Summary
Background
It remains unknown how ambient temperature impact pneumonia of various infectious causes.
Methods
Based on the national death registry covering all counties in Chinese mainland, we conducted an individual-level case-crossover study in China from 2013 to 2019. Exposures were assigned at residential addresses for each decedent. Conditional logistic regression model combined with distributed lag non-linear models were used to estimate the exposure-response associations. The attributable fractions due to non-optimum temperature were calculated after accounting for spatial and temporal patterns for the excess risks.
Findings
The exposure-response curves were inversely J-shaped with both low and high temperature increasing the risks, and the effect of low temperature was stronger. Extremely low temperature was associated with higher magnitude of influenza-related pneumonia [relative risk (RR): 2.46, 95% confidence interval (CI): 1.62–3.74], than viral pneumonia (RR: 1.89, 95% CI: 1.55–2.30) and bacterial pneumonia (RR: 1.81, 95% CI: 1.56–2.09). The magnitudes of RRs associated with extremely high temperature were similar among the three categories of pneumonia. The mortality attributable fraction for influenza-related pneumonia (29.78%) was the highest. The effects were stronger in people of low education level or residence in the north.
Interpretation
This nationwide study presents findings on the varied risk and burden of pneumonia mortality of various infectious causes, and highlights the susceptibility of influenza-related pneumonia to ambient low temperature.
Funding
This study is supported by the National Key Research and Development Program (2022YFC3702701), the Shanghai Municipal Science and Technology Commission (21TQ015) and Shanghai International Science and Technology Partnership Project (21230780200).
Keywords: Ambient temperature, Pneumonia, Case-crossover study, Attributable fraction, Infectious diseases
Research in context.
Evidence before this study
We searched PubMed and Web of Science up to June 8, 2023, using search terms “pneumonia AND (ambient temperature OR meteorological factors OR weather factors OR air temperature)” without language restrictions, to identify papers on the relationships between non-optimum ambient temperature and pneumonia of various infectious causes. The majority of prior discoveries associating ambient temperature with pneumonia were derived from ecological time-series investigations that depended on aggregated daily information of pneumonia hospitalizations or fatalities, posing challenges in accounting for confounding factors at the individual level. Besides, all previous studies used temperature data or their averages measured by one or a few fixed-site weather stations, resulting in somewhat exposure errors at a spatial scale. Furthermore, all prior research has evaluated the impacts of ambient temperature on overall or a specific etiological type of pneumonia. No investigations have attempted to differentiate the impacts on pneumonia with varied infectious causes, thereby presenting a challenge in the prevention and treatment of heterogeneous pneumonia under climate change.
Added value of this study
In this nationally-representative case-crossover study of 236,987 pneumonia deaths of specific infectious causes from all 31 province-level and 2844 county-level administrative units across the Chinese mainland, we provided the evidence that non-optimum ambient temperature could increase the risk of pneumonia mortality of various infectious causes and contribute a considerable burden. The impact of extremely low temperature on mortality of influenza-related pneumonia is higher than that of viral pneumonia and bacterial pneumonia. The effects were stronger in people of low education level or residence in the north.
Implications of all the available evidence
As pneumonia is a life-threatening respiratory disease, our results add to the growing body of evidence on modifiable environmental risk factors for pneumonia, and provide some valuable insights into the prevention and management of pneumonia with different infections, which will benefit public health policy formulation and prevention for susceptible subgroups.
Introduction
Despite the presence of reliable antibiotics and vaccines for prevention and treatment, pneumonia remains the single biggest infectious killer of children and the fourth leading cause of deaths globally.1,2 Moreover, pneumonia is also among the costliest ailments to treat when hospitalization is necessary, amounting to an approximate total expenditure of $6.4 billion in US hospital settings in 2017.3 Characterized as an acute infection affecting the lung parenchyma, pneumonia can be caused by an array of microorganisms such as viruses, bacteria and fungi.4 The epidemic season and clinical treatment of pneumonia could also differ by infectious causes.5 Pneumonia of bacterial infections has received the greatest concern for a long time, as bacteria contributed the largest proportion of adult pneumonia.6 Since the coronavirus (COVID-19) pandemic, pneumonia of respiratory viruses has received increasing attention as it may cause severe pneumonia with higher morbidity and poorer prognosis.7 Preventing pneumonia is of utmost importance, thus it is crucial to investigate the factors that could trigger the occurrence or exacerbation of pneumonia and take measures to shield the population from these detrimental elements.
The Global Burden of Disease (GBD) study in 2019 identified non-optimum ambient temperature (i.e., low temperature and high temperature) as a novel risk factor for premature deaths, further emphasizing climate change as the foremost global menace to human health.8 It has been well documented that non-optimum ambient temperature could elevate the likelihood of hospitalization and death for a range of respiratory diseases including pneumonia.9, 10, 11 Multiple research studies have shown a heightened susceptibility to pneumonia linked to both low and high temperature.12,13 GBD 2019 further estimated that 12.78% of the deaths from lower respiratory infections in China were attributable to non-optimum temperature.8 Nevertheless, the majority of prior discoveries associating ambient temperature with pneumonia were derived from ecological time-series investigations that depended on aggregated daily information of pneumonia hospitalizations or fatalities, posing challenges in accounting for confounding factors at the individual level. Besides, all previous studies used temperature data or their averages measured by one or a few fixed-site weather stations, resulting in somewhat exposure errors at a spatial scale. Furthermore, all prior research has evaluated the impacts of ambient temperature on overall or a specific etiological type of pneumonia. No investigations have attempted to differentiate the impacts on pneumonia with varied infectious causes, thereby presenting a challenge in the prevention and treatment of heterogeneous pneumonia under climate change.
By virtue of the most representative death registry of China, we therefore conducted an individual-level, time-stratified, case-crossover study to quantitatively assess the association between short-term exposure to non-optimum temperature and pneumonia mortality of various infectious causes.
