Abstract
Non–small cell lung cancer (NSCLC) is associated with a poor survival rate, even for patients with early-stage cancer. Identifying patients with pathological N0 NSCLC who may benefit from adjuvant chemotherapy treatment after surgery is essential. We conducted a retrospective cohort study used data from the Surveillance, Epidemiology, and End Results database and included 26,380 patients with pathological N0 NSCLC after surgery between January 2018, and December 2019. Among 26,380 patients, 24,273 patients received surgery alone and the other 2107 patients received surgery plus adjuvant chemotherapy. After 1:1 propensity score matching, both groups contained 2107 patients. Adjuvant chemotherapy did not show significantly better 24-month survival in T2aN0 NSCLC patients (83.41% vs. 82.91%, p = 0.067), although it did for T2bN0 patients (86.36% vs. 81.70%, p = 0.028). Poorly-differentiated NSCLC remained a high-risk factor for pT2N0, and adjuvant chemotherapy provided better 24-month survival after matching (86.36% vs. 81.70%, p = 0.029). In conclusion, when treating pN0 NSCLC, adjuvant chemotherapy had a beneficial effect when the tumor size was larger than 4 cm. The effect when the tumor size was between 3 and 4 cm was not remarkable. Poorly-differentiated NSCLC was a high-risk factor in the pT2N0 stage.
Subject terms: Cancer, Medical research, Oncology
Introduction
Lung cancer is identified as the leading cause of cancer death in both genders worldwide1. Around 85% of lung cancers are identified as non–small cell lung cancer (NSCLC). The survival rate still remains poor, even for patients with early-stage NSCLC. There is only a 75% 5-year overall survival (OS) rate for patients with a pathological N0 nodal stage2. Although complete surgical resection is vital to improve the survival rate in these patients, identifying patients with pathological N0 NSCLC who may benefit from adjuvant chemotherapy treatment after surgery is also essential3.
The National Comprehensive Cancer Network (NCCN) guidelines suggest that adjuvant chemotherapy should be applied in all patients with pathological N1 and N2 NSCLC4. Using adjuvant chemotherapy should be carefully considered due to its toxicity and immunosuppressive effects. In patients with pathological N0 NSCLC, adjuvant chemotherapy is not recommended when the tumor status is T1a to T1c, but it may have benefits at the T2a (3.1 cm to 4.0 cm) and T2b (4.1 cm to 5.0 cm) statuses when there is a high-risk factor such as a poorly-differentiated tumor, vascular invasion, wedge resection, visceral pleural involvement or unknown lymph node status. However, these recommendations are based on low levels of evidence and little supporting data5. The treatment effects of adjuvant chemotherapy in these high-risk patients are still unclear.
Traditionally, most large randomized trials considered tumor size alone. More recently, the pairings of tumor size with several high-risk factors have been proposed as more accurate indications for adjuvant chemotherapy in early-stage NSCLC patients3. Previous studies on the effectiveness of adjuvant chemotherapy used data based on 6th or 7th edition of the lung cancer staging system from the American Joint Committee on Cancer (AJCC) and converted the data to fit the 8th edition. That data might be too old to reflect current treatment statuses since surgical procedures have rapidly evolved and improved in recent years. Therefore, this study aimed to find out the effects of adjuvant chemotherapy for pathological N0 NSCLC patients with current data that reflects the 8th edition of the AJCC’s lung cancer staging system.
Patients and Methods
Patient population and selection
Population data were obtained from the Surveillance, Epidemiology, and End Results (SEER) database supported by the Surveillance Research Program in the Division of Cancer Control and Population Sciences of the National Cancer Institute in the US. All the details and data were obtained from the SEER public access database using version 8.4.0.1 of the SEER*Stat software. We collected information on NSCLC patients who were diagnosed between 2018 and 2019. Thus, the information was based on the 8th edition of the AJCC’s lung cancer staging system. We included pathological N0 patients who received surgical intervention. Patients were excluded if they were younger than eighteen years old, had a T0/Tis/Tmi status, had an unspecified histology or received systemic therapy before surgery. Patients were also excluded if their database records were missing values for therapy or T status. Our study was approved by our institutional review board (IRB-210808) (Changhua Christian Hospital, Changhua, Taiwan). Informed consent from all participants was waived with the understanding that the released information would be used strictly for research purposes.
