Abstract
Background
Diabetic patients on metformin therapy may be vulnerable to lactic acidosis during acute illness. This is particularly true since the comorbid conditions among patients with diabetes and the frequent use of renin-angiotensin system blockers increase the risk of renal dysfunction.
Case Description
We present two cases of metformin-associated lactic acidosis (MALA) occurred after abdominal surgery. A 74-year-old woman presented to emergency department (ED) for a transient loss of consciousness. She had vomiting, diarrhea, and lack of appetite in the last 3 days and she had had an abdominal adhesiolysis surgery 12 days before. A 78-year-old man, with history of right hemicolectomy performed 15 days before admission to ED. Patient presented with diffuse abdominal pain, diarrhea, nausea, and vomiting). Arterial blood gas analysis showed acidemia (pH 7.031), elevated anion gap (AG), lactate >15.5 mmol/L. Due to the patients’ critical condition, vasopressor infusion and fluid resuscitation were started and an urgent continuous veno-venous hemodialysis with citrate and calcium (CVVHD-CiCa) treatment was provided.
Conclusions
A promptly differential diagnosis at ED and early treatment with vital support and CVVHD-CiCa enabled the resolution of MALA, which can often be a fatal complication in diabetic patients taking metformin. Close collaboration with the surgical and endocrinological team would be necessary for the management of the postoperative period planning the reintroduction of metformin in patients undergoing major abdominal surgery, to avoid the possibility of the onset of MALA.
Keywords: Lactic acidosis, diabetes mellitus (DM), metformin, surgery, case report
Highlight box.
Key findings
• Occurrence of metformin-associated lactic acidosis (MALA), in patients who have recently undergone abdominal surgery with predisposing conditions, can be rapidly recognized and treated already in the early stages.
What is known and what is new?
• Urgent and aggressive supportive care and use of continuous renal replacement therapy (CRRT), after a careful differential diagnosis and predisposing conditions evaluation (recent surgery, sepsis, concomitant therapies, acute deterioration in renal functions, etc.), may help in improving outcomes.
• In patients with hemodynamic instability, the early use of continuous veno-venous hemodialysis with citrate and calcium (CVVHD-CiCa) proves to be safe and effective, allowing a more conservative resuscitation treatment.
What is the implication, and what should change now?
• Assessment and monitoring of renal function, associated therapies (e.g., renin-angiotensin system blockers) and postoperative hydration status should be mandatory for proper timing of metformin reintroduction. Multidisciplinary team would be necessary for the optimal management of the postoperative period up to discharge for planning the reintroduction of metformin in patients undergoing abdominal surgery.
Introduction
The dramatic incidence of diabetes growth represents a serious challenge for public health worldwide, affecting approximately 10–15% of surgical population with a mortality rate of 50% higher than non-diabetic patient population. Acute events such as surgery in elderly patients, renal dysfunctions, sepsis, and heart failure, could lead to adverse effects on co-administration of metformin as in the case of lactic acidosis (1).
Metformin, oral agent of biguanide class, is recommended as first-line treatment for type 2 diabetes, it has multiple mechanisms of action, such as reduction of gluconeogenesis, increases peripheral uptake of glucose and decreases fatty acid oxidation (2).
Metformin-associated lactic acidosis (MALA) is described as presence of lactic acidosis (blood arterial pH <7.35 and serum lactate >5 mmol/L) in patient on acute/chronic metformin therapy, which cannot be explained by other causes (2). Severe MALA is relatively rare with an incidence of 1/100,000 to 9/100,000 cases, and a mortality rate ranged of 10–50% (3).
The incidence of MALA has increased in recent years, especially after scientific societies have given approval to prescribe metformin also to patients with mild to moderate chronic renal failure. The increased risk of renal dysfunction is also exacerbated by frequent use of metformin in patients taking drugs that block renin-angiotensin system.
The scientific literature lacks, except for sporadic clinical cases, in discussing the relationship between surgery, anesthesia and MALA, which is considered as a perioperative complication despite its not well-recognized origin (4).
Even the same international guidelines differ in the providing guidance on the management of metformin therapy in patients undergoing to surgery. Moreover, the recommendations regarding the reintroduction of metformin in the perioperative period are not very clear, the decision to continue/discontinue or re-enter the drug, should be individualized on the patient and on the performed surgical procedure (5).
