Abstract
Background
Several randomized clinical trials provide evidence of the survival benefit of extended adjuvant tamoxifen in women with estrogen receptor (ER)-positive early breast cancer (BC). However, non-adherence may lead to underestimate treatment effects using intention to treat (ITT) methods. We reanalyzed a randomized trial using contemporary statistical methods adjusting for non-adherence.
Methods
The TAM01 study was a phase 3 trial including women with early BC, who had completed 2–3 years of adjuvant tamoxifen between 1986 and 1995. Participants were randomly assigned to continue tamoxifen up to 10 years or to discontinue the treatment at randomization. Invasive disease-free survival (iDFS) and overall survival (OS) were estimated using marginal structural models (MSM) and rank preserving structural failure time model (RPSFTM).
Results
Of 3830 patients enrolled, 2485 were randomized to extended tamoxifen, and 1345 to treatment discontinuation. The 10-year non-adherence rate in the extended group was 27.2%. Among women with ER-positive BC (n = 2402), extended tamoxifen was associated with a 45% and 21% relative improvement in iDFS by MSM and RPSFTM, respectively (Hazard Ratio (HR), 0.55; 95% Confidence Interval (CI), 0.48–0.64 and HR, 0.79; 95%CI, 0.67–0.95, respectively), a considerable greater benefit than in the ITT analysis (HR, 0.90; 95%CI, 0.81–0.99). The OS reanalysis revealed a substantial benefit of extended tamoxifen (MSM: HR, 0.70; 95%CI, 0.59–0.83; RPSFTM: HR, 0.85; 95%CI, 0.67–1.04), compared to the ITT analyses (HR, 0.94; 95%CI, 0.84–1.07).
Conclusion
This analysis emphasizes both the importance of adherence to hormonotherapy in hormone-receptor positive early BC and the usefulness of more complex statistical analyses.
Subject terms: Statistics, Breast cancer
Introduction
Despite meaningful incremental improvements in screening, local treatment and adjuvant therapies, including endocrine therapies (AETs), estrogen receptor (ER)-positive breast cancer (BC) remains associated with a significant long-term risk of late relapse. Recommendations for extended AETs after 5 years are evolving due to recently published trials results: in patients at intermediate and high risk of recurrence, current guidelines recommend 7 to 10 years of adjuvant endocrine therapy, including at least 5 years of aromatase inhibitors (AI) given either upfront or as part of an early switch strategy after 2–3 years of tamoxifen [1]. Recently, the final results of the GIM4 phase 3 trial [2] showed that at a median follow-up of more than 12 years, disease-free survival (DFS) and overall survival (OS) outcomes were significantly improved in postmenopausal patients with ER-positive BC who, after adjuvant tamoxifen for 2–3 years, received 5 years of letrozole extension compared to the standard 2–3 years of letrozole (hazard ratios (HRs), 0.78; 95% confidence interval (CI), 0.65–0.93 and 0.77; 0·60–0.98, respectively). Safety and tolerability issues may hinder adherence to chronic treatments, however, leading to either switching treatment to another endocrine therapy, or discontinuation, according to the clinician’s or patient’s decision. Cross-sectional, retrospective, and longitudinal studies estimated adherence to treatment as averaging 79% in the first year, decreasing to 56% in the fourth and fifth year [3]). Several studies [2, 4–6] showed that adherence to tamoxifen is often suboptimal, ranging from 53% to 86%, and that its proportion decreases over time. The factors involved in non-adherence to tamoxifen treatment can be patient-related, therapy-related (duration, side effects) or associated with health care system factors such as an unsatisfactory patient–health care provider relationship [7].
Suboptimal adherence to endocrine therapy can affect BC outcomes as soon as the patients become non-adherent. We previously showed that biochemical non-adherence to tamoxifen over the first year was associated with an absolute 5.9% increase in the risk of distant recurrences at 3 years [8] (HR, 2.31; 1.05-5.06; p = 0.036). Moreover, discontinuation of AET is associated with increased all-cause mortality [9] (HR, 1.26; 1.09–1.46), and related to reduced DFS [10] (HR, 1.45; 1.09–1.93).
Although the prognostic relevance of adherence is well established, survival outcomes in most trials are analyzed according to the intention-to-treat (ITT) principle, which means that all patients who were enrolled in the trial are included in the analyses and are analyzed according to their randomized treatment assignment. Where there is considerable non-adherence to assigned treatment and late survival events, a frequent situation in trials with long-term follow-up, the on-treatment effect may be underestimated [11]. Simple methods of adjustment for treatment switch have been used historically in health technology assessments, which are based on the exclusion of switchers from the analysis or censorship of data at the time of their switch. These methods can create bias in treatment effect assessment, because treatment switch can be related to prognosis [12]. Recent recommendations indicate that these simple approaches should be avoided in the estimation of survival outcomes and replaced with advanced statistical techniques that are either randomization- or observational-based, allowing a robust theoretical treatment effect to be estimated in the absence of non-adherence [13–16].
These recent techniques are structural methods, relying on counterfactual survival times (i.e. survival times that would have been observed in the absence of non-adherence [17, 18]). Adjusting for non-adherence thus appears to be crucial in order to provide reliable evidence of a treatment effect in an adherent population, and to drive evidence-based medical decisions. Our aim was to apply these methods (inverse probability of censoring weights (IPCW), marginal structural models (MSM) and rank preserving structural failure time model (RPSFTM)) to a previously published large scale randomized phase 3 trial regarding the duration of tamoxifen (TAM01 study [19]) in order to provide robust and reliable estimates of extended adjuvant tamoxifen treatment benefit on survival outcomes in early BC when patients actually take the drug as assigned over the whole treatment period.