The following patient characteristics were collected from the SEER database: age, gender, tumor characteristics (histologic grade, cell type, and T stage), treatment details (chemotherapy and radiotherapy) and surgical method. The histologic subtypes were classified into adenocarcinoma (AD), squamous cell carcinoma (SqCC), and other histologic types. The outcome measure for our study was 24-month OS rate. OS was calculated as the time from date of diagnosis to either death from any cause or the 2020 cutoff.
Statistical analyses
We examined two groups of patients: those that had surgery alone and those that had surgery plus adjuvant chemotherapy. Propensity score matching was used with age, gender, cell type and T status to minimize the bias. Histology grade could not be used in the matching due to there being too few patients with undifferentiated tumors. We used Wilcoxon rank-sum tests for continuous variables and Chi-squared or Fisher’s exact tests for categorical variables. Survival curves were plotted using the Kaplan–Meier method. Univariate and multivariate analyses were performed using a Cox proportional hazards regression model. The following clinical-pathologic factors were included in the analyses: age, gender, histologic grade, cell type, T stage, therapeutic method and surgical method. All calculations were performed using IBM SPSS Statistics for Windows, version 22.0 (IBM Corp., Armonk, NY). Statistical analysis with a p-value less than 0.05 was considered statistically significant.
Ethics approval
This is an observational study. The institutional review board in our institute has confirmed that no ethical approval is required.
Results
Data from 31,545 patients with NSCLC were analyzed. Based on the above exclusion criteria, 5165 patients were excluded. The remaining 26,380 patients were included in this study. There were 24,273 patients who received surgery alone, and the other 2107 patients received surgery plus adjuvant chemotherapy. The detailed baseline characteristics were shown in Table 1. All the variables were significantly different between the two groups.
Table 1.
Baseline characteristics before matching.
| Surgery alone (n = 24,273) | Surgery plus adjuvant chemotherapy (n = 2107) | p-value | |
|---|---|---|---|
| Age in years (mean ± SD) | 71.25 ± 9.80 | 67.17 ± 8.44 | < 0.001 |
| Sex | |||
| Male | 10,885 (44.8%) | 1055 (50.1%) | < 0.001 |
| Female | 13,388 (55.2%) | 1052 (49.9%) | |
| Histologic grade | |||
| Well differentiated | 2592 (10.7%) | 147 (7.0%) | < 0.001 |
| Moderately differentiated | 5252 (21.6%) | 632 (30.0%) | |
| Poorly differentiated | 2497 (10.3%) | 595 (28.2%) | |
| Undifferentiated | 51 (0.2%) | 43 (2.0%) | |
| Unknown | 13,881 (57.2%) | 690 (32.7%) | |
| Cell type | |||
| Adenocarcinoma | 12,592 (51.9%) | 889 (42.2%) | < 0.001 |
| Squamous cell carcinoma | 6540 (26.9%) | 626 (29.7%) | |
| Others | 5141 (21.2%) | 592 (28.1%) | |
| T status | |||
| T1a | 2336 (9.6%) | 68 (3.2%) | < 0.001 |
| T1b | 7561 (31.1%) | 164 (7.8%) | |
| T1c | 4701 (19.4%) | 121 (5.7%) | |
| T2a | 4586 (18.9%) | 454 (21.5%) | |
| T2b | 1200 (4.9%) | 275 (13.1%) | |
| T3 | 2065 (8.5%) | 609 (28.9%) | |
| T4 | 1824 (7.5%) | 416 (19.7%) | |
| Surgical methods | |||
| Wedge resection | 2376 (9.8%) | 208 (9.9%) | < 0.001 |
| Segmentectomy | 900 (3.7%) | 63 (3.0%) | |
| Lobectomy/bilobectomy | 8818 (36.3%) | 1323 (62.8%) | |
| Others or unknown | 12,179 (50.2%) | 513 (24.3%) | |
We used 1:1 propensity score matching to reduce the treatment assignment bias. Both groups contained 2107 patients after matching (Table 2). The mean age was approximately 67 years in each group. In both groups, there were roughly as many males as females. Due to the miniscule number of undifferentiated tumors, we could not match by histologic grade in this study. Patients who received surgery plus adjuvant chemotherapy had higher ratios of moderately-differentiated (n = 632, 30.0% vs. n = 619, 29.4%), poorly-differentiated (n = 595, 28.2% vs. n = 375, 17.8%) and undifferentiated (n = 43, 2.0% vs. n = 11, 0.5%) tumors. There were 919 patients (43.6%) with AD, 631 patients (29.9%) with SqCC and 557 patients (26.4%) with other cell types in the surgery alone group, while there were 889 patients (42.2%) with AD, 626 patients (29.7%) with SqCC and 592 patients (28.1%) with other cell types in the surgery plus adjuvant chemotherapy group. The T statuses were equally distributed in both groups with T2 statuses approximately accounting for 34% of the cases and merely 17% of patients having a T1 status. Most patients received lobectomy/bi-lobectomy in both groups (around 60%). Wedge resection and segmentectomy were in a tiny minority group.