Methods
Study population and health data
Individual death records were collected from the China Cause of deaths Reporting System (CDRS), a well-established system designed by the central government to collect information on all deaths occurring in Chinese Mainland. This national registry collected information on causes of deaths and basic demographic variables for all the deceased from all 31 province-level and 2844 county-level administrative units across the Chinese mainland. The data collection process adhered to stringent protocols, standard procedures, and meticulous quality control measures. Regular training and supervision were carried out at all administrative levels, from township to national, throughout the year to assure the quality of each death certificate reported. Experienced staff in each district- or county-level Center of Disease Control and Prevention assigned and coded the underlying cause of death using the International Classification of Diseases (ICD) coding system. As a routine quality control measure, a random sample of death certificates was selected monthly by the higher-level CDC to verify the underlying cause of death. The data from this system are widely used by the central government to produce official mortality statistics for informing health policy, as well as being a reliable data source for scientific research. Detailed descriptions of the registry were published elsewhere.14
In this particular analysis, we collected anonymous individual records of all pneumonia deaths from the CDRS between January 1, 2013 and December 31, 2019 of various specific infectious causes, including influenza-related pneumonia, viral pneumonia, and bacterial pneumonia. The diagnosis of pneumonia deaths was determined according to the underlying cause using the codes of the International Classification of Diseases, 10th revision (ICD-10). We assessed pneumonia of three kinds of infectious causes, including influenza-related pneumonia (J10.0 and J11.0), viral pneumonia (J12), and bacterial pneumonia (J13–J15). The date of death, geocoded residential addresses and demographic information (i.e., age, sex and education level) were also obtained. In China, the sex information is linked to the ID number. As ID numbers were mandated for the death registry, the data on sex was automatically collected. Due to the significantly lower number of cases, we did not take into account pneumonia caused by other specific infectious agents, such as mycoplasma pneumoniae, chlamydia pneumoniae, fungi, and parasites. We also did not evaluate pneumonia deaths without specific pathogens identified. This study followed the Reporting guidelines for Enhanced Observational Epidemiological Studies (STROBE).
Exposure assessment
Meteorological data, including temperature and relative humidity, were obtained from the European Centre for Medium-Range Weather Forecasts Reanalysis Fifth Generation (ERA5) reanalysis product with 0.1° × 0.1° spatial and hourly temporal resolutions.15 Hourly meteorological data were extracted from the nearest grid cell in the ERA5 dataset based on the residential address of the decedent. For each grid cell, the daily mean meteorological data was determined by calculating the average of all 24-h estimates. In addition, considering that air pollution could complicate the connections between temperature and pneumonia, we gathered the daily average concentrations of five criteria pollutants from the National Urban Air Quality Real-time Publishing Platform (http://106.37.208.233:20035/), including fine particulate matter (PM2.5), ozone (O3, maximum 8-h average), nitrogen dioxide (NO2), carbon monoxide (CO), sulfur dioxide (SO2). The platform is administered by China's Ministry of Ecology and Environment and displays real-time concentrations of criteria air pollutants in all state-controlled monitoring sites.16
Study design
We adopted a time-stratified case-crossover design to investigate the connections between pneumonia deaths caused by different infections and ambient temperature. This design allows each case to serve as his/her own control. Therefore, factors that are time-invariant or are stable (e.g., demographic, socioeconomic, and behavioral risk factors) within a short period of time (such as a month) are automatically controlled.17,18 For this analysis, the case period was defined as the date of death for each pneumonia fatality. The corresponding control periods were chosen as other days on the same day of week in the same month as the case period to control for potential confounding effect by day of week and seasonal patterns.19,20 For example, if a pneumonia death occurred on Monday, September 16, 2013, we would define this day as the case period and all other Mondays in September 2013 (i.e., September 2, 9, 23, and 30) as the control periods. We matched each case period to 3 or 4 control periods, depending on the number of the same days of week within this month.
Statistical analyses
Associations of temperature and pneumonia mortality
To estimate the links between pneumonia mortality caused by different infections and ambient temperature, we utilized a combination of conditional logistic regression models and the distributed lag nonlinear model (DLNM).21,22 Specifically, we firstly presented the temperature's cross-basis function constructed by the DLNM, which consisted of a natural cubic spline with three internal knots positioned at the 10th, 25th and 90th percentiles of temperature distributions; the cross-basis function also incorporated a lag-response space using a natural cubic spline with an intercept and three internal knots placed at equally spaced values in the logarithmic scale of lags; and we empirically selected a maximum of up to 21 lag days (i.e., three weeks duration) according to previous studies.23 Secondly, we incorporated the temperature's cross-basis function into the conditional logistic regression model to assess the associations between ambient temperature and pneumonia fatalities, adjusting for a binary indicator of public holidays and a natural cubic spline with 3 degrees of freedom for relative humidity.
We firstly plotted exposure-response relationship curves after truncating the temperature distribution from the 1st percentile to the 99th percentile. The main purpose of the truncation was to decrease the statistical uncertainty linked to the extremely limited sample size at the extremes of exposure. We then determined the reference temperature that corresponded to the lowest mortality relative risk (RR). We thereby plotted the lag patterns for the RRs at temperature extremes (i.e., the 1st or 99th percentile of the temperature distribution) compared with the reference temperature, and further calculated the cumulative risks and their 95% confidence intervals (CIs) from lag 0 day to a specific lag day that corresponds to a RR close to 1 in main analyses.
Based on the above main models, we conducted several subgroup analyses to explore the potential modification by region (north, south), sex (male, female), education level (low: junior high school and below, high: high school and above) and age (<75 years, ≥75 years). Statistical differences between stratum-specific estimates were tested using 2-sample z tests with the following formula:
| (1) |
where β1 and β2 were the stratum-specific regression coefficients (log-transformed RR), and SE12 and SE22 were the corresponding standard errors.18
Furthermore, during sensitivity analyses, we included all criteria air pollutants in the primary model to evaluate the reliability of the primary estimates after accounting for air pollution control.
Pneumonia mortality fractions attributable to non-optimum temperature
In our main analyses, we utilized a backward approach to calculate the attributable fraction (AF) of pneumonia fatalities associated with non-optimum ambient temperature along the lag days used.24 This approach could account for the additional temporal dimension in exposure-response associations, and thus provide more appropriate measures for AF with complex temporal patterns, which has been widely used in previous studies within the framework of DLNM.25,26 The AFx,t at time t was calculated using the following formula:
| (2) |
where AFx,t indicate the related fractions at time t that can be attributed to previous exposures to x in the period , …, , relative to the referent temperature, with the variables l0 and L corresponding to the lowest and highest time lags, respectively. Additionally, empirical confidence intervals (eCIs) were calculated through Monte Carlo simulations, assuming a multivariate normal distribution of the point estimate and (co)variance matrix obtained from the regression model.24 By simulating 5000 random samples, the 95% eCIs can be interpreted as the percentiles related to the 2.5th and 97.5th of such distributions.24,25
Analyses were performed using the “dlnm” and “survival” packages of the R software (version 4.1.2). The AF was calculated by the function “attrdl” provided by Gasparrini and colleagues.24 All tests were two-sided and a p-value <0.05 was considered statistically significant.
