Abstract
Background
This study analyzes the effect of frailty and Post-Operative Delirium (POD) on the functional status at hospital discharge and at 4-month follow-up in patients with hip fracture (HF).
Methods
Multicenter prospective observational study of older patients with HF admitted to 12 Italian Orthogeriatric centers (July 2019-August 2022). POD was assessed using the 4AT. A 26-item Frailty Index (FI) was created using data collected on admission. The outcome measures were Cumulated Ambulation Score (CAS) ≤ 2 at discharge and a telephone-administered CAS ≤ 2 after 4 months. Poisson regression models were used to assess the effect of frailty and POD on outcomes.
Results
984 patients (median age 84 years, IQR = 79–89) were recruited: 480 (48.7%) were frail at admission, 311 (31.6%) developed POD, and 158 (15.6%) had both frailty and POD. In a robust Poisson regression, frailty alone (Relative Risk, RR = 1.56, 95% Confidence Intervals, CI 1.19–2.04, p = 0.001) and its combination with POD (RR = 2.57, 95% CI 2.02–3.26, p < 0.001) were associated with poor functional status at discharge. At 4-month follow-up, the combination of frailty with POD (RR 3.65, 95% CI 1.85–7.2, p < 0.001) increased the risk of poor outcome more than frailty alone (RR 2.38, 95% CI 1.21–4.66, p < 0.001).
Conclusions
POD development exacerbates the negative effect that frailty exerts on functional outcomes in HF patients.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40520-023-02522-8.
Keywords: Delirium, Frailty, Functional outcome, Hip fracture, Orthogeriatric
Introduction
Hip fractures (HFs) are common in older people: yearly 1,600,000 HF occur worldwide, of which more than 123,000 in Italy [1]. The consequences are relevant: nearly one-third of patients die within one year after HF, and about half of the survivors do not regain their pre-fracture functional status [2, 3]. These figures threaten the sustainability of national healthcare systems [4] as the population is aging and the number of HF is expected to increase [5].
Frailty is a geriatric syndrome characterized by excessive vulnerability to stressors and impaired ability to maintain individual homeostasis. Frailty is a predisposing factor for postoperative delirium (POD) [6, 7].
Both frailty and POD are associated with an increased risk of negative outcomes, including poor functional status and disability, suggesting that these conditions may concur to affect the patient’s health status after HF [8–11].
However, studies focusing on the combined effect of frailty and POD on the functional status of HF patients after surgical repair are lacking [8, 9, 12–14].
The aim of this study is to explore the effect of frailty, POD, and their combination on the risk of poor functional status at hospital discharge and four months after discharge in an Italian multicenter cohort of patients with HF.
Methods
Setting and sample
The GIOG 2.0 is an unfunded, multicenter, prospective, observational study to evaluate the practice of care and key-performance indicators in 12 Italian orthogeriatric centers. The data presented in this study refer to the period between July 1, 2019, and August 31, 2022. Inclusion criteria for the study were: age ≥ 65 years, proximal HF requiring urgent surgical intervention, the willingness of the patient or his/her caregiver (if the patient was unable) to sign an informed consent form, and ability to speak Italian. Exclusion criteria were a diagnosis of distal HF, metastatic cancer, or a life expectancy of less than one month (according to the physician’s judgment). The study was conducted in accordance with the EU Regulation 2016/679 and the EU Directive 2016/680, and the protocol was approved by the Brianza Institutional Review Board. The RedCap Cloud platform was used to ensure data confidentiality, and data were anonymized (https://eulogin.redcapcloud.com/#cid=nph2020&act=list&studyId=343).
Frailty index
A Frailty Index (FI) of health deficits was operationalized according to a standard procedure [15], that includes the ascertainment of the presence of medical conditions, disabilities, signs, and symptoms based on the information documented in their medical records and reported by their family members with reference to the pre-fracture health status. The values (median and IQR or number and percentage) of the variables used to compute our 26-item FI are reported in Supplementary Table 1. A score of 0 for the absence of deficit and 1 for the presence of deficit was assigned for each variable. The FI score was calculated for each patient by dividing the sum of observed deficits by the sum of all measured variables. For example, if a person had 10/26 altered items, the corresponding FI score was 0.38. As in a previous study, a cut-off ≥ 0.25 was used to define frail patients [16].
Diagnosis of POD
From the first to the third day after surgery, each patient was evaluated daily for POD occurrence by a geriatrician using the 4AT test [17], a tool with a sensitivity of 88% and a specificity of 88% for the diagnosis of delirium [18]. All patients who scored > 4 at 4AT and showed symptoms of delirium for at least one day after surgery were classified as having POD. On holidays, when assessors were not at the hospital and could not assess 4AT, information on delirium was obtained from a review of daily medical and nursing notes, as in previous studies [14, 19].