Table 2.
Baseline characteristics after matching.
| Surgery alone (n = 2107) | Surgery plus adjuvant chemotherapy (n = 2107) | p-value | |
|---|---|---|---|
| Age in years (mean ± SD) | 67.64 ± 8.19 | 67.17 ± 8.44 | 0.065 |
| Sex | |||
| Male | 1015 (48.2%) | 1055 (50.1%) | 0.218 |
| Female | 1092 (51.8%) | 1052 (49.9%) | |
| Histologic grade | |||
| Well differentiated | 306 (14.5%) | 147 (7.0%) | < 0.001 |
| Moderately differentiated | 619 (29.4%) | 632 (30.0%) | |
| Poorly differentiated | 375 (17.8%) | 595 (28.2%) | |
| Undifferentiated | 11 (0.5%) | 43 (2.0%) | |
| Unknown | 796 (37.8%) | 690 (32.7%) | |
| Cell type | |||
| Adenocarcinoma | 919 (43.6%) | 889 (42.2%) | 0.453 |
| Squamous cell carcinoma | 631 (29.9%) | 626 (29.7%) | |
| Others | 557 (26.4%) | 592 (28.1%) | |
| T status | |||
| T1a | 68 (3.2%) | 68 (3.2%) | 0.999 |
| T1b | 173 (8.2%) | 164 (7.8%) | |
| T1c | 122 (5.8%) | 121 (5.7%) | |
| T2a | 456 (21.6%) | 454 (21.5%) | |
| T2b | 271 (12.9%) | 275 (13.1%) | |
| T3 | 606 (28.8%) | 609 (28.9%) | |
| T4 | 411 (19.5%) | 416 (19.7%) | |
| Surgical methods | |||
| Wedge resection | 214 (10.2%) | 208 (9.9%) | 0.235 |
| Segmentectomy | 61 (2.9%) | 63 (3.0%) | |
| Lobectomy/bilobectomy | 1264 (60.0%) | 1323 (62.8%) | |
| Others or unknown | 568 (27.0%) | 513 (24.3%) | |
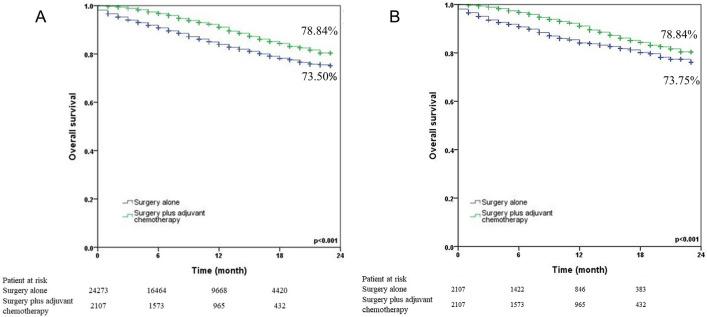
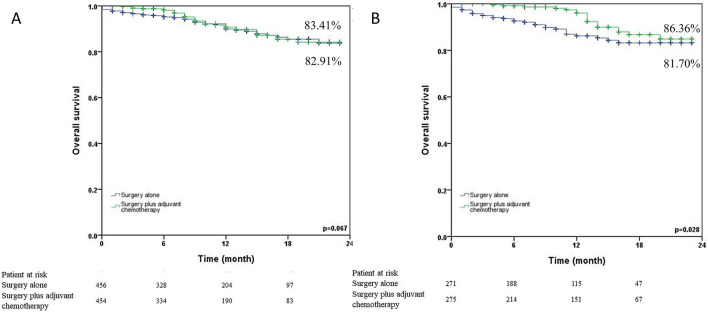
The 24-month survival curves for the surgery alone group and the surgery plus adjuvant chemotherapy group were shown in Fig. 1 (1a: before matching; 1b: after matching). Surgery plus adjuvant chemotherapy showed significantly better 24-month survival than surgery alone in N0 NSCLC patients both before (78.84% vs. 73.50%, p < 0.001) and after matching (78.84% vs. 73.75%, p < 0.001). We also analyzed the 24-month survival curves by T status (Fig. 2). Surgery plus adjuvant chemotherapy did not reveal significantly better 24-month survival than surgery alone in the T1 status (87.14% vs. 91.36%, p = 0.386) and T2 (83.52% vs. 82.75%, p = 0.079). On the other hand, surgery