrel and ticagrelor across the spectrum of bleeding risk.
Abbreviations: MACE = Major adverse cardiovascular event, NACE = Net adverse clinical event.
Discussion
In this observational study, we aimed to assess whether the benefits of more intensive anti-ischemic therapy outweigh the risks of major bleeding in MI patients who have a high bleeding risk. We found that ticagrelor was associated with a lower risk of recurrent ischemic events, while also being associated with an increased risk of bleeding compared to clopidogrel in HBR patients. However, we observed no differences in all-cause mortality and NACE between ticagrelor and clopidogrel in 25 042 MI patients with HBR, which is in line with the results of other studies on the use of less potent P2Y12 inhibitors in HBR patients.9,16 Additionally, when examining the relationship between antiplatelet therapy and bleeding risk in 69 040 MI patients, we found no statistically significant interactions between the PRECISE-DAPT score and treatment effect.
Studies suggest that the use of less intensive anti-ischemic regimens, such as shorter DAPT durations or P2Y12 monotherapy, may decrease the risk bleeding without significantly increasing the risk of adverse ischemic events.30,31 In contrast to these findings, we found that although the less potent P2Y12 inhibitor was associated with a lower risk of bleeding events, it was also associated with a higher risk of ischemic events than the potent P2Y12 inhibitor ticagrelor.32 This discrepancy may, in part, be explained by differences in study populations and the definitions used for HBR. Furthermore, it is important to bear in mind that these strategies of de-escalation are rather different and therefore may not necessarily have the same association with patient outcomes. More research is required to determine which de-escalation strategies might be most appropriate for HBR patients.
Currently, there is no consensus regarding the definition of HBR in patients with coronary heart disease.24,33 The PRECISE-DAPT score was used in this study because it has performed well in both external validations and comparisons with other classifications of HBR and is recommended in the current guidelines.23,34–37 There are, however, several differences between the derivation cohort of the PRECISE-DAPT score and the participants of this study. For instance, the PRECISE-DAPT score was conceived as a bleeding risk score for patients who undergo coronary stenting, whereas the present cohort also includes patients who were treated medically. Furthermore, while the derivation cohort of the PRECISE-DAPT score consisted of clinical trial participants, the current study was based on real-world data. These discrepancies in study demographics may have affected the performance of the PRECISE-DAPT score in this cohort, as previously demonstrated by Wester et al.34
Studies show that the pharmacological effect of P2Y12 inhibitors is subject to considerable inter-individual differences, which is particularly true for clopidogrel.38,39 In addition to bleeding risk assessments, pharmacogenomics and platelet function testing have also been proposed to guide the choice of antiplatelet therapy in MI patients.40,41 Given that we did not observe any statistically significant modification of treatment effect across bleeding risk groups in this study, one might speculate that other strategies for patient selection may be more appropriate in guiding the choice of antiplatelet therapy. More research is required to investigate the impact of patient selection on the effect of antiplatelet therapy in MI patients.
A limitation of this study is that DAPT duration was not accounted for in the study protocol, as this parameter was not recorded in the SWEDEHEART registry at the time of this study. Moreover, we were unable to adjust for differences in stent type as well as the type of anticoagulant, which was used in conjunction with PCI. An additional limitation of this study is that the cause of death was not known. There is also a risk of survival bias, as the protocol of this study required the exclusion of patients who died prior to hospital discharge. Furthermore, we acknowledge that the observational design of this study constitutes a major limitation, as it allows for the possibility of residual confounding. The fact that this was an observational study may, however, also be considered beneficial, as it allowed us to assess treatment outcomes in a real-world setting.
In conclusion, we found that ticagrelor was associated with a lower risk of recurrent ischemic events, but a higher risk of major bleeding compared to clopidogrel in HBR patients. We found no significant differences in all-cause mortality and NACE when comparing ticagrelor and clopidogrel in HBR patients. Furthermore, we found no statistically significant interactions between bleeding risk and the comparative effectiveness of clopidogrel and ticagrelor in a larger population of MI patients.
Supplementary Material
Contributor Information
Jonathan Tjerkaski, Department of Clinical Sciences, Danderyd University Hospital, Karolinska Institute, Stockholm, 18257 Danderyd, Sweden.
Tomas Jernberg, Department of Clinical Sciences, Danderyd University Hospital, Karolinska Institute, Stockholm, 18257 Danderyd, Sweden.