Other measurements
On admission, all patients underwent a Comprehensive Geriatric Assessment (CGA), which included data on demographics (age, sex, and living arrangements), mobility status (Standardised Audit of Hip Fracture in Europe-SAHFE) [20], and cognitive status (Short Portable Mental Status Questionnaire, SPMSQ) [21]. During hospitalization, we also assessed the American Society of Anesthesiologists (ASA) Classification score [22], time between admission and surgery, type of HF, and type of anesthesia.
Medical care and rehabilitation
At each center, patients were examined daily by an orthopedic surgeon and a geriatrician, and post-operative rehabilitation was provided by a team of physical therapists. Treatment protocols included standing and walking exercises aimed at improving the patient’s functional status.
Outcome measure
The outcome measure was evaluated with the Cumulated Ambulation Score (CAS) [23] at hospital discharge and at 4 months. We chose a 4-month follow-up period for assessing functional status because previous studies have indicated that most of functional improvement after hip fracture occurs within the first 3 months, with minimal further improvements expected beyond this time frame [24]. CAS is a score that assesses the patient’s independence in three essential functions: transfer in and out of bed, sit to stand from a chair, walking with or without aid. For each function, 2 points are given if the patient can complete the task without help, 1 point if the patient requires help, and 0 points if the patient cannot perform the task. Poor functional status at discharge was defined by a CAS ≤ 2.
The outcome measure at the 4-month follow-up was a telephone-administered CAS in which either the patient or the caregiver was asked to report the patient’s independence in the tasks evaluated by the CAS (i.e., transfer from sitting to supine to sitting, transfer from sitting-to-standing-to-sitting and walking with or without an appropriate aid). We used the same scoring system as the original CAS, and we defined the presence of poor functional status at 4 months with a total score ≤ 2.
Statistical analyses
Continuous variables are reported as median and interquartile range (IQR) because their distribution was not normal. Qualitative variables are reported as frequencies and percentages. Statistical significance between groups (frails vs non-frails) was evaluated using the Wilcoxon test for continuous variables and the Chi-square test for categorical variables.
To evaluate the association of frailty, POD, and their combination with the outcome measure at hospital discharge, a 4-group variable (frailty alone, POD alone, frailty plus POD, neither) was created and included in a robust Poisson regression [25], adjusting for confounders selected a priori based on their significance in univariate analysis (age, sex, type of fracture, 48-h surgical delay, type of anesthesia). A similar Poisson regression analysis was performed in the cohort of patients who had the 4-month follow-up data, using telephone-administered CAS as the outcome measure. Results were adjusted for the likelihood of patients being lost or dead at follow-up. Association estimates were reported as relative risk (RR) and corresponding 95% confidence intervals (95%CI). All tests were two-sided, and we considered a p value < 0.05 as significant. All analyses were performed using SAS software (version 9.4; SAS Institute).
Results
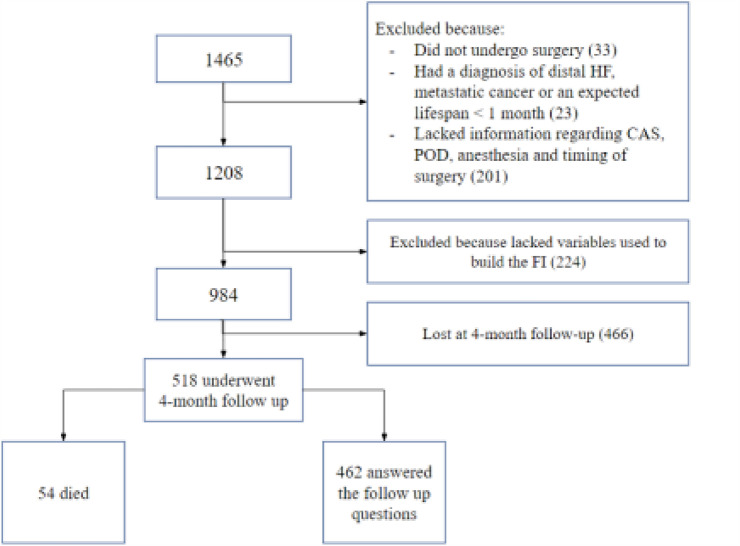
Twelve centers participated in the baseline recruitment. Figure 1 shows the flowchart of the patients in the study. A total of 1465 patients’ records were collected at hospital discharge. Of these, 481 had one or more exclusion criteria, leaving a final population of 984 patients. The 4-month follow-up was obtained in 518 patients, recruited from 8 centers. Of these patients, 54 died, leaving a final sample of 462 patients. Differences between patients who underwent the 4-month follow-up and those who did not are shown in Supplementary Table 2.