plus adjuvant chemotherapy provided a benefit in the T3 (81.63% vs. 63.65%, p < 0.001) and T4 (60.52% vs. 56.78%, p < 0.001) statuses. Figure 3 illustrated the subgroup analysis of T2a and T2b NSCLC patients after propensity matching. Surgery plus adjuvant chemotherapy did not show significantly better 24-month survival than surgery alone in T2aN0 NSCLC patients (83.41% vs. 82.91%, p = 0.067), but it did show improved survival in T2bN0 patients (86.36% vs. 81.70%, p = 0.028).
Figure 1.
Kaplan–Meier 24-month survival curves by treatment method for N0 NSCLC patients (A) before propensity matching (B) after propensity matching.
Figure 2.
Kaplan–Meier 24-month survival curves by T status and treatment method for N0 NSCLC patients after propensity matching (A) T1N0 (B) T2N0 (C) T3N0 (D) T4N0.
Figure 3.
Kaplan–Meier 24-month survival curves by treatment method for T2N0 NSCLC patients after propensity matching (A) T2aN0 (B) T2bN0.
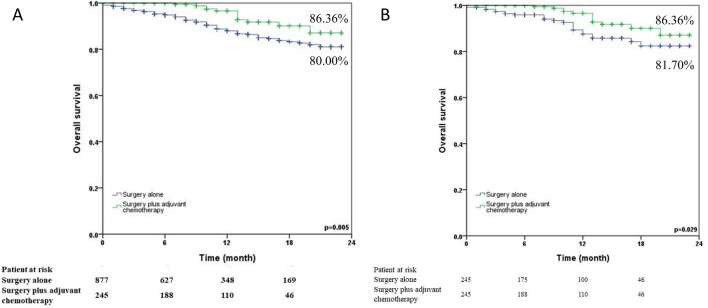
Whether a poorly-differentiated tumor is a high-risk factor in T2N0 NSCLC patients still remains controversial. We did a subgroup analysis of these patients in which 877 patients received surgery alone and 245 patients received surgery plus adjuvant chemotherapy (Fig. 4). Surgery plus adjuvant chemotherapy provided significantly better 24-month survival than surgery alone both before (86.36% vs. 80.00%, p = 0.005) and after matching (86.36% vs. 81.70%, p = 0.029).
Figure 4.
Kaplan–Meier 24-month survival curves by treatment method for poorly-differentiated T2N0 NSCLC patients (A) before propensity matching (B) after propensity matching.
Both univariate and multivariate Cox regression models were analyzed (Table 3). In univariate analysis, young age, female gender, well-differentiated histologic grade, AD cell type, early T stage and received adjuvant chemotherapy were statistically associated with better 24-month survival rates. Similar trends were also found in multivariate analysis.
Table 3.
Univariate and multivariate analyses of overall survival before matching.