Methods
TAM01 study [19]: design and data derivation
The TAM01 trial is a randomized multicenter open-label superiority phase 3 trial that assessed the effects of extended tamoxifen treatment on recurrence and mortality in early BC, the design and conduct of which have been described elsewhere [19]. Briefly, the double parallel design included 3793 women in 20 cancer centers (mainly in France), enrolled between September 1986 and May 1995, aged up to 75 years, with early BC without evidence of local-regional or distant recurrence, and 2 to 3 years of tamoxifen exposure at randomization: patients were randomly allocated to the short term (ST) arm (tamoxifen was to be stopped immediately after randomization) or to the long term (LT) arm (patients continued tamoxifen for a further 10 years). Daily doses of tamoxifen ranged from 20 to 40 mg. The adherence to treatment was assessed at the patients’ follow-up visits based on patient declaration and recorded by the case report forms. The protocol was approved by the Caen Committee for the Protection of Persons in Biomedical Research.
TAM01 trial’s primary endpoint was DFS while OS was a secondary. DFS was defined as the time elapsed between inclusion and local or regional recurrence, distant metastases or death from any cause. Patients who did not experience any recurrence, metastases or died were censored at date of last news. OS was defined as the time elapsed between inclusion and death, regardless of its cause. Patients who were still alive (including those lost to follow-up) were censored at the last known date they were alive. In the original trial publication [19], an ITT analysis found that the LT arm was associated with a significantly better DFS compared to the ST arm (7-year DFS were 78% and 72%, respectively), but no substantial OS advantage was noted (7-years OS was 79% in both groups). The current analyses consider actual adherence to the assigned arm: adherence was reported by patients through scheduled hospital visits.
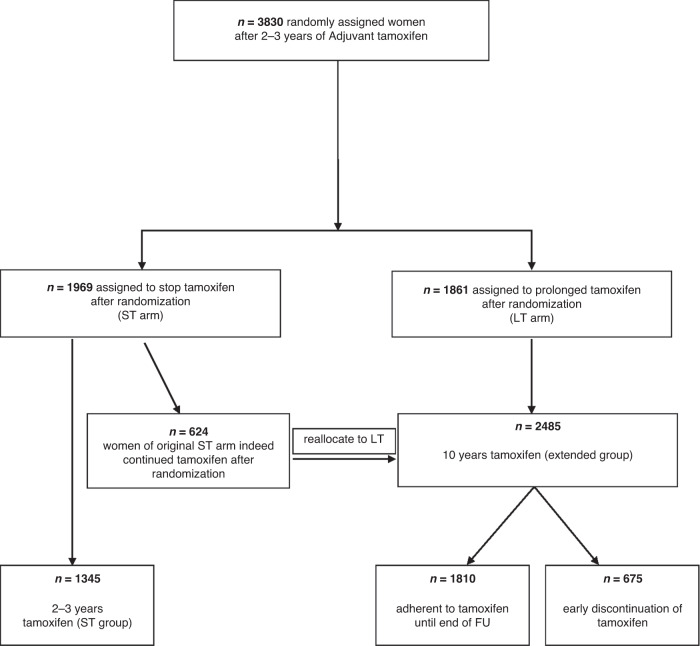
Patients from the LT arm and patients from the ST arm, with prolonged exposure after treatment, were therefore, allocated according to true tamoxifen exposure to a cohort of “12 years tamoxifen (extended group)”, while patients from the ST arm or LT arm who discontinued at randomization were included in a cohort of “2–3 years tamoxifen (short-term group)”.
The revised definition of invasive DFS (iDFS) reported by DATECAN initiative [20] has been considered as regards endpoints: invasive/in situ contra lateral BC and second primary invasive cancer were included in the composite event. The survival data (for OS) were updated in September 2015.
Statistical analysis
Statistical analyses were first performed on the ITT population, which included all randomized patients. The endpoints in this post-hoc analysis were iDFS and OS: HRs and associated 95% CIs were estimated using an unadjusted Cox proportional hazards model. We used the Schoenfeld test to check the proportionality assumption. Median follow-up was estimated with a reverse Kaplan-Meier estimator [21].The presence of tamoxifen discontinuation in the “treated patients” cannot be considered random and can create a bias in treatment effect estimation if not properly accounted for. The purpose of causal inference methods when treatment is stopped relies on the construction of counterfactual patients (“pseudo-population”), in order to mimic the conditions of perfect randomization throughout the trial duration. These methods, called “structural methods”, allow to estimate the true benefit of the experimental drug on survival endpoints that would have been estimated if there were no early tamoxifen discontinuation. There are several methods. Considering the specificities of the TAM01 trial (average proportion of switchers, variety of prognostic data collected and large sample size [22]), the IPCW, MSM, and RPSFTM were used to adjust survival estimates for non-adherence to treatment. Table 1 summarizes models characteristics. The following baseline covariates were used in order to calculate the probability of treatment adherence in the complete MSM: age, year of initial treatment, surgery (yes/no), nodal status (positive/negative), estrogen receptor status (positive/negative), radiotherapy (yes/no), chemotherapy (yes/no) and dose of tamoxifen (≤20 mg/>20 mg). Several sensitivity analyses were performed to assess the robustness of the results, either regarding methodological implementation of methods (optimal discretization of time for MSM, recensoring of follow-up and exploration of the underlying assumption of RPSFTM) or clinical questions (reallocation of patients between arms, exploratory subgroups analyses). Details are reported in Supplementary Methods 1.