We describe two cases of post-operative MALA occurred in diabetics’ patients with common clinical characteristics and reviewed in literature. We present these two cases in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-22-100/rc).
Case presentation
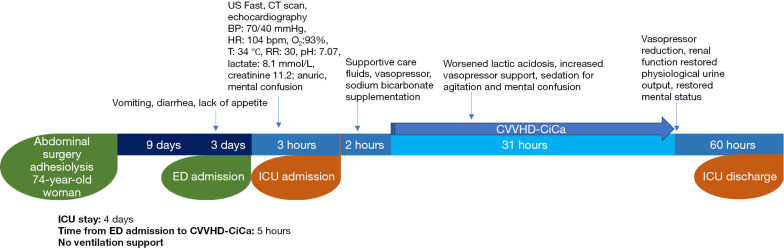
A 74-year-old woman presented to the emergency department (ED) for a transient loss of consciousness. She had been experiencing vomiting, diarrhea, and lack of appetite for the last 3 days. Her medical history was summarized in Table 1. She had had an abdominal adhesiolysis surgery 12 days before. On arrival at ED, she was conscious, slightly disoriented, dehydrated and anuric, with a normal abdominal examination; vital signs were unstable [blood pressure (BP) =70/40 mmHg, heart rate (HR) =104 bpm, O2 saturation 93%, T=34 ℃, respiratory rate (RR) =30]. No clinical signs of acute abdomen obstruction, free fluid in abdominal compartment and septic localizations were detected by ultrasound (US) fast and subsequent computed tomography (CT) scan. A heart exam with echocardiography and electrocardiography excluded cardiac injury. The laboratory finding revealed severe kidney injury and arterial blood analysis showed severe acidosis with elevated anion gap (AG) (Table 1). Baseline estimated glomerular filtration rate (eGFR) was 3 mL/min/1.73 m2 (stage V of chronic kidney disease). MALA was suspected. Supportive treatment was promptly started with fluids, sodium bicarbonate and vasopressor without results. An urgent continuous veno-venous hemodialysis with citrate and calcium (CVVHD-CiCa) treatment was started. As the patient was hemodynamically unstable, she was admitted to the intensive care unit (ICU) and hemodynamic parameters were closely monitored. During the following hours lactic acidosis had worsened to the point that an increased vasopressor support was necessary. Even though the patient was still conscious, a mild sedation was performed to relieve her agitation and confusing state. In about 36 hours, the patient was alert and oriented. Lactic acidosis, hemodynamic instability and renal failure resolved as well (Table 1). Physiologic urine output on the third day was restored, CVVHD-CiCa was discontinued, and patient was discharged from ICU (Figure 1).
Table 1. Main laboratory data, blood gas analysis, ICU scores and noradrenaline dosage of Case 1, over the acute phase.
| Case 1 | ED (T=0) | ICU (Adm) T1 (3 h) | T2 (6 h) | T3 (12 h) | T4 (24 h) | T5 (30 h) | T6 (36 h) | T7 (48 h) | T8 (60 h) | T9 (72 h) | ICU (D) T11 (96 h) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lactate (mmol/L) | 8.1 | 9.6 | 11.2 | 13.5 | 9.9 | 8.5 | 4.6 | 2.33 | 1.4 | 1.11 | 0.9 |
| BE (mmol/L) | −21.3 | −23.3 | −25.8 | −23.3 | −18.6 | −15.7 | −11 | −1.2 | 0.8 | 2 | 1.8 |
| PCO2 (mmHg) | 22.3 | 16.1 | 11.7 | 14.3 | 19.5 | 19.8 | 25.8 | 33.3 | 41.1 | 41.9 | 42.8 |
| HCO3− (mmol/L) | 6.4 | 5 | 4 | 5.1 | 7.4 | 9.3 | 13.9 | 22.8 | 25.8 | 26.7 | 26.4 |
| pH | 7.07 | 7.06 | 6.99 | 7.08 | 7.2 | 7.29 | 7.34 | 7.45 | 7.41 | 7.42 | 7.41 |
| AG (mEq/L) | 24 | 27 | 27 | 30 | 28 | 26 | 21 | 14 | 8 | 7 | 9 |