Role of the funding source
The funders of this study had no roles in the study design, data collection, analysis, result interpretation, and in drafting the manuscript.
Results
Descriptive statistics
A total of 236,987 pneumonia deaths from all areas of Chinese mainland were included in this analysis. Table 1 shows the demographic characteristics of pneumonia deaths of different infectious causes during the study period. Among the included pneumonia deaths, 68.5% were aged over 75, 57.1% were male, 57.9% were from the South region and 83.3% have a junior middle-school education or below. Table 2 presents summary statistics on weather conditions and ambient air pollution. The average daily mean temperature was 13.7 °C, ranging from −19.8 to 31.2 °C.
Table 1.
Numbers of infectious pneumonia deaths classified by age, sex, geographic zones and education level.
| Influenza-related pneumonia | Viral pneumonia | Bacterial pneumonia | All three typesc | Proportion (%)d | |
|---|---|---|---|---|---|
| Total | 15,480 | 83,582 | 137,925 | 236,987 | 100.0 |
| Age | |||||
| <75 | 4426 | 29,382 | 40,869 | 74,677 | 31.5 |
| ≥75 | 11,054 | 54,200 | 97,056 | 162,310 | 68.5 |
| Sex | |||||
| Male | 7940 | 46,688 | 80,690 | 135,318 | 57.1 |
| Female | 7540 | 36,894 | 57,235 | 101,669 | 42.9 |
| Region | |||||
| South | 8938 | 49,324 | 78,825 | 137,087 | 57.9 |
| North | 6542 | 34,258 | 59,100 | 99,900 | 42.2 |
| Education | |||||
| Lowa | 14,044 | 72,362 | 110,942 | 197,348 | 83.3 |
| Highb | 1436 | 11,220 | 26,983 | 39,639 | 16.7 |
Low: Junior high school and below.
High: High school and above.
All three types: The sum of three types of pneumonia.
Proportion (%): The proportion of each stratified person to the sum of three types of pneumonia.
Table 2.
Descriptive statistics on annual-average weather conditions and ambient air pollution in all counties (N = 2844) of Chinese Mainland during the study period (2013–2019).
| Mean ± SD | Minimum | Percentile |
Maximum | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| P1 | P2.5 | P25 | P50 | P75 | P97.5 | P99 | ||||
| Weather condition | ||||||||||
| Temperature (°C) | 13.7 ± 10.2 | −19.8 | −14.1 | −9.2 | 6.6 | 14.8 | 22.1 | 28.7 | 29.6 | 31.2 |
| Relative humidity (%) | 66.7 ± 16.6 | 19.3 | 25.2 | 29.8 | 55.6 | 70.1 | 79.9 | 90.3 | 92.3 | 95.2 |
| Air pollutants (μg/m3) | ||||||||||
| PM2.5 | 51.1 ± 40.4 | 4.8 | 7.3 | 9.4 | 24.1 | 39.3 | 64.4 | 164.0 | 207.0 | 323.1 |
| O3a | 80.7 ± 44.7 | 3.7 | 7.9 | 12.4 | 47.6 | 74.1 | 107.0 | 186.0 | 207.0 | 244.2 |
| NO2 | 33.5 ± 19.9 | 2.5 | 4.8 | 6.7 | 18.4 | 29.3 | 44.5 | 82.5 | 94.3 | 120.0 |
| CO | 1.0 ± 0.6 | 0.1 | 0.2 | 0.3 | 0.7 | 0.9 | 1.2 | 2.6 | 3.2 | 4.5 |
| SO2 | 21.0 ± 22.9 | 1.5 | 2.1 | 2.8 | 7.9 | 13.6 | 24.8 | 89.3 | 126.0 | 206.6 |
Abbreviations: SD, standard deviation; PM2.5, fine particulate matter, particles with aerodynamic diameters less than or equal to 2.5 μm; O3, ozone; NO2, nitrogen dioxide; CO, carbon monoxide; SO2, sulfur dioxide.
O3: Maximum daily 8-h.
The association between temperature and pneumonia mortality
Fig. 1 demonstrates the exposure-response curves for the relationships between ambient temperature and pneumonia mortality. The curves were inversely J-shaped with both low and high temperature increasing the risks. The effects of extreme low temperature were much stronger than those of extreme high temperature. There appears to be a higher cold effect on influenza-related pneumonia than the other two types. The minimum-mortality temperature was 23.3 °C for influenza-related pneumonia, 22.3 °C for viral pneumonia, and 21.9 °C for bacterial pneumonia.
Fig. 1.
Cumulative exposure-response curves for the associations of temperature with the mortality of influenza-related pneumonia (a), viral pneumonia (b) and bacterial pneumonia (c). Note: The associations were presented as relative risks and 95% confidence intervals of mortality comparing a given temperature to the reference temperatures. The lag period for calculating the cumulative risks was 0–14 days for (a), 0–14 days for (b), and 0–21 days for (c). The blue solid lines are mean risk estimates and the blue areas are their 95% confidence intervals. The pink vertical lines denote the referent temperatures (23.3 °C, 22.3 °C, 21.9 °C, respectively) that showed the lowest mortality risk. Abbreviations: P1, the 1st percentile of temperature; P99, the 99th percentile of temperature.
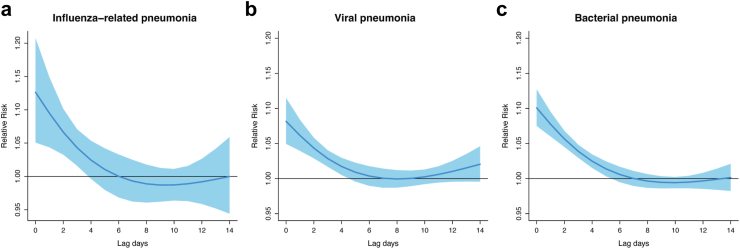
As illustrated in Fig. 2, the risks of influenza-related pneumonia and viral pneumonia associated with extreme low temperature were present at lag 2 d, persisted till lag 13 d, and thereafter became insignificant. The risks of bacterial pneumonia occurred later (at lag 7 d) and disappeared at lag 18 d. Fig. 3 depicts the lag patterns for the RRs associated with extreme high temperature, which are consistent for the three kinds of pneumonia. Specifically, the RR was strongest on the present day (i.e., the day of exposure, lag 0 d), attenuated drastically until lag 5 d, and became non-significant thereafter. Accordingly, in subsequent analyses we used lags 0–14 d to calculate cumulative RRs related to low temperature for influenza-related pneumonia and viral pneumonia, using lags 0–21 d for bacterial pneumonia; and lags 0–5 d for all analyses of extreme high temperature.