Fig. 1.
Flow chart of the patients’ selection process
Table 1 shows clinical features and outcomes at discharge of patients recruited at baseline, for the entire population and according to the presence of frailty. The median age was 84 (IQR 79–89) years and only 241 participants were males (24.5%). Almost all patients (938, 95.3%) lived at home and only 46 (4.7%) were institutionalized. The median number of drugs taken daily was 4 (IQR 2–6). Before the fracture, more than half of the sample already had walking impairment, and the median SPMSQ score was 3 (IQR 1–6), suggesting mild cognitive impairment. Inter‐trochanteric fractures were more common (45%) than intracapsular fractures (43.6%), which corresponded to a prevalent use of intramedullary nails for osteosynthesis (51.4%). Regional anesthesia was used in 879 (87.6%) patients, and surgical delay (i.e., ≥ 48 h from hospital admission to surgery) occurred in 21.7% of patients. POD developed in 311 (31.6%) patients and the median length of hospital stay was 9 (IQR 7–12) days. At discharge, 31.7% of patients had poor functional status (i.e., CAS score ≤ 2); most of them were discharged to a rehabilitation facility (58%), 33.6% returned home, and 6.3% to a nursing home.
Table 1.
Clinical features and outcomes of the patients recruited, as a whole sample and according to Frailty Index (FI) score
| Variable | Full sample (n = 984) | FI < 0.25 (n = 504) | FI ≥ 0.25 (n = 480) | p value |
|---|---|---|---|---|
| Collected at hospital admission | ||||
| Age, years | 84 (79–89) | 82 (77–87) | 86 (81–90) | < .0001 |
| Male | 241 (24.5) | 113 (22.4) | 128 (26.7) | 0.122 |
| Living at home | 938 (95.3) | 498 (98.8) | 440 (91.6) | < .0001 |
| Number of daily drugs | 4 (2–6) | 3 (1–5) | 5 (3–7) | < .0001 |
| Unable to walk | 15 (1.6) | 1 (0.2) | 14 (3) | < .0001 |
| Able to walk only indoor (with aid) | 521 (54.4) | 152 (31.0) | 369 (78.9) | |
| Able to walk outdoor with or without aid | 422 (44.0) | 337 (68.8) | 85 (18.2) | |
| SPMSQ score | 3 (1–6) | 1 (0–3) | 5 (2–10) | < .0001 |
| Hemoglobin serum levels (g/dl) | 12 (11–13) | 12.4 (11.4–13.5) | 11.7 (10.5–12.5) | < .0001 |
| Related to intervention | ||||
| Fracture type | ||||
| Intracapsular | 429 (43.6) | 241 (47.8) | 188 (39.2) | 0.015 |
| Inter‐trochanteric | 446 (45.3) | 207 (41.1) | 239 (49.8) | |
| Other | 109 (11.1) | 56 (11.1) | 53 (11) | |
| ASA score | 3 (2–3) | 3 (2–3) | 3 (2–3) | < .0001 |
| Regional anesthesia | 862 (87.6) | 446 (88.5) | 416 (86.7) | 0.385 |
| Hip arthroplasty | 400 (40.6) | 221 (43.8) | 179 (37.3) | < .0001 |
| Intramedullary nail | 506 (51.4) | 230 (45.6) | 276 (57.5) | |
| Other | 78 (7.9) | 53 (10.6) | 25 (5.1) | |
| Surgical delay (≥ 48 h) | 214 (21.7) | 98 (19.4) | 116 (24.2) | 0.072 |
| Related to post‐surgical course | ||||
| Postoperative delirium | 311 (31.6) | 83 (16.5) | 228 (47.5) | < .0001 |
| Outcomes collected at discharge | ||||
| CAS | 3 (2–3) | 3 (3–4) | 3 (1–3) | < .0001 |
| Length of hospital stay, days | 9 (7–12) | 9 (7–12) | 9 (7–13) | 0.126 |
| Discharged to home | 330 (33.6) | 186 (37) | 144 (30.1) | < .0001 |
| Discharged to rehabilitation | 570 (58) | 304 (60.4) | 266 (55.5) | |
| Discharged to nursing home | 62 (6.3) | 10 (2) | 52 (10.9) | |
| Other discharge | 20 (2) | 3 (0.6) | 17 (3.5) | |
Values are reported as median and (Interquartile range) or number (%)
Wilcoxon test for continuous variables and chi-square test for categorical variables were used to compare frail and non-frail patients
MNA mini nutritional assessment, SAHFE scottish audit hip fracture classification, ADL activities of daily living, NMS new mobility score, NEWS national early warning score, SPMSQ short portable mental status questionnaire, ASA american society of anesthesiologists, CAS cumulated ambulation score, FI frailty index
Overall, 421 (42.8%) were non frail and didn’t develop delirium, 252 (25.7%) had frailty alone without delirium, 83 (8.4%) were non frail and didn’t develop delirium and 228 (23.1%) had both frailty and delirium. At discharge, the proportion of patients with CAS score ≤ 2 was higher in frail patients (43.3% vs 20.6%), whereas there were no significant differences in the discharge setting.