| Creatinine (mg/dL) | 11.2 | 5.8 | – | – | 2.6 | – | – | 2.6 | – | – | 1.9 |
| Glycemia (mg/dL) | 146 | 172 | 186 | 188 | 194 | 182 | 178 | 124 | 161 | 140 | 146 |
| BUN (mg/dL) | 56 | 37.8 | – | – | 28.5 | – | – | 28.5 | – | – | 24.7 |
| eGFR (mL/min/1.73 m2) | 3 | 6.6 | – | – | 18 | – | – | 17.3 | – | – | 25.3 |
| Urine output (mL/kg/h) | – | – | – | – | 0.1 | 0.3 | 0.5 | 0.8 | >1.0 | >1.0 | >1.0 |
| Lipase (U/L) | 268 | 1,830 | – | – | – | – | – | – | – | – | 145 |
| Amylase (U/L) | 78 | 400 | – | – | – | – | – | – | – | – | 76 |
| INR (ratio) | 1.3 | 1.34 | – | – | – | – | – | – | – | – | 1.01 |
| PT (sec) | 1.2 | 14.6 | – | – | – | – | – | – | – | – | 11.4 |
| PTT (sec) | 33 | 27 | – | – | – | – | – | – | – | – | 21 |
| PCT (ng/mL) | 0.41 | 0.5 | – | – | 0.5 | – | – | 0.5 | – | – | 0.3 |
| APACHE II score | 22 | 19 | – | – | – | – | – | 15 | – | – | 13 |
| SOFA score | 10 | 11 | – | – | – | – | – | 9 | – | – | 4 |
| Noradrenaline (mcg/kg/min) | – | 0.24 | 0.35 | 0.42 | 0.35 | 0.28 | – | – | 0.03 | – | |
| Albumin (g/dL) | 3.2 | – | – | – | – | – | – | – | – | – | – |
ICU, intensive care unit; ED, emergency department; Adm, admission; D, discharge; BE, base excess; PCO2, partial pressure of carbon dioxide; AG, anion gap; BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate; INR, international normalized ratio; PT, prothrombin time; PTT, partial thromboplastin time; PCT, procalcitonin; APACHE, Acute Physiologic Assessment and Chronic Health Evaluation Scoring System; SOFA, Sequential Organ Failure Assessment.
Figure 1.
Timeline of Case 1. ICU, intensive care unit; ED, emergency department; US Fast, ultrasound focused assessment with sonography in trauma; CT scan, computed tomography scan; BP, blood pressure; HR, heart rate; RR, respiratory rate; CVVHD-CiCa, continuous veno-venous hemodialysis with citrate and calcium.
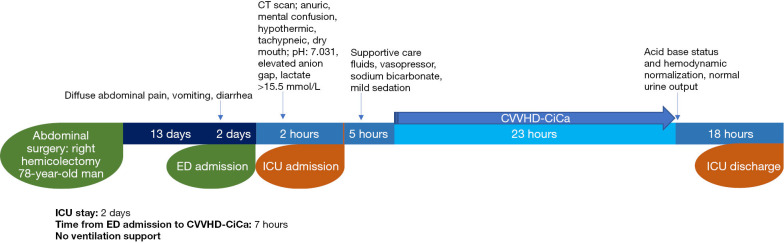
A 78-year-old man, with history of right hemicolectomy performed 15 days before admission to ED, was described. Patient presented with diffuse abdominal pain, diarrhea, nausea, and vomiting in the last 3 days. Suspecting intestinal obstruction, the patient was quickly transferred to diagnostics. The CT scan proved negative. In the meanwhile, mental status change occurred, and ICU was alerted. At first, he was able to talk, but was confused and hypotensive (BP: 70/39 mmHg). On physical examination, he was hypothermic, tachypneic, confused, had dry mouth, and reduced bowel sounds. Nevertheless, heart and chest examination were normal. Arterial blood gas (ABG) analysis showed acidemia (pH 7.031), elevated AG, lactate >15.5 mmol/L (maximum detectable value) (Table 2). Baseline eGFR was 7.6 mL/min/1.73 m2 (stage V of chronic kidney disease). Due to the patient’s critical condition, he was rapidly transferred to ICU. Norepinephrine infusion and fluid resuscitation with sodium bicarbonate was started. As the degree of lactic acidemia was out of range, MALA was suspected, and renal replacement therapy was urgently started with CVVHD-CiCa. The hemodialysis was stopped with acid base status and hemodynamic normalization (Table 2, Figure 2).