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age in years | 1.04 | 1.03 ~ 1.05 | < 0.001 | 1.03 | 1.02 ~ 1.03 | < 0.001 |
| Gender | ||||||
| Male (reference) | 1 | – | 1 | |||
| Female | 0.54 | 0.46 ~ 0.65 | < 0.001 | 0.75 | 0.71 ~ 0.81 | < 0.001 |
| Grade | ||||||
| Well (reference) | 1 | 1 | ||||
| Moderately | 2.91 | 1.49 ~ 5.68 | 0.002 | 1.88 | 1.45 ~ 2.43 | < 0.001 |
| Poorly differentiated | 3.20 | 1.63 ~ 6.28 | 0.001 | 2.54 | 1.95 ~ 3.31 | < 0.001 |
| Undifferentiated | 4.74 | 1.64 ~ 13.70 | 0.004 | 3.38 | 1.79 ~ 6.41 | < 0.001 |
| Unknown | 10.67 | 5.64 ~ 20.18 | < 0.001 | 1.87 | 1.44 ~ 2.43 | < 0.001 |
| Cell type | ||||||
| AD | 1 | 1 | ||||
| SqCC | 2.24 | 1.84 ~ 2.73 | < 0.001 | 1.46 | 1.36 ~ 1.28 | < 0.001 |
| Others | 1.20 | 0.96 ~ 1.50 | 0.112 | 1.55 | 1.41 ~ 1.69 | < 0.001 |
| T status | ||||||
| T1a (reference) | 1 | 1 | ||||
| T1b | 0.59 | 0.28 ~ 1.26 | 0.174 | 1.14 | 0.95 ~ 1.37 | 0.150 |
| T1c | 0.76 | 0.35 ~ 1.64 | 0.481 | 1.38 | 1.15 ~ 1.66 | 0.001 |
| T2a | 1.06 | 0.56 ~ 2.00 | 0.851 | 2.06 | 1.72 ~ 2.46 | < 0.001 |
| T2b | 1.60 | 0.84 ~ 3.05 | 0.150 | 2.95 | 2.43 ~ 3.60 | < 0.001 |
| T3 | 1.87 | 1.01 ~ 3.46 | 0.045 | 3.06 | 2.55 ~ 3.67 | < 0.001 |
| T4 | 3.96 | 2.15 ~ 7.29 | < 0.001 | 4.50 | 3.76 ~ 5.39 | < 0.001 |
| Surgical methods | ||||||
| Wedge resection | 1 | |||||
| Segmentectomy | 0.73 | 0.51 ~ 1.05 | 0.099 | 0.79 | 0.55 ~ 1.14 | 0.204 |
| Lobectomy/bilobectomy | 0.89 | 0.74 ~ 1.07 | 0.203 | 0.82 | 0.68 ~ 0.98 | 0.033 |
| Others or unknown | 4.90 | 4.15 ~ 5.80 | < 0.001 | 3.40 | 2.79 ~ 4.16 | < 0.001 |
| Adjuvant chemotherapy | ||||||
| Yes (reference) | 1 | 1 | ||||
| No | 2.60 | 2.17 ~ 3.11 | < 0.001 | 1.86 | 1.60 ~ 2.16 | < 0.001 |
HR hazard ratio, CI confidence interval, AD adenocarcinoma, SqCC squamous cell carcinoma.
Discussion
This study is a retrospective investigation of the treatment effect of adjuvant chemotherapy in pathological N0 NSCLC patients. We suggest that adjuvant chemotherapy has a benefit for such patients when their T status is T2b or higher. The effect for stage T2aN0 is not remarkable. Poorly-differentiated NSCLC is still a high-risk factor in stage T2N0, and adjuvant chemotherapy is recommended in such cases.
The effect of adjuvant chemotherapy on pathological N0 NSCLC tumors between 3 and 5 cm in size has been controversial. The debate has changed as chemotherapy drugs and surgical methods have evolved. The original randomized control trial dates back to CALGB 96336. A statistically significant survival advantage was noted for 344 patients with tumors larger than 4 cm who had adjuvant paclitaxel/carboplatin. On the contrary, a later JBR-10 trial reported that adjuvant chemotherapy did not provide a survival benefit for pathological N0 NSCLC patients with a tumor size between 3 and 5 cm7. The regimen of vinorelbine/cisplatin could be used in pathological N1 NSCLC. Other large randomized control trials like the ALPI, IALT, BLT and ANITA trials combined stage I to IIIA patients and did not focus on the effect of adjuvant chemotherapy8–12. Based on these trials during the late 2000s, tumor size has become a common single criterion for the use of adjuvant chemotherapy on pathological N0 NSCLC patients13. The recent JCOG 0707 trial demonstrated the benefit of tegafur-uracil (UFT) in N0 NSCLC14. It suggested that for tumors without ground-glass attenuation and size greater than 3 cm, patients with adjuvant UFT therapy had significantly longer survival than those without.
