Abstract
Peripheral nerve injury (PNI) is a structural event with harmful consequences worldwide. Due to the limited intrinsic regenerative capacity of the peripheral nerve in adults, neural restoration after PNI is difficult. Neurological remodeling has a crucial effect on the repair of the form and function during the regeneration of the peripheral nerve after the peripheral nerve is injured. Several studies have demonstrated that acupuncture is effective for PNI-induced neurologic deficits, and the potential mechanisms responsible for its effects involve the nervous system remodeling in the process of nerve repair. Moreover, acupuncture promotes neural regeneration and axon sprouting by activating related neurotrophins retrograde transport, such as nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), glial cell-derived neurotrophic factor (GDNF), N-cadherin, and MicroRNAs. Peripheral nerve injury enhances the perceptual response of the central nervous system to pain, causing central sensitization and accelerating neuronal cell apoptosis. Together with this, the remodeling of synaptic transmission function would worsen pain discomfort. Neuroimaging studies have shown remodeling changes in both gray and white matter after peripheral nerve injury. Acupuncture not only reverses the poor remodeling of the nervous system but also stimulates the release of neurotrophic substances such as nerve growth factors in the nervous system to ameliorate pain and promote the regeneration and repair of nerve fibers. In conclusion, the neurological remodeling at the peripheral and central levels in the process of acupuncture treatment accelerates nerve regeneration and repair. These findings provide novel insights enabling the clinical application of acupuncture in the treatment of PNI.
Keywords: acupuncture, peripheral nerve injury, mechanism, nerve system remodeling, nerve repair
Graphical abstract
1. Introduction
Peripheral nerve injury caused by compression, traction, ischemia, and laceration can cause motor impairment of the limb, disuse atrophy of the limb muscles, and other nutritional disorders and sensory disturbances in the damaged innervated areas. With a 13–23 per 100,000 person-years estimated incidence rate, peripheral nerve damage (PNI) can cause sensory loss, persistent discomfort, motor impairment, or even amputation (Asplund et al., 2009; Sullivan et al., 2016). The most popular nerve injury categorization system is Seddon’s original one based on neurophysiological alterations: grade 1 nerve damage is a neurapraxia disease, grade 2 is axonal degeneration, and grade 3 is nerve transection. Peripheral nerve injury functional recovery is a slow process. The amount of time required for functional reconstruction and neural regeneration may be the reason for this, but the treatment method used also may be a factor in how long it takes to recover. Before the nerve injury becomes irreparable, early detection enables the start of neuroanastomosis, a suitable rehabilitation program, and adjustment of biomechanics, all of which benefit to encourage nerve repair. For instance, peripheral nerve injuries are more frequent in the upper extremities than the lower, tend to be sport-specific, and frequently include a biomechanical component. According to earlier research, people with peripheral nerve injury of the upper limbs did not have a high quality of life because of the acute neuropathic pain and potential for impairment (Pan et al., 2019). At present, there are gradually reconstructive operations clinically, but many patients experience a loss of their upper limbs’ normal function after the operation. Long-term nerve injury and inability to recover will also result in a lifetime impairment of patients. Therefore, further postoperative recovery is required to treat them (Bruyns et al., 2003; Magistroni et al., 2020). The main strategies for the treatment of PNI include pharmacologic interventions, behavioral therapy, physical stimulation, and supportive therapy in addition to surgery. In the numerous interventions for peripheral nerve injury, we need to carry out precision treatment according to the patient’s condition. Among them, acupuncture has its unique advantages in that acupuncture plays an important role in anti-inflammation, analgesia, and speeding up nerve repair. This makes the sequence of postoperative rehabilitation treatment of patients can be acupuncture first and rehabilitation manipulation later, to reduce the pain during rehabilitation manipulation and maximize the analgesic efficiency of acupuncture. Moreover, acupuncture is a safe, effective, and less painful treatment method. But there is still much uncertainty about how acupuncture works in PNI.
Acupuncture is a procedure involving the insertion of a fine needle into the skin or deeper tissues at specific locations of the body (acupoints) to prevent and treat diseases. Several lines of neuroanatomical and neurological evidence have demonstrated the abundant distribution of nerve endings in human meridians and acupoints, and the involvement of the nervous system is indispensable for the effects of acupuncture. Acupuncture is regarded as a potential adjuvant treatment for acupuncture analgesia, neural regeneration, and functional restoration in addition to conventional therapy of nerve anastomosis (Hao et al., 1995; Balogun et al., 1998; Zhang et al., 2018). Some studies showed that electroacupuncture (EA) helped individuals with peripheral nerve injuries improve their motor or sensory function (Dimitrova et al., 2017). Some researchers have indicated that rehabilitation training together with Jiaji electroacupuncture can greatly facilitate the recovery of muscle group function and improve the quality of life of patients with upper limb peripheral nerve injury (Li H. et al., 2021). With the rapid development of research on acupuncture’s central effect, accumulating evidence showed acupuncture or electro-acupuncture also played a key role in brain remodeling, which suggested a potentially positive impact on axon regeneration and synapse formation (Xiao et al., 2018). Some research suggests acupuncture and electroacupuncture can encourage nerve regeneration and enhance nerve function (Inoue et al., 2003; Lu et al., 2008; Chen et al., 2013). What’s more, acupuncture is green and harmless, which has this beauty, that accelerates the recovery after PNI.
Research on the mechanism of acupuncture in peripheral nerve injury is currently mostly concentrated at the level of individual molecules and/or signaling cascades. Acupuncture has the potential to control a complex network of several signaling molecules and pathways due to the wide range of interaction communication that exists between various signaling pathways. This notion is in line with acupuncture’s holistic regulation features, which involve numerous targets, linkages, techniques, and levels. And more research is needed to describe how acupuncture affects this intricate network. Therefore, this paper reviews the effects and mechanisms of acupuncture on nerve regeneration and repair from the perspective of nervous system remodeling.
2. Clinical efficacy of acupuncture or electro-acupuncture on nerve regeneration of PNI patients
Acupuncture or EA affects nerve system according to several studies. Researchers showed that low-intensity ES at the ST36 site could activate brainstem vagal efferent neurons or drive catecholamine release from adrenal glands to achieve the target that suppressed systemic inflammation induced by bacterial endotoxins (Liu et al., 2021). Some results suggested that electroacupuncture may be able to modulate extracellular adenosine triphosphate (ATP) levels in the prefrontal cortex of depressive-like maternal separation rats, potentially contributing to its antidepressant effects (Zheng et al., 2023). It is demonstrated that EA pretreatment resulted in increased ambient endocannabinoid (eCB) levels and subsequent activation of ischemic penumbral astroglial cannabinoid type 1 receptors (CB1R) in a middle cerebral artery occlusion (MCAO) model which led to moderate upregulation of extracellular glutamate that protected neurons from cerebral ischemic injury (Yang et al., 2021). Researchers found that EA markedly improved acute lumbar sprain, and EA better improved the rehabilitation and regeneration of force-displacement values and temperature index of the infrared thermogram of the muscle (Fan and Wu, 2015). Acupuncture or EA has been found to have a beneficial clinical effect in treating PNI, according to some researchers. Accelerated nerve regeneration caused by electroacupuncture with intermittent direct current may contribute to the recovery of PNI (Inoue et al., 2011). Some studies demonstrated that short-term acupuncture treatment may result in long-term improvement in mild-to-moderate idiopathic CTS. Acupuncture treatment can be considered an alternative therapy to other conservative treatments for those who do not opt for early surgical decompression (Yang et al., 2011). Some findings indicated that electroacupuncture could improve symptomatology for CTS patients, as evidenced by improved symptomatology, grip strength, electrophysiological function, and physical provocation sign (Ho et al., 2014). The grip strength of CTS patients’ primary symptomatic side dramatically increased following acupuncture treatment indicating an improvement in median nerve motor function obstacle (Mathiowetz et al., 1985). As CTS-induced paresthesias constitute diffuse, synchronized, multidigit symptomatology, researchers found that maladaptive change and correction are consistent with Hebbian plasticity mechanisms. Acupuncture shows promise in inducing beneficial cortical plasticity manifested by more focused digital representations (Napadow et al., 2007b). The acupoints for PNI treatment are listed in Tables 1–5 and shown in Figures 1–5. Table 1 summarizes the evidence of the effect of acupuncture on the repair of peripheral nerve injury.
Table 1.
Effect of acupuncture on the repair of peripheral nerve injury.
| Study | Model | Acupoints | Acupuncture type/parameter | Major effects |
|---|---|---|---|---|
| Hoang et al. (2012) | Sciatic nerve crush | GB30, GB34 | EA/0.8–1 mA, 2 Hz, 15 min | motor recovery↑, target re-innervation pain↓ |
| Ho et al. (2013) | Transected Median Nerve | PC7, PC3 | EA/1 mA, 2 Hz, 15 min | Electrophysiological Measurements (Latency, amplitude, MAPs, NCV)↑, Morphologically (axon number, endoneurial area, total nerve area, blood vessel number)↑, Functionally (Grasping Test)↑ |
| Cheng et al. (2001) | Rat sciatic nerve | GB30, GB34 | EA/0.8-1 mA, 2 Hz, 15 min | axon density↑, blood vessel area↑, percentage of blood vessel area↑ |
| Li H. et al. (2021) | Upper limb peripheral nerve injury | C6-T1 Jiaji points | EA/once a day, 30 min | Barthel index↑, Fugl-Meyer assessment score↑, motor nerve conduction velocity↑, sensory nerve conduction velocity and amplitude↑, SF-36↑ |
| Napadow et al. (2007a,b) | CTS | Common:TW-5, PC-7 matching aupoint: HT-3, PC-3, SI-4, LI-5, LI-10, LU-5 | EA/2 Hz, 10 min | BCTSQ ↑, nerve sensory latencies ↑, grip strength↑, fMRI: inducing beneficial cortical plasticity manifested ↑ |
| Inoue et al. (2011) | Peripheral nerve damage | Anode electrode–proximal to the injured part, cathode electrode–innervated muscle | DCEA/100 Hz, 20 min | functional↑: neurapraxia 2, axonotmesis 3; No functional: axonotmesis 1, neuromesis 1 |
| Ho et al. (2014) | CTS | PC7, PC6 | EA/0.8 mA, 2 Hz, 15 min | Elec-Acu group: short clinical questionnaire by Lo and Chiang↑; Acu group: grip strength↑, distal median motor amplitude of the palm-wrist segment↑, Tinel’s sign↓; No changes: two-point discrimination |
| Yang et al. (2011) | CTS | PC-7, PC-6 | MA/30 min | modified GSS↑, DML↑, CMAP↑, MNCV↑, DSL↑, SNAP↑, W-P SNCV↑ |
| Fan and Wu (2015) | Acute lumbar muscle sprain | Bilateral SI-3, Jiaji (EX-B2), Ashi points | EA/10-25 Hz, 20 min | FDVs of the bilateral lumbar muscle↑, temperature index of the lumbar skin↑ |
MAPs, muscle action potentials; NCV, nerve conduction velocity; CTS, carpal tunnel syndrome; BCTSQ, Boston Carpal Tunnel Syndrome Questionnaire; GSS, Global symptom score; DML, distal motor latency; CMAP, compound muscle action potential; MNCV, motor nerve conduction velocity; DSL, distal sensory latency; SNAP, sensory nerve action potential; W-P SNCV, wrist-palm sensory nerve conduction velocity; FDV, force-displacement value.
Table 5.
Mechanism of acupuncture on the regulation of factors associated with neuronal response in peripheral nerve injury.
| Animal study | Model | Acupoints | Acupuncture type/parameter | Major effects |
|---|---|---|---|---|
| Liu et al. (2015) | MS | GV6 GV9 |
EA/1-2 mA, 2/60 Hz, 20 min | NT-3↑, differentiation of oligodendrocyte-like cells from grafted NR-MSCs in the demyelinated spinal cord↑ |
| Hu et al. (2018) | Sciatic nerve injury | GB30 ST36 |
EA/20 mA, 5 Hz, 15 min | Compare to model-only group: sciatic functional index↑, recovery rate of conduction velocity↑, diameter recovery of the gastrocnemius muscle fibers↑, S100-immunoreactive cells↑, nerve growth factor↑; treatment groups: not differ |
| Zhao X. et al. (2018) | MCAO | GV20 | EA/1-2 mA, 2/10 Hz, 30 min | miR-132↑, SOX2↓ |
| Cui et al. (2017) | SD rats | ST36 SP6 |
EA/3 mA, 2/15 Hz, 30 min | miR-7a-5p↓, miR-148a-3p ↓, miR-124-3p↑, miR-204-5p ↓, miR-370-3p↓, miR-221-3p ↑, miR-107-3p↑ |
| Du and Liu (2015) | Diabetic models | ST36 | EA/1 mA, 10/100 Hz, 30 min | The mRNA and protein level of the enteric neurons↑, GDNF in colon↑, p-Akt in colon↑ |
| Yu et al. (2017) | SNI models | GB30 ST36 | EA/2 mA, 5 Hz, 30 min | Agrin ↑, AChR-ε↑, AChR-γ↓ |
MS, multiple sclerosis; NT-3, neurotrophin-3; NR-MSCs, neurotrophin-3 and retinoic acid preinduced mesenchymal stem cells; MCAO, middle cerebral artery occlusion; GDNF, glia cell line-derived neurotrophic factor; SNI, sciatic nerve injury; AChR, acetylcholine receptor; SFI, sciatic nerve functional index; CSA, cross-sectional area.
Figure 1.
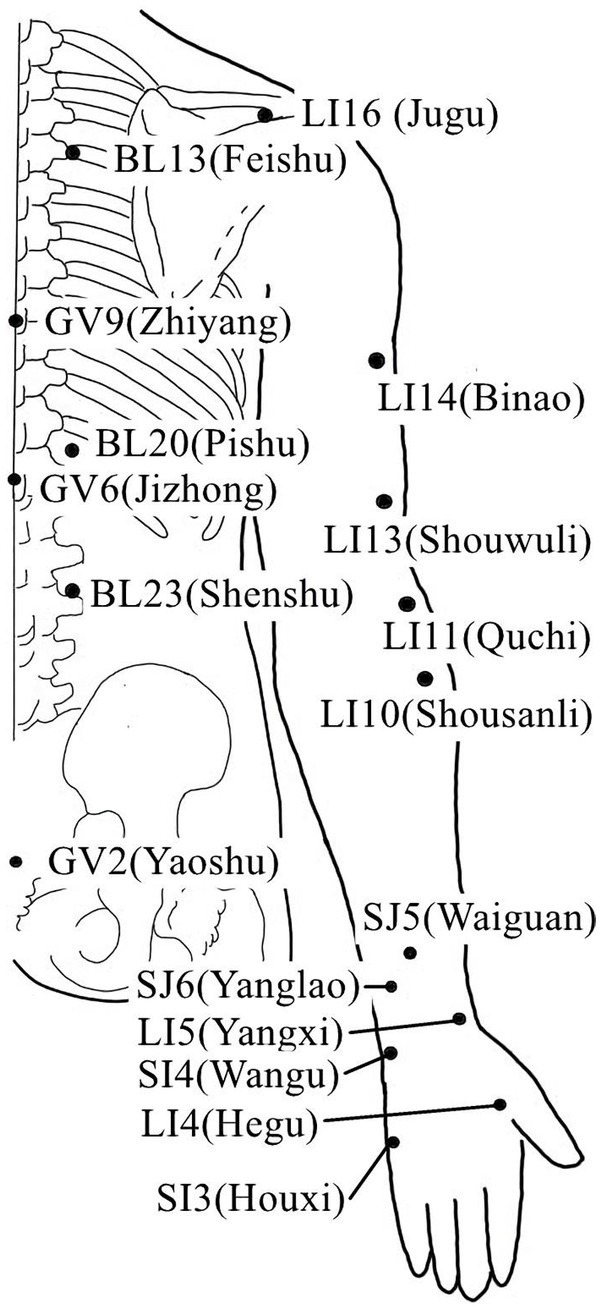
Acupoints on the upper limb lateral and back lumbar.
Figure 5.