Table 2. Main laboratory data, blood gas analysis, ICU scores and noradrenaline dosage of Case 2, over the acute phase.
| Case 2 | ED (T=0) | ICU Adm T1 (2 h) | T2 (6 h) | T3 (12 h) | T4 (24 h) | T5 (30 h) | T6 (36 h) | ICU (D) T7 (48 h) |
|---|---|---|---|---|---|---|---|---|
| Lactate (mmol/L) | >15.5 | >1.5 | >15.5 | 8.3 | 0.9 | 0.6 | 0.6 | 0.8 |
| BE (mmol/L) | −23.3 | −25.9 | −21.4 | −1.4 | 2.7 | 8.1 | 1.5 | 9.1 |
| PCO2 (mmHg) | 7.2 | 10.5 | 12.1 | 12.1 | 29.5 | 40.5 | 30.1 | 40.6 |
| HCO3− (mmol/L) | 2.6 | 2.8 | 5 | 7.1 | 2.7 | 31.7 | 23.9 | 32.6 |
| pH | 7.17 | 7.03 | 7.24 | 7.39 | 7.53 | 7.5 | 7.51 | 7.52 |
| AG (mEq/L) | 61 | 62 | 55 | 37 | 12 | 4 | 14 | 7 |
| Creatinine (mg/dL) | 6.4 | – | – | – | 2.2 | – | – | 1.8 |
| Glycemia (mg/dL) | 83 | 87 | 221 | 148 | 146 | 140 | 139 | 162 |
| BUN (mg/dL) | 77.5 | – | – | – | 41.1 | – | – | 39.2 |
| eGFR (mL/min/1.73 m2) | 7.6 | – | – | – | 27.7 | – | – | 35.3 |
| Urine output (mL/kg/ h) | – | – | – | – | 1.13 | >1.2 | >1.2 | >1.2 |
| Amylase (U/L) | 37 | – | – | – | – | – | – | – |
| INR (ratio) | 1.48 | – | – | – | 1.72 | – | – | 1.63 |
| PT (sec) | 17 | – | – | – | 19.7 | – | – | 17.6 |
| PTT (sec) | 27 | – | – | – | 32 | – | – | 30 |
| PCT (ng/mL) | 0.3 | – | – | – | 0.86 | – | – | 0.85 |
| APACHE II score | 28 | – | – | – | 16 | – | – | 13 |
| SOFA score | 12 | – | – | – | 7 | – | – | 6 |
| Noradrenaline (mcg/kg/min) | 0.35 | – | – | – | – | – | – | – |
| Albumin (g/dL) | 2.3 | – | – | – | – | – | – | – |
ICU, intensive care unit; ED, emergency department; Adm, admission; D, discharge; BE, base excess; PCO2, partial pressure of carbon dioxide; AG, anion gap; BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate; INR, international normalized ratio; PT, prothrombin time; PTT, partial thromboplastin time; PCT, procalcitonin; APACHE, Acute Physiologic Assessment and Chronic Health Evaluation Scoring System; SOFA, Sequential Organ Failure Assessment.
Figure 2.
Timeline of Case 2. ICU, intensive care unit; ED, emergency department; CT scan, computed tomography scan; CVVHD-CiCa, continuous veno-venous hemodialysis with citrate and calcium.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). As stated in the CARE reporting guidelines (6), written informed consent was obtained from the patients for data collection and publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Clinical presentation of MALA is often non-specific. It can mimic various severe conditions such as sepsis, gastrointestinal disorders, heart failure and hypovolemic shock. Presenting symptoms in many cases were nausea, vomiting, diarrhea, abdominal pain and loss of appetite. The initial symptoms are consistent with both infectious gastroenteric syndrome and initial accumulation of metformin, so it is unclear what the trigger is. Disturbances of consciousness up to coma and circulatory instability have been reported in exacerbations of lactic acidosis.