Clinical characteristics and outcomes of patients who underwent the4-month follow-up are shown in Table 2. Overall, 54 (10.4%) patients died, and 71 (15.4%) of the survivors had poor functional status. Most of the patients were living at home (71.5%), 12.8% were in a rehabilitation ward, and 8.8% were in a nursing home. The mortality of frail patients was threefold higher than in non-frail patients (16.7% vs 4.8% p < 0.001). The percentage of those with poor functional status was 27.2% in frail and 5.9% in non-frail patients. There was a higher proportion of frail patients living in nursing homes compared to non-frail patients.
Table 2.
Outcomes of the patients who underwent the 4-month follow-up, as a whole sample and according to Frailty Index (FI) score
| Variable | Full sample (n = 518) | FI < 0.25 (n = 272) | FI ≥ 0.25 (n = 246) | p value |
|---|---|---|---|---|
| Mortality | 54 (10.4) | 13 (4.8) | 41 (16.7) | < .0001 |
| Telephone-administered CAS ≤ 2 (N = 462) | 71 (15.4) | 15 (5.9) | 56 (27.2) | < .0001 |
| Residence status (N = 462) | ||||
| Home | 291 (71.5) | 164 (81.2) | 127 (61.9) | < .0001 |
| Nursing home | 36 (8.8) | 7 (3.5) | 29 (14.1) | |
| Rehabilitation | 52 (12.8) | 26 (12.9) | 26 (12.7) | |
| Other | 28 (6.9) | 5 (2.5) | 23 (11.2) | |
Values are reported as median and (Interquartile range) or number (%)
Wilcoxon test for continuous variables and chi-square test for categorical variables were used to compare frail and non-frail patients
CAS cumulated ambulation score, FI frailty index
Table 3 shows the results of two Poisson regression models to estimate the risk of poor functional status at discharge (panel A) and after 4 months (panel B) according to the presence of frailty and POD, alone or in combination. Frailty alone (RR = 1.56, 95% CI 1.19–2.04, p = 0.01) and frailty plus POD (RR = 2.57, 95% CI 2.21–3.26, p < 0.001) were significantly associated with poor functional status at discharge. At the 4-month follow-up, the interaction between frailty and POD (RR 3.65, 95% CI 1.85—7.2, p < 0.001) increased the risk of negative outcomes more than frailty alone (RR = 2.38, 95% CI 1.21–4.66, p = 0.01.
Table 3.
Robust Poisson regression models of the variables associated with poor functional status at discharge (CAS ≤ 2) and at 4-month follow up (telephone administered CAS ≤ 2)
| Variable | Panel A | Panel B | ||
|---|---|---|---|---|
| At discharge (N = 984) | At 4-month follow-up (N = 462) | |||
| RR (95% CI) | p value | RR (95% CI) | p value | |
| Frailty index and post‐operative delirium | ||||
| Frailty no/delirium no | 1 | 1 | ||
| Frailty yes/delirium no | 1.56 (1.19–2.04) | 0.0012 | 2.38 (1.21–4.66) | 0.0116 |
| Frailty no/delirium yes | 1.37 (0.92–2.02) | 0.1197 | 0.22 (0.03–1.56) | 0.1307 |
| Frailty yes/delirium yes | 2.57 (2.02–3.26) | < .0001 | 3.65 (1.85–7.2) | 0.0002 |
| Socio‐demographic variables | ||||
| Age | 0.0059 | 0.0039 | ||
| Female sex | 0.9465 | 0.4702 | ||
| Fracture and intervention covariates | ||||
| Inter‐trochanteric/subtrochanteric fracture | 0.2304 | 0.5928 | ||
| Other types of fracture | < .0001 | 0.4014 | ||
| 48‐h delay in intervention | 0.0946 | 0.5399 | ||
| General anesthesia/Sedation | 0.1821 | 0.2001 | ||
RR relative risk, 95% CI confidence intervals, CAS cumulated ambulation score
Discussion
This large, multicenter, prospective study of patients with HF recruited in Italian orthogeriatric centers, shows that frailty alone and its combination with POD significantly affect functional status at discharge. Furthermore, the development of POD exacerbates the negative effect that frailty exerts on patient function at 4-month follow-up.