Table 1.
Methodology: Summary description and key assumptions of selected statistical methods to adjust for non-adherence to assigned treatment.
| Advanced Model | Summary Description | Key assumptions |
|---|---|---|
|
Inverse Probability of Censoring Weighting (IPCW) |
Main principle: The IPCW method uses patient data to create an artificial analysis set of fully-adherent patients. To adjust for treatment discontinuation, remaining patients who continued the treatment but have similar characteristics are reweighted according to the inverse probability of non-stop of treatment. Steps - calculation of probability of treatment adherence using baseline covariates that predict both treatment discontinuation and outcomes (iDFS and OS) - a ‘pseudo-population’ is created using the adherence weights - treatment effects were estimated with a weighted Cox proportional hazards model to calculate hazard ratios (HRs) and 95 % confidence intervals (CIs). |
No unmeasured confounders: all baseline covariates and time-dependent confounders that predict stop of treatment and survival outcomes are included. |
|
Marginal Structural Model (MSM) |
Main principle: combination of IPCW and weighting for follow-up censorship MSM recreates the population that would be seen without dropouts and without stopping treatment Steps - calculation of the probability of treatment adherence and follow-up using baseline covariates that predict treatment stop, drop-out and outcomes (iDFS and OS) - a ‘pseudo-population’ is created using the final weights defined by multiplying the adherence weights and the censoring weights - treatment effects were estimated with a weighted Cox proportional hazards model to calculate HRs and associated 95% CIs |
No unmeasured confounders: all baseline covariates and time-dependent confounders that predict stop of treatment and survival outcomes, and drop-out are included. |
|
Rank Preserving Structural Failure Time Model (RPSFTM) |
Main principle: estimation of treatment effect using grid-search process, based on CTE assumption Steps: – survival is defined in treatment group according to periods of treatment (with treatment effect ψ) and periods of non-treatment (without any treatment effect) – ψ is estimated through g-estimation, and the treatment effect for fully-adherent patients is derived Treatment effects were estimated with a Cox proportional hazards model to calculate HRs. |
Perfect randomization assumption: the two groups are comparable beyond chance and if they had both received control treatment then their survival would be the same on average -Common treatment effect assumption: experimental treatment effect is the same regardless of when it is given (in this case, the no-treatment effect is the same at randomization or after tamoxifen discontinuation) |
CTE common treatment effect.
Missing data
Some clinical variables were incomplete in the original TAM01 trial. ER receptor status in particular had a considerable amount of missing information. It cannot be ensured that data are missing completely at random [23], and so two approaches were considered for ER status and clinical/demographic variables with sporadic missingness in order to consider missingness at random (Supplementary Results: Table S1 and Fig. S1). In the first approach, presented as the main result in this study, missing data were imputed using multiple imputation using the chained equations (MICE) technique [24]. Details about missingness by variable and implementation of MICE [25, 26] are reported in Supplementary Methods 2. The second simple approach consists of considering missing data as a specific category of the variable (analysis reported in Supplementary Results).
All statistical analyses were performed using R software version 4.0.2 (2020) and StataCorp LP. Stata statistical software (14.2). The statistical packages used for all analysis analyses performed are reported in Supplementary Methods 3.
Results
Population and tamoxifen adherence
Figure 1 shows patient allocation in the reanalysis. Of 3830 patients enrolled, 2485 actually received extended tamoxifen, and only 1345 stopped the treatment. Table 2 shows the characteristics of the two cohorts: “2–3 years tamoxifen (ST group)” and “10 years tamoxifen (extended group)” patients. Although the newly defined groups do not correspond to randomization arms, the clinico-pathological parameters are well balanced between groups. The median duration of follow-up was 8.1 (interquartile range (IQR): 4.7–11.0) years for iDFS and 10.4 (IQR: 8.0–12.8) years for OS.
Fig. 1. Study flow chart.
Diagram of the study reporting randomization and reallocation of patients enrolled in the trial according to tamoxifen treatment.
Table 2.
Clinical and pathological characteristics of enrolled patients.
| Groups | ||
|---|---|---|
| 2–3 years tamoxifen (ST group) n = 1345 | 10 year tamoxifen (extended group) n = 2485 | |
| Age (years) | ||
| Median (IQR) | 63 (57–68) | 62 (57–68) |
| Years of initial treatment | ||
| <1990 | 848 (63.0%) | 1614 (64.9%) |
| ≥1990 | 496 (36.9%) | 863 (34.7%) |
| Unknown | 1 (0.07%) | 8 (0.3%) |
| Type of breast surgery | ||
| No surgery | 48 (3.6 %) | 78 (3.1%) |
| Lumpectomy | 579 (43.0%) | 1265 (50.9%) |
| Mastectomy | 715 (53.2%) | 1130 (45.5%) |
| Unknown | 3 (0.2%) | 12 (0.5%) |
| Tumor size | ||
| pT0 | 31 (2.3%) | 102 (4.1%) |
| pT1 | 288 (21.4%) | 633 (25.5%) |
| pT2 | 719 (53.5%) | 1209 (48.7%) |
| pT3-4 | 204 (15.2%) | 352 (14.2%) |
| Unknown | 103 (7.7%) | 189 (7.6%) |
| Nodal status | ||
| Negative | 381 (28.3%) | 846 (34.0%) |
| Positive | 961(71.5%) | 1634 (65.8%) |
| Unknown | 3 (0.2%) | 5 (0.2 %) |
| Estrogen receptor status | ||
| Positive | 806 (59.9%) | 1596 (64.2%) |
| Negative | 180 (13.4%) | 248 (10.0%) |
| Unknown | 359 (26.7%) | 641 (25.8%) |
| Tamoxifen dosage | ||
| ≤20 mg | 427 (31.8%) | 1355 (54.5%) |
| > 20 mg | 876 (65.1%) | 1108 (44.6%) |
| Unknown | 42 (3.1%) | 22 (0.9%) |
| Adjuvant chemotherapy | ||
| No | 956 (71.1%) | 1674 (67.4%) |
| Yes | 384 (28.6%) | 785 (31.6%) |
| Unknown | 5 (0.3%) | 26 (1.0%) |
| Adjuvant radiotherapy | ||
| No | 184 (13.7%) | 296 (11.9%) |
| Yes | 1158 (86.1%) | 2175 (87.5%) |
| Unknown | 3 (0.2%) | 14 (0.6%) |
IQR interquartile range.