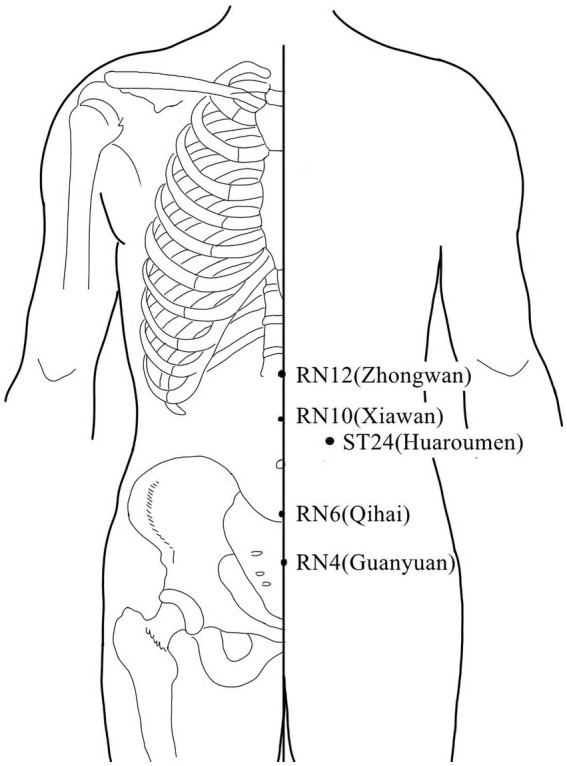
Acupoints in the chest and abdomen.
Figure 2.
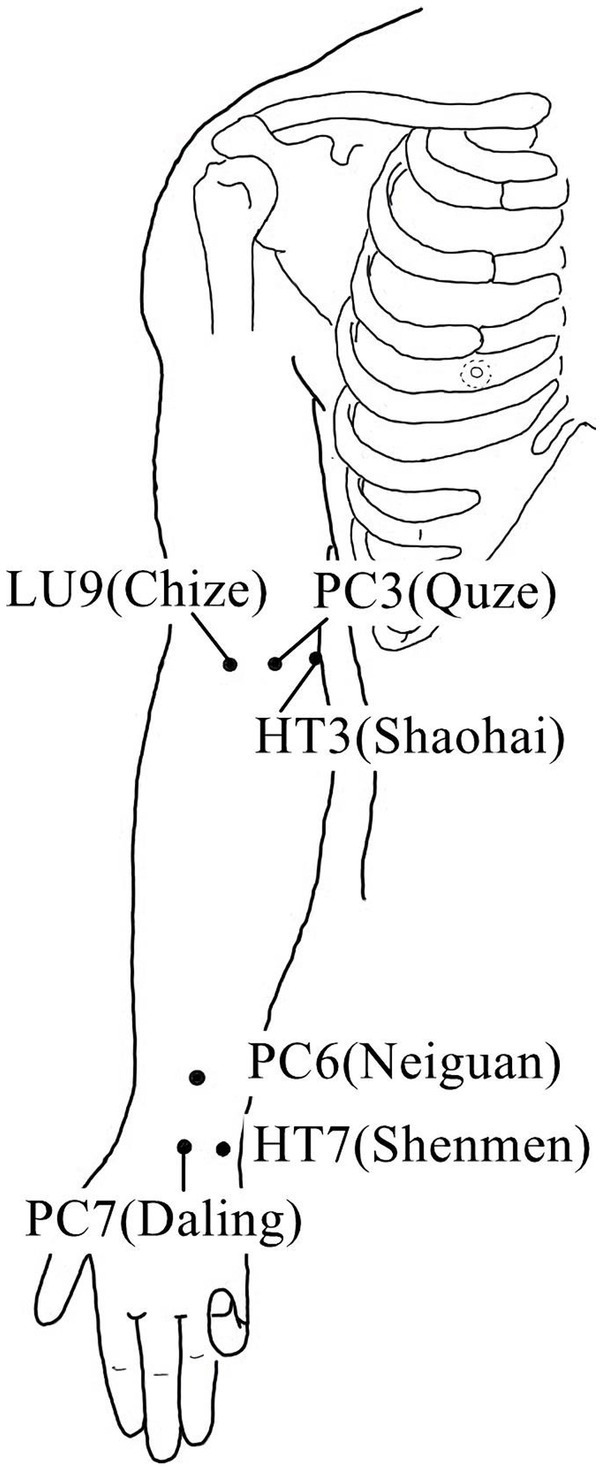
Acupoints on the inside of the upper limb.
Figure 3.
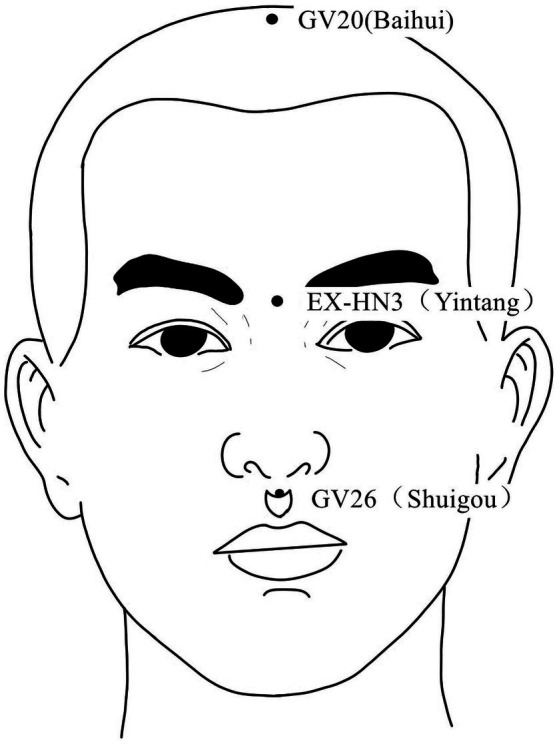
Acupoints on the head and face According to the newest National Standard of the People’s Republic of China GB/T 12346–2021 Nomenclature and Location of Meridia Points, Yintang belongs to Governor Vein (GV), in order to be consistent with the literature cited in this paper, Yintang is still labeled EX-HN3.
Figure 4.
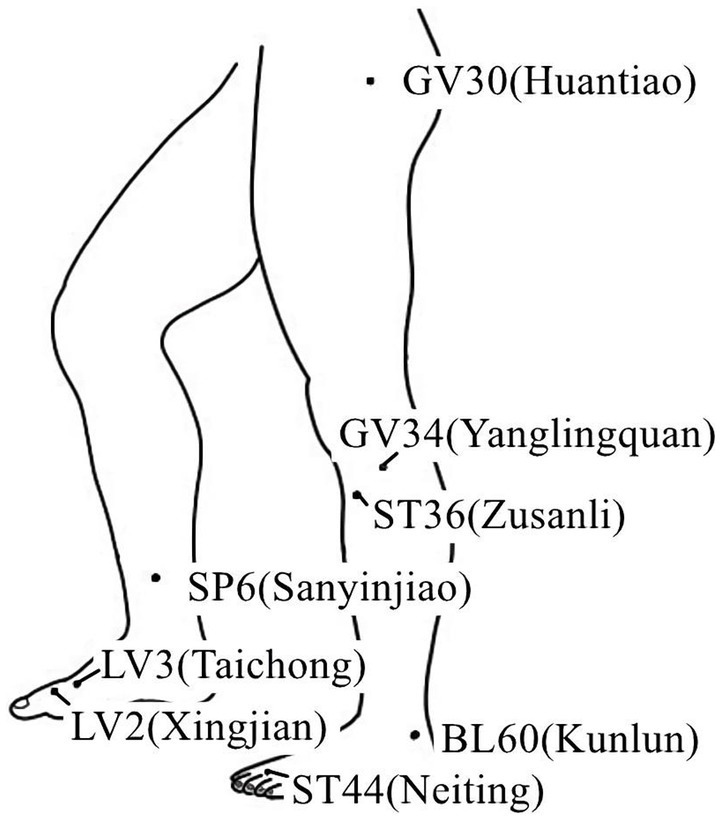
Acupoints on the lower extremity.
3. Nerve system remodeling-related mechanisms of acupuncture or electroacupuncture for the treatment of peripheral nerve injury
3.1. Peripheral nerve fiber regeneration and repair
The primary obstacles to saving neurological function following damage are repair, regeneration, and neuroprotection (Ribeiro et al., 2016; Tallon and Farah, 2017). The potential for regeneration after nerve fiber injury is reflected by the de novo expression or upregulation of a massive variety of molecules in the distal nerve fiber tracts (Gordon and Borschel, 2017). So, it is vital to maintain the integrity of the nerve fibers. Electrical stimulation can facilitate peripheral nerve regeneration and target reinnervation (Wenjin et al., 2011; Liu et al., 2013; Zhao et al., 2013). Investigators believe that a weak electric field may affect the regeneration of mammalian peripheral nerves (Schmidt et al., 1997), and extracellular electric fields could influence the orientation of neurite development (Patel and Poo, 1982). Under the influence of a weak electrical field, neurite outgrowth grew more quickly in the direction of the negative pole, but the growth in the direction of the positive pole was suppressed (Song et al., 2004). Studies have discovered that needles used for direct electrical stimulation of the damaged ulnar nerve together with rehabilitation expedite nerve regeneration (Tang et al., 2016). Additionally, electro-needling could increase the number of blood vessels and axon densities in rat peripheral nerves regenerated within a silicone rubber tube (Cheng et al., 2001), after nerve transection, EA could promote the recovery of transected median nerve morphology and function (Ho et al., 2013). According to the experimental results, electroacupuncture can upregulate β-endorphin expression, which reduces neuropathic pain and aid in the healing of damaged brachial plexus nerves (Zhang et al., 2014). According to some research, acupuncture therapy could enhance motor and sensory nerve conduction, scores for the sciatic nerve function index, compound muscle action potential, and motor nerve conduction velocity as well as better promote sciatic nerve regeneration and reduce muscle atrophy while causing less mechanical damage to nerve trunk (Yu et al., 2019). EA was reported to effectively improve functional outcomes following PNI (Zhi et al., 2017). After nerve reconstruction, the regeneration of the motion axis takes a long time (Pagnussat et al., 2012; Sturma et al., 2018). Therefore, EA can both serve as a foundation for nerve regeneration and has the potential to speed up the healing process for damaged nerves.
3.2. Mechanism of spinal cord regulation in peripheral nerve injury
3.2.1. Central sensitization
Central sensitization, where the input of noxious stimuli enhances the perceptual response of the central nervous system to pain, is caused by tissue inflammation or peripheral nerve damage and plays an important role in persistent pain (Van Griensven et al., 2020). Previous research has shown that a local cutaneous injury can produce primary hyperalgesia within the injured area and secondary hyperalgesia in the normal surrounding skin, for example, an intradermal injection of capsaicin in humans causes intense pain and hyperalgesia to heat and mechanical stimuli in the surrounding skin. Psychophysical studies in humans supported the conclusions that hyperalgesia was predominantly the secondary type and depended on one set of neurons sensitizing another (“neurogenic hyperalgesia”) and that the latter set of neurons is located in the central and not the peripheral nervous system (Baumann et al., 1991). Central sensitization is accountable for spreading pain and hyperalgesia to uninjured tissue, the process is involved in pain-transmission neurons in the central nervous system (CNS), often in the dorsal horn of the spinal cord (Kim et al., 2009). Second-level sensory neurons, or the relay stations of the conduction of the sensory system, are represented by the neurons of the spinal dorsal horn. The ascending fiber tracts are created by the spinal dorsal horn neurons’ axons as they enter the ipsilateral or contralateral white matter. These ascending fiber tracts then transmit the dorsal root afferents’ nerve impulses to the third-level neurons in the ventroposterolateral thalamic nucleus of the brain, which then transmits them to the cerebral cortex. Some results suggested that acupuncture may simultaneously modulate the resting state functional connectivity (rsFC) of key regions in the descending pain modulation (periaqueductal gray, PAG) and reward systems (ventral tegmental area, VTA), and the amygdala may be a key node linking the two systems to produce antinociceptive effects (Yu et al., 2020). This makes sense because it is widely known that ipsilateral acupuncture has an impact on numerous descending inhibitory networks from the amygdala, periaqueductal gray (PAG), and rostroventral medulla (RVM) which primarily modulate nociceptive signals entering the ipsilateral spinal cord and are influenced by ipsilateral acupuncture (Levine et al., 1991; Van Bockstaele et al., 1991; Manning, 1998; Urban et al., 1999). Acupuncture is demonstrated to activate the ascending sensory pathways, such as the spinal dorsal horn and thalamus, or the descending pain inhibitory mechanisms, such as opioid, adrenergic, and serotonergic pathways, however, the precise areas where EA affects pain are not fully established (Skrabanek, 1984; Tian et al., 2006; Li et al., 2007; Koo et al., 2008).
3.2.2. Neuronal apoptosis
According to previous findings, PNI not only results in localized damage but also trigger the apoptosis of certain spinal cord neurons in corresponding spinal cord segments (Inquimbert et al., 2018). The survival of central neurons plays a key role in determining the effectiveness of peripheral nerve regeneration (Kim and Choi, 2016). After PNI, the corresponding neuron cell bodies will undergo apoptosis because of neurotrophin transmission disorders. And we discovered that glial cell-derived neurotrophic factors and brain-derived neurotrophic factors could alleviate PNI in animal models, and decrease apoptosis and autophagy in vitro cell and cell injury model studies (Chou et al., 2014; Kingham et al., 2014). PNI causes apoptosis in spinal motor neurons and sensory spinal ganglion neurons, but it is not extensive and does not affect the entire spinal cord, it is associated with neurons that are connected to the injured peripheral nerves (Ahmad et al., 2015). According to a previous study, acupuncture can ensure and enhance the continuity between peripheral and central nerves, speeding up the process of repairing injured nerves, laying the groundwork for regeneration, and effectively restoring motor conduction (He et al., 2015), and electroacupuncture could dramatically boost facial nerve regeneration by increasing the expression of GDNF and N-cadherin in neurons, preventing neuronal apoptosis (Fei et al., 2019). Some results indicate that EA suppresses spinal nerve ligation (SNL)-induced neuropathic pain by improving neuronal plasticity via upregulating the adenosine A2A receptor (A2AR) and the cyclic adenosine monophosphate (cAMP)/protein kinase A (PKA) signaling pathway (Wu et al., 2021a), but more research is still needed to understand the underlying mechanism. Table 2 summarizes the evidence of the mechanism of acupuncture on the repair of spinal cord regulation in peripheral nerve injury.
Table 2.
Mechanism of acupuncture on the repair of spinal cord regulation in peripheral nerve injury.
| Study | Model | Acupoints | Acupuncture type/parameter | Major effects |
|---|---|---|---|---|
| Pan et al. (2019) | DPN | BL13, BL20, BL23, LI4, LR3, ST36, SP6 | EA/frequency 3 Hz, 20 min | GRP78↓, caspase-12↓, sciatic nerves cell apoptosis↓ |
| Kim et al. (2009) | Capsaicin-induced secondary hyperalgesia | GB30-GB34, BL40-BL60, GV2-GV6, LI3-LI6, SI3-TE8 | EA/intensity 3 mA, frequency 2/100 Hz, 30 min | Endogenous spinal mu- and delta-opioid receptors↑ |
| Koo et al. (2008) | Ankle sprain | SI6, LI4 | EA/intensity 2 mA, frequency 2/100 Hz, 30 min | Spinal alpha (2)-adrenoceptor↑ |
| Li et al. (2007) | Inflammatory pain | GB30 | EA/intensity 3 mA, frequency 10 Hz, 20 min | Supraspinal neurons↑ |
| Mayer et al. (1977) | Ho-Ku’ | MA/rotated for 2 min out of 5 min, 30 min | Release of an endogenous substance with narcotic analgesic activity↑ |
DPN, diabetic peripheral neuropathy; GRP78, glucose-regulated protein 78.
3.3. Synaptic remodeling
The place where neurons join and where information is transmitted is called a synapse. Synapses, which are mostly found on dendritic spines and/or soma, serve a crucial role in the functional connections between neurons and are essential parts of information transmission. The synaptic region contains a variety of enzymes that break down neurotransmitters. Neurotransmitters transmit more slowly and are more degraded when there is a larger synaptic distance. As a result, the structural basis for the remodeling of synaptic transmission function may be the increase in the number of synapses, synaptic vesicle density, and the narrowing of synaptic space. A prior study suggested that the analgesic effect of EA might be connected to the suppression of inflammatory factors and dendritic spine/synaptic remodeling (Wu et al., 2021b).