Constant increase of type 2 diabetes mellitus (DM) in surgical population and the widespread treatment with metformin, require the anesthetist to know drug pharmacokinetics and pharmacodynamics as well as its potential interactions. It proves to be essential to better understand MALA pathophysiology to prevent fatal complications. Real incidence of postoperative MALA is not estimable; however, we need to be able to recognize patients at risk of MALA preemptively, in order to diagnose and treat promptly. Metformin is absorbed in the proximal small intestine and excreted unchanged by the renal tubules, it blocks respiratory chain complex 1 in liver cells mitochondria causing an energy deficiency and activation of AMP-activated protein kinase. The blocking of gluconeogenesis and lipogenesis stimulates glucose absorption and fatty acids oxidation (7) with passage from aerobic to anaerobic metabolism and increase in lactic acid production. Consequently, the pyruvate, produced by glycolysis, is no longer metabolized by the mitochondria. Reduced hepatic gluconeogenesis leads to lactate accumulation, a precursor of the glucose-generating metabolic pathway. Furthermore, metformin, in the splanchnic blood flow of the small bowel, supports glucose conversion into lactate thus increasing its production (7).
AG was useful in the differential diagnosis. In diarrhea there is a bicarbonate loss which causes non-AG hyperchloremic acidosis; instead in MALA we find a normal or slightly elevated AG value. Metabolic acidosis with increased AG in MALA is only caused by type B lactic acidosis. In fact, while type B is caused by inhibition of mitochondrial cellular respiration, the type A is due to hypoperfusion during hemodynamic instability (8).
Metformin is eliminated unchanged via renal tubules. Indeed, worsening of kidney function decreases the clearance of metformin leading to its accumulation. Clinical conditions such as acute renal failure, liver failure, dehydration, hypoxia, sepsis, trauma, shock, myocardial infarction, and use of nephrotoxic drugs appear to be considered risk factors for MALA (2). Only kidneys are responsive of metformin elimination, it follows that in patients with an eGFR below 30 mL/min, serum levels will progressively raise, hence current guidelines suggest to revise metformin dosage in those patients (9).
However, as there are no specific signs and/or instrumental analysis, except for the plasma concentration of metformin, MALA always remains a diagnosis of exclusion. MALA is likely to affect patients who develop acute kidney injury (AKI) due to vomiting, diarrhea, surgery and in any state of dehydration. In fact, dehydration could cause AKI reducing metformin clearance. It results in an increase of plasmatic level of metformin, especially when the drug is continuously administered.
Both cases described above were severely dehydrated, oligo-anuric and with high levels of creatinine and lactate. Both had been subjected to abdominal surgery during their recent recovery, with discontinuation on surgery days, but no changes in metformin dosage were made in postoperative period. This results in accumulation of reintroduced metformin and consequent severe lactic acidosis as describe and summarized in Table 3 in other few cases report (10-14). Electrolyte imbalances and severe dehydration occurred more frequently in postoperative period. Our patients underwent diuretic and antihypertensive therapy with angiotensin-converting enzyme (ACE) inhibitors. Diuretics worsen dehydration and electrolyte depletion, leading to pre-renal AKI (2).
Table 3. Review of post-operative MALA cases.
| Cases | Comorbidity | Therapy | MALA onset from surgery/type of surgery | Treatment (type and time from ED admission) | ICU stay | Outcome | Preoperative metformin last administration |
|---|---|---|---|---|---|---|---|
| Case 1, female, 74-year-old | Diabetes type 2, hypertension, dyslipidemia, hyperuricemia | Metformin 2,500 mg, perindopril tert-butylamine 8 mg, doxazosin 2 mg, atorvastatin 20 mg, allopurinol 300 mg |
12 days/abdominal adenolysis | CVVHD-CiCa, supportive care, vasopressor, mild sedation, sodio bicarbonate infusion, 5 hours | 96 hours | Alive | 48 hours |
| Case 2, male, 78-year-old | Diabetes type II, ischemic heart disease, paroxysmal atrial fibrillation |
Metformin 1,000 mg, lansoprazole 15 mg, folic acid 5 mg, amiodarone 200 mg, clopidogrel 75 mg, aspirin 100 mg, rosuvastatin 20 mg, amlodipine 10 mg |
15 days/right hemicolectomy | CVVHD-CiCa, supportive care, vasopressor, mild sedation, sodio bicarbonate infusion, 7 hours | 48 hours | Alive | 48 hours |