Joakim Alfredsson, Department of Health, Medicine and Caring Sciences and Department of Cardiology, Linköping University, 581 83 Linköping, Sweden.
David Erlinge, Department of Clinical Sciences, Cardiology, Lund University, 221 85 Lund, Sweden.
Stefan James, Department of Medical Sciences, Uppsala University, 751 85 Uppsala, Sweden; Uppsala Clinical Research Center, Uppsala University, 751 85 Uppsala, Sweden.
Bertil Lindahl, Department of Medical Sciences, Uppsala University, 751 85 Uppsala, Sweden; Uppsala Clinical Research Center, Uppsala University, 751 85 Uppsala, Sweden.
Moman Aladdin Mohammad, Department of Clinical Sciences, Cardiology, Lund University, 221 85 Lund, Sweden.
Elmir Omerovic, Department of Cardiology, Sahlgrenska University Hospital, Institute of Medicine, Department of Molecular and Clinical Medicine, Sahlgrenska Academy at University of Gothenburg, 41345 Gothenburg, Sweden.
Dimitrios Venetsanos, Department of Health, Medicine and Caring Sciences and Department of Cardiology, Linköping University, 581 83 Linköping, Sweden.
Karolina Szummer, Section of Cardiology, Department of Medicine, Karolinska Institutet, Huddinge, 171 77 Stockholm, Sweden.
Funding
K.S. research time is funded by the Stockholm County council.
Conflicts of interest: K.S. has received speaker fee compensation from Bayer and Vifor.
Data availability
The data underlying this article cannot be shared publicly due to ethical and privacy reasons.
References
- 1. Angiolillo DJ, Galli M, Collet JP, Kastrati A, O'Donoghue ML. Antiplatelet therapy after percutaneous coronary intervention. EuroIntervention 2022;17:e1371–e1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Collet J-P, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, Dendale P, Dorobantu M, Edvardsen T, Folliguet T, Gale CP, Gilard M, Jobs A, Jüni P, Lambrinou E, Lewis BS, Mehilli J, Meliga E, Merkely B, Mueller C, Roffi M, Rutten FH, Sibbing D, Siontis GCM; ESC Scientific Document Group . 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2021;42:1289–1367. [DOI] [PubMed] [Google Scholar]
- 3. Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, Jüni P, Kastrati A, Kolh P, Mauri L, Montalescot G, Neumann FJ, Petricevic M, Roffi M, Steg PG, Windecker S, Zamorano JL, Levine GN; ESC Scientific Document Group; ESC Committee for Practice Guidelines (CPG) ; ESC National Cardiac Societies . 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2018;39:213–260. [DOI] [PubMed] [Google Scholar]
- 4. Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, Granger CB, Lange RA, Mack MJ, Mauri L, Mehran R, Mukherjee D, Newby LK, O'gara PT, Sabatine MS, Smith PK, Smith SC. 2016 ACC/AHA Guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol 2016;68:1082–1115. [DOI] [PubMed] [Google Scholar]
- 5. Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009;361:1045–1057. [DOI] [PubMed] [Google Scholar]
- 6. Sahlén A, Varenhorst C, Lagerqvist B, Renlund H, Omerovic E, Erlinge D, Wallentin L, James SK, Jernberg T. Outcomes in patients treated with ticagrelor or clopidogrel after acute myocardial infarction: experiences from SWEDEHEART registry. Eur Heart J 2016;37:3335–3342. [DOI] [PubMed] [Google Scholar]
- 7. Capodanno D, Alfonso F, Levine GN, Valgimigli M, Angiolillo DJ. ACC/AHA versus ESC Guidelines on dual antiplatelet therapy: JACC guideline comparison. J Am Coll Cardiol 2018;72:2915–2931. [DOI] [PubMed] [Google Scholar]
- 8. Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann F-J, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007;357:2001–2015. [DOI] [PubMed] [Google Scholar]
- 9. Zocca P, Kok MM, van der Heijden LC, van Houwelingen KG, Hartmann M, de Man FHAF, Stoel MG, Louwerenburg J(H)W, Knottnerus IL, Linssen GCM, Doggen CJM, Von Birgelen C. High bleeding risk patients with acute coronary syndromes treated with contemporary drug-eluting stents and clopidogrel or ticagrelor: insights from CHANGE DAPT. Int J Cardiol 2018;268:11–17. [DOI] [PubMed] [Google Scholar]