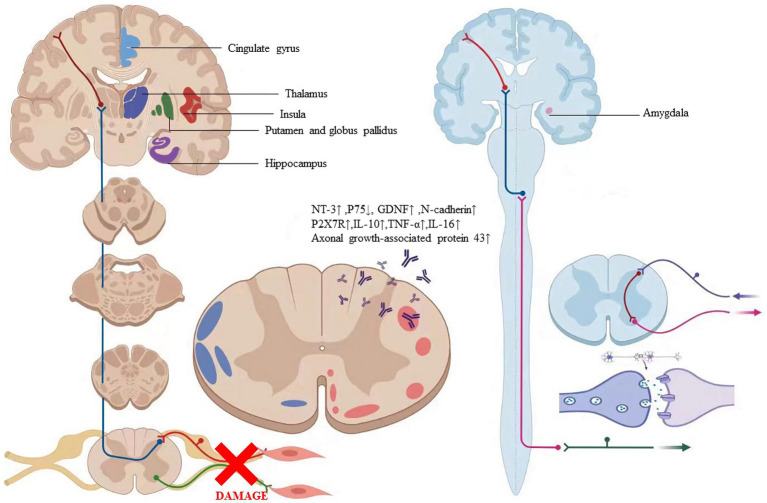
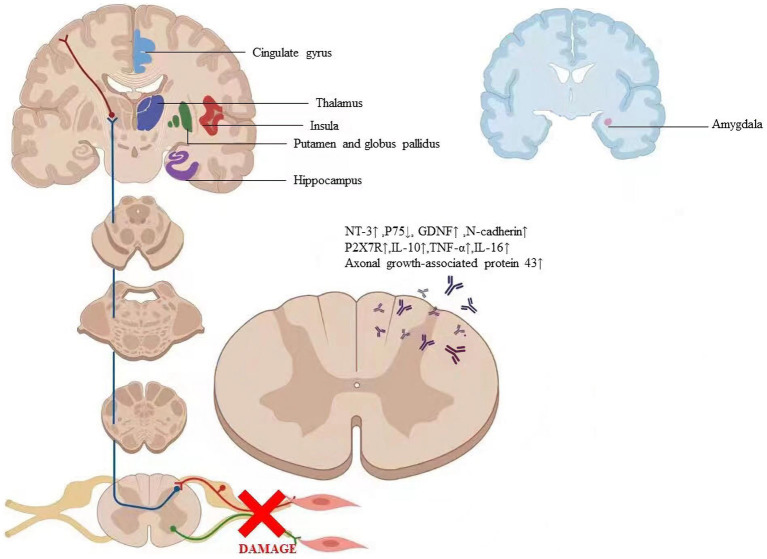
Microglial cells, the smallest glial cells in the nervous system with polysynaptic and plasticity, are the innate immunological effector cells in the central nervous system. Previous research has shown that microglial cells are a crucial target for analgesia by EA (Shan et al., 2007; Sun et al., 2008), EA can reduce neuropathic pain by promoting the production of IL-10 in spinal microglial cells (Ali et al., 2020). EA-induced anti-hyperalgesia may be partially associated with the reduced expression of Phospho-p38 MAPK (p-p38 MAPK) on microglia, and subsequently reducing the activation of oxytocin-42 (OX-42, marker of microglia) in neuropathic pain (Liang et al., 2016). P2X7R overexpression in neuropathic pain models can result in microglia activation and overexpression of TNF-a and IL-1b. Long-term potentiation is avoided and the pain threshold is raised by downregulating P2X7R with P2X7-specific siRNA (Chu et al., 2010; Vadivelu et al., 2015; Shen et al., 2018). Following chronic constriction injury, there was an increase in P2X7R expression in the spinal cord and a corresponding drop in the rat pain threshold (Lin et al., 2018). Therefore, EA may reduce aberrant dendritic spine/synaptic remodeling, and inflammation by downregulating P2X7R to improve neuropathic pain and promote nerve repair (Figure 6; Wu et al., 2021b). Table 3 summarizes the evidence of the mechanism of acupuncture on the regulation of synaptic in peripheral nerve injury.
Figure 6.
The labeled regions revealed where the particular alterations in the brain are caused by acupuncture. The factors regulated by acupuncture include axonal growth-associated protein 431, NT-31, P751, GDNF1, N-cadherin, P2X7R1, IL-101, TNF-at, and IL-161. Abbreviation: NT-3: Neurotrophins-3, GDNF: Glial cell line-derived neurotrophic factor, P2X7R: P2X7 receptor, IL- 10: Interleukin 10, TNF-α: Tumor necrosis factor-alpha, IL-16: Interleukin 16.
Table 3.
Mechanism of acupuncture on the regulation of brain regions in peripheral nerve injury.
| Study | Acupoint | Intervention and acupuncture type/parameters | Control intervention | Method | Major effects |
|---|---|---|---|---|---|
| Matsumoto-Miyazaki et al. (2016) | GV 26, Ex-HN 3, bilateral LI 4, ST 36 | MA/10 min | No treatment | TMS | MEP amplitude↑, MEP/Mmax ↑, CMCTs↓, the CST activity of patients with chronic DOC after severe TBI↑ |
| Zhao N. et al. (2018) | At the middle 2/5 of the MS6 line in the affected hemisphere | SA/40 min + LF-rTMS 20 min | SA/40 min | DTI | FMA↑, MBI↑, FAvalue↑, MDvalue↓ |
| Yang et al. (2017) | LI-11, LI-10, TB-5, LI-4, ST-36, GB-34, SP-6, EX-UE9 | MA/Deqi, later hold the needle still for 30 min | No stimulation | TMS | Left MEP↓, right MEP↑, IHI↑ |
| Chen et al. (2015) | GB34 | MA/1.5 Hz, 1 min baseline-30 s Stimulation-three blocks | Non-acupoint | fMRI | Motor-cognition connectivity↑, compensation of unaffected motor cortex and homolateral synkinesis↓ |
| Napadow et al. (2007a,b) | LI-4 | MA/1 Hz, 2 min rest-1 min stimulation-7-min block paradigm | Non-insertive cutaneous stimulation | fMRI | Functional connectivity between the hypothalamus and amygdala: amygdala deactivation↓, hypothalamus activation↑, and vice versa |
| Wang et al. (2016) | RN12, RN10, RN6, RN4, KL17, ST24, Qipang | Abdominal acupuncture/20 min | Non-insertive cutaneous stimulation | fMRI | MADRS scores↓, SDS scores↓, rsFC between the left amygdala and sgACC/ pgACC↑ |
| Napadow et al. (2005) | ST-36 | MA/1 Hz; EA/2 Hz, 100 Hz, 2 min rest-1 min stimulation-7-min block paradigm | Tactile control stimulation | fMRI | Acu, EA: anterior insula hemodynamic signal↑, limbic and paralimbic structures hemodynamic signal ↓ only EA: anterior middle cingulate cortex signal↑, pontine raphe area signal↑ |
| Yan et al. (2005) | Liv3 LI4 | MA/1 Hz | Non-acupoints | fMRI | Liv3, LI4: middle temporal gyrus and cerebellum↑, middle frontal gyrus and inferior parietal lobule↓ Liv3: postcentral gyrus, posterior cingulate, parahippocampal gyrus, BA 7, 19 and 41↑, inferior frontal gyrus, anterior cingulate, BA 17 and 18↓ LI4: temporal pole↑, precentral gyrus, superior temporal gyrus, pulvinar and BA 8, 9 and 45↓ |
| Kong et al. (2002) | LI4 | EA, MA/3 Hz, 1 min baseline-1 min stimulation-5-min block paradigm | fMRI | EA: precentral gyrus, postcentral gyrus/inferior parietal lobule, and putamen/insula fMRI signal ↑ Acu: posterior cingulate, superior temporal gyrus, putamen/insula fMRI signal↓ | |
| Hui et al. (2000) | LI4 | MA/120 times per min, 2 min baseline-2 min stimulation-4 min rest-16 min scan time | Tactile stimulation | fMRI | Modulates the activity of the limbic system and subcortical structures |
| Hui et al. (2005) | ST36 | MA/1 Hz, 2 min baseline-2 min stimulation-3 min rest-10 min scan time | Sensory control stimulation | fMRI | An integrated response of the human cerebro-cerebellar and limbic systems to acupuncture stimulation that correlates with the psychophysical response |
| Li et al. (2015) | SJ5 | MA/twirled ± 180°, 60 times per minutes, 30s stimulation-30s rest-6 min scan time | fMRI | The clinical effect of Deqi during acupuncture is based on brain functional changes | |
| Lu et al. (2014) | ST36 | MA | Non-point | PET | Bilateral amygdalae activation↑, left temporal lobe activation↑, blood perfusion↑, glycol metabolism↑ |
| Shi et al. (2016) | BL40 | MA/depth 2 mm, 5 min | MA/depth 10–20 mm, 5 min | fMRI | Acupuncture modulates the limbic-paralimbic-neocortical network to produce its Deqi effects; The similarity of LPNN and DMN suggests that deep needing may mobilize an important intrinsic brain network for its multiple modulation effects |
| Wang et al. (2013) | LV3 | MA/rotated 180°, 1 Hz, 2 min baseline-2 min stimulation-3 min rest-10 min scan time | Tactile stimulation | fMRI | Pressure was contributing to negative activation of a LPNN; modulatory effects of different needling sensations contribute to acupuncture modulations of LPNN network |
| Napadow et al. (2009) | PC6 | MA/0.5 Hz, 30s stimulation-30s rest-5.5 min scan time | Non-invasive cutaneous stimulation | fMRI | Cognitive load↑, dmPFC activity↑ |
| Fang et al. (2009) | LV3, LV2, ST44 | MA/160 times per min, 180°, 1 min stimulation- 1 min rest-6 min scan time | Sham acupoint stimulation | fMRI | Limbic-paralimbic-neocortical system extensive deactivation↓; sensorimotor cortices, thalamus and occasional paralimbic structures activated↑ |
| Dhond et al. (2008) | PC6 | MA/twirled ± 180°, 0.5 Hz, 5.5 min baseline-5.5 min stimulation-31.5 min scan time | Non-insertive cutaneous stimulation | fMRI | DMN connectivity↑, SMN connectivity↑, post-stimulation spatial extent of resting brain networks to include anti-nociceptive, memory, and affective brain regions↑ |
| He et al. (2014) | LI4 | MA/Deqi, 10 min baseline-10 min stimulation-10 min postacupuncture resting state | fMRI | Connectivity in the primary somatosensory region of both early and late recovery groups↑ | |
| Zhang et al. (2016) | LR3, KI3 | MA/90–180°, 60–90 times per min, lifted and thrust 0.3–0.5 cm, 30 min | Non-acupoint | fMRI | Number of brain regions with altered brain activity after acupuncture at acupoint combinations↑ |
| Jung et al. (2015) | PC6, HT7 | MA/1 Hz | Pseudo-stimulation | fMRI | Salience network↑, default mode network↓ |
| Lee et al. (2013) | ST36 | MA/1 min baseline-30s stimulation-30s rest-5 min scan time | Non-acupoint | fMRI | Blood oxygenation level-dependent signal intensity in basal ganglia, limbic system, and cerebellum↓ |
| Napadow et al. (2009) | PC6 | MA/0.5 Hz, 30s baseline-30s stimulation-30s rest-5.5 min scan time | Non-insertive cutaneous stimulation | fMRI | Anterior dmPFC activity↑, posterior dmPFC activity↑ |
| Long et al. (2016) | ST36 | MA/6 min stimulation-6 min rest-4 min break | Non-acupoint | fMRI | Centrality in parahippocampal gyrus↑, centrality in middle temporal gyrus↑, DMN↑ |
| Lin et al. (2016) | LI4 | SNA + MS/twirled rotated, 180°, 1 Hz, 20s for15min | SNA, TNA/15 min; TENS/1 Hz, 20s for 15 min | fMRI | Enhance the acupuncture dose induce different DMN modulatory effects; TNA induces the most extensive DMN modulation |
| Dhond et al. (2008) | PC6 | MA/twirled (∼ ± 180°), 0.5 Hz, 5.5 min rest-5.5 min stimulation-31.5 min scan time | Non-insertive cutaneous stimulation | fMRI | DMN connectivity with pain, affective and memory related brain regions↑, SMN connectivity with pain-related brain regions ↑ |
| Ren et al. (2008) | Neiguan, Waiguan, Sanyinjiao, Zusanli | EA/1 mA, 10 Hz, 30 min | Dendritic spine density↑, ephrin-A5↑, neural plasticity at the peri-infarct cerebral cortex in acute cerebral ischemia rat↑ | ||
| Liang et al. (2017) | ST36, LI11 | EA/2 mA, 1/20 Hz, 30 min | The modified neurologic severity scores↑, neural activity of motor function-related brain regions↑ | ||
| Wu et al. (2018a,b) | GB30, ST36 | EA/0.2 mA, 1/20 Hz, 15 min | Limbic/paralimbic areas fluctuated↑ | ||
| Maeda et al. (2017) | TW5 PC7 + three additional acupoints | EA/2 Hz, 20 min | Sham acupuncture | fMRI | Primary somatosensory cortex somatotopy↑ |
MA, manual acupuncture; TMS, transcranial magnetic stimulation; MEP, motor-evoked potential; CMCT, Central motor conduction time; CST, cortico spinal tract; DOC, disorders of consciousness; TBI, traumatic brain injury; DTI, Diffusion tensor imaging; FA, fractional anisotropy; MD, mean diffusion; LF-rTMS, low frequency repetitive transcranial magnetic stimulation; IHI, interhemispheric inhibition; SA, scalp acupuncture; Deqi, a characteristic sensation of aching and tingling; 7-min block paradigm including a 2-min rest, 1-min stimulation, 2-min rest, 1-min stimulation, 1-min rest block; MADRS, Montgomery–Åsberg Depression Rating Scale scores; SDS, Self-Rating Depression Scale scores; sgACC, subgenual anterior cingulate cortex; pgACC, preguenual anterior cingulate cortex; rsFC, resting state functional connectivity; DMN, default mode networks; LPNN, limbic-paralimbic-neocortical network; dmPFC, dorsomedial prefrontal cortex; SMN, sensorimotor network; SNA, single needle acupuncture; TENS, transcutaneous electrical nerve stimulation; TNA, three needle acupuncture; MS, manual stimulation.
3.4. Regulation of brain regions in peripheral nerve injury
Plasticity following peripheral nerve transection has been demonstrated throughout the neuroaxis in animal models of nerve injury (Fawcett and Keynes, 1990). However, little research has demonstrated the brain changes that occur following peripheral nerve transection and surgical repair in humans. Furthermore, the extent to which peripheral nerve regeneration influences functional and structural brain changes has not been characterized (Taylor et al., 2009). Numerous studies on neural imaging extensively revealed the particular alterations in the brain caused by acupuncture (Figure 6; Kong et al., 2009; Napadow et al., 2009; Lee et al., 2013). Due to the tight relationship between behavioral performance and cortical plasticity, acupuncture may have an impact on cortical plasticity (Klingner et al., 2014; Jung et al., 2015; Zhang et al., 2016). Therefore, we asked whether acupuncture treatment of peripheral nerve injury is accompanied by gray and/or white matter structural changes and whether these changes relate to nerve system remodeling.
3.4.1. The endogenous opioid peptide system network
Numerous brain regions and nuclei, including the caudate nucleus, septal area, arcuate nucleus, periaqueductal gray, and nucleus raphe magnus, all of which contain opioid peptides, and μ, δ, and κ receptors, are involved in the transmission of acupuncture signals (Zhao, 2008). The hypothalamic arcuate nucleus is a significant structure in the endogenous opioid peptide system and a crucial region that mediates low-frequency electroanalgesia (Takeshige et al., 1991). Researchers found that acupuncture at HT7 points attenuates behavioral manifestation of alcohol dependence by activating endorphinergic input to the nucleus accumbens (NAc) from the arcuate nucleus(ARC) (Chang et al., 2019). The arcuate nucleus may control electroanalgesia based on the fact that its axons branch out to the adjacent nucleus accumbens, septum, periaqueductal gray, and locus coeruleus. In praxiology and electrophysiology, activating the arcuate nucleus will boost analgesic effects, increased the activity of neurons in the dorsal raphe nucleus and decreased the activity of neurons in the locus coeruleus (Kwon et al., 2004; Yoon et al., 2013). According to studies, more genes were differentially regulated by low-frequency EA than high-frequency EA (154 vs. 66 regulated genes/ESTs) in Arc, especially those related to neurogenesis (Wang et al., 2012). These findings suggest that the opioid-system network that includes the dorsal horn, periaqueductal gray, nucleus raphe magnus, and the arcuate nucleus is crucial for electroanalgesia (Mayer et al., 1977; Han et al., 1991).