Two recent systematic reviews that included older patients undergoing HF surgery, predominantly examined the association of frailty with mortality, length of hospital stay, risk of complications after surgery, and risk of institutionalization, whereas functional status was relatively understudied [26, 27].
Using a modified 19-item FI, Inoue et al. found that frailty was independently associated with an increased likelihood of poor functional recovery at discharge [15]. In another study, Low et al. found that frailty (measured by the Clinical Frailty Scale) was the strongest independent predictor of poor Functional Independence Measure (FIM) efficiency at discharge, inability to regain pre-fracture mobility, and return home [28]. Furthermore, in a multicenter study of 36,192 patients, frailty, measured by the Hospital Frailty Risk Score (HFRS) based on ICD-10 reports, was associated with a higher risk of poor Barthel Index score at hospital discharge [29]. However, none of these studies examined the patient’s functional status after discharge.
The relationship between POD and functional outcome is supported by a large body of evidence. Ouellet et al. [30] showed that patients who developed POD after HF surgery had a higher risk of poor Barthel Index at discharge. Shi et al. [31] found that patients who developed POD experienced a greater decline in ADL score at 24 and 36 months after surgery compared to their counterparts. The negative effect of POD on functional status was also found in studies that included patients discharged to rehabilitation and long-term care facilities after HF surgery [32–34]. However, none of these studies examined the combined effect of frailty and delirium on subsequent functional status. This is an interesting issue because frailty may predispose to delirium and should act as a confounding variable when examining the association between delirium and functional outcomes.
To our knowledge, only one previous study [12] has examined the effect of frailty and POD on patients’ functional status at hospital discharge. In a cohort of 988 patients undergoing HF surgery, the authors found that frailty, POD, and their combination were independently associated with low CAS scores at discharge. However, this study was based on data from a single center and did not collect information on patients’ status after hospital discharge.
Our work contributes to the evidence in this field by demonstrating that the effect of frailty on functional status extends beyond hospital discharge and that POD interacts with frailty to increase the risk of poor functional outcome 4 months after hospital discharge.
Overall, these data suggest at least three possible interpretations. The most suitable is that POD superimposed on frailty may trigger a downward spiral leading to a negative chain of reactions (i.e., neuroinflammation, brain metabolic insufficiency, neurotransmitters’ imbalance, and others) that self-maintain after discharge and threaten the patient’s recovery [35].
However, it could also be hypothesized that POD hampers functional recovery through various mechanisms, such as a delayed onset of the rehabilitation process and reduced patient engagement. Additionally, POD might represent a marker of poor resilience, suggesting that it could be used as a condition to stratify risk in frail patients [36]. The lack of effect of POD alone on the outcomes at discharge and at 4-month follow-up might be due to the scarcity of patients without frailty who developed POD. The difference between this study and our previous study [12] regarding the effect of POD on functional status at discharge after HF surgery may be explained by the different methods used to assess POD and by the multicenter design of the present study.
Previous systematic reviews have shown that multicomponent non-pharmacological interventions can prevent delirium, decreasing its incidence by more than 40% [37]. Since functional improvement in HF patients occurs mainly within 3 months after HF, the results of the present study suggest that it is important to screen for frailty on hospital admission and to prevent and treat POD not only to improve functional status at discharge but also to reduce the risk of further decline in the longer term [24].
The strengths of this study are the large sample size, the prospective and multicenter design, the use of a standardized approach to evaluate the patient’s clinical status, and the method used to assess POD. Indeed, all patients were assessed using the 4AT, which has shown, good overall performance in diagnostic accuracy for delirium detection in a recent systematic review and meta-analysis [18].
A limitation of the study is that we lost a relevant proportion of patients at the 4-month assessment, which may bias our results at follow-up. However, it may be considered that the multivariate Poisson regression model used to determine the variables associated with poor functional status at 4 months was adjusted for the patient’s likelihood of being lost or dead at follow-up. A second limitation is that functional status at 4 months was determined with a surrogate CAS assessed by telephone interviews because of limited resources (the GIOG 2.0. is not supported by any funding source). Third, the number of items used to compute the FI was lower than that suggested by Searle et al. [38]. However, a growing number of studies have recently been published using FIs that include 20–25 variables and show a good ability to predict negative outcomes in different patient cohorts [39, 40]. Lastly, we must acknowledge the potential for bias in our study results due to the impact of post-hospitalization development of COVID-19 on the extent of functional recovery in certain patients.