In developing countries, most pathological N0 NSCLC patients with tumors measuring 3 to 5 cm cannot afford adjuvant target therapy or immunotherapy. They are left with the choice of adjuvant chemotherapy or no further treatment after operation. Several recent retrospective studies have reviewed adjuvant chemotherapy outcomes in these patients. Some studies supported that adjuvant chemotherapy should be applied when the tumor size is 3 to 5 cm15–17. Other studies focused on a tumor size of 3 to 4 cm and suggested a positive effect18–20. On the contrary, others claimed that adjuvant chemotherapy did not have a positive effect on disease-free survival or OS in stage IB NSCLC patients, even for patients with multiple risk factors21,22. All of these studies converted data based on the 7th edition of the AJCC’s lung cancer staging system to fit analyses based on the 8th edition, which might introduce some bias and indicates that the data is old. Our study is the first study to analyze the effects of adjuvant chemotherapy on pathological N0 NSCLC patients based solely on the 8th edition of the AJCC’s lung cancer staging system. This data can best reflect the current treatments of these patients. We suggest that adjuvant chemotherapy should be applied when the tumor size is 4 to 5 cm, and it could be applied when the tumor is 3 to 4 cm in size and has a poorly-differentiated histologic grade.
In recent research, tumor size alone was not associated with improved efficacy of adjuvant chemotherapy in patients with early-stage NSCLC3. Improved efficacy occurs when tumor size is considered along with high-risk factors such as poorly-differentiated tumors, vascular invasion, wedge resection, visceral pleural involvement and unknown lymph node status4. A tumor size > 4 cm did not appear on the list of high-risk factors found in NCCN guidelines released in 2023. However, several studies provided evidence against the idea of these high-risk factors. Moon et al. reported that lymphovascular invasion was the only prognostic factor identified in patients with stage IB NSCLC23. Choi et al. suggested that visceral pleural invasion and vascular invasion are more relevant to OS and adjuvant therapy should be given even when the tumor size is less than 3 cm24. A previous meta-analysis reported that visceral pleural invasion and tumor size should be taken into account simultaneously. Patients with tumors larger than 3 cm with visceral pleural invasion might be considered for adjuvant chemotherapy25. We believe lymphovascular invasion and visceral pleural involvement still remain high-risk factors and highly recommended adjuvant chemotherapy for these patients.
As for patients with poorly-differentiated tumors, the concept was proposed two decades ago that they had a high recurrence rate and unfavorable prognosis26. A retrospective study including 179 patients with resected pathological stage I NSCLC supported that adjuvant chemotherapy might be beneficial in these patients27. However, more recent studies did not find the treatment benefit of adjuvant chemotherapy in poorly-differentiated tumors23,24,28. We did a subgroup analysis of pathological N0 NSCLC patients who had poorly-differentiated tumors measuring 3 to 5 cm. We suggest that adjuvant chemotherapy still plays a positive effect in these patients.
Regarding surgical methods, there were seldom trials that compared lobectomy to sublobar resection in pathological N0 NSCLC patients with a tumor size between 3 and 5 cm. Lobectomy still remained the standard treatment in early-stage NSCLC. The recent large randomized control trials JCOG 0802 and CALGB 140583 both supported that wedge resection and segmentectomy do not provide inferior survival compared to lobectomy for pathological N0 NSCLC when the tumor size is less than 2 cm29,30. No study compared sublobar resection to lobectomy when the tumor size was larger than 2 cm. Thus, patients who received sublobar resection in these groups may have contributed to selection bias pertaining to poorer comorbidities, performance status, and pulmonary function and might have less OS initially. A prospective randomized control trial is needed to determine the risk level for sublobar resection when the tumor size is larger than 2 cm.