3.4.2. Cortical remodeling
3.4.2.1. Remodeling pattern of areas of sensorimotor integration
Maintaining synergy and appropriate sensory and motor function depends on the sensorimotor circuit’s integrity (Yao et al., 2022). PNI disrupts the sensorimotor circuit’s integrity and results in the loss of sensory and motor feedback, which may result in changes to the sensory-motor network’s intrinsic activity (Taylor et al., 2009). However, the adult brain is capable of profound plasticity (Li C. et al., 2021). The fMRI study showed that insertion of the acupuncture needle at acupoint ST 36 significantly affected the proprioceptive brain activation by decreasing blood oxygenation level-dependent signal intensity in basal ganglia, limbic system, and cerebellum (Lee et al., 2013), and EA could reduce chronic pain, so it can be used to treat the paresthesia brought on by PNI persisted (Lu et al., 2021). Current research showed that acupuncture at local versus distal sites may improve median nerve function at the wrist by somatotopically distinct neuroplasticity in the primary somatosensory cortex following therapy (Maeda et al., 2017). Some studies found that after sciatic nerve injury, local synaptic activity increased in the ipsilateral somatosensory cortex and decreased in the contralateral somatosensory cortex (Zuo et al., 2010; Nugent et al., 2015), and the plasticity changes following PNI were situated in homologous regions of the ipsilateral hemisphere in addition to the contralateral hemisphere (Fornander et al., 2016). Acupuncture at GB34 may increase motor-cognition connectivity meanwhile decrease compensation of unaffected motor cortex and homolateral synkinesis (Chen et al., 2015). EA at ST 36 and LI 11 could enhance the neural activity of motor function-related brain regions, including the motor cortex, dorsal thalamus, and striatum in rats (Liang et al., 2017). The results of resting-state functional MRI connectivity show that acupuncture induces significant connectivity changes in the primary somatosensory region of recovery patients with Bell’s palsy (He et al., 2014). Acupuncture increased sensorimotor network (SMN) connectivity with pain-related brain regions (ACC, cerebellum) (Dhond et al., 2008). Nervous damage causes the sensorimotor circuit to lose its integrity, which may prompt the brain to reorganize itself to make up for the damaged sensorimotor circuit. However, these alterations in the brain could be beneficial or harmful (Mohan and Vanneste, 2017). Although EA treatment might reverse the maladaptive cortical plasticity, the status of the brain has not recovered to a level consistent with normalcy (Wu et al., 2018a).
3.4.2.2. Pain network of brain region
It has been demonstrated in both a human and a rat model that nerve transection in PNI typically causes paresthesia or pain before reinnervation (Campbell and Meyer, 2006; Gu et al., 2016; Peng et al., 2016; Lim et al., 2017). Researchers found that the activity pattern of the region associated with pain was remarkably coordinated (Lefaucheur et al., 2009). The changes in regions of the brain, such as the thalamus, amygdala, and cingulate, involved in pain modulation were observed under EA stimulation. For Carpal Tunnel Syndrome patients responding to acupuncture, functional connectivity was found between the hypothalamus and amygdala--the less deactivation in the amygdala, the greater the activation in the hypothalamus, and vice versa. Furthermore, hypothalamic response correlated positively with the degree of maladaptive cortical plasticity in Carpal Tunnel Syndrome patients (inter-digit separation distance; Napadow et al., 2007a). The thalamus, which relays sensory data to the cerebral cortex and is the most significant sensory integration region, has a strong correlation with pain (Çetin et al., 2014; Zitnik et al., 2014). It is well known that the amygdala, hippocampus, and parahippocampus are crucial for regulating emotions, motivation, memory, and the affective aspect of pain (LeDoux, 2000; Zubieta et al., 2001; Mears and Pollard, 2016). Some findings demonstrate the additive effect of acupuncture on antidepressant treatment and suggest that this effect may be achieved through the amygdala and the anterior cingulate cortex (ACC) (Wang et al., 2016). Researchers found higher centrality in the parahippocampal gyrus and middle temporal gyrus after ST36 stimulation. These regions are positively correlated to major hubs of the default mode network, which might be the primary network affected by chronic pain (Long et al., 2016). Studies showed that conventional methods to enhance the acupuncture dose induce different DMN modulatory effects. Conventional methods of enhancing the acupuncture dose could potentially be applied as a means of modulating brain activity (Lin et al., 2016).
3.4.2.3. Limbic-paralimbic system
A group of brain regions known as the limbic system have been linked to emotion, memory, and sensation. Numerous earlier research showed that acupuncture not only stimulated sensorimotor regions of the brain but also deactivated extensive regions of the brain, including the limbic-paralimbic system (Fang et al., 2009; Napadow et al., 2009; Chae et al., 2013). The “Deqi” sensation has been described as a phenomenon of concerted attenuation of signal strength in the para-limbic regions through fMRI investigations. Additionally, acupuncture’s “Deqi” effects may be mediated by modulation of the limbic-paralimbic-neocortical network (Hui et al., 2005; Shi et al., 2016). Acupuncture’s effects on depression, schizophrenia, ischemic stroke, and Alzheimer’s disease may be brought on by the limbic system, according to some animal and clinical investigations (Lu et al., 2014; Bosch et al., 2015; Li et al., 2015; Wang et al., 2016). Researchers discovered that, while treating PNI following surgical repair, the brain plasticity generated by longitudinal EA intervention also occurred in limbic/paralimbic areas. The fMRI experiments of manual acupuncture at LI4, ST36, and LV3 showed the weakness of signal in several limbic, paralimbic, and neocortical areas (Kong et al., 2002; Hui et al., 2005; Napadow et al., 2005; Yan et al., 2005). Some hypothesize that voluntary slow deep breathing functionally resets the autonomic nervous system through stretch-induced inhibitory signals and hyperpolarization currents propagated through both neural and non-neural tissue which synchronizes neural elements in the heart, lungs, limbic system, and cortex (Jerath et al., 2006). The anterior cingulate cortex and nearby medial prefrontal cortex, insula, hippocampus, amygdala, and midcingulate cortices were consistently linked to peripheral markers of autonomic nervous system activity, according to recent meta-analyzes and other reviews (Thayer et al., 2012; Beissner et al., 2013; Gianaros and Wager, 2015; Myers, 2017). According to the hypothesis, the limbic system’s fluctuating behavior was caused by a conflict between the up-regulating effects of paresthesia or pain and the down-regulating effects of EA intervention (Beissner and Henke, 2011). Previous research suggested that acupuncture could promote the regeneration of peripheral nerves (He et al., 2015). It demonstrated the changes in the brain caused by the long-term therapeutic effects of EA, which were manifested as a synchronized pattern of activation in the somatosensory and pain-related areas, as well as a fluctuating pattern in the limbic/paralimbic system (Wu et al., 2018b).
3.4.3. White matter remodeling
According to some studies, corticospinal tract(CST) and spinothalamic tract (STT) fibers are similarly altered when peripheral nerve injury occurs (Zhang et al., 2019). Previous research demonstrated that neural pathway injuries, such as brain and spinal cord injuries, caused STT fibers to adapt or be maladapt (Wang and Thompson, 2008; Wasner et al., 2008). According to findings from a rat study, chronic pain stimuli of the sciatic nerve could lead to the hyperexcitability of ventral posterior thalamus neurons, which were the major termination of STT fibers and responsible for the signal relay (Miki et al., 2000). Neuronal hyperexcitability could be an inappropriate reaction to a reduction or absence of sensory input. Although there was little evidence that acupuncture improved sensory function, some researchers thought that the plasticity alteration in STT may also be predicted to restore sensory function. Previous studies found that acupuncture treatment has a complex effect on the corticospinal system for a variety of disorders (Chen et al., 2015; Yang et al., 2017). It is demonstrated that based on routine rehabilitation treatment, scalp acupuncture plus low-frequency rTMS can promote white matter tracts repair better than scalp acupuncture alone for stroke hemiplegic patients (Zhao N. et al., 2018). Prior research changed the focus of plasticity from gray matter, which includes the motor cortex, to white matter, which includes the CST. Cortical alterations were anticipated to lead to modifications in efferent neural pathways (Matsumoto-Miyazaki et al., 2016). Acupuncture also aided in the recovery process and ameliorated the efferent neural pathway’s longitudinal prognosis (Zou et al., 2019). Table 4 summarizes the evidence of the mechanism of acupuncture on the regulation of brain regions in peripheral nerve injury.
Table 4.
Mechanism of acupuncture on the regulation of factors associated with neuronal response in peripheral nerve injury.
| Animal study | Model | Acupoints | Acupuncture type/parameter | Major effects |
|---|---|---|---|---|
| Liu et al. (2015) | MS | GV6 GV9 |
EA/1-2 mA, 2/60 Hz, 20 min | NT-3↑, differentiation of oligodendrocyte-like cells from grafted NR-MSCs in the demyelinated spinal cord↑ |
| Hu et al. (2018) | Sciatic nerve injury | GB30 ST36 |
EA/20 mA, 5 Hz, 15 min | Compare to model-only group: sciatic functional index↑, recovery rate of conduction velocity↑, diameter recovery of the gastrocnemius muscle fibers↑, S100-immunoreactive cells↑, nerve growth factor↑; treatment groups: not differ |
| Zhao X. et al. (2018) | MCAO | GV20 | EA/1-2 mA, 2/10 Hz, 30 min | miR-132↑, SOX2↓ |
| Cui et al. (2017) | SD rats | ST36 SP6 |
EA/3 mA, 2/15 Hz, 30 min | miR-7a-5p↓, miR-148a-3p ↓, miR-124-3p↑, miR-204-5p ↓, miR-370-3p↓, miR-221-3p ↑, miR-107-3p↑ |
| Du and Liu (2015) | Diabetic models | ST36 | EA/1 mA, 10/100 Hz, 30 min | The mRNA and protein level of the enteric neurons↑, GDNF in colon↑, p-Akt in colon↑ |
| Yu et al. (2017) | SNI models | GB30 ST36 | EA/2 mA, 5 Hz, 30 min | Agrin ↑, AChR-ε↑, AChR-γ↓ |
| Yu et al. (2017) | Sciatic nerve injury | GB30 ST36 |
EA/2 mA, 5 Hz, 30 min | SFI↑, tibialis anterior muscle weight↑, muscle fibre CSA↑, agrin expression levels↑, AChR-ε expression levels↑, AChR-γ expression levels↓ |
| Liang et al. (2016) | SD rats | ST36 BL60 |
EA/1-2 mA, 2/100 Hz, 30 min | PWTs↑, p-p38 MAPK↓, OX-42↓ |
| Shan et al. (2007) | SD rats | GB30 GB34 |
EA/ranging from 1–2-3 mA, 2/100 Hz, 30 min | Microglial activation↓, MA-induced up-regulation of IL-1β, IL-6, and TNFα mRNA ↓, OX-42-IR↓ |
| Sun et al. (2008) | SD rats | GB30 GB34 |
EA/1–3 mA, 2/100 Hz, 30 min | Spinal glial activation↓ |
| Ali et al. (2020) | Wistar rats | ST36 SP6 |
EA/2–3 mA, 2 Hz, 20 min | IL-10↑, β-endorphin↑, neuropathic pain↓ |
| Zhang et al. (2014) | Avulsion injury to the left brachial plexus root | LI11, LI04 ST36, GB34 |
EA/8 mA, 2–100 Hz, 15–20 min | Mechanical stimulation pain threshold↑, Autotomy scoring↓, β-endorphin↑ |
| Wang et al. (2018) | Sciatic nerve CCI | ST36, GB34 Both ilateral |
EA/1 mA, 2/15 Hz, 30 min | Hyperalgesia↓, glial cell activation of the lumbar spinal cord↓, microgliacytes of astrocytes↓, astrocytes activity↓, GFAP protein↓ |
MS, multiple sclerosis; NT-3, neurotrophin-3; NR-MSCs, neurotrophin-3 and retinoic acid preinduced mesenchymal stem cells; MCAO, middle cerebral artery occlusion; GDNF, glia cell line-derived neurotrophic factor; SNI, sciatic nerve injury; AChR, acetylcholine receptor; SFI, sciatic nerve functional index; CSA, cross-sectional area; PWTs, paw withdrawal thresholds; p38 MAPK, p38 mitogen-activated protein kinase; OX-42, oxycocin-42 (marker of microglia); TNFα, tumor necrosis factor-alpha; IL, interleukin; CCI, chronic constrictive injury; GFAP, glial fibrillary acidic protein.
3.5. Regulation of neurotrophic factor associated with PNI by acupuncture
Peripheral nerves in mammals can regenerate after being damaged (Menorca et al., 2013; Scheib and Höke, 2013). Schwann cell proliferation is frequently thought to be related to the mechanism behind peripheral nerve regeneration. In particular, neurotrophic substances released by Schwann cells are crucial for the regeneration of peripheral nerves (Jessen et al., 2015). According to research, neurotrophic factors secreted by Schwann cells as well as extracellular matrix and cell adhesion molecules can induce, stimulate, and modulate axon regeneration and the development of myelin sheaths (Madduri and Gander, 2010). Based on this discovery, a proposal was put forth that the distal portions of the nerve guided the regeneration of peripheral nerves, helping to extend neurites and restore innervation through identification and communication between neurons and neurogliocytes. After the sciatic nerve cut off, some researchers found that Schwann cells produced S100, suggesting central nervous system damage. For peripheral nerve injury, low-frequency EA (5 Hz) had the better effect, and the results were most pronounced in the early stages (Cheng et al., 2014; Liao et al., 2017). Neurotrophic factors, cell adhesion molecules (including L1, NCAM, and N-cadherin), transcription factors, growth-stimulating agents, extracellular matrix components, intracellular signaling enzymes, and proteins regulating cell-surface cytoskeletal interactions are related to neuronal response (Figure 6).
3.5.1. Neurotrophic factors
Nerve growth factor (NGF) is the earliest discovered neurotrophic factor and can nourish neurons and promote neurite sprouting (Yu et al., 2014).In response to nerve damage, NGF expression was upregulated, creating the ideal conditions for axon regeneration, the restoration of the connection between axons and Schwann cells, and the reinnervation of target locations to enhance nerve growth (Tang et al., 2013). After peripheral nerve damage, EA may strengthen new axonal connections, which would ultimately accelerate the transfer of NGF. Additionally, EA was able to promote the growth of Schwann cells, which greatly increased the amount of NGF released (Hu et al., 2018).
In the peripheral nervous system, Schwann cells (SCs) are the predominant glial cells. Following PNI, SC migration and proliferation result in the release of a variety of neurotrophic factors (NTFs), which are beneficial for preserving the survival of injured neurons, encouraging nerve fiber regeneration, and fostering the formation of new synapses (Dai et al., 2013). It has been demonstrated that EA may promote the recovery of neuroplasticity in rats subjected to spinal cord transection. This could be attributed to the systematic regulation of NTFs and their receptors after EA.EA stimulation at ST36 in rats increases the expression of axonal growth-associated protein 43 in neurons of the dorsal root ganglia, promoting axonal development (Wang et al., 2017). EA may alleviate tibialis anterior muscle atrophy induced by sciatic nerve injection injury by upregulating agrin and AChR-ε and downregulating AChR-γ (Yu et al., 2017). Some studies found that EA increased NT-3 levels and promoted differentiation of oligodendrocyte-like cells from grafted NR-MSCs in the demyelinated spinal cord and induced MSC transplantation combined with EA treatment not only increased MSC differentiation into oligodendrocyte-like cells forming myelin sheaths but also promoted remyelination and functional improvement of nerve conduction in the demyelinated spinal cord (Liu et al., 2015). Some researchers indicate that electroacupuncture and moxibustion promoted nerve regeneration and functional recovery, which mechanism might be associated with the enhancement of Schwann cell proliferation and upregulation of nerve growth factor (Hu et al., 2018).
As one of the most significant NTFs, persistent exogenous BDNF release in the damaged peripheral nerve may be a helpful tool to boost axonal density (Lopes et al., 2017). In the recovery process after peripheral nerve injury in rats, electrical muscle stimulation increases intramuscular BDNF levels (Willand et al., 2016). Studies have shown that BDNF actively contributes to the regeneration of motor neurons and the restoration of motor function. The amount of the protein BDNF, which has been identified as a crucial regulator of axon regeneration, was significantly enhanced in the spinal cord and DRG following PNI (Santos et al., 2016; Sanna et al., 2017; McGregor and English, 2018).