| Mears et al. (10), male, 82-year-old | Diabetes type II, hypothyroidism, benign prostatic hypertrophy |
Metformin 500 mg, levothyroxine 0.1 mg | 36 hours/cervical decompression | Supportive care | 96 hours | Alive | 24 hours |
| Gonçalves et al. (11), female, 65-year-old | Diabetes type II, diabetic retinopathy, chronic kidney disease, dyslipidemia, hypertension, hyperuricemia, anemia, peripheral venous insufficiency | Metformin 500 mg, levothyroxine 0.1 mg | 37 hours/cervical decompression | Supportive care | 97 hours | Alive | 12 hours |
| Margiani et al. (12), male, 70-year-old | Diabetes type II, benign prostatic hypertrophy, hypertension |
Metformin 3,000 mg, irbesartan/hydrochlorothiazide 150 mg/12.5 mg, tamsulosin 0.4 mg |
14 days/low anterior rectal resection with ileostomy |
CVVHD-CiCa, supportive care | 8 days | Alive | 72 hours |
| Mercker et al. (13), male, 66-year-old | Diabetes type II, hypertension, peripheral vascular disease, previous pulmonary embolism | Metformin 500 mg, nifedipine, isosorbide mononitrate, phenprocoumon |
96 hours/abdominal hernia repair | Supportive care, 2 hours |
49 days | Dead | 24 hours |
| Cotae et al. (14), female, 90-year-old | Diabetes type II, hypertension, atrial fibrillation | Metformin, sitagliptin, gliquidone | 72 hours/left hip hemiarthroplasty and great trochanter osteosynthesis | CVVHD-CiCa, supportive care | – | Alive | – |
MALA, metformin-associated lactic acidosis; ED, emergency department; ICU, intensive care unit; CVVHD-CiCa, continuous veno-venous hemodialysis with citrate and calcium.
As in our hospital it was not possible to confirm MALA diagnosis with metformin blood test, it was made by exclusion. Our patients have always remained conscious, and despite psychomotor agitation, they have always maintained good airways protection, so intubation was not necessary.
There is no clear consensus on MALA treatment. The EXTRIP (Extracorporeal Treatment in Poisoning) working group has published the guidelines for the management of MALA, in which supportive treatment (fluids, sodium bicarbonate, and electrolyte balance) is mentioned as the most important measure. Dialysis is indicated in cases of lactic acidosis >20 mmol/L, pH <7, hypotension, reduced level of consciousness, renal insufficiency, lack of response to supportive treatment (15). Intermittent hemodialysis is the first choice due to its better ability to remove metformin. Serum lactate >20 mmol/L is an important prognostic factor of mortality related to MALA, so it is an alarm signal that should lead to more aggressive treatments.
The benefits of extracorporeal treatment, both for hemodialysis and continuous renal replacement therapy (CRRT), have been reported (16). Hemodialysis is effective not only in reducing lactate levels and regulating electrolyte damage, but also in reducing blood level of metformin. In our cases, instead of hemodialysis, we preferred CVVHD with calcium-citrate system due to the patients’ hemodynamic instability.
As it was not possible for us to assess blood levels of metformin as a parameter of CVVHD efficacy, we used lactate, pH, trends in hemodynamic values and vasopressor dose. After the first hours of CVVHD, there was an improvement in the parameters until they completely normalised (Tables 1,2).
There was a complete recovery of urinary capacity with a decrease in blood creatinine levels.
In our case, MALA was most likely caused by dehydration, which resulted in an alteration in renal function and thus an increase in the serum concentration of metformin. This led to a positive feedback loop and critical lactic acidosis. After diagnosis, treatment should include alkalization, support of vital signs, treatment of primitive causes, forced diuresis, and/or hemodialysis for rapid drug elimination (16). The preoperative evaluation must always be carried out in a meticulous way, concentrating on recognizing patients with DM and risk of stress-induced hyperglycemia, diabetes-specific complications, such as gastroparesis, diabetic cardiopathy and/or nephropathy. Perioperative management of diabetic chronic renal failure is important to avoid the administration of nephrotoxic agents. As stated before, metformin in patients with chronic renal failure amplify the risk of MALA. It follows that before surgery it is mandatory to identify risk factors [creatinine clearance (CrCl) <60 mL/min], minimize all those situations that can cause a reduction in renal function such as dehydration, prolonged fasting, heart failure and administration of iodinated contrast.