Fig. 2.
Lag-response curve for the associations of extremely low temperature with the mortality of influenza-related pneumonia (a), viral pneumonia (b) and bacterial pneumonia (c). Note: The associations were presented as relative risks and 95% confidence intervals of mortality at extremely low (P1, −12.9 °C, −16.7 °C, −16.9 °C, respectively) compared to the corresponding referent temperatures (23.3 °C, 22.3 °C, 21.9 °C, respectively) on different lag days. The blue solid lines are mean risk estimates and the blue areas are their 95% confidence intervals. Abbreviations: P1, the 1st percentile of temperature.
Fig. 3.
Lag-response curve for the associations of extremely high temperature with the mortality of influenza-related pneumonia (a), viral pneumonia (b) and bacterial pneumonia (c). Note: The associations were presented as relative risks and 95% confidence intervals of mortality at extremely high (P99, 30.7 °C, 31.0 °C, 30.9 °C, respectively) compared to the corresponding referent temperatures (23.3 °C, 22.3 °C, 21.9 °C, respectively) on different lag days. The blue solid lines are mean risk estimates and the blue areas are their 95% confidence intervals. Abbreviations: P99, the 99th percentile of temperature.
Table 3 presents the cumulative RRs of pneumonia mortality associated with non-optimum temperature with and without the adjustment of air pollutants. In the main analyses, extremely low temperature was associated with higher magnitude of influenza-related pneumonia (RR: 2.46, 95% CI: 1.62–3.74), than viral pneumonia (RR: 1.89, 95% CI: 1.55–2.30) and bacterial pneumonia (RR: 1.81, 95% CI: 1.56–2.09). The RRs comparing extreme high temperature to the reference temperatures were similar among the three categories of pneumonia, which were 1.42 (95% CI: 1.19–1.69) for influenza-related pneumonia, 1.26 (95% CI: 1.17–1.37) for viral pneumonia and 1.35 (95% CI: 1.27–1.44) for bacterial pneumonia. In our sensitivity analyses, all associations remained stable after additionally controlling for all criteria air pollutants in the primary model.
Table 3.
Relative risks (95% CIs) of pneumonia mortality from different infectious causes due to non-optimum temperature, with and without the adjustment of air pollutants.
| Extremely low temperaturea |
Extremely high temperatureb |
|||
|---|---|---|---|---|
| Without adjustment | With adjustment | Without adjustment | With adjustment | |
| Influenza-related pneumonia | 2.46 (1.62, 3.74) | 2.23 (1.37, 3.64) | 1.42 (1.19, 1.69) | 1.49 (1.19, 1.86) |
| Viral pneumonia | 1.89 (1.55, 2.30) | 2.09 (1.64, 2.66) | 1.26 (1.17, 1.37) | 1.27 (1.16, 1.39) |
| Bacterial pneumonia | 1.81 (1.56, 2.09) | 1.79 (1.51, 2.11) | 1.35 (1.27, 1.44) | 1.27 (1.19, 1.36) |
Abbreviations: CIs, confidence intervals.
Extremely low temperature: the 1st percentile of temperature distribution (−12.9 °C, −16.7 °C, −16.9 °C), and the referent temperature is the minimum risk temperature (23.3 °C, 22.3 °C, 21.9 °C).
Extremely high temperature: the 99th percentile of temperature distribution (30.7 °C, 31.0 °C, 30.9 °C), and the referent temperature is the minimum risk temperature (23.3 °C, 22.3 °C, 21.9 °C).
Table 4 shows estimates of the attributable fraction (AF) for infectious pneumonia mortality attributed to non-optimum temperature, by temperature category. There were higher AF for influenza-related pneumonia (29.78%) than the others (19.00% for viral pneumonia and 15.64% for bacterial pneumonia). Of all deaths for influenza-related pneumonia, 29.78% (95% eCI, 21.32%–36.39%) could be attributable to non-optimum temperature, of which 28.47% (95% eCI, 19.73%–35.50%) was related to all low temperatures (i.e., temperatures below the minimum-mortality temperature) and 1.31% (95% eCI, −0.22% to 2.65%) to all high temperatures (i.e., temperatures beyond the minimum-mortality temperature). Notably, the attributable fraction of influenza-related pneumonia to all high temperatures was quite smaller and statistically insignificant, compared with that of viral pneumonia and bacterial pneumonia.
Table 4.
Attributable fractions (%, means and 95% eCIs) of pneumonia mortality from different infectious causes due to non-optimum temperature.
| Influenza-related pneumonia | Viral pneumonia | Bacterial pneumonia | |
|---|---|---|---|
| Overall | 29.78 (21.32, 36.39) | 19.00 (15.65, 22.10) | 15.64 (13.10, 18.04) |
| Heata | 1.31 (−0.22, 2.65) | 1.79 (0.96, 2.60) | 1.96 (1.25, 2.65) |
| Coldb | 28.47 (19.73, 35.50) | 17.21 (13.80, 20.35) | 13.69 (11.16, 16.13) |
Abbreviations: eCIs, empirical confidence intervals.
Heat: Components attributable to heat were computed with temperature higher than the referent temperature (23.3 °C, 22.3 °C, 21.9 °C).
Cold: Components attributable to cold were computed with temperature lower than the referent temperature (23.3 °C, 22.3 °C, 21.9 °C).
Table 5 summarizes the results of stratified analyses by age, sex, region and education level. There are generally similar RRs between age subgroups for both low temperature and high temperature. The risk estimates among males and females were comparable for both low temperature and high temperature. Larger RRs for extreme high temperature were found in the north than in the south, especially for bacterial pneumonia (p-value for difference <0.01), whereas the between-region differences for extreme low temperature were not significant. We generally found stronger associations of low temperature among patients with junior middle-school level or below with significant between-group difference for viral pneumonia (p-value for difference = 0.02), while the associations among patients with high school level or above were much weaker or insignificant.