Previous research demonstrated that glial cell-derived neurotrophic factor (GDNF) was the most effective survival factor described for motoneurons in vitro (Höke et al., 2002). Recent investigations have proven GDNF’s function in neuronal protection and axonal regeneration in vivo (Allen et al., 2013; Kopra et al., 2015). Researchers discovered that GDNF mRNA is expressed in the cytoplasm membrane, notably in the neuromuscular junctions, and less in the axons and Schwann cells (Suzuki et al., 1998). To protect neurons, GDNF binds to N-cadherin and initiates the intracellular PI3K/Akt signaling pathway (Wang et al., 2014). Researchers found that EA with high-frequency and long-term stimuli at acupoint ST-36 can induce regeneration of lost enteric neurons in diabetic rats, and GDNF and PI3K/Akt signal pathway may play an important role in EA-induced regeneration of impaired enteric neurons (Du and Liu, 2015).
3.5.2. N-cadherin
Cadherins tend to form dimers or multimers, and the extracellular peptide chain partially folds to form five or six repeat domains (Stevens et al., 2023). Calcium ions are bound between the repeat domains, thus imparting rigidity and strength to the cadherin molecule (Oroz et al., 2011). The more calcium ions are bound, the more rigid the cadherin is, so when calcium ions are removed, the rigidity of the extracellular part of the cadherin is lost. Researchers revealed that electroacupuncture may encourage nerve regeneration through an increase in calcium concentration, which activates neuron outgrowth and repair (McCaig et al., 2002). Similarly to NCAM and integrin 1, N-cadherin is a calcium-dependent neuronal cell surface protein that facilitates adhesion and signal transduction (Neugebauer et al., 1988). Ret has a cadherin-like domain in its extracellular region that interacts with the GDNF/GDNF family receptor α1 (Oppenheim et al., 1995; Kjaer and Ibáñez, 2003). Some studies have confirmed that electroacupuncture promotes regeneration of peripheral facial nerve injury in rabbits, inhibits neuronal apoptosis, and reduces peripheral inflammatory response, resulting in the recovery of facial muscle function, which is achieved by up-regulating the expression of GDNF and N-cadherin in central facial neurons (Fei et al., 2019). GDNF binds to N-cadherin, a transmembrane cell adhesion molecule, to exercise its neuroprotective impact on dopaminergic neurons (Cao et al., 2010; Patil et al., 2011; Zuo et al., 2013).
3.5.3. MicroRNAs
MicroRNAs (miRNAs), which comprise between 2 and 3 percent of all human genes and primarily function to silence genes by binding to people’s targets’ 3′ untranslated regions (3′ UTR), are a vital and significant component of the regulation over a variety of signaling pathways and biological functions in the restoration of PNI (Wanke et al., 2018; Treiber et al., 2019), so MiRNAs have gained growing interest in the field of peripheral nerve regeneration (Yu et al., 2015). Numerous studies have shown that miRNAs play a key role in the recovery process after PNI. For instance, miRNA expression in rat dorsal root ganglia (DRG) tissues was drastically altered following sciatic nerve damage (Li et al., 2012). To increase rat DRG neurons’ survival and prevent apoptosis following sciatic nerve damage, miR142-3p can target CDKN1B and TIMP3. MiR-7 influences repair following PNI by targeting cdc42 and preventing neural stem cell migration and proliferation. By targeting GF1-A-binding protein 1, miR-221-3p may prevent SCs from maturing into myelin sheaths when they are cocultured with DRG neurons (Nab1) (Zhao L. et al., 2018; Zhou et al., 2018). Researchers have demonstrated miR-1-targeting BDNF in the regulation of SC proliferation and migration following nerve injury; in addition, miR-1 modulated chronic neuropathic pain in rats by targeting cx43 and BDNF, and miR-206 can target BDNF to the regulation of the MERK-ERK signaling pathway to influence neuro stress pain (Neumann et al., 2015; Yi et al., 2016; Sun et al., 2017). Liu et al. (2018) have discovered that miR-1b overexpression inhibited RSC96’s migration and proliferation while boosting cell apoptosis. Similar targets and expression profiles are used to identify miRNAs that belong to the same family. Numerous miRNAs, including miR-129 and miR-195, had altered expression following PNI, according to studies (Shi et al., 2013; Zhu et al., 2018). Using miR-132 to target SOX2-mediated axonal regeneration, it has been found that EA improved neurobehavioral functional recovery after ischemic stroke (Zhao X. et al., 2018). According to another study, let-7b-5p, miR-148a-3p, miR-124-3p, miR-107-3p, and miR-370-3p were confirmed to participate in EA tolerance probably through the functional categories related to nerve impulse transmission, receptor signal pathways, and gene expression regulation, as well as pathways related to MAPK, neurotrophin, fatty acid metabolism, lysosome, and the degradation of valine, leucine, and isoleucine (Cui et al., 2017). MiR-1b expression was initially found to be highly downregulated in the local nerve following EA in a prior investigation, and EA may promote the proliferation, migration of SC, and nerve repair after PNI by regulating miR-1b, which targets BDNF (Liu et al., 2020). Table 5 summarizes the evidence of the mechanism of acupuncture on the regulation of factors associated with neuronal response in peripheral nerve injury.
4. Conclusion and consideration
With in-depth research on the mechanism underlying the effects of acupuncture in treating PNI, we now fully appreciate the importance of nerve system remodeling in this process. we have found that after peripheral nerve injury, not only is the injury limited to the local area of the nerve, but the entire nervous system is altered accordingly. Acupuncture not only reverses the poor remodeling of the nervous system but also activates the central opioid system and the innate immunological effector cells in the nervous system to ameliorate pain. Acupuncture can promote the proliferation and migration of Schwann cells and the release of neurotrophic substances such as nerve growth factors, accelerating the regeneration and repair of nerve fibers. Based on the view that acupuncture affects the remodeling of the nervous system, it is believed that in the treatment of peripheral nerve injury, acupuncture should not only be applied to the local acupoint of peripheral nerve injury but also be combined with scalp acupuncture and Jiaji acupoints. At present, there are many studies on the mechanism of acupuncture affecting nerve remodeling, but there are still many limitations. First, the reduction of inflammatory pain by acupuncture may be related to the interaction between glial cells and neurons in the spinal cord, but the mechanisms of action of microglia and astrocytes after acupuncture are different. It is not known which factors influence their activation timing and molecular signaling within the cell. Secondly, in the process of acupuncture treatment of peripheral nerve injury, the related neural circuit, functional area, and network connection at the central nervous system level are still not clear. Thirdly, it is known that neurotrophic factors are involved in the repair process of peripheral nerve injury, but the production site, production time, and transport route of neurotrophic factors during acupuncture treatment need to be further improved. Finally, current animal models have drawbacks because they show an acute inflammatory response and short-lived hyperalgesia after PNI, both of which become attenuated over time. Most studies have only examined the protective effect of acupuncture in the initial phase of PNI. Better models or clinical trials are needed to explore the effectiveness of acupuncture in the process of chronic nerve repair. Overall, this review of studies provides strong evidence for the usefulness of acupuncture in the treatment of PNI. The elucidation of the mechanisms underlying the effects of acupuncture in the treatment of PNI from the perspective of nervous system remodeling will open a variety of opportunities for further applications of acupuncture and a combination of acupuncture and drugs for treating, managing, and accelerating nerve repair. Therefore, the continuation of research on this topic is extremely important.
Author contributions
YY: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. CR: Writing – review & editing. TY: Writing – review & editing. SW: Writing – review & editing. HS: Conceptualization, Methodology, Writing – review & editing. XY: Methodology, Writing – review & editing. LZ: Funding acquisition, Writing – review & editing. XM: Writing – review & editing. WG: Writing – review & editing. YD: Writing – review & editing. FH: Funding acquisition, Supervision, Validation, Writing – review & editing.
Funding Statement
This work was supported by the National Natural Science Foundation of China, No. 82205257.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Ahmad I., Fernando A., Gurgel R., Jason Clark J., Xu L., Hansen M. R. (2015). Merlin status regulates p75(NTR) expression and apoptotic signaling in Schwann cells following nerve injury. Neurobiol. Dis. 82, 114–122. doi: 10.1016/j.nbd.2015.05.021, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali U., Apryani E., Wu H. Y., Mao X. F., Liu H., Wang Y. X. (2020). Low frequency electroacupuncture alleviates neuropathic pain by activation of spinal microglial IL-10/β-endorphin pathway. Biomed. Pharmacother. 125:109898. doi: 10.1016/j.biopha.2020.109898, PMID: [DOI] [PubMed] [Google Scholar]
- Allen S. J., Watson J. J., Shoemark D. K., Barua N. U., Patel N. K. (2013). GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol. Ther. 138, 155–175. doi: 10.1016/j.pharmthera.2013.01.004, PMID: [DOI] [PubMed] [Google Scholar]
- Asplund M., Nilsson M., Jacobsson A., Von Holst H. (2009). Incidence of traumatic peripheral nerve injuries and amputations in Sweden between 1998 and 2006. Neuroepidemiology 32, 217–228. doi: 10.1159/000197900, PMID: [DOI] [PubMed] [Google Scholar]
- Balogun J. A., Biasci S., Han L. (1998). The effects of acupuncture, electroneedling and transcutaneous electrical stimulation therapies on peripheral haemodynamic functioning. Disabil. Rehabil. 20, 41–48. doi: 10.3109/09638289809166052, PMID: [DOI] [PubMed] [Google Scholar]
- Baumann T. K., Simone D. A., Shain C. N., LaMotte R. H. (1991). Neurogenic hyperalgesia: the search for the primary cutaneous afferent fibers that contribute to capsaicin-induced pain and hyperalgesia. J. Neurophysiol. 66, 212–227. doi: 10.1152/jn.1991.66.1.212, PMID: [DOI] [PubMed] [Google Scholar]
- Beissner F., Henke C. (2011). Methodological problems in FMRI studies on acupuncture: a critical review with special emphasis on visual and auditory cortex activations. Evid. Based Complement. Alternat. Med. 2011:607637. doi: 10.1093/ecam/nep154, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beissner F., Meissner K., Bär K. J., Napadow V. (2013). The autonomic brain: an activation likelihood estimation meta-analysis for central processing of autonomic function. J. Neurosci. 33, 10503–10511. doi: 10.1523/JNEUROSCI.1103-13.2013, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch P., van den Noort M., Staudte H., Lim S. (2015). Schizophrenia and depression: a systematic review of the effectiveness and the working mechanisms behind acupuncture. Explore (N.Y.) 11, 281–291. doi: 10.1016/j.explore.2015.04.004, PMID: [DOI] [PubMed] [Google Scholar]
- Bruyns C. N., Jaquet J. B., Schreuders T. A., Kalmijn S., Kuypers P. D., Hovius S. E. (2003). Predictors for return to work in patients with median and ulnar nerve injuries. J. Hand Surg. Am. 28, 28–34. doi: 10.1053/jhsu.2003.50026, PMID: [DOI] [PubMed] [Google Scholar]
- Campbell J. N., Meyer R. A. (2006). Mechanisms of neuropathic pain. Neuron 52, 77–92. doi: 10.1016/j.neuron.2006.09.021, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J. P., Li F. Z., Zhu Y. Y., Yuan H. H., Yu Z. Q., Gao D. S. (2010). Expressions and possible roles of GDNF receptors in the developing dopaminergic neurons. Brain Res. Bull. 83, 321–330. doi: 10.1016/j.brainresbull.2010.09.009, PMID: [DOI] [PubMed] [Google Scholar]
- Çetin M. S., Christensen F., Abbott C. C., Stephen J. M., Mayer A. R., Cañive J. M., et al. (2014). Thalamus and posterior temporal lobe show greater inter-network connectivity at rest and across sensory paradigms in schizophrenia. NeuroImage 97, 117–126. doi: 10.1016/j.neuroimage.2014.04.009, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae Y., Chang D. S., Lee S. H., Jung W. M., Lee I. S., Jackson S., et al. (2013). Inserting needles into the body: a meta-analysis of brain activity associated with acupuncture needle stimulation. J. Pain 14, 215–222. doi: 10.1016/j.jpain.2012.11.011, PMID: [DOI] [PubMed] [Google Scholar]
- Chang S., Kim D. H., Jang E. Y., Yoon S. S., Gwak Y. S., Yi Y. J., et al. (2019). Acupuncture attenuates alcohol dependence through activation of endorphinergic input to the nucleus accumbens from the arcuate nucleus. Sci. Adv. 5:eaax1342. doi: 10.1126/sciadv.aax1342, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. Y., Ke M. D., Kuo C. D., Huang C. H., Hsueh Y. H., Chen J. R. (2013). The influence of electro-acupuncture stimulation to female constipation patients. Am. J. Chin. Med. 41, 301–313. doi: 10.1142/S0192415X13500225, PMID: [DOI] [PubMed] [Google Scholar]
- Chen X., Zhang H., Zou Y. (2015). A functional magnetic resonance imaging study on the effect of acupuncture at GB34 (Yanglingquan) on motor-related network in hemiplegic patients. Brain Res. 1601, 64–72. doi: 10.1016/j.brainres.2015.01.011, PMID: [DOI] [PubMed] [Google Scholar]
- Cheng Y. S., Cheng W. C., Yao C. H., Hsieh C. L., Lin J. G., Lai T. Y., et al. (2001). Effects of buyang huanwu decoction on peripheral nerve regeneration using silicone rubber chambers. Am. J. Chin. Med. 29, 423–432. doi: 10.1142/S0192415X01000447, PMID: [DOI] [PubMed] [Google Scholar]
- Cheng C. Y., Lin J. G., Tang N. Y., Kao S. T., Hsieh C. L. (2014). Electroacupuncture-like stimulation at the Baihui (GV20) and Dazhui (GV14) acupoints protects rats against subacute-phase cerebral ischemia-reperfusion injuries by reducing S100B-mediated neurotoxicity. PLoS One 9:e91426. doi: 10.1371/journal.pone.0091426, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou A. K., Yang M. C., Tsai H. P., Chai C. Y., Tai M. H., Kwan A. L., et al. (2014). Adenoviral-mediated glial cell line-derived neurotrophic factor gene transfer has a protective effect on sciatic nerve following constriction-induced spinal cord injury. PLoS One 9:e92264. doi: 10.1371/journal.pone.0092264, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y. X., Zhang Y., Zhang Y. Q., Zhao Z. Q. (2010). Involvement of microglial P2X7 receptors and downstream signaling pathways in long-term potentiation of spinal nociceptive responses. Brain Behav. Immun. 24, 1176–1189. doi: 10.1016/j.bbi.2010.06.001, PMID: [DOI] [PubMed] [Google Scholar]
- Cui L., Ding Y., Feng Y., Chen S., Xu Y., Li M., et al. (2017). MiRNAs are involved in chronic electroacupuncture tolerance in the rat hypothalamus. Mol. Neurobiol. 54, 1429–1439. doi: 10.1007/s12035-016-9759-8, PMID: [DOI] [PubMed] [Google Scholar]