Of the 2485 patients in the extended group, 675 (27.2%) stopped tamoxifen intake during the follow-up. Figure 2 shows the prevalence of declared use of tamoxifen over time (since randomization) in this population. Treatment adherence decreased at a constant rate over time: overall, 82.6% and 62.9% of patients were still under therapy, respectively, at 5 and 10 years from randomization.
Fig. 2. Tamoxifen adherence in women taking tamoxifen for 10 years (extended group, n = 2485 women).
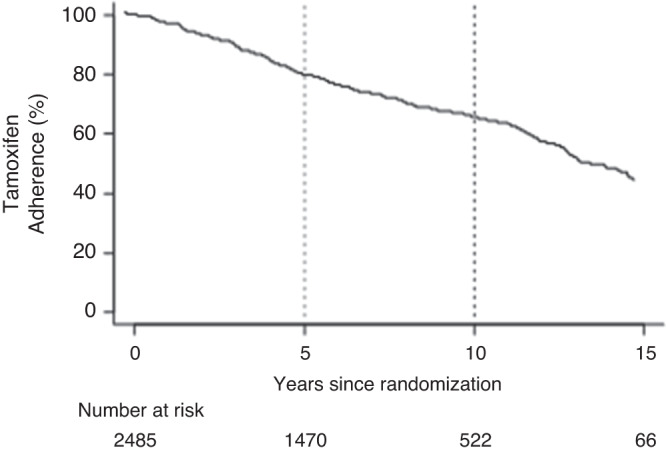
Dotted lines: 5-years and 10-years tamoxifen adherence.
Survival analysis
iDFS outcome
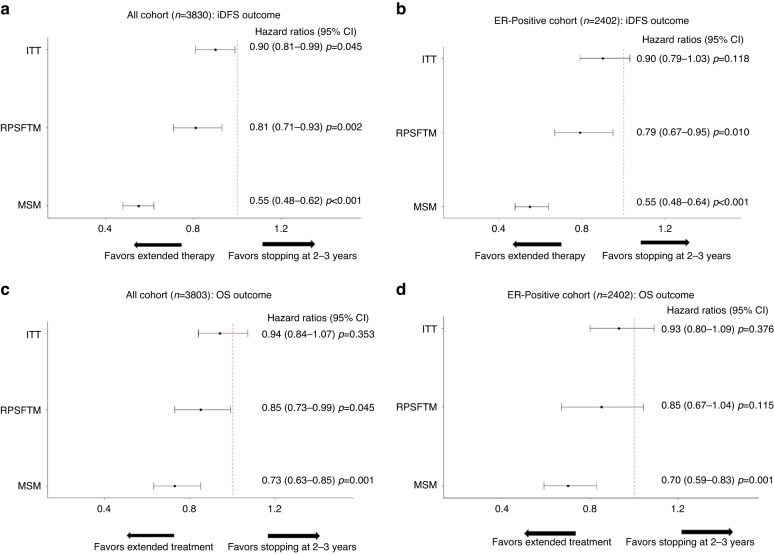
The ITT unadjusted analysis for the whole cohort, and among women with ER positive BC, showed that iDFS was slightly improved for patients assigned to 12 years tamoxifen compared to those assigned to 2–3 years (HR = 0.90 (95% CI: 0.81–0.99), p = 0.045 and HR = 0.90 (0.79–1.03), p = 0.118, respectively).
The results of the adherence-adjusted analyses are presented in Fig. 3A, B. All causal inference methods suggest a stronger benefit of prolonged tamoxifen on iDFS: in ER-positive women, the HRs using MSM and RPSFT modeling were 0.55 (0.48–0.64) and 0.79 (0.67–0.95), respectively (p < 0.001 for MSM and p = 0.010 for RPSFTM).
Fig. 3. Forest plots showing ITT, RPSFTM and MSM estimates of tamoxifen treatment effect on iDFS and OS.
Forest plots of hazards ratios for iDFS in all cohort (A), in estrogen positive breast cancers (B) and for OS in all cohort (C) and in estrogen positive breast cancers (D). Hazard ratios were calculated by comparing the extended tamoxifen group (12 years) and the short-term group (2–3 years) for all methods (intention to treat, marginal structural models and rank preserving structural failure time models). ITT intention-to-treat, iDFS invasive disease-free survival, OS overall survival, MSM marginal structural models, RPSFTM Rank Preserving Structural Failure Time Model; CI confidence interval, p p value.