Table 5.
Relative risks (95% CIs) of pneumonia mortality from different infectious causes due to non-optimum temperature, classified by age, sex, geographic zones and education level.
| Influenza-related pneumonia | p-value | Viral pneumonia | p-value | Bacterial pneumonia | p-value | |
|---|---|---|---|---|---|---|
| Extremely low temperaturea | ||||||
| Age | ||||||
| <75 | 2.98 (1.29, 6.91) | 0.62 | 1.69 (1.21, 2.35) | 0.44 | 1.78 (1.36, 2.33) | 0.95 |
| ≥75 | 2.31 (1.43, 3.72) | 1.99 (1.57, 2.51) | 1.80 (1.52, 2.12) | |||
| Sex | ||||||
| Male | 2.49 (1.41, 4.42) | 0.94 | 2.06 (1.58, 2.68) | 0.29 | 1.80 (1.49, 2.18) | 0.97 |
| Female | 2.40 (1.30, 4.44) | 1.67 (1.26, 2.22) | 1.81 (1.44, 2.28) | |||
| Region | ||||||
| South | 1.77 (1.10, 2.84) | 0.31 | 1.73 (1.44, 2.06) | 0.67 | 1.69 (1.48, 1.93) | 1.00 |
| North | 2.68 (1.43, 5.02) | 1.86 (1.43, 2.41) | 1.69 (1.40, 2.03) | |||
| Education | ||||||
| Juniorc | 2.31 (1.52, 3.52) | 0.40 | 2.05 (1.66, 2.54)e | 0.02 | 1.82 (1.55, 2.13) | 0.77 |
| Highd | 4.77 (0.96, 23.72) | 1.18 (0.77, 1.81)e | 1.72 (1.25, 2.37) | |||
| Extremely high temperatureb | ||||||
| Age | ||||||
| <75 | 1.23 (0.97, 1.56) | 0.30 | 1.16 (1.02, 1.32) | 0.11 | 1.25 (1.11, 1.41) | 0.13 |
| ≥75 | 1.46 (1.18, 1.81) | 1.32 (1.20, 1.45) | 1.39 (1.30, 1.49) | |||
| Sex | ||||||
| Male | 1.55 (1.21, 1.99) | 0.33 | 1.24 (1.12, 1.37) | 0.51 | 1.30 (1.20, 1.41) | 0.12 |
| Female | 1.30 (1.01, 1.67) | 1.31 (1.16, 1.47) | 1.43 (1.31, 1.56) | |||
| Region | ||||||
| South | 1.23 (1.00, 1.50) | 0.11 | 1.22 (1.11, 1.34) | 0.11 | 1.24 (1.16, 1.34)e | 0.0001 |
| North | 1.66 (1.23, 2.22) | 1.39 (1.22, 1.58) | 1.60 (1.45, 1.78)e | |||
| Education | ||||||
| Juniorc | 1.49 (1.23, 1.80) | 0.07 | 1.26 (1.16, 1.37) | 0.90 | 1.39 (1.30, 1.49) | 0.10 |
| Highd | 0.98 (0.65, 1.47) | 1.23 (0.89, 1.71) | 1.22 (1.06, 1.40) |
Abbreviations: CIs, confidence intervals.
Extremely low temperature: the 1st percentile of temperature distribution (−12.9 °C, −16.7 °C, −16.9 °C), and the referent temperature is the minimum risk temperature (23.3 °C, 22.3 °C, 21.9 °C).
Extremely high temperature: the 99th percentile of temperature distribution (30.7 °C, 31.0 °C, 30.9 °C), and the referent temperature is the minimum risk temperature (23.3 °C, 22.3 °C, 21.9 °C).
Junior: Junior high school and below.
High: High school and above.
Significant between-stratum difference.
Discussion
By virtue of the nationwide death registry of China, this is the largest study in the world to explore the association between non-optimum temperature and pneumonia mortality. We also provide more robust evidence for the risks and burden of pneumonia associated with both low temperature and high temperature using an individual-level case-crossover design. In addition, to our knowledge, no previous studies have investigated the effect of ambient temperature on pneumonia of different infectious causes. The largest risk and burden associated with low temperature was found among influenza-related pneumonia, followed by viral pneumonia and bacterial pneumonia. There were higher AF of influenza-related pneumonia (29.78%) than the viral pneumonia (19.00%) and bacterial pneumonia (15.64%) in relation to non-optimum temperature. Besides, patients of low education level were vulnerable to these risks.
Climatic factors have been widely recognized to influence host, air-borne pathogen, and environmental–human interactions, thereby affecting the seasonality of respiratory diseases. Our findings indicated that both low temperature and high temperature were linked to higher pneumonia mortality, and low temperature showed the larger risks, which are consistent with previous studies.12,13,27 Further, some evidences indicated that positive tests for viral pathogens are higher in the cold season compared to the warm season,28 and epidemics of these viruses usually occur typically during late autumn, winter, and early spring.29 For instance, both respiratory syncytial virus and influenza virus exhibited comparable seasonal trends, with a preference for spreading in colder temperature.30,31 Significant seasonal variation was also observed in bacterial pathogens. Pneumococcal infection was found to be negatively correlated with ambient temperature, that is, the lower the temperature, the higher the incidence of pneumococcal infection.5 A population-based study in Taiwan Province showed that the monthly hospitalization rate of community-acquired pneumonia (per 10,000 people) increased by about 0.03 for every 1 °C decrease in ambient temperature.32 As streptococcus pneumoniae is the prevailing bacterial source of community-acquired pneumonia, it can be deduced that the connection between reduced ambient temperature and elevated hospitalization rate for community-acquired pneumonia was a result of the inverse correlation mentioned herein. Besides, the effects of low temperature on influenza-related pneumonia and viral pneumonia appear immediately in the shorter lag period (lag 2 day) and persist until lag 12 day. In contrast, the effect of low temperature on bacterial pneumonia seems to occur with a lag of about one week, and lasts for another week. These findings may be related to the long incubation period and late detection of the outbreak of bacterial pneumonia.33,34 Nevertheless, in accord with previous studies, the impact of high temperature was found to be restricted to the first several days.23 Through this nationwide individual-level case-crossover design, we provide more reliable evidence on the lag pattern and exposure-response curves for the relationships between non-optimum temperature and pneumonia.