MALA is a rare complication however; it is associated with high mortality, which is why anesthetists should be experienced in treating diabetic patients. It is crucial to pay attention on the perioperative evaluation and identifying among all patients on metformin therapy, those who are most susceptible to MALA.
Metformin in the perioperative period remains controversial, although in elective surgery it is better to interrupt administration, monitor intraoperative blood glucose and correct hyperglycemia, if deemed necessary, with fast-acting insulin. Some guidelines available for the use of metformin recommend discontinuing the treatment perioperatively. However, firm evidence for discontinuation is lacking and there is evidence that perioperative continuation of metformin may be safe. Therefore, metformin should be discontinued if there is pre-existing renal impairment or a significant risk that the patient will develop acute kidney injury. Anesthesiologists should be alert to the dangers of nephrotoxic agents or the use of contrast agents and the type of surgery to be performed on the patient (17).
In the post-operative period, patients should restart oral intake and home-therapy. According to the Association of Anesthesiologists of Great Britain and Ireland, if the eGFR is superior to 50 mL/min/1.73 m2, metformin should be recommenced. However, French guidelines suggest that in patients with HbA1c 8% who are already taking metformin, the drug should be resumed at the same dose 48 h after surgery, if provided creatinine levels are CrCl >60 mL/min (5,18).
The Food and Drug Administration (FDA) recently indicated that metformin can be started in patients with an eGFR of 45 to 60 mL/min and continued in patients with an eGFR of 30 and 45 mL/min provided that renal function is closely monitored.
Metformin continues, however, to be contraindicated in the presence of an eGFR below 30 mL/min.
Lactic acidosis in patients with severe chronic kidney disease is likely mediated by the accumulation of excessive metformin levels, which is eliminated renally, but metformin per se does not appear to be renally toxic (19,20).
About 25% of patients treated with metformin experience side effects such as nausea and diarrhea. Some experiences suggest starting with a dose of 500 mg/day, slowly titrating and/or using prolonged-release formulations until the anti-hyperglycemic effect dosage is reached. This recommended attitude may mitigate gastrointestinal adverse effects, and if these occur, physicians should work with patients to find a dose that balances glucose reduction with adverse effects. This aspect is equally important in the reintroduction of therapy in patients undergoing major surgery, especially abdominal surgery, as shown by our clinical experience (19).
Treatment with ACE-inhibitors contributed to postoperative AKI, with metformin accumulation and toxicity. In the early stages it manifests itself with diarrhea and vomiting, which aggravate hypovolemia and renal failure, ending in severe MALA.
Conclusions
Early recognition of MALA differential diagnosis is crucial for its management. Unfortunately, the clinical presentation does not always correlate with the severity of the disease or the laboratory alterations. For this reason, even initially stable patients may experience a rapid deterioration, which is why immediate consultation of intensive care and nephrology is necessary. In fact, a prompt diagnosis in ED and intensive care treatment with early support of CVVHD-CiCa hemofiltration can be crucial in MALA. Close collaboration with the surgical and endocrinological team for optimal postoperative management and therapeutic discharge planning would be desirable. Paying attention to peri-operative assessment by optimizing treatments and identifying patients and surgical procedures at highest risk of complications such as MALA in diabetics should be imperative.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). As stated in the CARE reporting guidelines, written informed consent was obtained from the patients for data collection and publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Footnotes
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-22-100/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-22-100/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-22-100/coif). The authors have no conflicts of interest to declare.