OS outcome
compared to the original TAM01 trial, and according to the ITT principle, the OS endpoint did not achieve statistical significance. When implementing an unadjusted Cox model, the relative hazards comparing the extended tamoxifen group and the ST group were 0.94 (0.84–1.07) in all cohort and 0.93(0.80–1.09) in ER positive women. The counterfactual statistical methods revealed that the observed survival benefit was likely to be underestimated (Fig. 3C, D): HRs were statistically significant across structural methods: in the whole cohort the estimates of the HRs given by MSM and RPSFTM were 0.73 (0.63–0.85) and 0.85 (0.73–0.99), respectively. In women with ER positive BC, HRs ranged from 0.70 (0.59–0.83) to 0.85 (0.64–1.04) using the MSM and RPSFT models, respectively.
Sensitivity analyses
Several sensitivity analyses were conducted in the whole cohort in order to evaluate the robustness of the counterfactual methods. In the MSM model, estimate accuracy increases as interval length decreases: the HRs which calculate weights using 180 days as the time-interval (compared to 50 days selected for the main analysis) for the extended group compared to the ST group, were 0.57 (0.51–0.65) and 0.77 (0.66–0.89), evaluating iDFS and OS outcomes, respectively. The RPSFTM method produced an iDFS HR of 0.72 (0.59–0.88) and 0.81 (0.71–0.93) and an OS HR of 0.76 (0.58–0.99) and 0.85 (0.73-99) with and without recensoring, respectively (Supplementary Table S2, Fig. S2). The investigation of survival outcomes in ER negative patients (n = 428) confirmed a stronger treatment effect from tamoxifen in the ER positive extended cohort: in terms of OS the analysis performed for ER-negative patients showed no differences between the ST and 12-year tamoxifen groups (HR = 0.74 (0.50–1.11), p = 0.144 and HR = 0.78 (0.51–1.18), p = 0.233, applying the MSM and RPSFTM methods, respectively). Sensitivity analyses results are reported in Supplementary Results (Supplementary Table S2).
Discussion
Tamoxifen is a common and established adjuvant therapy, particularly for premenopausal patients with ER-positive BC [27]. Traditionally, treatment has been given for around 5 years, but many women remain at risk of relapse for 10 years or more.
In this reanalysis of a large randomized trial of women with ER-positive BC, at a median follow-up of more than 8 years, and after adjustment for treatment adherence, the patients in the extended therapy group (12 years) had a significantly improved iDFS and OS than those who received ST therapy (2–3 years) (iDFS HR of 0.55 (0.48–0.64) and of 0.79 (0.67–0.95); OS HR of 0.70 (0.59–0.83) and of 0.85 (0.64–1.04) using MSMs and RPSFTM, respectively). These estimates indicated a significantly larger treatment effect with respect to ITT analysis.
This analysis is still significantly relevant, provided that adjuvant endocrine therapy for 5 years or longer remains a cornerstone in the treatment of patients with HR + BC.
The Adjuvant Tamoxifen, To Offer More? (aTTom [28]) and Adjuvant Tamoxifen: Longer against Shorter (ATLAS [29]) studies are the most recent trials to investigate the question of extended tamoxifen treatment (5 versus 10 years) without any other AIs. Results show a time-dependent reduction in the risk of recurrence and death, with a beneficial effect that was small during treatment uptake and started to increase from year 10 onwards, and emerging in the second decade, after treatment discontinuation. Survival outcomes were investigated in both trials with ITT analysis, although adherence to extended tamoxifen therapy dropped to 75–80% as early as 2–3 years after randomization.
To our knowledge, the treatment benefits for survival have not been estimated in any of the previous published studies investigating extended tamoxifen, adjusting for adherence. Colleoni et al. [30] analyzed the BIG 1–98 breast trial data using the IPCW modeling method, but for the issue of crossover treatment. The authors also implemented IPCW only, and no other causal inference methods.
Some important issues need to be addressed: nowadays, tamoxifen 20 mg/day for 5 years is still considered the standard of care to reduce the risk of recurrence/death in premenopausal women with ER-positive BC who are at low risk of recurrence [31]. In the TAM01 study, only half of patients received the current standard dose of tamoxifen. Patient characteristics and treatment dosing are related to the enrollment period: in the late 1980s and early 1990s, tamoxifen was widely used in treating postmenopausal receptor-negative BC [32] and women could receive tamoxifen dose of 40 mg/day [33]. Similarly, it is common in old trials investigating adjuvant tamoxifen treatment effect, to include women with unknown receptor status [27]. TAM01 was well performed in its historical context and our aim is not to adapt it to current treatment guidelines but apply of statistical methods that properly estimate tamoxifen treatment effect in the presence of non-adherence.