- Dai L. G., Huang G. S., Hsu S. H. (2013). Sciatic nerve regeneration by cocultured Schwann cells and stem cells on microporous nerve conduits. Cell Transplant. 22, 2029–2039. doi: 10.3727/096368912X658953, PMID: [DOI] [PubMed] [Google Scholar]
- Dhond R. P., Yeh C., Park K., Kettner N., Napadow V. (2008). Acupuncture modulates resting state connectivity in default and sensorimotor brain networks. Pain 136, 407–418. doi: 10.1016/j.pain.2008.01.011, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrova A., Murchison C., Oken B. (2017). Acupuncture for the treatment of peripheral neuropathy: a systematic review and Meta-analysis. J. Altern. Complement. Med. 23, 164–179. doi: 10.1089/acm.2016.0155, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du F., Liu S. (2015). Electroacupuncture with high frequency at acupoint ST-36 induces regeneration of lost enteric neurons in diabetic rats via GDNF and PI3K/AKT signal pathway. Am. J. Physiol. Regul. Integr. Comp. Physiol. 309, R109–R118. doi: 10.1152/ajpregu.00396.2014, PMID: [DOI] [PubMed] [Google Scholar]
- Fan Y., Wu Y. (2015). Effect of electroacupuncture on muscle state and infrared thermogram changes in patients with acute lumbar muscle sprain. J. Tradit. Chin. Med. 35, 499–506. doi: 10.1016/s0254-6272(15)30131-x, PMID: [DOI] [PubMed] [Google Scholar]
- Fang J., Jin Z., Wang Y., Li K., Kong J., Nixon E. E., et al. (2009). The salient characteristics of the central effects of acupuncture needling: limbic-paralimbic-neocortical network modulation. Hum. Brain Mapp. 30, 1196–1206. doi: 10.1002/hbm.20583, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett J. W., Keynes R. J. (1990). Peripheral nerve regeneration. Annu. Rev. Neurosci. 13, 43–60. doi: 10.1146/annurev.ne.13.030190.000355, PMID: [DOI] [PubMed] [Google Scholar]
- Fei J., Gao L., Li H. H., Yuan Q. L., Li L. J. (2019). Electroacupuncture promotes peripheral nerve regeneration after facial nerve crush injury and upregulates the expression of glial cell-derived neurotrophic factor. Neural Regen. Res. 14, 673–682. doi: 10.4103/1673-5374.247471, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornander L., Nyman T., Hansson T., Brismar T., Engström M. (2016). Inter-hemispheric plasticity in patients with median nerve injury. Neurosci. Lett. 628, 59–66. doi: 10.1016/j.neulet.2016.06.018, PMID: [DOI] [PubMed] [Google Scholar]
- Gianaros P. J., Wager T. D. (2015). Brain-body pathways linking psychological stress and physical health. Curr. Dir. Psychol. Sci. 24, 313–321. doi: 10.1177/0963721415581476, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon T., Borschel G. H. (2017). The use of the rat as a model for studying peripheral nerve regeneration and sprouting after complete and partial nerve injuries. Exp. Neurol. 287, 331–347. doi: 10.1016/j.expneurol.2016.01.014, PMID: [DOI] [PubMed] [Google Scholar]
- Gu N., Peng J., Murugan M., Wang X., Eyo U. B., Sun D., et al. (2016). Spinal microgliosis due to resident microglial proliferation is required for pain hypersensitivity after peripheral nerve injury. Cell Rep. 16, 605–614. doi: 10.1016/j.celrep.2016.06.018, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J. S., Chen X. H., Sun S. L., Xu X. J., Yuan Y., Yan S. C., et al. (1991). Effect of low- and high-frequency TENS on met-enkephalin-Arg-Phe and dynorphin a immunoreactivity in human lumbar CSF. Pain 47, 295–298. doi: 10.1016/0304-3959(91)90218-M, PMID: [DOI] [PubMed] [Google Scholar]
- Hao J., Zhao C., Cao S., Yang S. (1995). Electric acupuncture treatment of peripheral nerve injury. J. Tradit. Chin. Med. 15, 114–117. PMID: [PubMed] [Google Scholar]
- He G. H., Ruan J. W., Zeng Y. S., Zhou X., Ding Y., Zhou G. H. (2015). Improvement in acupoint selection for acupuncture of nerves surrounding the injury site: electro-acupuncture with governor vessel with local meridian acupoints. Neural Regen. Res. 10, 128–135. doi: 10.4103/1673-5374.150720, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Zhu Y., Li C., Park K., Mohamed A. Z., Wu H., et al. (2014). Acupuncture-induced changes in functional connectivity of the primary somatosensory cortex varied with pathological stages of Bell’s palsy. Neuroreport 25, 1162–1168. doi: 10.1097/WNR.0000000000000246, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho C. Y., Lin H. C., Lee Y. C., Chou L. W., Kuo T. W., Chang H. W., et al. (2014). Clinical effectiveness of acupuncture for carpal tunnel syndrome. Am. J. Chin. Med. 42, 303–314. doi: 10.1142/S0192415X14500207, PMID: [DOI] [PubMed] [Google Scholar]
- Ho C. Y., Yao C. H., Chen W. C., Shen W. C., Bau D. T. (2013). Electroacupuncture and acupuncture promote the Rat's transected median nerve regeneration. Evid. Based Complement. Alternat. Med. 2013:514610. doi: 10.1155/2013/514610, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höke A., Gordon T., Zochodne D. W., Sulaiman O. A. (2002). A decline in glial cell-line-derived neurotrophic factor expression is associated with impaired regeneration after long-term Schwann cell denervation. Exp. Neurol. 173, 77–85. doi: 10.1006/exnr.2001.7826, PMID: [DOI] [PubMed] [Google Scholar]
- Hu L. N., Tian J. X., Gao W., Zhu J., Mou F. F., Ye X. C., et al. (2018). Electroacupuncture and moxibustion promote regeneration of injured sciatic nerve through Schwann cell proliferation and nerve growth factor secretion. Neural Regen. Res. 13, 477–483. doi: 10.4103/1673-5374.228731, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang N. S., Sar C., Valmier J., Sieso V. (2012). Scamps, Electro-acupuncture on functional peripheral nerve regeneration in mice: a behavioural study. BMC Complement Altern Med. 12: 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui K. K., Liu J., Makris N., Gollub R. L., Chen A. J., Moore C. I., et al. (2000). Acupuncture modulates the limbic system and subcortical gray structures of the human brain: evidence from fMRI studies in normal subjects. Hum Brain Mapp. 9, 13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui K. K., Liu J., Marina O., Napadow V., Haselgrove C., Kwong K. K., et al. (2005). The integrated response of the human cerebro-cerebellar and limbic systems to acupuncture stimulation at ST 36 as evidenced by fMRI. NeuroImage 27, 479–496. doi: 10.1016/j.neuroimage.2005.04.037, PMID: [DOI] [PubMed] [Google Scholar]
- Inoue M., Hojo T., Yano T., Katsumi Y. (2003). The effects of electroacupuncture on peripheral nerve regeneration in rats. Acupunct. Med. 21, 9–17. doi: 10.1136/aim.21.1-2.9, PMID: [DOI] [PubMed] [Google Scholar]
- Inoue M., Katsumi Y., Itoi M., Hojo T., Nakajima M., Ohashi S., et al. (2011). Direct current electrical stimulation of acupuncture needles for peripheral nerve regeneration: an exploratory case series. Acupunct. Med. 29, 88–93. doi: 10.1136/aim.2010.003046, PMID: [DOI] [PubMed] [Google Scholar]
- Inquimbert P., Moll M., Latremoliere A., Tong C. K., Whang J., Sheehan G. F., et al. (2018). NMDA receptor activation underlies the loss of spinal dorsal horn neurons and the transition to persistent pain after peripheral nerve injury. Cell Rep. 23, 2678–2689. doi: 10.1016/j.celrep.2018.04.107, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerath R., Edry J. W., Barnes V. A., Jerath V. (2006). Physiology of long pranayamic breathing: neural respiratory elements may provide a mechanism that explains how slow deep breathing shifts the autonomic nervous system. Med. Hypotheses 67, 566–571. doi: 10.1016/j.mehy.2006.02.042, PMID: [DOI] [PubMed] [Google Scholar]
- Jessen K. R., Mirsky R., Lloyd A. C. (2015). Schwann cells: development and role in nerve repair. Cold Spring Harb. Perspect. Biol. 7:a020487. doi: 10.1101/cshperspect.a020487, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung W. M., Lee I. S., Wallraven C., Ryu Y. H., Park H. J., Chae Y. (2015). Cortical activation patterns of bodily attention triggered by acupuncture stimulation. Sci. Rep. 5:12455. doi: 10.1038/srep12455, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Choi J. Y. (2016). The effect of subthreshold continuous electrical stimulation on the facial function of patients with Bell’s palsy. Acta Otolaryngol. 136, 100–105. doi: 10.3109/00016489.2015.1083121, PMID: [DOI] [PubMed] [Google Scholar]
- Kim H. Y., Wang J., Lee I., Kim H. K., Chung K., Chung J. M. (2009). Electroacupuncture suppresses capsaicin-induced secondary hyperalgesia through an endogenous spinal opioid mechanism. Pain 145, 332–340. doi: 10.1016/j.pain.2009.06.035, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingham P. J., Kolar M. K., Novikova L. N., Novikov L. N., Wiberg M. (2014). Stimulating the neurotrophic and angiogenic properties of human adipose-derived stem cells enhances nerve repair. Stem Cells Dev. 23, 741–754. doi: 10.1089/scd.2013.0396, PMID: [DOI] [PubMed] [Google Scholar]
- Kjaer S., Ibáñez C. F. (2003). Identification of a surface for binding to the GDNF-GFR alpha 1 complex in the first cadherin-like domain of RET. J. Biol. Chem. 278, 47898–47904. doi: 10.1074/jbc.M309772200, PMID: [DOI] [PubMed] [Google Scholar]
- Klingner C. M., Volk G. F., Brodoehl S., Witte O. W., Guntinas-Lichius O. (2014). The effects of deefferentation without deafferentation on functional connectivity in patients with facial palsy. Neuroimage Clin. 6, 26–31. doi: 10.1016/j.nicl.2014.08.011, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J., Kaptchuk T. J., Polich G., Kirsch I., Vangel M., Zyloney C., et al. (2009). An fMRI study on the interaction and dissociation between expectation of pain relief and acupuncture treatment. NeuroImage 47, 1066–1076. doi: 10.1016/j.neuroimage.2009.05.087, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J., Ma L., Gollub R. L., Wei J., Yang X., Li D., et al. (2002). A pilot study of functional magnetic resonance imaging of the brain during manual and electroacupuncture stimulation of acupuncture point (LI-4 Hegu) in normal subjects reveals differential brain activation between methods. J. Altern. Complement. Med. 8, 411–419. doi: 10.1089/107555302760253603, PMID: [DOI] [PubMed] [Google Scholar]
- Koo S. T., Lim K. S., Chung K., Ju H., Chung J. M. (2008). Electroacupuncture-induced analgesia in a rat model of ankle sprain pain is mediated by spinal alpha-adrenoceptors. Pain 135, 11–19. doi: 10.1016/j.pain.2007.04.034, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopra J., Vilenius C., Grealish S., Härma M. A., Varendi K., Lindholm J., et al. (2015). GDNF is not required for catecholaminergic neuron survival in vivo. Nat. Neurosci. 18, 319–322. doi: 10.1038/nn.3941, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon Y. B., Han H. J., Beitz A. J., Lee J. H. (2004). Bee venom acupoint stimulation increases Fos expression in catecholaminergic neurons in the rat brain. Mol. Cells 17, 329–333. doi: 10.1016/j.jmb.2004.02.047 PMID: [DOI] [PubMed] [Google Scholar]
- LeDoux J. E. (2000). Emotion circuits in the brain. Annu. Rev. Neurosci. 23, 155–184. doi: 10.1146/annurev.neuro.23.1.155, PMID: [DOI] [PubMed] [Google Scholar]
- Lee S. H., Jahng G. H., Choe I. H., Choi C. B., Kim D. H., Kim H. Y. (2013). Neural pathway interference by retained acupuncture: a functional MRI study of a dog model of Parkinson's disease. CNS Neurosci. Ther. 19, 585–595. doi: 10.1111/cns.12108, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefaucheur J. P., Drouot X., Cunin P., Bruckert R., Lepetit H., Créange A., et al. (2009). Motor cortex stimulation for the treatment of refractory peripheral neuropathic pain. Brain 132, 1463–1471. doi: 10.1093/brain/awp035, PMID: [DOI] [PubMed] [Google Scholar]
- Levine R., Morgan M. M., Cannon J. T., Liebeskind J. C. (1991). Stimulation of the periaqueductal gray matter of the rat produces a preferential ipsilateral antinociception. Brain Res. 567, 140–144. doi: 10.1016/0006-8993(91)91446-8, PMID: [DOI] [PubMed] [Google Scholar]
- Li M. K., Li Y. J., Zhang G. F., Chen J. Q., Zhang J. P., Qi J., et al. (2015). Acupuncture for ischemic stroke: cerebellar activation may be a central mechanism following Deqi. Neural Regen. Res. 10, 1997–2003. doi: 10.4103/1673-5374.172318, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Liu S. Y., Pi W., Zhang P. X. (2021). Cortical plasticity and nerve regeneration after peripheral nerve injury. Neural Regen. Res. 16, 1518–1523. doi: 10.4103/1673-5374.303008, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A., Wang Y., Xin J., Lao L., Ren K., Berman B. M., et al. (2007). Electroacupuncture suppresses hyperalgesia and spinal Fos expression by activating the descending inhibitory system. Brain Res. 1186, 171–179. doi: 10.1016/j.brainres.2007.10.022, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Yu B., Wang S., Gu Y., Yao D., Wang Y., et al. (2012). Identification and functional analysis of novel micro-RNAs in rat dorsal root ganglia after sciatic nerve resection. J. Neurosci. Res. 90, 791–801. doi: 10.1002/jnr.22814, PMID: [DOI] [PubMed] [Google Scholar]
- Li H., Yu L., Ye D., Chang L., Zhao F., Wang H., et al. (2021). Rehabilitation training combined with Jiaji Electroacupuncture can promote the recovery of muscle group function and improve the quality of life in patients with upper limb peripheral nerve injury. J. Healthc. Eng. 2021:3621568. doi: 10.1155/2021/3621568, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y., Du J. Y., Qiu Y. J., Fang J. F., Liu J., Fang J. Q. (2016). Electroacupuncture attenuates spinal nerve ligation-induced microglial activation mediated by p38 mitogen-activated protein kinase. Chin. J. Integr. Med. 22, 704–713. doi: 10.1007/s11655-015-2045-1, PMID: [DOI] [PubMed] [Google Scholar]
- Liang S., Lin Y., Lin B., Li J., Liu W., Chen L., et al. (2017). Resting-state functional magnetic resonance imaging analysis of brain functional activity in rats with ischemic stroke treated by electro-acupuncture. J. Stroke Cerebrovasc. Dis. 26, 1953–1959. doi: 10.1016/j.jstrokecerebrovasdis.2017.06.018, PMID: [DOI] [PubMed] [Google Scholar]
- Liao H. Y., Hsieh C. L., Huang C. P., Lin Y. W. (2017). Electroacupuncture attenuates CFA-induced inflammatory pain by suppressing Nav1.8 through S100B, TRPV1, opioid, and adenosine pathways in mice. Sci. Rep. 7:42531. doi: 10.1038/srep42531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim H., Lee H., Noh K., Lee S. J. (2017). IKK/NF-κB-dependent satellite glia activation induces spinal cord microglia activation and neuropathic pain after nerve injury. Pain 158, 1666–1677. doi: 10.1097/j.pain.0000000000000959, PMID: [DOI] [PubMed] [Google Scholar]
- Lin J. P., Chen C. Q., Huang L. E., Li N. N., Yang Y., Zhu S. M., et al. (2018). Dexmedetomidine attenuates neuropathic pain by inhibiting P2X7R expression and ERK phosphorylation in rats. Exp. Neurobiol. 27, 267–276. doi: 10.5607/en.2018.27.4.267, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y. J., Kung Y. Y., Kuo W. J., Niddam D. M., Chou C. C., Cheng C. M., et al. (2016). Effect of acupuncture 'dose' on modulation of the default mode network of the brain. Acupunct. Med. 34, 425–432. doi: 10.1136/acupmed-2016-011071, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Grumbles R. M., Thomas C. K. (2013). Electrical stimulation of embryonic neurons for 1 hour improves axon regeneration and the number of reinnervated muscles that function. J. Neuropathol. Exp. Neurol. 72, 697–707. doi: 10.1097/NEN.0b013e318299d376, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., He B., Zhang R. Y., Zhang K., Ding Y., Ruan J. W., et al. (2015). Electroacupuncture promotes the differentiation of transplanted bone marrow mesenchymal stem cells Preinduced with Neurotrophin-3 and retinoic acid into oligodendrocyte-like cells in demyelinated spinal cord of rats. Cell Transplant. 24, 1265–1281. doi: 10.3727/096368914X682099, PMID: [DOI] [PubMed] [Google Scholar]