References
- 1.Eppenga WL, Lalmohamed A, Geerts AF, et al. Risk of lactic acidosis or elevated lactate concentrations in metformin users with renal impairment: a population-based cohort study. Diabetes Care 2014;37:2218-24. 10.2337/dc13-3023 [DOI] [PubMed] [Google Scholar]
- 2.Visconti L, Cernaro V, Ferrara D, et al. Metformin-related lactic acidosis: is it a myth or an underestimated reality? Ren Fail 2016;38:1560-5. 10.1080/0886022X.2016.1216723 [DOI] [PubMed] [Google Scholar]
- 3.DeFronzo R, Fleming GA, Chen K, et al. Metformin-associated lactic acidosis: Current perspectives on causes and risk. Metabolism 2016;65:20-9. 10.1016/j.metabol.2015.10.014 [DOI] [PubMed] [Google Scholar]
- 4.Vreven R, De Kock M. Metformin lactic acidosis and anaesthesia: myth or reality? Acta Anaesthesiol Belg 2005;56:297-302. [PubMed] [Google Scholar]
- 5.Cosson E, Catargi B, Cheisson G, et al. Practical management of diabetes patients before, during and after surgery: A joint French diabetology and anaesthesiology position statement. Diabetes Metab 2018;44:200-16. 10.1016/j.diabet.2018.01.014 [DOI] [PubMed] [Google Scholar]
- 6.Gagnier JJ, Kienle G, Altman DG, et al. The CARE Guidelines: Consensus-based Clinical Case Reporting Guideline Development. Glob Adv Health Med 2013;2:38-43. 10.7453/gahmj.2013.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Apostolova N, Iannantuoni F, Gruevska A, et al. Mechanisms of action of metformin in type 2 diabetes: Effects on mitochondria and leukocyte-endothelium interactions. Redox Biol 2020;34:101517. 10.1016/j.redox.2020.101517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blumenberg A, Benabbas R, Sinert R, et al. Do Patients Die with or from Metformin-Associated Lactic Acidosis (MALA)? Systematic Review and Meta-analysis of pH and Lactate as Predictors of Mortality in MALA. J Med Toxicol 2020;16:222-9. 10.1007/s13181-019-00755-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.NICE guideline. Type 2 diabetes in adults: Management; 2015. Available online: nice.org.uk/guidance/ng28
- 10.Mears SC, Lipsett PA, Brager MD, et al. Metformin-associated lactic acidosis after elective cervical spine fusion: a case report. Spine (Phila Pa 1976) 2002;27:E482-4. 10.1097/00007632-200211150-00019 [DOI] [PubMed] [Google Scholar]
- 11.Gonçalves BM, Coelho D. Metformin-associated lactic acidosis: A case reporting a serious complication in the perioperative period. Rev Esp Anestesiol Reanim (Engl Ed) 2019;66:483-6. [DOI] [PubMed] [Google Scholar]
- 12.Margiani C, Zorcolo L, Mura P, et al. Metformin-associated lactic acidosis and temporary ileostomy: a case report. J Med Case Rep 2014;8:449. 10.1186/1752-1947-8-449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mercker SK, Maier C, Neumann G, et al. Lactic acidosis as a serious perioperative complication of antidiabetic biguanide medication with metformin. Anesthesiology 1997;87:1003-5. 10.1097/00000542-199710000-00043 [DOI] [PubMed] [Google Scholar]
- 14.Cotae A, Melente O, Costache M, et al. Rare case of life threatening metformin -associated acidosis without hyperlactatemia in postoperative period. Romanian Journal of Clinical Research 2022. doi: 10.33695/rjcr.v4i2.80. [DOI]
- 15.Calello DP, Liu KD, Wiegand TJ, et al. Extracorporeal Treatment for Metformin Poisoning: Systematic Review and Recommendations From the Extracorporeal Treatments in Poisoning Workgroup. Crit Care Med 2015;43:1716-30. 10.1097/CCM.0000000000001002 [DOI] [PubMed] [Google Scholar]
- 16.Mariano F, Pozzato M, Inguaggiato P, et al. Metformin-Associated Lactic Acidosis Undergoing Renal Replacement Therapy in Intensive Care Units: A Five-Million Population-Based Study in the North-West of Italy. Blood Purif 2017;44:198-205. 10.1159/000471917 [DOI] [PubMed] [Google Scholar]
- 17.FDA. Label information: Glucophage tablets and Glucophage XR extended-release tablets. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/020357s037s039,021202s021s023lbl.pdf
- 18.Membership of the Working Party , Barker P, Creasey PE, et al. Peri-operative management of the surgical patient with diabetes 2015: Association of Anaesthetists of Great Britain and Ireland. Anaesthesia 2015;70:1427-40. Erratum in: Anaesthesia 2019;74:810. 10.1111/anae.13233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flory J, Lipska K. Metformin in 2019. JAMA 2019;321:1926-7. 10.1001/jama.2019.3805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonnet F, Scheen A. Understanding and overcoming metformin gastrointestinal intolerance. Diabetes Obes Metab 2017;19:473-81. 10.1111/dom.12854 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as