Conclusions
This study shows that in older patients undergoing HF surgery, frailty alone and its combination with POD are significantly associated with poor functional status at discharge. Furthermore, the development of POD exacerbates the negative effect that frailty exerts on functional outcomes after 4 months. The results of the present study highlight the importance of screening for frailty and of preventing and treating POD in patients undergoing HF surgery, in order to improve their functional status at discharge and reduce the risk of medium- and long-term disability.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The following investigators should be mentioned as authors for the GIOG study group: Maria Lia Lunardelli, Enrico Benvenuti, Stefania Maggi, Alberto Pilotto, Antonella Barone, Amedeo Zurlo, Monica Pizzonia, Raffaele Antonelli Incalzi, Luigi Residori, Paola Cena, Paolo Mazzola, Maurizio Corsi, Alessio Greco, Riccardo Galluccio, Alice Riccò, Luca Molteni, Andrea Poli, Chiara Bendini, Alice Ceccofiglio, Gaia Rubbieri, Giulio Mannarino, Alessandro Cartei, Eleonora Barghini, Ilaria Del Lungo, Silvia Tognelli, Chiara Bandinelli, Emilio Martini, Giulia Venturelli, Alberto Cella, Chiara Ceolin, Labjona Haxhiaj, Alice Laudisio, Luigi Residori, Martina Bonetto, Maria Grazia Valsecchi.
Author contributions
CMG, AZ and GB: designed the study. MCF, GC, FC, CM, EM, GS, AC, GZ, CT, SV, AU and GB: collected the data. ET and AZ: performed the statistical analyses. CMG, AZ, MCF and GB: drafted the manuscript. All authors reviewed the manuscript.
Funding
Open access funding provided by Università degli Studi di Milano - Bicocca within the CRUI-CARE Agreement. The authors did not receive support from any organization for the submitted work.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
All Authors declare no conflict of interest.
Ethical approval
The study protocol was approved by the hospital Institutional Review Board. The study was compliant with the declaration of Helsinki and no identifiable personal data were used for this study.
Statement of human and animal rights
The study was approved by the Brianza Institutional Review Board and was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. No identifiable personal data were used for this study.
Informed consent
Informed consent for participation in clinical studies was obtained from all patients.
Footnotes
The members of GIOG 2.0 Study Group, Società Italiana di Gerontologia e Geriatria (SIGG) are listed in Acknowledgements.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Giuseppe Bellelli, Email: giuseppe.bellelli@unimib.it.
GIOG 2.0 Study Group, Società Italiana di Gerontologia e Geriatria (SIGG):
Maria Lia Lunardelli, Enrico Benvenuti, Stefania Maggi, Alberto Pilotto, Antonella Barone, Amedeo Zurlo, Monica Pizzonia, Raffaele Antonelli Incalzi, Luigi Residori, Paola Cena, Paolo Mazzola, Maurizio Corsi, Alessio Greco, Riccardo Galluccio, Alice Riccò, Luca Molteni, Andrea Poli, Chiara Bendini, Alice Ceccofiglio, Gaia Rubbieri, Giulio Mannarino, Alessandro Cartei, Eleonora Barghini, Ilaria Del Lungo, Silvia Tognelli, Chiara Bandinelli, Giulia Venturelli, Alberto Cella, Chiara Ceolin, Labjona Haxhiaj, Alice Laudisio, Luigi Residori, Martina Bonetto, and Maria Grazia Valsecchi
References
- 1.Agenas - Agenzia Nazionale per i servizi sanitari Regionali AGENAS. http://pne2017.agenas.it/. Accessed 18 Jan 2023
- 2.Maggi S, Siviero P, Wetle T, Besdine RW, Saugo M, Crepaldi G. A multicenter survey on profile of care for hip fracture: predictors of mortality and disability. Osteoporos Int. 2010;21:223–231. doi: 10.1007/s00198-009-0936-8. [DOI] [PubMed] [Google Scholar]
- 3.Bellelli G, Mazzola P, Corsi M, et al. The combined effect of ADL impairment and delay in time from fracture to surgery on 12-month mortality: an observational study in orthogeriatric patients. J Am Med Dir Assoc. 2012;13:e9–664.e14. doi: 10.1016/j.jamda.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Kanis JA, Odén A, McCloskey EV, Johansson H, Wahl DA, Cooper C, IOF Working Group on Epidemiology and Quality of Life A systematic review of hip fracture incidence and probability of fracture worldwide. Osteoporos Int. 2012;23:2239–2256. doi: 10.1007/s00198-012-1964-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gullberg B, Johnell O, Kanis JA. World-wide projections for hip fracture. Osteoporos Int. 1997;7:407–413. doi: 10.1007/pl00004148. [DOI] [PubMed] [Google Scholar]