The associations of non-optimum temperature with pneumonia with different pathogens are biologically plausible. Previous studies have documented that hypothermia may have an inhibitory effect on human immune function.35 An in vitro study by Luo and colleagues found that the phagocytosis function and cell vitality of alveolar macrophages decreased under cold stress, and the degree of decline was negatively correlated with temperature.36 Under normal conditions, the respiratory tract has filtration, humidification and heating functions for inhaled gas, and IgA and cilia in the airway can prevent bacterial adhesion in the airway. However, in cold environment, blood vessels shrink, blood velocity decreases, and cilia activity of the tracheal mucosal epithelium slows down, thus reducing immune proteins, and bacterial adhesion in the respiratory tract becomes easier to colonize.37 Besides, people stay indoors longer in winter, and thus the indoor crowding combined with insufficient ventilation will increase the spread of respiratory viruses, increasing the risk of pneumonia infection.38 Coupled with severe air pollution in winter, it further aggravates respiratory virus infection. A newly published study shows that PM2.5 may pave the way for the spread of the flu virus in animals, acting like a Trojan horse to transport the virus into cells while making it easier for the virus to enter the deep lungs.39 In addition, cold temperature has been shown to facilitate the spread of influenza viruses,40 which may partly explain the effect of non-optimum temperature on the deaths of influenza-related pneumonia is higher than that of other pneumonia. In terms of hot weather, when the body exceeds its thermoregulatory threshold, heat can trigger a series of acute reactions. The hot weather is always accompanied by high humidity in summer, which provides a suitable environment for the spread of a portion of airborne bacteria and viruses and thus cause pneumonia in susceptible individuals.41 It is worth noting that the high temperature is not conducive to the spread of influenza viruses,40 this may partly explain the results that a much smaller and insignificant proportion of influenza pneumonia was attributed to all high temperatures, compared with that of viral and bacterial pneumonia.
In the present study, subjects with lower education level exhibited a considerably higher vulnerability to the impacts of low temperature. This vulnerability may stem from a weaker initial state of health, restricted availability of healthcare, and unfavorable housing situations like the absence of air conditioning.42 Further, we observed the relationship between high temperature and pneumonia were more evident in the north regions, which could be due to the insufficient heat adaptation for northern population. In addition, according to the China Statistical Yearbook 2020, the proportion of elder population in northern China is higher than that in southern China; the number of beds in township hospitals per thousand rural population in northern China is significantly less than that in southern China.43 These statistics may be used to explain the greater risk of pneumonia deaths in northern China in relation to non-optimal ambient temperature. Our results suggest that prevention and control measures for pneumonia should be strengthened for these vulnerable subgroups.
Our findings had notable policy significance for various stakeholders and help to better consider climatic risk factors in clinical management and public health interventions for pneumonia. Firstly, we estimated considerable burdens of infectious pneumonia deaths that can be attributed to non-optimum temperature. The AF was higher in influenza-related pneumonia (29.78%) than viral pneumonia (19.00%) and bacterial pneumonia (15.64%). As far as we know, no prior research has quantified the burden of pneumonia mortality of various infectious causes attributable to non-optimum temperature. However, our data showed a consistently higher AF compared to the AF reported in the GBD Study 2019 for the lower respiratory infection deaths in China, which estimated a significant burden (AF: 12.78%) attributed to non-optimum temperature.8 The heterogeneity was mainly due to differences in estimations of exposure-response relationships. By virtue of the individual-level case-crossover design and the most representative death registry of China, we provided more reliable estimation on the exposure-response relationships. We also considered the spatial heterogeneity and cumulative effects over days, yielding a more accurate estimation on the burden of diseases. These estimates provide solid support for including non-optimum temperature in prevention and control of pneumonia. Secondly, the varied lag patterns of high and low temperature suggest the necessity for immediate and timely safeguards in order to mitigate the heat-related exacerbation of pneumonia, while prolonged preventive measures may be beneficial in addressing cold-related pneumonia risks. Thirdly, with the aging of the world's population, the number of pneumonia deaths would continue to increase, resulting in a greater economic burden; and therefore, the government should create a healthy social support and living environment for susceptible populations, including establishing cold and hot weather warning systems, strengthening the construction of refrigeration and heating facilities in public places, and proactively reminding susceptible populations to adopt precautionary measures. Fourthly, community education is crucially important to raise public awareness of climatic risk factors of pneumonia to minimize the health consequences caused by extreme weather conditions by individual-level protective measures. Fifthly, the susceptible populations need to take appropriate preventive measures when encountering low and high temperature.
The limitations of this study should also be noted. Firstly, although our study covers all areas in mainland China, one of the largest countries in the world, our results could not be easily generalized to other countries with varied climatic and healthcare characteristics. Secondly, as done in most previous epidemiological studies on temperature,23,44 we only evaluated the health impact of ambient but not personal temperature, so exposure errors are inevitable, but this kind of exposure misclassification could not result in substantial bias in time-series or case crossover studies. Thirdly, our study did not include pneumonia deaths of unknown or unspecific causes, which could have incorporated a considerable proportion of pneumonia deaths with infectious causes but could not be identified by the death registry. However, we deemed the occurrence of missed diagnoses to be random and not related to variations in weather conditions, so they were not likely to bias our results. Fourthly, we could not conduct a deep analysis on pneumonia of specific infectious pathogens due to the few numbers of cases with such detailed diagnoses. Fifthly, given that influenza vaccination is one of the most effective measures to prevent influenza and reduce the burden of influenza-related disease,45 we believe that this kind of factor could not vary in a short time (e.g., a month) and thus do not have any confounding effects on our results due to our self-matching design, but only serve as possible effect modifiers. It is a great pity that we could not explore the possible modification effects of geographic difference of flu vaccination rate on our results due to the data unavailability. Finally, although we used a time-stratified, case-crossover study design, residual confounding may still exist, especially from those time-varying risk factors (such as allergens in the home environment).46
In conclusion, this nationwide, case-crossover study provides compelling epidemiological evidence that non-optimum ambient temperature could increase the risk of pneumonia mortality of various infectious causes in China. We also provide the evidence that non-optimum ambient temperature could account for a considerable proportion of pneumonia deaths, and highlight the susceptibility of influenza-related pneumonia to ambient low temperature. As pneumonia is a life-threatening respiratory disease, our results add to the growing body of evidence on modifiable environmental risk factors for pneumonia, and provide some valuable insights into the prevention and management of pneumonia with different infections, which will benefit public health policy formulation and prevention for susceptible subgroups.