- Liu Y. P., Luo Z. R., Wang C., Cai H., Zhao T. T., Li H., et al. (2020). Electroacupuncture promoted nerve repair after peripheral nerve injury by regulating miR-1b and its target brain-derived neurotrophic factor. Front. Neurosci. 14:525144. doi: 10.3389/fnins.2020.525144, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Wang Z., Su Y., Qi L., Yang W., Fu M., et al. (2021). A neuroanatomical basis for electroacupuncture to drive the vagal-adrenal axis. Nature 598, 641–645. doi: 10.1038/s41586-021-04001-4, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. P., Xu P., Guo C. X., Luo Z. R., Zhu J., Mou F. F., et al. (2018). miR-1b overexpression suppressed proliferation and migration of RSC96 and increased cell apoptosis. Neurosci. Lett. 687, 137–145. doi: 10.1016/j.neulet.2018.09.041, PMID: [DOI] [PubMed] [Google Scholar]
- Long X., Huang W., Napadow V., Liang F., Pleger B., Villringer A., et al. (2016). Sustained effects of acupuncture stimulation investigated with centrality mapping analysis. Front. Hum. Neurosci. 10:510. doi: 10.3389/fnhum.2016.00510, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes C. D. F., Gonçalves N. P., Gomes C. P., Saraiva M. J., Pêgo A. P. (2017). BDNF gene delivery mediated by neuron-targeted nanoparticles is neuroprotective in peripheral nerve injury. Biomaterials 121, 83–96. doi: 10.1016/j.biomaterials.2016.12.025, PMID: [DOI] [PubMed] [Google Scholar]
- Lu M. C., Ho C. Y., Hsu S. F., Lee H. C., Lin J. H., Yao C. H., et al. (2008). Effects of electrical stimulation at different frequencies on regeneration of transected peripheral nerve. Neurorehabil. Neural Repair 22, 367–373. doi: 10.1177/1545968307313507, PMID: [DOI] [PubMed] [Google Scholar]
- Lu Y., Huang Y., Tang C., Shan B., Cui S., Yang J., et al. (2014). Brain areas involved in the acupuncture treatment of AD model rats: a PET study. BMC Complement. Altern. Med. 14:178. doi: 10.1186/1472-6882-14-178, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z., Wang Q., Sun X., Zhang W., Min S., Zhang J., et al. (2021). Transcutaneous electrical acupoint stimulation before surgery reduces chronic pain after mastectomy: a randomized clinical trial. J. Clin. Anesth. 74:110453. doi: 10.1016/j.jclinane.2021.110453, PMID: [DOI] [PubMed] [Google Scholar]
- Madduri S., Gander B. (2010). Schwann cell delivery of neurotrophic factors for peripheral nerve regeneration. J. Peripher. Nerv. Syst. 15, 93–103. doi: 10.1111/j.1529-8027.2010.00257.x, PMID: [DOI] [PubMed] [Google Scholar]
- Maeda Y., Kim H., Kettner N., Kim J., Cina S., Malatesta C., et al. (2017). Rewiring the primary somatosensory cortex in carpal tunnel syndrome with acupuncture. Brain 140, 914–927. doi: 10.1093/brain/awx015, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistroni E., Parodi G., Fop F., Battiston B., Dahlin L. B. (2020). Cold intolerance and neuropathic pain after peripheral nerve injury in upper extremity. J. Peripher. Nerv. Syst. 25, 184–190. doi: 10.1111/jns.12376, PMID: [DOI] [PubMed] [Google Scholar]
- Manning B. H. (1998). A lateralized deficit in morphine antinociception after unilateral inactivation of the central amygdala. J. Neurosci. 18, 9453–9470. doi: 10.1523/JNEUROSCI.18-22-09453.1998, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiowetz V., Kashman N., Volland G., Weber K., Dowe M., Rogers S. (1985). Grip and pinch strength: normative data for adults. Arch. Phys. Med. Rehabil. 66, 69–74. PMID: [PubMed] [Google Scholar]
- Matsumoto-Miyazaki J., Asano Y., Yonezawa S., Nomura Y., Ikegame Y., Aki T., et al. (2016). Acupuncture increases the excitability of the Cortico-spinal system in patients with chronic disorders of consciousness following traumatic brain injury. J. Altern. Complement. Med. 22, 887–894. doi: 10.1089/acm.2014.0356, PMID: [DOI] [PubMed] [Google Scholar]
- Mayer D. J., Price D. D., Rafii A. (1977). Antagonism of acupuncture analgesia in man by the narcotic antagonist naloxone. Brain Res. 121, 368–372. doi: 10.1016/0006-8993(77)90161-5, PMID: [DOI] [PubMed] [Google Scholar]
- McCaig C. D., Rajnicek A. M., Song B., Zhao M. (2002). Has electrical growth cone guidance found its potential? Trends Neurosci. 25, 354–359. doi: 10.1016/S0166-2236(02)02174-4, PMID: [DOI] [PubMed] [Google Scholar]
- McGregor C. E., English A. W. (2018). The role of BDNF in peripheral nerve regeneration: activity-dependent treatments and Val66Met. Front. Cell. Neurosci. 12:522. doi: 10.3389/fncel.2018.00522, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mears D., Pollard H. B. (2016). Network science and the human brain: using graph theory to understand the brain and one of its hubs, the amygdala, in health and disease. J. Neurosci. Res. 94, 590–605. doi: 10.1002/jnr.23705, PMID: [DOI] [PubMed] [Google Scholar]
- Menorca R. M., Fussell T. S., Elfar J. C. (2013). Nerve physiology: mechanisms of injury and recovery. Hand Clin. 29, 317–330. doi: 10.1016/j.hcl.2013.04.002, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki K., Iwata K., Tsuboi Y., Morimoto T., Kondo E., Dai Y., et al. (2000). Dorsal column-thalamic pathway is involved in thalamic hyperexcitability following peripheral nerve injury: a lesion study in rats with experimental mononeuropathy. Pain 85, 263–271. doi: 10.1016/s0304-3959(99)00279-1, PMID: [DOI] [PubMed] [Google Scholar]
- Mohan A., Vanneste S. (2017). Adaptive and maladaptive neural compensatory consequences of sensory deprivation-from a phantom percept perspective. Prog. Neurobiol. 153, 1–17. doi: 10.1016/j.pneurobio.2017.03.010, PMID: [DOI] [PubMed] [Google Scholar]
- Myers B. (2017). Corticolimbic regulation of cardiovascular responses to stress. Physiol. Behav. 172, 49–59. doi: 10.1016/j.physbeh.2016.10.015, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napadow V., Dhond R. P., Kim J., LaCount L., Vangel M., Harris R. E., et al. (2009). Brain encoding of acupuncture sensation--coupling on-line rating with fMRI. NeuroImage 47, 1055–1065. doi: 10.1016/j.neuroimage.2009.05.079, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napadow V., Kettner N., Liu J., Li M., Kwong K. K., Vangel M., et al. (2007a). Hypothalamus and amygdala response to acupuncture stimuli in carpal tunnel syndrome. Pain 130, 254–266. doi: 10.1016/j.pain.2006.12.003, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napadow V., Liu J., Li M., Kettner N., Ryan A., Kwong K. K., et al. (2007b). Somatosensory cortical plasticity in carpal tunnel syndrome treated by acupuncture. Hum. Brain Mapp. 28, 159–171. doi: 10.1002/hbm.20261, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napadow V., Makris N., Liu J., Kettner N. W., Kwong K. K., Hui K. K. (2005). Effects of electroacupuncture versus manual acupuncture on the human brain as measured by fMRI. Hum. Brain Mapp. 24, 193–205. doi: 10.1002/hbm.20081, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer K. M., Tomaselli K. J., Lilien J., Reichardt L. F. (1988). N-cadherin, NCAM, and integrins promote retinal neurite outgrowth on astrocytes in vitro. J. Cell Biol. 107, 1177–1187. doi: 10.1083/jcb.107.3.1177, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann E., Hermanns H., Barthel F., Werdehausen R., Brandenburger T. (2015). Expression changes of microRNA-1 and its targets Connexin 43 and brain-derived neurotrophic factor in the peripheral nervous system of chronic neuropathic rats. Mol. Pain 11:39. doi: 10.1186/s12990-015-0045-y, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent A. C., Martinez A., D'Alfonso A., Zarate C. A., Theodore W. H. (2015). The relationship between glucose metabolism, resting-state fMRI BOLD signal, and GABAA-binding potential: a preliminary study in healthy subjects and those with temporal lobe epilepsy. J. Cereb. Blood Flow Metab. 35, 583–591. doi: 10.1038/jcbfm.2014.228, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim R. W., Houenou L. J., Johnson J. E., Lin L. F., Li L., Lo A. C., et al. (1995). Developing motor neurons rescued from programmed and axotomy-induced cell death by GDNF. Nature 373, 344–346. doi: 10.1038/373344a0, PMID: [DOI] [PubMed] [Google Scholar]
- Oroz J., Valbuena A., Vera A. M., Mendieta J., Gómez-Puertas P., Carrión-Vázquez M. (2011). Nanomechanics of the cadherin ectodomain: "canalization" by Ca2+ binding results in a new mechanical element. J. Biol. Chem. 286, 9405–9418. doi: 10.1074/jbc.M110.170399, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagnussat A. S., Michaelsen S. M., Achaval M., Ilha J., Hermel E. E., Back F. P., et al. (2012). Effect of skilled and unskilled training on nerve regeneration and functional recovery. Braz. J. Med. Biol. Res. 45, 753–762. doi: 10.1590/S0100-879X2012007500084, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H., Huang H., Zhang L., Ma S., Yang H., Wang H. (2019). "adjusting internal organs and dredging channel" electroacupuncture treatment prevents the development of diabetic peripheral neuropathy by downregulating glucose-related protein 78 (GRP78) and caspase-12 in streptozotocin-diabetic rats. J. Diabetes 11, 928–937. doi: 10.1111/1753-0407.12916, PMID: [DOI] [PubMed] [Google Scholar]
- Patel N., Poo M. M. (1982). Orientation of neurite growth by extracellular electric fields. J. Neurosci. 2, 483–496. doi: 10.1523/JNEUROSCI.02-04-00483.1982, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil S. B., Brock J. H., Colman D. R., Huntley G. W. (2011). Neuropathic pain- and glial derived neurotrophic factor-associated regulation of cadherins in spinal circuits of the dorsal horn. Pain 152, 924–935. doi: 10.1016/j.pain.2011.01.017, PMID: [DOI] [PubMed] [Google Scholar]
- Peng J., Gu N., Zhou L., Murugan B. E. U., Gan W. B., Wu L. J. (2016). Microglia and monocytes synergistically promote the transition from acute to chronic pain after nerve injury. Nat. Commun. 7:12029. doi: 10.1038/ncomms12029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren L., Zhang W. A., Fang N. Y., Wang J. X. (2008). The influence of electro-acupuncture on neural plasticity in acute cerebral infarction. Neurol Res 30, 985–9, PMID: [DOI] [PubMed] [Google Scholar]
- Ribeiro F. F., Xapelli S., Miranda-Lourenço C., Tanqueiro S. R., Fonseca-Gomes J., Diógenes M. J., et al. (2016). Purine nucleosides in neuroregeneration and neuroprotection. Neuropharmacology 104, 226–242. doi: 10.1016/j.neuropharm.2015.11.006, PMID: [DOI] [PubMed] [Google Scholar]
- Sanna M. D., Ghelardini C., Galeotti N. (2017). HuD-mediated distinct BDNF regulatory pathways promote regeneration after nerve injury. Brain Res. 1659, 55–63. doi: 10.1016/j.brainres.2017.01.019, PMID: [DOI] [PubMed] [Google Scholar]
- Santos D., González-Pérez F., Giudetti G., Micera S., Udina E., Del Valle J., et al. (2016). Preferential enhancement of sensory and motor axon regeneration by combining extracellular matrix components with neurotrophic factors. Int. J. Mol. Sci. 18:65. doi: 10.3390/ijms18010065, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheib J., Höke A. (2013). Advances in peripheral nerve regeneration. Nat. Rev. Neurol. 9, 668–676. doi: 10.1038/nrneurol.2013.227, PMID: [DOI] [PubMed] [Google Scholar]
- Schmidt C. E., Shastri V. R., Vacanti J. P., Langer R. (1997). Stimulation of neurite outgrowth using an electrically conducting polymer. Proc. Natl. Acad. Sci. U. S. A. 94, 8948–8953. doi: 10.1073/pnas.94.17.8948, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan S., Qi-Liang M. Y., Hong C., Tingting L., Mei H., Haili P., et al. (2007). Is functional state of spinal microglia involved in the anti-allodynic and anti-hyperalgesic effects of electroacupuncture in rat model of monoarthritis? Neurobiol. Dis. 26, 558–568. doi: 10.1016/j.nbd.2007.02.007, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y., Guan S., Ge H., Xiong W., He L., Liu L., et al. (2018). Effects of palmatine on rats with comorbidity of diabetic neuropathic pain and depression. Brain Res. Bull. 139, 56–66. doi: 10.1016/j.brainresbull.2018.02.005, PMID: [DOI] [PubMed] [Google Scholar]
- Shi G., Shi J., Liu K., Liu N., Wang Y., Fu Z., et al. (2013). Increased miR-195 aggravates neuropathic pain by inhibiting autophagy following peripheral nerve injury. Glia 61, 504–512. doi: 10.1002/glia.22451, PMID: [DOI] [PubMed] [Google Scholar]
- Shi Y., Zhang S., Li Q., Liu Z., Guo S., Yang J., et al. (2016). A study of the brain functional network of Deqi via acupuncturing stimulation at BL40 by rs-fMRI. Complement. Ther. Med. 25, 71–77. doi: 10.1016/j.ctim.2016.01.004, PMID: [DOI] [PubMed] [Google Scholar]
- Skrabanek P. (1984). Acupuncture and endorphins. Lancet 1:220. doi: 10.1016/s0140-6736(84)92137-8, PMID: [DOI] [PubMed] [Google Scholar]
- Song B., Zhao M., Forrester J., McCaig C. (2004). Nerve regeneration and wound healing are stimulated and directed by an endogenous electrical field in vivo. J. Cell Sci. 117, 4681–4690. doi: 10.1242/jcs.01341, PMID: [DOI] [PubMed] [Google Scholar]
- Stevens A. J., Harris A. R., Gerdts J., Kim K. H., Trentesaux C., Ramirez J. T., et al. (2023). Programming multicellular assembly with synthetic cell adhesion molecules. Nature 614, 144–152. doi: 10.1038/s41586-022-05622-z, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturma A., Hruby L. A., Prahm C., Mayer J. A., Aszmann O. C. (2018). Rehabilitation of upper extremity nerve injuries using surface EMG biofeedback: protocols for clinical application. Front. Neurosci. 12:906. doi: 10.3389/fnins.2018.00906, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan R., Dailey T., Duncan K., Abel N., Borlongan C. V. (2016). Peripheral nerve injury: stem cell therapy and peripheral nerve transfer. Int. J. Mol. Sci. 17:22101. doi: 10.3390/ijms17122101, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S., Cao H., Han M., Li T. T., Zhao Z. Q., Zhang Y. Q. (2008). Evidence for suppression of electroacupuncture on spinal glial activation and behavioral hypersensitivity in a rat model of monoarthritis. Brain Res. Bull. 75, 83–93. doi: 10.1016/j.brainresbull.2007.07.027, PMID: [DOI] [PubMed] [Google Scholar]
- Sun W., Zhang L., Li R. (2017). Overexpression of miR-206 ameliorates chronic constriction injury-induced neuropathic pain in rats via the MEK/ERK pathway by targeting brain-derived neurotrophic factor. Neurosci. Lett. 646, 68–74. doi: 10.1016/j.neulet.2016.12.047, PMID: [DOI] [PubMed] [Google Scholar]
- Suzuki H., Hase A., Miyata Y., Arahata K., Akazawa C. (1998). Prominent expression of glial cell line-derived neurotrophic factor in human skeletal muscle. J. Comp. Neurol. 402, 303–312. doi: , PMID: [DOI] [PubMed] [Google Scholar]
- Takeshige C., Tsuchiya M., Guo S. Y., Sato T. (1991). Dopaminergic transmission in the hypothalamic arcuate nucleus to produce acupuncture analgesia in correlation with the pituitary gland. Brain Res. Bull. 26, 113–122. doi: 10.1016/0361-9230(91)90195-P, PMID: [DOI] [PubMed] [Google Scholar]
- Tallon C., Farah M. H. (2017). Beta secretase activity in peripheral nerve regeneration. Neural Regen. Res. 12, 1565–1574. doi: 10.4103/1673-5374.217319, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y. J., Wu M. H., Tai C. J. (2016). Direct electrical stimulation on the injured ulnar nerve using acupuncture needles combined with rehabilitation accelerates nerve regeneration and functional recovery-a case report. Complement. Ther. Med. 24, 103–107. doi: 10.1016/j.ctim.2015.12.003, PMID: [DOI] [PubMed] [Google Scholar]