In future research, we plan to complement the current analysis using the contemporary CANTO cohort [34], which includes a fine-tuned data collection of prognostic factors for treatment adherence, management of adverse effects and quality of life. Finally, although adjustment methods such as MSM and RPSFTM are likely to produce less bias than naive per‐protocol adjustments, it is important to assess the validity of underlying assumptions [35]. The most demanding assumption for MSM is the absence of unmeasured confounders in the treatment allocation and switching process. Even if several potential confounders have been considered, it cannot be ensured that all confounders are known or measured, especially as time-dependent covariates were not available. The absence of high level of outliers in the weight estimation, however, works in favor of the absence of unmeasured confounding. The RPSFTM method relies on the untestable assumption that common treatment effect (CTE), that is treatment effect vs non-treatment, is the same whatever the time of treatment switch. This hypothesis is somehow related to the reasons for switching (a patient switching after progression probably received less benefit from the treatment), which are unknown in this study. Sensitivity analyses do not provide strong elements for the invalidity of the CTE hypothesis, however. The variable effect size obtained using MSM and RPSFTM for the iDFS outcome, with a higher estimated treatment effect for MSM, might be related to the different underlying assumptions. The MSM method may be more appropriate in trials with a relatively large sample size, in which non-adherence is observed in a moderate proportion of patients, and sufficient information regarding potential confounding factors is available [36]. The RPSFTM would be preferable for smaller trials with relatively little information on covariates and is also suitable for trials in which massive non-adherence is observed [37]. The published data of several cancer trials [30, 37–39] recently showed that using the adherence-adjusted methods resulted in the experimental treatment having a less biased effect on OS than reported in the ITT analysis. However, causal methods are still rarely used in clinical trials settings [40]: there could be several reasons for this. First, they require data transformations and modeling steps, each including methodological choices, that may have limited their easy use and can be computationally intensive. Second, assumptions underpinning causal methods are untestable. Third, the counterfactual approach is more difficult to explain than the more common ITT approach. Notwithstanding, Latimer and al., using a simulation study [41] concluded that the MSM and RPSFTM methods consistently produced less bias than ITT analysis.
Conclusions
Conventional ITT analysis is a valid and universally accepted analytic approach to test two treatment strategies, but it does not control for potential biases due to treatment discontinuation. Additional analyses should be considered in long-term trials with substantial non-adherence to randomized treatment, so as to assess the effect of non-adherence on the estimate from the ITT analysis. In the context of the TAM01 trial, adjusting for treatment adherence using the MSM and RPSFT methods, reveals that long-term tamoxifen has a greater protective effect on iDFS and OS in ER-positive early BC patients. These structural methods emphasize the benefit of adhering to hormonotherapy in hormone-receptor positive early BC.
Supplementary information
Author contributions
Study design: AB, SM; Acquisition of clinical data: SM; Formal analyses: AB, FG; Methodology: AB, FG, SM; Software: AB, FG; Supervision: SM, BP; Interpretation of results: all authors; Writing- original draft: AB, FG; Writing- review & editing: all authors; Approval of final version: all authors.
Data availability
The data that support the findings of this study can be requested from the corresponding author upon requests that comply with the local data protection regulations.
Code availability
The R code for the analysis used in this article is available upon request.
Competing interests
AB declares consulting fees for ROCHE SAS. SD reports, outside the submitted work, grants and non-financial support from Pfizer, grants from Novartis, grants and non-financial support from AstraZeneca, grants and non-financial support from Roche Genentech, grants from Lilly, grants from Puma, grants from Myriad, grants from Orion, grants from Amgen, grants from Sanofi, grants from Genomic Health, grants from GE, grants from Servier, grants from MSD, grants from BMS, grants from Pierre Fabre, grants from Seagen, grants from Exact Sciences, grants from Rappta, grants from Besins, grants from European Commission grants, grants from French government grants, grants from Fondation ARC grants, grants from Taiho, grants from Elsan, SM. reports, outside the scope of the submitted work, receiving fees for statistical advice to IDDI, Amaris, and Roche, and for data and safety monitoring membership of clinical trials: IQVIA, Sensorion, Biophytis, Servier, Yuhan. BP reports, outside the scope of the submitted work, receiving personal fees from Novartis, AstraZeneca, MSD Oncology and Pfizer; receiving research funding from Daiichi, Puma Biotechnology, Novartis, Merus, Pfizer, and AstraZeneca; and serving as consultant/advisor for Puma Biotechnology, Novartis, Myriad Genetics and Pierre Fabre. IVLI reports, outside the scope of the submitted work, receiving speaker honoraria from Amgen, AstraZeneca, Pfizer/Edimark, Novartis, Sandoz; writing engagement from Pfizer/Edimark, Research funding from Resilience Care. The other co-authors declare no conflict of interest.
Ethics approval and consent to participate
The protocol of TAM01 study was approved by the Caen Committee for the Protection of Persons in Biomedical Research. The need for informed consent was waived due to nature of this study, i.e., a reanalysis of a previous published clinical trial.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-023-02420-w.