- 6.Geriatric Medicine Research Collaborative Delirium is prevalent in older hospital inpatients and associated with adverse outcomes: results of a prospective multi-centre study on World Delirium Awareness Day. BMC Med. 2019;17:229. doi: 10.1186/s12916-019-1458-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Persico I, Cesari M, Morandi A, et al. Frailty and delirium in older adults: a systematic review and meta-analysis of the literature. J Am Geriatr Soc. 2018;66:2022–2030. doi: 10.1111/jgs.15503. [DOI] [PubMed] [Google Scholar]
- 8.Boissonneault A, Mener A, Schwartz A, Wilson J, Staley C, Schenker M. Impact of frailty on 30-day morbidity and mortality of patients with intertrochanteric femur fractures. Orthopedics. 2019;42:344–348. doi: 10.3928/01477447-20191001-05. [DOI] [PubMed] [Google Scholar]
- 9.Wilson JM, Boissonneault AR, Schwartz AM, Staley CA, Schenker ML. Frailty and malnutrition are associated with inpatient postoperative complications and mortality in hip fracture patients. J Orthop Trauma. 2019;33:143–148. doi: 10.1097/BOT.0000000000001386. [DOI] [PubMed] [Google Scholar]
- 10.Chen C-L, Chen C-M, Wang C-Y, et al. Frailty is associated with an increased risk of major adverse outcomes in elderly patients following surgical treatment of hip fracture. Sci Rep. 2019;9:19135. doi: 10.1038/s41598-019-55459-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gleason LJ, Benton EA, Alvarez-Nebreda ML, Weaver MJ, Harris MB, Javedan H. FRAIL questionnaire screening tool and short-term outcomes in geriatric fracture patients. J Am Med Dir Assoc. 2017;18:1082–1086. doi: 10.1016/j.jamda.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gandossi CM, Zambon A, Oliveri G, et al. Frailty, post-operative delirium and functional status at discharge in patients with hip fracture. Int J Geriatr Psychiatry. 2021;36:1524–1530. doi: 10.1002/gps.5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383:911–922. doi: 10.1016/S0140-6736(13)60688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bellelli G, Mazzola P, Morandi A, et al. Duration of postoperative delirium is an independent predictor of 6-month mortality in older adults after hip fracture. J Am Geriatr Soc. 2014;62:1335–1340. doi: 10.1111/jgs.12885. [DOI] [PubMed] [Google Scholar]
- 15.Inoue T, Misu S, Tanaka T, et al. Frailty defined by 19 items as a predictor of short-term functional recovery in patients with hip fracture. Injury. 2019;50:2272–2276. doi: 10.1016/j.injury.2019.10.011. [DOI] [PubMed] [Google Scholar]
- 16.Song X, Mitnitski A, Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc. 2010;58:681–687. doi: 10.1111/j.1532-5415.2010.02764.x. [DOI] [PubMed] [Google Scholar]
- 17.Bellelli G, Morandi A, Davis DHJ, et al. Validation of the 4AT, a new instrument for rapid delirium screening: a study in 234 hospitalised older people. Age Ageing. 2014;43:496–502. doi: 10.1093/ageing/afu021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tieges Z, MacLullich AMJ, Anand A, et al. Diagnostic accuracy of the 4AT for delirium detection in older adults: systematic review and meta-analysis. Age Ageing. 2021;50:733–743. doi: 10.1093/ageing/afaa224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bellelli G, Carnevali L, Corsi M, et al. The impact of psychomotor subtypes and duration of delirium on 6-month mortality in hip-fractured elderly patients. Int J Geriatr Psychiatry. 2018;33:1229–1235. doi: 10.1002/gps.4914. [DOI] [PubMed] [Google Scholar]
- 20.Parker MJ, Currie CT, Mountain JA, et al. Standardised Audit of hip fracture in Europe (SAHFE) Hip Int. 1998;8:10–15. doi: 10.1007/s002640000152. [DOI] [Google Scholar]