- Tang S., Zhu J., Xu Y., Xiang A. P., Jiang M. H., Quan D. (2013). The effects of gradients of nerve growth factor immobilized PCLA scaffolds on neurite outgrowth in vitro and peripheral nerve regeneration in rats. Biomaterials 34, 7086–7096. doi: 10.1016/j.biomaterials.2013.05.080, PMID: [DOI] [PubMed] [Google Scholar]
- Taylor K. S., Anastakis D. J., Davis K. D. (2009). Cutting your nerve changes your brain. Brain 132, 3122–3133. doi: 10.1093/brain/awp231, PMID: [DOI] [PubMed] [Google Scholar]
- Thayer J. F., Ahs F., Fredrikson M., Sollers J. J., 3rd, Wager T. D. (2012). A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci. Biobehav. Rev. 36, 747–756. doi: 10.1016/j.neubiorev.2011.11.009, PMID: [DOI] [PubMed] [Google Scholar]
- Tian X. Y., Bian Z. X., Hu X. G., Zhang X. J., Liu L., Zhang H. (2006). Electro-acupuncture attenuates stress-induced defecation in rats with chronic visceral hypersensitivity via serotonergic pathway. Brain Res. 1088, 101–108. doi: 10.1016/j.brainres.2006.03.014, PMID: [DOI] [PubMed] [Google Scholar]
- Treiber T., Treiber N., Meister G. (2019). Regulation of microRNA biogenesis and its crosstalk with other cellular pathways. Nat. Rev. Mol. Cell Biol. 20, 5–20. doi: 10.1038/s41580-018-0059-1, PMID: [DOI] [PubMed] [Google Scholar]
- Urban M. O., Zahn P. K., Gebhart G. F. (1999). Descending facilitatory influences from the rostral medial medulla mediate secondary, but not primary hyperalgesia in the rat. Neuroscience 90, 349–352. doi: 10.1016/S0306-4522(99)00002-0, PMID: [DOI] [PubMed] [Google Scholar]
- Vadivelu N., Kai A., Maslin B., Kodumudi G., Legler A., Berger J. M. (2015). Tapentadol extended release in the management of peripheral diabetic neuropathic pain. Ther. Clin. Risk Manag. 11, 95–105. doi: 10.2147/TCRM.S32193, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bockstaele E. J., Aston-Jones G., Pieribone V. A., Ennis M., Shipley M. T. (1991). Subregions of the periaqueductal gray topographically innervate the rostral ventral medulla in the rat. J. Comp. Neurol. 309, 305–327. doi: 10.1002/cne.903090303, PMID: [DOI] [PubMed] [Google Scholar]
- Van Griensven H., Schmid A., Trendafilova T., Low M. (2020). Central sensitization in musculoskeletal pain: lost in translation? J. Orthop. Sports Phys. Ther. 50, 592–596. doi: 10.2519/jospt.2020.0610, PMID: [DOI] [PubMed] [Google Scholar]
- Wang X., Chan S. T., Fang J., Nixon E. E., Liu J., Kwong K. K., et al. (2013). Neural encoding of acupuncture needling sensations: evidence from a FMRI study, Evidence-based complementary and alternative medicine. eCAM, 483105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. Y., Gao Y. H., Qiao L. N., Zhang J. L., Duan-Mu C. L., Yan Y. X., et al. (2018). Repeated electroacupuncture treatment attenuated hyperalgesia through suppression of spinal glial activation in chronic neuropathic pain rats. BMC Complement Altern Med, 18:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Ju S., Chen S., Gao W., Ding J., Wang G., et al. (2017). Effect of electro-acupuncture on neuroplasticity of spinal cord-transected rats. Med. Sci. Monit. 23, 4241–4251. doi: 10.12659/MSM.903056, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Thompson S. M. (2008). Maladaptive homeostatic plasticity in a rodent model of central pain syndrome: thalamic hyperexcitability after spinothalamic tract lesions. J. Neurosci. 28, 11959–11969. doi: 10.1523/JNEUROSCI.3296-08.2008, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Wang Z., Liu J., Chen J., Liu X., Nie G., et al. (2016). Repeated acupuncture treatments modulate amygdala resting state functional connectivity of depressive patients. Neuroimage Clin. 12, 746–752. doi: 10.1016/j.nicl.2016.07.011, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Wang H., Pang J., Li L., Zhang S., Song G., et al. (2014). Glial cell-derived neurotrophic factor attenuates neuropathic pain in a mouse model of chronic constriction injury: possible involvement of E-cadherin/p120ctn signaling. J. Mol. Neurosci. 54, 156–163. doi: 10.1007/s12031-014-0266-y, PMID: [DOI] [PubMed] [Google Scholar]
- Wang K., Zhang R., He F., Lin L. B., Xiang X. H., Ping X. J., et al. (2012). Electroacupuncture frequency-related transcriptional response in rat arcuate nucleus revealed region-distinctive changes in response to low- and high-frequency electroacupuncture. J. Neurosci. Res. 90, 1464–1473. doi: 10.1002/jnr.23028, PMID: [DOI] [PubMed] [Google Scholar]
- Wanke K. A., Devanna P., Vernes S. C. (2018). Understanding neurodevelopmental disorders: the promise of regulatory variation in the 3'UTRome. Biol. Psychiatry 83, 548–557. doi: 10.1016/j.biopsych.2017.11.006, PMID: [DOI] [PubMed] [Google Scholar]
- Wasner G., Lee B. B., Engel S., McLachlan E. (2008). Residual spinothalamic tract pathways predict development of central pain after spinal cord injury. Brain 131, 2387–2400. doi: 10.1093/brain/awn169, PMID: [DOI] [PubMed] [Google Scholar]
- Wenjin W., Wenchao L., Hao Z., Feng L., Yan W., Wodong S., et al. (2011). Electrical stimulation promotes BDNF expression in spinal cord neurons through ca(2+)- and Erk-dependent signaling pathways. Cell. Mol. Neurobiol. 31, 459–467. doi: 10.1007/s10571-010-9639-0, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willand M. P., Rosa E., Michalski B., Zhang J. J., Gordon T., Fahnestock M., et al. (2016). Electrical muscle stimulation elevates intramuscular BDNF and GDNF mRNA following peripheral nerve injury and repair in rats. Neuroscience 334, 93–104. doi: 10.1016/j.neuroscience.2016.07.040, PMID: [DOI] [PubMed] [Google Scholar]
- Wu Q., Chen J., Yue J., Ying X., Zhou Y., Chen X., et al. (2021a). Electroacupuncture improves neuronal plasticity through the A2AR/cAMP/PKA signaling pathway in SNL rats. Neurochem. Int. 145:104983. doi: 10.1016/j.neuint.2021.104983, PMID: [DOI] [PubMed] [Google Scholar]
- Wu J. J., Lu Y. C., Hua X. Y., Ma S. J., Shan C. L., Xu J. G. (2018a). Cortical remodeling after electroacupuncture therapy in peripheral nerve repairing model. Brain Res. 1690, 61–73. doi: 10.1016/j.brainres.2018.04.009, PMID: [DOI] [PubMed] [Google Scholar]
- Wu J. J., Lu Y. C., Hua X. Y., Ma S. J., Xu J. G. (2018b). A longitudinal mapping study on cortical plasticity of peripheral nerve injury treated by direct anastomosis and Electroacupuncture in rats. World Neurosurg. 114, e267–e282. doi: 10.1016/j.wneu.2018.02.173, PMID: [DOI] [PubMed] [Google Scholar]
- Wu Q., Yue J., Lin L., Yu X., Zhou Y., Ying X., et al. (2021b). Electroacupuncture may alleviate neuropathic pain via suppressing P2X7R expression. Mol. Pain 17:7654. doi: 10.1177/1744806921997654, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L. Y., Wang X. R., Yang Y., Yang J. W., Cao Y., Ma S. M., et al. (2018). Applications of acupuncture therapy in modulating plasticity of central nervous system. Neuromodulation 21, 762–776. doi: 10.1111/ner.12724, PMID: [DOI] [PubMed] [Google Scholar]
- Yan B., Li K., Xu J., Wang W., Li K., Liu H., et al. (2005). Acupoint-specific fMRI patterns in human brain. Neurosci. Lett. 383, 236–240. doi: 10.1016/j.neulet.2005.04.021, PMID: [DOI] [PubMed] [Google Scholar]
- Yang Y., Eisner I., Chen S., Wang S., Zhang F., Wang L. (2017). Neuroplasticity changes on human motor cortex induced by acupuncture therapy: a preliminary study. Neural Plast. 2017:4716792. doi: 10.1155/2017/4716792, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Liu J., Wang J., Yin A., Jiang Z., Ye S., et al. (2021). Activation of astroglial CB1R mediates cerebral ischemic tolerance induced by electroacupuncture. J. Cereb. Blood Flow Metab. 41, 2295–2310. doi: 10.1177/0271678X21994395, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C. P., Wang N. H., Li T. C., Hsieh C. L., Chang H. H., Hwang K. L., et al. (2011). A randomized clinical trial of acupuncture versus oral steroids for carpal tunnel syndrome: a long-term follow-up. J. Pain 12, 272–279. doi: 10.1016/j.jpain.2010.09.001, PMID: [DOI] [PubMed] [Google Scholar]
- Yao M., Fang J., Li J., Ng A. C. K., Liu J., Leung G. K. K., et al. (2022). Modulation of the proteoglycan receptor PTPσ promotes white matter integrity and functional recovery after intracerebral hemorrhage stroke in mice. J. Neuroinflammation 19:207. doi: 10.1186/s12974-022-02561-4, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi S., Yuan Y., Chen Q., Wang X., Gong L., Liu J., et al. (2016). Regulation of Schwann cell proliferation and migration by miR-1 targeting brain-derived neurotrophic factor after peripheral nerve injury. Sci. Rep. 6:29121. doi: 10.1038/srep29121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon Y. S., Lee J. S., Lee H. S. (2013). Retrograde study of CART- or NPY-neuronal projection from the hypothalamic arcuate nucleus to the dorsal raphe and/or the locus coeruleus in the rat. Brain Res. 1519, 40–52. doi: 10.1016/j.brainres.2013.04.039, PMID: [DOI] [PubMed] [Google Scholar]
- Yu H., Liu J., Ma J., Xiang L. (2014). Local delivery of controlled released nerve growth factor promotes sciatic nerve regeneration after crush injury. Neurosci. Lett. 566, 177–181. doi: 10.1016/j.neulet.2014.02.065, PMID: [DOI] [PubMed] [Google Scholar]
- Yu S., Ortiz A., Gollub R. L., Wilson G., Gerber J., Park J., et al. (2020). Acupuncture treatment modulates the connectivity of key regions of the descending pain modulation and reward Systems in Patients with chronic Low Back pain. J. Clin. Med. 9:1719. doi: 10.3390/jcm9061719, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu A. P., Shen Y. J., Qiu Y. Q., Li J., Shen Y. D., Wang X. M., et al. (2019). Comparative effects of implanted electrodes with differing contact patterns on peripheral nerve regeneration and functional recovery. Neurosci. Res. 145, 22–29. doi: 10.1016/j.neures.2018.08.007, PMID: [DOI] [PubMed] [Google Scholar]
- Yu J., Wang M., Liu J., Zhang X., Yang S. (2017). Effect of electroacupuncture on the expression of agrin and acetylcholine receptor subtypes in rats with tibialis anterior muscular atrophy induced by sciatic nerve injection injury. Acupunct. Med. 35, 268–275. doi: 10.1136/acupmed-2015-011005, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B., Zhou S., Yi S., Gu X. (2015). The regulatory roles of non-coding RNAs in nerve injury and regeneration. Prog. Neurobiol. 134, 122–139. doi: 10.1016/j.pneurobio.2015.09.006, PMID: [DOI] [PubMed] [Google Scholar]
- Zhang C. H., Ma Z. Z., Huo B. B., Lu Y. C., Wu J. J., Hua X. Y., et al. (2019). Diffusional plasticity induced by electroacupuncture intervention in rat model of peripheral nerve injury. J. Clin. Neurosci. 69, 250–256. doi: 10.1016/j.jocn.2019.08.088, PMID: [DOI] [PubMed] [Google Scholar]
- Zhang S., Tang H., Zhou J., Gu Y. (2014). Electroacupuncture attenuates neuropathic pain after brachial plexus injury. Neural Regen. Res. 9, 1365–1370. doi: 10.4103/1673-5374.137589, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Zhang Y., Bian Y., Fu H., Xu Y., Guo Y. (2018). Effect of long-term electroacupuncture stimulation on recovery of sensorimotor function after peripheral nerve anastomosis. Acupunct. Med. 36, 170–175. doi: 10.1136/acupmed-2017-011367, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Zheng Y., Wang Y., Qu S., Zhang S., Wu C., et al. (2016). Evidence of a synergistic effect of Acupoint combination: a resting-state functional magnetic resonance imaging study. J. Altern. Complement. Med. 22, 800–809. doi: 10.1089/acm.2016.0016, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z. Q. (2008). Neural mechanism underlying acupuncture analgesia. Prog. Neurobiol. 85, 355–375. doi: 10.1016/j.pneurobio.2008.05.004, PMID: [DOI] [PubMed] [Google Scholar]
- Zhao X., Bai F., Zhang E., Zhou D., Jiang T., Zhou H., et al. (2018). Electroacupuncture improves neurobehavioral function through targeting of SOX2-mediated axonal regeneration by MicroRNA-132 after ischemic stroke. Front. Mol. Neurosci. 11:471. doi: 10.3389/fnmol.2018.00471, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F., He W., Zhang Y., Tian D., Zhao H., Yu K., et al. (2013). Electric stimulation and decimeter wave therapy improve the recovery of injured sciatic nerves. Neural Regen. Res. 8, 1974–1984. doi: 10.3969/j.issn.1673-5374.2013.21.006, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Yuan Y., Li P., Pan J., Qin J., Liu Y., et al. (2018). miR-221-3p inhibits Schwann cell myelination. Neuroscience 379, 239–245. doi: 10.1016/j.neuroscience.2018.03.019, PMID: [DOI] [PubMed] [Google Scholar]
- Zhao N., Zhang J., Qiu M., Wang C., Xiang Y., Wang H., et al. (2018). Scalp acupuncture plus low-frequency rTMS promotes repair of brain white matter tracts in stroke patients: a DTI study. J. Integr. Neurosci. 17, 61–69. doi: 10.31083/JIN-170043, PMID: [DOI] [PubMed] [Google Scholar]
- Zheng Y., Pan L., He J., Yan J., Xia Y., Lin C., et al. (2023). Electroacupuncture-modulated extracellular ATP levels in prefrontal cortex ameliorated depressive-like behavior of maternal separation rats. Behav. Brain Res. 452:114548. doi: 10.1016/j.bbr.2023.114548, PMID: [DOI] [PubMed] [Google Scholar]
- Zhi M. J., Liu K., Zheng Z. L., He X., Li T., Sun G., et al. (2017). Application of the chronic constriction injury of the partial sciatic nerve model to assess acupuncture analgesia. J. Pain Res. 10, 2271–2280. doi: 10.2147/JPR.S139324, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N., Hao S., Huang Z., Wang W., Yan P., Zhou W., et al. (2018). MiR-7 inhibited peripheral nerve injury repair by affecting neural stem cells migration and proliferation through cdc42. Mol. Pain 14:1744806918766793. doi: 10.1177/1744806918766793, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H., Xue C., Yao M., Wang H., Zhang P., Qian T., et al. (2018). miR-129 controls axonal regeneration via regulating insulin-like growth factor-1 in peripheral nerve injury. Cell Death Dis. 9:720. doi: 10.1038/s41419-018-0760-1, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitnik G. A., Clark B. D., Waterhouse B. D. (2014). Effects of intracerebroventricular corticotropin releasing factor on sensory-evoked responses in the rat visual thalamus. Brain Res. 1561, 35–47. doi: 10.1016/j.brainres.2014.02.048, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y., Tang W., Li X., Xu M., Li J. (2019). Acupuncture reversible effects on altered default mode network of chronic migraine accompanied with clinical symptom relief. Neural Plast. 2019:5047463. doi: 10.1155/2019/5047463, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta J. K., Smith Y. R., Bueller J. A., Xu Y., Kilbourn M. R., Jewett D. M., et al. (2001). Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science 293, 311–315. doi: 10.1126/science.1060952, PMID: [DOI] [PubMed] [Google Scholar]
- Zuo X. N., Di Martino A., Kelly C., Shehzad Z. E., Gee D. G., Klein D. F., et al. (2010). The oscillating brain: complex and reliable. NeuroImage 49, 1432–1445. doi: 10.1016/j.neuroimage.2009.09.037, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo T., Qin J. Y., Chen J., Shi Z., Liu M., Gao X., et al. (2013). Involvement of N-cadherin in the protective effect of glial cell line-derived neurotrophic factor on dopaminergic neuron damage. Int. J. Mol. Med. 31, 561–568. doi: 10.3892/ijmm.2013.1226, PMID: [DOI] [PubMed] [Google Scholar]