References
- 1.Burstein HJ, Temin S, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of Clinical Oncology clinical practice guideline focused update. J Clin Oncol. 2014;32:2255–69. doi: 10.1200/JCO.2013.54.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Del Mastro L, Mansutti M, Bisagni G, Ponzone R, Durando A, Amaducci L, et al. Extended therapy with letrozole as adjuvant treatment of postmenopausal patients with early-stage breast cancer: a multicentre, open-label, randomized, phase 3 trial. Lancet Oncol. 2021;2045:00352–1. doi: 10.1016/S1470-2045(21)00352-1. [DOI] [PubMed] [Google Scholar]
- 3.Moon Z, Moss-Morris R, Hunter MS, Carlisle S, Hughes LD. Barriers and facilitators of adjuvant hormone therapy adherence and persistence in women with breast cancer: a systematic review. Patient Pref Adher. 2017;11:305. doi: 10.2147/PPA.S1266518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huiart L, Ferdynus C, Giorgi R. A meta-regression analysis of the available data on adherence to adjuvant hormonal therapy in breast cancer: summarizing the data for clinicians. Breast Cancer Res Treat. 2013;138:325–8. doi: 10.1007/s10549-013-2422-4. [DOI] [PubMed] [Google Scholar]
- 5.Huiart L, Bouhnik AD, Rey D, Tarpin C, Cluze C, Bendiane MK, et al. Early discontinuation of tamoxifen intake in younger women with breast cancer: is it time to rethink the way it is prescribed? Eur J Cancer. 2012;48:1939–46. doi: 10.1016/j.ejca.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Van Herk-sukel MPP, van Del Poll-franse LV, Voogd AC, Nieuwenhuijzen GAP, Coebergh JWW, Herings RMC. Half of breast cancer patients discontinue tamoxifen and any endocrine treatment before the end of the recommended treatment period of 5 years: a population-based analysis. Breast Cancer Res Treat. 2010;122:843–51. doi: 10.1007/s10549-009-0724-3. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, Malin JL, Diamant AL, Thind A, Maly RC. Adherence to adjuvant hormone therapy in low-income women with breast cancer: the role of provider-patient communication. Breast Cancer Res Treat. 2013;137:829–36. doi: 10.1007/s10549-012-2387-8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pistilli B, Paci A, Ferreira AR, Di Meglio A, Poinsignon V, Bardet A, et al. Serum detection of nonadherence to adjuvant tamoxifen and breast cancer recurrence risk. Clin Oncol. 2020;38:2762–72. doi: 10.1200/JCO.19.01758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hershman DL, Shao T, Kushi LH, Buono D, Tsai WY, Fehrenbacher L, et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat. 2011;126:529–37. doi: 10.1007/s10549-010-1132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chirgwin JH, Giobbie-Hurder A, Coates AS, Price KN, Ejlertsen B, Debled M, et al. 1-98 Trial of Tamoxifen and Letrozole, alone and in sequence. J Clin Oncol. 2016;34:2452–9. doi: 10.1200/JCO.2015.63.8619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheiner LB, Rubin DB. Intention-to-treat analysis and the goals of clinical trials. Clin Pharm Ther. 1995;57:6–15. doi: 10.1016/0009-9236(95)90260-0. [DOI] [PubMed] [Google Scholar]
- 12.Latimer NR, Abrams KR, Lambert PC, Crowther MJ, Wailoo AJ, Morden JP, et al. Adjusting survival time estimates to account for treatment switching in randomized controlled trials-an economic evaluation context: methods, limitations, and recommendations. Med Decis Mak. 2014;34:387–402. doi: 10.1177/0272989X13520192. [DOI] [PubMed] [Google Scholar]
- 13.Latimer N, Abrams K. NICE DSU Technical Support Document 16: Adjusting survival time estimates in the presence of treatment switching, Report by the Decision Support Unit. 2014. London: National Institute for Health and Care Excellence (NICE); Available from https://www.ncbi.nlm.nih.gov/books/NBK310374/pdf/Bookshelf_NBK310374.pdf. [PubMed]
- 14.Robins JM, Finkelstein DM. Correcting for noncompliance and dependent censoring in an AIDS Clinical Trial with inverse probability of censoring weighted (IPCW) log-rank tests. Biometrics. 2000;56:779–88. doi: 10.1111/j.0006-341x.2000.00779.x. [DOI] [PubMed] [Google Scholar]
- 15.Robins JM, Tsiatis AA. Correcting for noncompliance in randomized trials using rank preserving structural failure time models. Commun Stat Theory Methods. 1991;20:2609–31. doi: 10.1080/03610929108830654. [DOI] [Google Scholar]
- 16.Sullivan TR, Latimer NR, Gray J, Sorich MJ, Salter AB, Karnon J. Adjusting for treatment switching in oncology trials: a systematic review and recommendations for reporting. Value Health. 2020;23:388–96. doi: 10.1016/j.jval.2019.10.015. [DOI] [PubMed] [Google Scholar]
- 17.Alshreef A, Latimer N, Tappenden P, Wong R, Hughes D, Fotheringham J, et al. Statistical methods for adjusting estimates of treatment effectiveness for patient nonadherence in the context of time-to-event outcomes and health technology assessment: a systematic review of methodological papers. Med Decis Mak. 2019;39:910–25. doi: 10.1177/0272989X19881654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mostazir M, Taylor RS, Henley W, Watkins E. An overview of statistical methods for handling nonadherence to intervention protocol in randomized control trials: a methodological review. J Clin Epidemiol. 2019;108:121–31. doi: 10.1016/j.jclinepi.2018.12.002.. [DOI] [PubMed] [Google Scholar]
- 19.Delozier T, Spielmann M, Macé-Lesec’h J, Janvier M, Hill C, Asselain B, et al. Tamoxifen adjuvant treatment duration in early breast cancer: initial results of a randomized study comparing short-term treatment with long-term treatment. Fédération Nationale des Centres de Lutte Contre le Cancer Breast Group. J Clin Oncol. 2000;18:3507–12. doi: 10.1200/JCO.2000.18.20.3507.. [DOI] [PubMed] [Google Scholar]
- 20.Gourgou-Bourgade S, Cameron D, Poortmans P, Asselain B, Azria D, Cardoso F, et al. Guidelines for time-to-event end point definitions in breast cancer trials: results of the DATECAN initiative (Definition for the Assessment of Time-to-event Endpoints in CANcer trials. Ann Oncol. 2015;26:2505–6. doi: 10.1093/annonc/mdv478. [DOI] [PubMed] [Google Scholar]
- 21.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343–6. doi: 10.1016/0197-2456(96)00075-x. [DOI] [PubMed] [Google Scholar]
- 22.Latimer NR, Henshall C, Siebert U, Bell H. Treatment switching: statistical and decision-making challenges and approaches. Int J Technol Assess Health Care. 2016;32:160–6. doi: 10.1017/S026646231600026X. [DOI] [PubMed] [Google Scholar]
- 23.Missing data in confirmatory clinical trials | European Medicines Agency. 2010 EMA/CPMP/EWP/1776/99 Rev 1. https://www.ema.europa.eu/en/missing-data-confirmatory-clinical-trials.