A strength of our study is that it includes only data from after the 2017 release of the 8th edition of the AJCC’s lung cancer staging system. In addition, we analyzed T2a (tumor size: 3 to 4 cm) and T2b (tumor size: 4 to 5 cm) NSCLC separately since there have been recent disputes concerning tumor sizes > 4 cm. However, there were several limitations of our study. First, the retrospective design may contribute to selection bias, which could affect the results. Second, the study period was limited to data available between 2018 and 2019, so we were limited to 24-month OS. A future study with an observation period allowing for to 5-year or 10-year OS should be performed. Third, the SEER database did not provide detailed data on tumor subtypes and invasion status. We did not evaluate other high-risk factors about these patients, which might have caused a selection bias. The SEER data does not capture specific comorbidities, performance status, or pulmonary function, which could affect the decision to provide adjuvant chemotherapy. The data of chemotherapy regimen could not provide in this study either which might influent the effect of adjuvant chemotherapy. Despite these limitations, our findings contribute to the treatment strategy for these pathological N0 NSCLC patients.
In conclusion, adjuvant chemotherapy had a benefit against pN0 NSCLC when the tumor size was larger than 4 cm. Its effect on tumors between 3 and 4 cm was not remarkable. Poorly-differentiated NSCLC was still a high-risk factor in the pT2N0 stage, and adjuvant chemotherapy provided a benefit in such cases.
Consent to participate
Informed consent from all participants was waived with the understanding that the released information would be used strictly for research purposes.
Author contributions
Y.F.C: Writing- Original draft preparation. Y.L.C: Methodology, Software, Validation. C.C.L: Data curation. C.M.L: Visualization, Investigation. S.S.T: Visualization, Investigation. B.Y.W: Conceptualization, Writing- Reviewing and Editing.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Asamura H, et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the revision of the N descriptors in the forthcoming 8th edition of the TNM classification for lung cancer. J. Thorac. Oncol. 2015;10:1675–1684. doi: 10.1097/JTO.0000000000000678. [DOI] [PubMed] [Google Scholar]
- 3.Pathak R, et al. Association of survival with adjuvant chemotherapy among patients with early-stage non-small cell lung cancer with vs without high-risk clinicopathologic features. JAMA Oncol. 2020;6:1741–1750. doi: 10.1001/jamaoncol.2020.4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.David, S. E. et al. NCCN Clinical Practice Guidelines in Oncology: Non-Small Cell Lung Cancer. Version 2. (NCCN.org., 2023). Accessed March 9, 2023.
- 5.Donington J, et al. American College of Chest Physicians and Society of Thoracic Surgeons consensus statement for evaluation and management for high-risk patients with stage I non-small cell lung cancer. Chest. 2012;142:1620–1635. doi: 10.1378/chest.12-0790. [DOI] [PubMed] [Google Scholar]
- 6.Strauss GM, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J. Clin. Oncol. 2008;26:5043–5051. doi: 10.1200/JCO.2008.16.4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butts CA, et al. Randomized phase III trial of vinorelbine plus cisplatin compared with observation in completely resected stage IB and II non-small-cell lung cancer: Updated survival analysis of JBR-10. J. Clin. Oncol. 2010;28:29–34. doi: 10.1200/JCO.2009.24.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scagliotti GV, et al. Randomized study of adjuvant chemotherapy for completely resected stage I, II, or IIIA non-small-cell lung cancer. J. Natl. Cancer Inst. 2003;95:1453–1461. doi: 10.1093/jnci/djg059. [DOI] [PubMed] [Google Scholar]
- 9.Arriagada R, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N. Engl. J. Med. 2004;350:351–360. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 10.Arriagada R, et al. Long-term results of the international adjuvant lung cancer trial evaluating adjuvant Cisplatin-based chemotherapy in resected lung cancer. J. Clin. Oncol. 2010;28:35–42. doi: 10.1200/JCO.2009.23.2272. [DOI] [PubMed] [Google Scholar]
- 11.Waller D, et al. Chemotherapy for patients with non-small cell lung cancer: The surgical setting of the Big Lung Trial. Eur. J. Cardiothorac. Surg. 2004;26:173–182. doi: 10.1016/j.ejcts.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 12.Douillard JY, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): A randomised controlled trial. Lancet Oncol. 2006;7:719–727. doi: 10.1016/S1470-2045(06)70804-X. [DOI] [PubMed] [Google Scholar]