Contributors
Qinglin He, Yunning Liu, Yanming Li and Renjie Chen designed the study. The underlying data were verified by Qinglin He, Yunning Liu, Yanming Li and Renjie Chen. Qinglin He and Yunning Liu analysed the data. Qinglin He and Yunning Liu wrote the first draft of the manuscript, which was revised by Yanming Li, Renjie Chen, Maigeng Zhou, Haidong Kan, Peng Yin and Ya Gao. All authors interpreted data, provided critical review and revision of the text, and approved the final version of the manuscript. All authors had access to the data underlying the study and accept responsibility for the decision to submit for publication. All authors have read and approved the final version of the manuscript. All authors have final responsibility for the decision to submit for publication.
Data sharing statement
The death data that support the findings of this study are obtained from the National Center for Chronic Non-communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, and the license for the use of the data was restricted only to the current study, so the death data are not publicly available. Mortality data are, however, available from the authors upon reasonable request and with permission of the National Center for Chronic Non-communicable Disease Control and Prevention. The environmental data could be available upon reasonable request.
Declaration of interests
All authors declare no potential conflicts of interest.
Acknowledgements
This study is supported by the National Key Research and Development Program (2022YFC3702701), the Shanghai Municipal Science and Technology Commission (21TQ015) and Shanghai International Science and Technology Partnership Project (21230780200).
Contributor Information
Renjie Chen, Email: chenrenjie@fudan.edu.cn.
Yanming Li, Email: liyanming2632@bjhmoh.cn.
References
- 1.Walker C.L.F., Rudan I., Liu L., et al. Global burden of childhood pneumonia and diarrhoea. Lancet. 2013;381(9875):1405–1416. doi: 10.1016/S0140-6736(13)60222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cillóniz C., Greenslade L., Dominedò C., Garcia-Vidal C. Promoting the use of social networks in pneumonia. Pneumonia (Nathan) 2020;12:3. doi: 10.1186/s41479-020-00066-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liang L., Moore B., Soni A. Agency for Healthcare Research and Quality (US); Rockville (MD): 2006. National inpatient hospital costs: the most expensive conditions by payer, 2017. Healthcare Cost and Utilization Project (HCUP) statistical briefs. [PubMed] [Google Scholar]
- 4.Mandell L.A., Wunderink R.G., Anzueto A., et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 Suppl 2(Suppl 2):S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nascimento-Carvalho C.M., Cardoso M.R., Barral A., et al. Seasonal patterns of viral and bacterial infections among children hospitalized with community-acquired pneumonia in a tropical region. Scand J Infect Dis. 2010;42(11-12):839–844. doi: 10.3109/00365548.2010.498020. [DOI] [PubMed] [Google Scholar]
- 6.Ruuskanen O., Lahti E., Jennings L.C., Murdoch D.R. Viral pneumonia. Lancet. 2011;377(9773):1264–1275. doi: 10.1016/S0140-6736(10)61459-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Z., Chen Q., Feng L., et al. Active case finding with case management: the key to tackling the COVID-19 pandemic. Lancet. 2020;396(10243):63–70. doi: 10.1016/S0140-6736(20)31278-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collaborators GRF Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1223–1249. doi: 10.1016/S0140-6736(20)30752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haines A., McMichael A.J., Epstein P.R. Environment and health: 2. Global climate change and health. CMAJ. 2000;163(6):729–734. [PMC free article] [PubMed] [Google Scholar]
- 10.Braga A.L., Zanobetti A., Schwartz J. The time course of weather-related deaths. Epidemiology. 2001;12(6):662–667. doi: 10.1097/00001648-200111000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Curriero F.C., Heiner K.S., Samet J.M., Zeger S.L., Strug L., Patz J.A. Temperature and mortality in 11 cities of the eastern United States. Am J Epidemiol. 2002;155(1):80–87. doi: 10.1093/aje/155.1.80. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y., Kan H., Xu J., et al. Temporal relationship between hospital admissions for pneumonia and weather conditions in Shanghai, China: a time-series analysis. BMJ Open. 2014;4(7) doi: 10.1136/bmjopen-2014-004961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bunker A., Wildenhain J., Vandenbergh A., et al. Effects of air temperature on climate-sensitive mortality and morbidity outcomes in the elderly; a systematic review and meta-analysis of epidemiological evidence. eBioMedicine. 2016;6:258–268. doi: 10.1016/j.ebiom.2016.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu S., Wu X., Lopez A.D., et al. An integrated national mortality surveillance system for death registration and mortality surveillance, China. Bull World Health Organ. 2016;94(1):46–57. doi: 10.2471/BLT.15.153148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muñoz-Sabater J., Dutra E., Agustí-Panareda A., et al. ERA5-Land: a state-of-the-art global reanalysis dataset for land applications. Earth Syst Sci Data. 2021;13(9):4349–4383. [Google Scholar]
- 16.Chen R., Yin P., Meng X., et al. Fine particulate air pollution and daily mortality. A Nationwide Analysis in 272 Chinese cities. Am J Respir Crit Care Med. 2017;196(1):73–81. doi: 10.1164/rccm.201609-1862OC. [DOI] [PubMed] [Google Scholar]
- 17.Guo Y., Barnett A.G., Pan X., Yu W., Tong S. The impact of temperature on mortality in Tianjin, China: a case-crossover design with a distributed lag nonlinear model. Environ Health Perspect. 2011;119(12):1719–1725. doi: 10.1289/ehp.1103598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y., Pan J., Fan C., et al. Short-term exposure to ambient air pollution and mortality from myocardial infarction. J Am Coll Cardiol. 2021;77(3):271–281. doi: 10.1016/j.jacc.2020.11.033. [DOI] [PubMed] [Google Scholar]
- 19.Bateson T.F., Schwartz J. Control for seasonal variation and time trend in case-crossover studies of acute effects of environmental exposures. Epidemiology. 1999;10(5):539–544. [PubMed] [Google Scholar]