- 10. Völz S, Petursson P, Odenstedt J, Ioanes D, Haraldsson I, Angerås O, Dworeck C, Hirlekar G, Myredal A, Albertsson P, Råmunddal T, Redfors B, Omerovic E. Ticagrelor is not superior to clopidogrel in patients with acute coronary syndromes undergoing PCI: a report from Swedish coronary angiography and angioplasty registry. J Am Hear Assoc Cardiovasc Cerebrovasc Dis 2020;9:e015990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Valgimigli M, Costa F, Lokhnygina Y, Clare RM, Wallentin L, Moliterno DJ, Armstrong PW, White HD, Held C, Aylward PE, Van de Werf F, Harrington RA, Mahaffey KW, Tricoci P. Trade-off of myocardial infarction vs. bleeding types on mortality after acute coronary syndrome: lessons from the Thrombin Receptor Antagonist for Clinical Event Reduction in Acute Coronary Syndrome (TRACER) randomized trial. Eur Heart J 2017;38:804–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eikelboom JW, Mehta SR, Anand SS, Xie C, Fox KAA, Yusuf S. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation 2006;114:774–782. [DOI] [PubMed] [Google Scholar]
- 13. Alexopoulos D, Xanthopoulou I, Deftereos S, Hamilos M, Sitafidis G, Kanakakis I, Pentara I, Vavouranakis M, Davlouros P, Hahalis G, Goudevenos J. Contemporary antiplatelet treatment in acute coronary syndrome patients undergoing percutaneous coronary intervention: 1-year outcomes from the GReek AntiPlatElet (GRAPE) Registry. J Thromb Haemost 2016;14:1146–1154. [DOI] [PubMed] [Google Scholar]
- 14. Sahlén A, Varenhorst C, Lagerqvist B, Renlund H, Wallentin L, James SK, Jernberg T. Contemporary use of ticagrelor in patients with acute coronary syndrome: insights from Swedish Web System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies (SWEDEHEART). Eur Heart J Cardiovasc Pharmacother 2016;2:5–12. [DOI] [PubMed] [Google Scholar]
- 15. Beigel R, Iakobishvili Z, Shlomo N, Segev A, Witberg G, Zahger D, Atar S, Alcalai R, Kapeliovich M, Gottlieb S, Goldenberg I, Asher E, Matetzky S . Real-world use of novel P2Y12 inhibitors in patients with acute myocardial infarction: a treatment paradox. Cardiology 2017;136:21–28. [DOI] [PubMed] [Google Scholar]
- 16. Gragnano F, Moscarella E, Calabrò P, Cesaro A, Pafundi PC, Ielasi A, Ielasi A, Patti G, Cavallari I, Antonucci E, Cirillo P, Pignatelli P, Palareti G, Pelliccia F, Gaudio C, Sasso FC, Pengo V, Gresele P, Marcucci R, Fimiani F, Vitale RA, Schiavo A, Conte M, Di Maio D, Pastori D, Menichelli D, Grossi G, Di Serafino L, Taglialatela V, Galiero R, Acierno C, Del Pinto M, Gugliemini G. Clopidogrel versus ticagrelor in high-bleeding risk patients presenting with acute coronary syndromes: insights from the multicenter START-ANTIPLATELET registry. Intern Emerg Med 2021;16:379–387. [DOI] [PubMed] [Google Scholar]
- 17. Wang HY, Li Y, Xu XM, Li J, Han YL. Impact of baseline bleeding risk on efficacy and safety of ticagrelor versus clopidogrel in Chinese patients with acute coronary syndrome undergoing percutaneous coronary intervention. Chin Med J (Engl) 2018;131:2017–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Szummer K, Montez-Rath ME, Alfredsson J, Erlinge D, Lindahl B, Hofmann R, Ravn-Fischer A, Svensson P, Jernberg T. Comparison between ticagrelor and clopidogrel in elderly patients with an acute coronary syndrome: insights from the SWEDEHEART registry. Circulation 2020:142;1700–1708. [DOI] [PubMed] [Google Scholar]