- 24.van Buuren S, Groothuis-Oudshoorn K. mice: Multivariate Imputation by Chained Equations in R. J Stat Softw. 2011;45:1–67. doi: 10.18637/jss.v045.i03. [DOI] [Google Scholar]
- 25.Sterne JAC, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Little RJA, Rubin DB. Statistical analysis with missing data. New York, NY: John Wiley & Sons, Inc; 2002.
- 27.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–717. doi: 10.1016/S0140-6736(05)66544-0.. [DOI] [PubMed] [Google Scholar]
- 28.Gray RG, Rea D, Handley K, Bowden SG, Perry P, Hearl HM, et al. aTTom: long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6953 women with early breast cancer. J Clin Oncol. 2013;31:5. doi: 10.1200/jco.2013.31.18_suppl.5. [DOI] [Google Scholar]
- 29.Davies C, Pan H, Godwin J, Gray R, Arriagada R, Raina V, Adjuvant Tamoxifen: Longer Against Shorter (ATLAS) Collaborative Group et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of estrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381:805–16. doi: 10.1016/S0140-6736(12)61963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Colleoni M, Giobbie-Hurder A, Regan MM, Thürlimann B, Mouridsen H, Mauriac L, et al. Analyses adjusting for selective crossover show improved overall survival with adjuvant letrozole compared with tamoxifen in the BIG 1-98 study. J Clin Oncol. 2011;29:1117–24. doi: 10.1200/JCO.2010.31.6455.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Visvanathan K, Fabian CJ, Bantug E, Brewster AM, Davidson NE, DeCensi A, et al. Use of endocrine therapy for breast cancer risk reduction: ASCO clinical practice guideline update. J Clin Oncol. 2019;37:3152–65. doi: 10.1200/JCO.19.01472. [DOI] [PubMed] [Google Scholar]
- 32.International Agency for Research on Cancer Working Group on the evaluation of carcinogenic risks to humans. Volume 66: 1–514 Lyon (FR); 1996. Available from: http://monographs.iarc.fr/ENG/Classification/index.php. [PMC free article] [PubMed]
- 33.Swedish Breast Cancer Cooperative Group. Randomized trial of two versus five years of adjuvant tamoxifen for postmenopausal early stage breast cancer. J Natl Cancer Inst. 1996;88:1543–9. doi: 10.1093/jnci/88.21.1543. [DOI] [PubMed] [Google Scholar]
- 34.Vaz-Luis I, Cottu P, Mesleard C, Martin AL, Dumas A, Dauchy S, et al. French prospective cohort study of treatment-related chronic toxicity in women with localised breast cancer (CANTO) ESMO Open. 2019;4:e000562. doi: 10.1136/esmoopen-2019-000562.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Halabi S Michiels S. Textbook of clinical trials in oncology: a statistical perspective. Chapman & Hall, CRC Press Taylor & Francis Group; 2020.
- 36.Jönsson L, Sandin R, Ekman M, Ramsberg J, Charbonneau C, Huang X, et al. Analyzing overall survival in randomized controlled trials with crossover and implications for economic evaluation. Value Health. 2014;17:707–13. doi: 10.1016/j.jval.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 37.Evans R, Hawkins N, Dequen-O’Byrne P, McCrea C, Muston D, Gresty C, et al. Exploring the impact of treatment switching on overall survival from the PROfound Study in Homologous Recombination Repair (HRR)-Mutated Metastatic Castration-Resistant Prostate Cancer (mCRPC). Target Oncol. 2021;16:613–23. doi: 10.1007/s11523-021-00837-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skaltsa K, Ivanescu C, Naidoo S, Phung D, Holmstrom S, Latimer NR. Adjusting overall survival estimates after treatment switching: a case study in metastatic castration-resistant prostate cancer. Target Oncol. 2017;12:111–21. doi: 10.1007/s11523-016-0472-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Latimer NR, Bell H, Abrams KR, Amonkar MM, Casey M. Adjusting for treatment switching in the METRIC study shows further improved overall survival with trametinib compared with chemotherapy. Cancer Med. 2016;5:806–15. doi: 10.1002/cam4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farmer RE, Kounali D, Walker AS, Savović J, Richards A, May MT, et al. Application of causal inference methods in the analyses of randomised controlled trials: a systematic review. Trials. 2018;19:23. 10.1186/s13063-017-2381-x. [DOI] [PMC free article] [PubMed]
- 41.Latimer NR, Abrams KR, Lambert PC, Morden JP, Crowther MJ. Assessing methods for dealing with treatment switching in clinical trials: a follow-up simulation study. Stat Methods Med Res. 2018;27:765–84. doi: 10.1177/0962280216642264.:. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study can be requested from the corresponding author upon requests that comply with the local data protection regulations.
The R code for the analysis used in this article is available upon request.