- 20.Levy D., Lumley T., Sheppard L., Kaufman J., Checkoway H. Referent selection in case-crossover analyses of acute health effects of air pollution. Epidemiology. 2001;12(2):186–192. doi: 10.1097/00001648-200103000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Gasparrini A. Modeling exposure-lag-response associations with distributed lag non-linear models. Stat Med. 2014;33(5):881–899. doi: 10.1002/sim.5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gasparrini A., Guo Y., Hashizume M., et al. Mortality risk attributable to high and low ambient temperature: a multicountry observational study. Lancet. 2015;386(9991):369–375. doi: 10.1016/S0140-6736(14)62114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang Y., Hu J., Peng L., et al. Non-optimum temperature increases risk and burden of acute myocardial infarction onset: a nationwide case-crossover study at hourly level in 324 Chinese cities. eClinicalMedicine. 2022;50 doi: 10.1016/j.eclinm.2022.101501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gasparrini A., Leone M. Attributable risk from distributed lag models. BMC Med Res Methodol. 2014;14:55. doi: 10.1186/1471-2288-14-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tian L., Qiu H., Sun S., Lin H. Emergency cardiovascular hospitalization risk attributable to cold temperatures in Hong Kong. Circ Cardiovasc Qual Outcomes. 2016;9(2):135–142. doi: 10.1161/CIRCOUTCOMES.115.002410. [DOI] [PubMed] [Google Scholar]
- 26.Fu S.H., Gasparrini A., Rodriguez P.S., Jha P. Mortality attributable to hot and cold ambient temperatures in India: a nationally representative case-crossover study. PLoS Med. 2018;15(7) doi: 10.1371/journal.pmed.1002619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li M., Zhou M., Yang J., Yin P., Wang B., Liu Q. Temperature, temperature extremes, and cause-specific respiratory mortality in China: a multi-city time series analysis. Air Qual Atmos Health. 2019;12(5):539–548. [Google Scholar]
- 28.Liu Y.N., Zhang Y.F., Xu Q., et al. Infection and co-infection patterns of community-acquired pneumonia in patients of different ages in China from 2009 to 2020: a national surveillance study. Lancet Microbe. 2023;4(5):e330–e339. doi: 10.1016/S2666-5247(23)00031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clementi N., Ghosh S., De Santis M., et al. Viral respiratory pathogens and lung injury. Clin Microbiol Rev. 2021;34(3) doi: 10.1128/CMR.00103-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.du Prel J.B., Puppe W., Gröndahl B., et al. Are meteorological parameters associated with acute respiratory tract infections? Clin Infect Dis. 2009;49(6):861–868. doi: 10.1086/605435. [DOI] [PubMed] [Google Scholar]
- 31.Xu B., Wang J., Li Z., et al. Seasonal association between viral causes of hospitalised acute lower respiratory infections and meteorological factors in China: a retrospective study. Lancet Planet Health. 2021;5(3):e154–e163. doi: 10.1016/S2542-5196(20)30297-7. [DOI] [PubMed] [Google Scholar]
- 32.Lin H.C., Lin C.C., Chen C.S., Lin H.C. Seasonality of pneumonia admissions and its association with climate: an eight-year nationwide population-based study. Chronobiol Int. 2009;26(8):1647–1659. doi: 10.3109/07420520903520673. [DOI] [PubMed] [Google Scholar]
- 33.Ancel Meyers L., Newman M.E., Martin M., Schrag S. Applying network theory to epidemics: control measures for Mycoplasma pneumoniae outbreaks. Emerg Infect Dis. 2003;9(2):204–210. doi: 10.3201/eid0902.020188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Michelow I.C., Olsen K., Lozano J., et al. Epidemiology and clinical characteristics of community-acquired pneumonia in hospitalized children. Pediatrics. 2004;113(4):701–707. doi: 10.1542/peds.113.4.701. [DOI] [PubMed] [Google Scholar]
- 35.Shephard R.J., Shek P.N. Cold exposure and immune function. Can J Physiol Pharmacol. 1998;76(9):828–836. doi: 10.1139/cjpp-76-9-828. [DOI] [PubMed] [Google Scholar]
- 36.Luo B., Liu J., Fei G., et al. Impact of probable interaction of low temperature and ambient fine particulate matter on the function of rats alveolar macrophages. Environ Toxicol Pharmacol. 2017;49:172–178. doi: 10.1016/j.etap.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 37.Martens W.J. Climate change, thermal stress and mortality changes. Soc Sci Med. 1998;46(3):331–344. doi: 10.1016/s0277-9536(97)00162-7. [DOI] [PubMed] [Google Scholar]
- 38.Hajat S., Haines A. Associations of cold temperatures with GP consultations for respiratory and cardiovascular disease amongst the elderly in London. Int J Epidemiol. 2002;31(4):825–830. doi: 10.1093/ije/31.4.825. [DOI] [PubMed] [Google Scholar]
- 39.Dong Z., Ma J., Qiu J., et al. Airborne fine particles drive H1N1 viruses deep into the lower respiratory tract and distant organs. Sci Adv. 2023;9(23) doi: 10.1126/sciadv.adf2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lowen A.C., Mubareka S., Steel J., Palese P. Influenza virus transmission is dependent on relative humidity and temperature. PLoS Pathog. 2007;3(10):1470–1476. doi: 10.1371/journal.ppat.0030151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paynter S., Weinstein P., Ware R.S., et al. Sunshine, rainfall, humidity and child pneumonia in the tropics: time-series analyses. Epidemiol Infect. 2013;141(6):1328–1336. doi: 10.1017/S0950268812001379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McGeehin M.A., Mirabelli M. The potential impacts of climate variability and change on temperature-related morbidity and mortality in the United States. Environ Health Perspect. 2001;109 Suppl 2(Suppl 2):185–189. doi: 10.1289/ehp.109-1240665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.China statistical Yearbook. http://www.stats.gov.cn/tjsj/ndsj
- 44.Zhou Y., Gao Y., Yin P., et al. Assessing the burden of suicide death associated with nonoptimum temperature in a changing climate. JAMA Psychiatr. 2023;80(5):488–497. doi: 10.1001/jamapsychiatry.2023.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nichol K.L., Nordin J.D., Nelson D.B., Mullooly J.P., Hak E. Effectiveness of influenza vaccine in the community-dwelling elderly. N Engl J Med. 2007;357(14):1373–1381. doi: 10.1056/NEJMoa070844. [DOI] [PubMed] [Google Scholar]
- 46.Norbäck D., Lu C., Zhang Y., et al. Common cold among pre-school children in China–associations with ambient PM(10) and dampness, mould, cats, dogs, rats and cockroaches in the home environment. Environ Int. 2017;103:13–22. doi: 10.1016/j.envint.2017.03.015. [DOI] [PubMed] [Google Scholar]