- 19. Simonsson M, Winell H, Olsson H, Szummer K, Alfredsson J, Hall M, Dondo TB, Gale CP, Jernberg T. Development and validation of a novel risk score for in-hospital major bleeding in acute myocardial infarction:-The SWEDEHEART score. J Am Heart Assoc 2019;8:e012157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Subherwal S, Bach RG, Chen AY, Gage BF, Rao S V., Newby LK, Wang TY, Gibler WB, Ohman EM, Roe MT, Pollack CV, Peterson ED, Alexander KP. Baseline risk of major bleeding in non—st-segment elevation myocardial infarction: the CRUSADE bleeding score. Circulation 2009;119:1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mehran R, Pocock SJ, Nikolsky E, Clayton T, Dangas GD, Kirtane AJ, Parise H, Fahy M, Manoukian SV, Feit F, Ohman ME, Witzenbichler B, Guagliumi G, Lansky AJ, Stone GW. A risk score to predict bleeding in patients with acute coronary syndromes. J Am Coll Cardiol 2010;55:2556–2566. [DOI] [PubMed] [Google Scholar]
- 22. Mathews R, Peterson ED, Chen AY, Wang TY, Chin CT, Fonarow GC, Cannon CP, Rumsfeld JS, Roe MT, Alexander KP. In-hospital major bleeding during ST-Elevation and Non—ST-Elevation myocardial infarction care: derivation and validation of a model from the ACTION registry®-GWTGTM. Am J Cardiol 2011;107:1136–1143. [DOI] [PubMed] [Google Scholar]
- 23. Costa F, van Klaveren D, James S, Heg D, Räber L, Feres F, Pilgrim T, Hong M-Ki, Kim H-S, Colombo A, Steg PG, Zanchin T, Palmerini T, Wallentin L, Bhatt DL, Stone GW, Windecker S, Steyerberg EW, Valgimigli M. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet North Am Ed 2017;389:1025–1034. [DOI] [PubMed] [Google Scholar]
- 24. Urban P, Mehran R, Colleran R, Angiolillo DJ, Byrne RA, Capodanno D, Cuisset T, Cutlip D, Eerdmans P, Eikelboom J, Farb A, Gibson CM, Gregson J, Haude M, James SK, Kim H-S, Kimura T, Konishi A, Laschinger J, Leon MB, Magee PFA, Mitsutake Y, Mylotte D, Pocock S, Price MJ, Rao SV, Spitzer E, Stockbridge N, Valgimigli M, Varenne O, Windhoevel U, Yeh RW, Krucoff MW, Morice M-C. Defining high bleeding risk in patients undergoing percutaneous coronary intervention: a consensus document from the Academic Research Consortium for High Bleeding Risk. Eur Heart J 2019;40:2632–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jernberg T, Attebring MF, Hambraeus K, Ivert T, James S, Jeppsson A, Lagerqvist B, Lindahl B, Stenestrand U, Wallentin L. The Swedish Web-system for enhancement and development of evidence-based care in heart disease evaluated according to recommended therapies (SWEDEHEART). Heart 2010;96:1617–1621. [DOI] [PubMed] [Google Scholar]
- 26. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976;16:31–41. [DOI] [PubMed] [Google Scholar]
- 27. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011;46:399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol 2008;168:656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Austin PC. The performance of different propensity-score methods for estimating differences in proportions (risk differences or absolute risk reductions) in observational studies. Statist Med 2010;29:2137–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Valgimigli M, Frigoli E, Heg D, Tijssen J, Jüni P, Vranckx P, Ozaki Y, Morice M-C, Chevalier B, Onuma Y, Windecker S, Tonino PAL, Roffi M, Lesiak M, Mahfoud F, Bartunek J, Hildick-Smith D, Colombo A, Stanković G, Iñiguez A, Schultz C, Kornowski R, Ong PJL, Alasnag M, Rodriguez AE, Moschovitis A, Laanmets P, Donahue M, Leonardi S, Smits PC. Dual antiplatelet therapy after PCI in patients at high bleeding risk. N Engl J Med 2021;385:1643–1655. [DOI] [PubMed] [Google Scholar]
- 31. Escaned J, Cao D, Baber U, Nicolas J, Sartori S, Zhang Z, Dangas G, Angiolillo DJ, Briguori C, Cohen DJ, Collier T, Dudek D, Gibson M, Gil R, Huber K, Kaul U, Kornowski R, Krucoff MW, Kunadian V, Mehta S, Moliterno DJ, Ohman EM, Oldroyd KG, Sardella G, Sharma SK, Shlofmitz R, Weisz G, Witzenbichler B, Pocock S, Mehran R . Ticagrelor monotherapy in patients at high bleeding risk undergoing percutaneous coronary intervention: TWILIGHT-HBR. Eur Heart J 2021;42:4624–4634. [DOI] [PubMed] [Google Scholar]