- 21.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23:433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 22.Owens WD, Felts JA, Spitznagel EL. ASA Physical status classifications: a study of consistency of ratings. Anesthesiology. 1978;49:239–243. doi: 10.1097/00000542-197810000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Foss NB, Kristensen MT, Kehlet H. Prediction of postoperative morbidity, mortality and rehabilitation in hip fracture patients: the cumulated ambulation score. Clin Rehabil. 2006;20:701–708. doi: 10.1191/0269215506cre987oa. [DOI] [PubMed] [Google Scholar]
- 24.Brown K, Cameron ID, Keay L, Coxon K, Ivers R. Functioning and health-related quality of life following injury in older people: a systematic review. Inj Prev. 2017;23:403–411. doi: 10.1136/injuryprev-2016-042192. [DOI] [PubMed] [Google Scholar]
- 25.Chen W, Qian L, Shi J, Franklin M. Comparing performance between log-binomial and robust Poisson regression models for estimating risk ratios under model misspecification. BMC Med Res Methodol. 2018;18:1–12. doi: 10.1186/s12874-018-0519-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan B, Sun W, Wang W, Wu J, Wang G, Dou Q. Prognostic significance of frailty in older patients with hip fracture: a systematic review and meta-analysis. Int Orthop. 2022;46:2939–2952. doi: 10.1007/s00264-022-05605-9. [DOI] [PubMed] [Google Scholar]
- 27.Lemos JL, Welch JM, Xiao M, Shapiro LM, Adeli E, Kamal RN. Is frailty associated with adverse outcomes after orthopaedic surgery?: A systematic review and assessment of definitions. JBJS Rev. 2021 doi: 10.2106/JBJS.RVW.21.00065. [DOI] [PubMed] [Google Scholar]
- 28.Low S, Wee E, Dorevitch M. Impact of place of residence, frailty and other factors on rehabilitation outcomes post hip fracture. Age Ageing. 2021;50:423–430. doi: 10.1093/ageing/afaa131. [DOI] [PubMed] [Google Scholar]
- 29.Shimizu A, Maeda K, Fujishima I, et al. Hospital Frailty Risk Score predicts adverse events in older patients with hip fractures after surgery: analysis of a nationwide inpatient database in Japan. Arch Gerontol Geriatr. 2022;98:104552. doi: 10.1016/j.archger.2021.104552. [DOI] [PubMed] [Google Scholar]
- 30.Ouellet JA, Ouellet GM, Romegialli AM, et al. Functional outcomes after hip fracture in independent community-dwelling patients. J Am Geriatr Soc. 2019;67:1386–1392. doi: 10.1111/jgs.15870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi Z, Mei X, Li C, et al. Postoperative delirium is associated with long-term decline in activities of daily living. Anesthesiology. 2019;131:492–500. doi: 10.1097/ALN.0000000000002849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 33.Kiely DK, Jones RN, Bergmann MA, Murphy KM, Orav EJ, Marcantonio ER. Association between delirium resolution and functional recovery among newly admitted postacute facility patients. J Gerontol A Biol Sci Med Sci. 2006;61:204–208. doi: 10.1093/gerona/61.2.204. [DOI] [PubMed] [Google Scholar]
- 34.Bielza R, Zambrana F, de la Puente EF, et al. Impact of delirium on short-term outcomes in hip fracture patients under a program of approach to delirium. Geriatr Gerontol Int. 2020;20:130–137. doi: 10.1111/ggi.13838. [DOI] [PubMed] [Google Scholar]
- 35.Amoretti M, Amsler C, Bonomi G, et al. Production and detection of cold antihydrogen atoms. Nature. 2002;419:456–459. doi: 10.1038/nature01096. [DOI] [PubMed] [Google Scholar]
- 36.Mazzola P, Tassistro E, Di Santo S, et al. The relationship between frailty and delirium: insights from the 2017 Delirium Day study. Age Ageing. 2021;50:1593–1599. doi: 10.1093/ageing/afab042. [DOI] [PubMed] [Google Scholar]
- 37.Hshieh TT, Yue J, Oh E, et al. Effectiveness of multicomponent nonpharmacological delirium interventions: a meta-analysis. JAMA Intern Med. 2015;175:512–520. doi: 10.1001/jamainternmed.2014.7779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:1–10. doi: 10.1186/1471-2318-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vetrano DL, Zucchelli A, Onder G, et al. Frailty detection among primary care older patients through the primary care frailty index (PC-FI) Sci Rep. 2023;13:3543. doi: 10.1038/s41598-023-30350-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cao W, Cao C, Zheng X, et al. Factors Associated with Medication Adherence among Community-Dwelling Older People with Frailty and Pre-Frailty in China. Int J Environ Res Public Health. 2022;19:16001. doi: 10.3390/ijerph192316001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.