- 13.Postmus PE, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017;28:iv1–vi21. doi: 10.1093/annonc/mdx222. [DOI] [PubMed] [Google Scholar]
- 14.Shukuya T, et al. Efficacy of adjuvant chemotherapy with tegafur-uracil in patients with completely resected, node-negative NSCLC-real-world data in the era of molecularly targeted agents and immunotherapy. JTO Clin. Res. Rep. 2022;3(5):100320. doi: 10.1016/j.jtocrr.2022.100320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park SY, et al. Efficacy of platinum-based adjuvant chemotherapy in T2aN0 stage IB non-small cell lung cancer. J. Cardiothorac. Surg. 2013;8:151. doi: 10.1186/1749-8090-8-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang T, Guo Q, Zhang Y, Liu Z, Zhou S, Xu S. Meta-analysis of adjuvant chemotherapy versus surgery alone in T2aN0 stage IB non-small cell lung cancer. J. Cancer Res. Ther. 2018;14:139–144. doi: 10.4103/jcrt.JCRT_862_17. [DOI] [PubMed] [Google Scholar]
- 17.Morgensztern D, et al. Adjuvant chemotherapy for patients with T2N0M0 NSCLC. J. Thorac. Oncol. 2016;11:1729–1735. doi: 10.1016/j.jtho.2016.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou K, et al. Adjuvant chemotherapy may improve long-term outcomes in stage IB non-small cell lung cancer patients with previous malignancies: A propensity score-matched analysis. Front. Oncol. 2022;12:938195. doi: 10.3389/fonc.2022.938195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J, Wu N, Lv C, Yan S, Yang Y. Should patients with stage IB non-small cell lung cancer receive adjuvant chemotherapy? A comparison of survival between the 8th and 7th editions of the AJCC TNM staging system for stage IB patients. J. Cancer Res. Clin. Oncol. 2019;145:463–469. doi: 10.1007/s00432-018-2801-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu Y, et al. Effect of adjuvant chemotherapy on survival of patients with 8th edition stage IB non-small cell lung cancer. Front. Oncol. 2021;11:784289. doi: 10.3389/fonc.2021.784289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng S, Li X, Wang Y, Liu J. Effect of adjuvant chemotherapy on DFS for patients with stage I NSCLC. Zhongguo Fei Ai Za Zhi. 2017;20:485–489. doi: 10.3779/j.issn.1009-3419.2017.07.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park HJ, et al. Efficacy of adjuvant chemotherapy for completely resected stage IB non-small cell lung cancer: A retrospective study. J. Thorac. Dis. 2018;10:2279–2287. doi: 10.21037/jtd.2018.03.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moon Y, Choi SY, Park JK, Lee KY. Prognostic factors in stage IB non-small cell lung cancer according to the 8(th) edition of the TNM staging system after curative resection. J. Thorac. Dis. 2019;11:5352–5361. doi: 10.21037/jtd.2019.11.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi J, et al. Clinical efficacy of adjuvant chemotherapy in stage IB (< 4 cm) non-small cell lung cancer patients with high-risk factors. Korean J. Intern Med. 2022;37:127–136. doi: 10.3904/kjim.2020.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang L, et al. The impact of visceral pleural invasion in node-negative non-small cell lung cancer: A systematic review and meta-analysis. Chest. 2015;148:903–911. doi: 10.1378/chest.14-2765. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi N, et al. Risk factors for recurrence and unfavorable prognosis in patients with stage I non-small cell lung cancer and a tumor diameter of 20 mm or less. J. Thorac. Oncol. 2007;2:808–812. doi: 10.1097/JTO.0b013e31814617c7. [DOI] [PubMed] [Google Scholar]
- 27.Liu CH, et al. Heterogeneous prognosis and adjuvant chemotherapy in pathological stage I non-small cell lung cancer patients. Thorac. Cancer. 2015;6:620–628. doi: 10.1111/1759-7714.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hou X, et al. Who are the real high-risk patients with pathological T2N0M0 non-small-cell lung cancer that can benefit from adjuvant chemotherapy? ESMO Open. 2022;7:100508. doi: 10.1016/j.esmoop.2022.100508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saji H, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): A multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet. 2022;399:1607–1617. doi: 10.1016/S0140-6736(21)02333-3. [DOI] [PubMed] [Google Scholar]
- 30.Altorki N, et al. Lobar or sublobar resection for peripheral stage IA non-small-cell lung cancer. N. Engl. J. Med. 2023;388:489–498. doi: 10.1056/NEJMoa2212083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.