- 32. James SK, Roe MT, Cannon CP, Cornel JH, Horrow J, Husted S, Katus H, Morais J, Steg PG, Storey RF, Stevens S, Wallentin L, Harrington RA. Ticagrelor versus clopidogrel in patients with acute coronary syndromes intended for non-invasive management: substudy from prospective randomised PLATelet inhibition and patient Outcomes (PLATO) trial. BMJ 2011;342:d3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Baber U, Mehran R, Giustino G, Cohen DJ, Henry TD, Sartori S, Ariti C, Litherland C, Dangas G, Gibson CM, Krucoff MW, Moliterno DJ, Kirtane AJ, Stone GW, Colombo A, Chieffo A, Kini AS, Witzenbichler B, Weisz G, Steg PG, Pocock S. Coronary thrombosis and major bleeding after PCI with drug-eluting stents: risk scores from PARIS. J Am Coll Cardiol 2016;67:2224–2234. [DOI] [PubMed] [Google Scholar]
- 34. Wester A, Mohammad MA, Olivecrona G, Holmqvist J, Yndigegn T, Koul S. Validation of the 4-item PRECISE-DAPT score: a SWEDEHEART study. JAHA 2021;10:20974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ueki Y, Bär S, Losdat S, Otsuka T, Zanchin C, Zanchin T, Gragnano F, Gargiulo G, Siontis GCM, Praz F, Lanz J, Hunziker L, Stortecky S, Pilgrim T, Heg D, Valgimigli M, Windecker S, Räber L. Validation of the Academic Research Consortium for High Bleeding Risk (ARC-HBR) criteria in patients undergoing percutaneous coronary intervention and comparison with contemporary bleeding risk scores. EuroIntervention 2020;16:371–379. [DOI] [PubMed] [Google Scholar]
- 36. Choi SY, Kim MH, Lee KM, Ko YG, Yoon CH, Jo MK, Yun S-C. Comparison of performance between ARC-HBR criteria and PRECISE-DAPT score in patients undergoing percutaneous coronary intervention. JCM 2021;10:2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fujii T, Ikari Y. Predictive ability of academic research consortium for high bleeding risk criteria in ST-Elevation myocardial infarction patients undergoing primary coronary intervention. Circ J 2021;85:159–165. [DOI] [PubMed] [Google Scholar]
- 38. Aradi D, Kirtane A, Bonello L, Gurbel PA, Tantry US, Huber K, Freynhofer MK, Ten Berg J, Janssen P, Angiolillo DJ, Siller-Matula JM, Marcucci R, Patti G, Mangiacapra F, Valgimigli M, Morel O, Palmerini T, Price MJ, Cuisset T, Kastrati A, Stone GW, Sibbing D. Bleeding and stent thrombosis on P2Y12-inhibitors: collaborative analysis on the role of platelet reactivity for risk stratification after percutaneous coronary intervention. Eur Heart J 2015;36:1762–1771. [DOI] [PubMed] [Google Scholar]
- 39. Sibbing D, Aradi D, Alexopoulos D, ten Berg J, Bhatt DL, Bonello L, Collet J-P, Cuisset T, Franchi F, Gross L, Gurbel P, Jeong Y-H, Mehran R, Moliterno DJ, Neumann F-J, Pereira NL, Price MJ, Sabatine MS, So DYF, Stone GW, Storey RF, Tantry U, Trenk D, Valgimigli M, Waksman R, Angiolillo DJ. Updated expert consensus statement on platelet function and genetic testing for guiding P2Y12 receptor inhibitor treatment in percutaneous coronary intervention. JACC Cardiovasc Interv 2019;12:1521–1537. [DOI] [PubMed] [Google Scholar]
- 40. Galli M, Ortega-Paz L, Franchi F, Rollini F, Angiolillo DJ. Precision medicine in interventional cardiology: implications for antiplatelet therapy in patients undergoing percutaneous coronary intervention. Pharmacogenomics 2022;23:723–737. [DOI] [PubMed] [Google Scholar]
- 41. Galli M, Benenati S, Franchi F, Rollini F, Capodanno D, Biondi-Zoccai G, Vescovo GM, Cavallari LH, Bikdeli B, Ten Berg J, Mehran R, Gibson CM, Crea F, Pereira NL, Sibbing D, Angiolillo DJ. Comparative effects of guided vs. potent P2Y12 inhibitor therapy in acute coronary syndrome: a network meta-analysis of 61 898 patients from 15 randomized trials. Eur Heart J 2022;43:959–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly due to ethical and privacy reasons.