Abstract
STUDY QUESTION
Is the abundance of certain biochemical compounds in human cumulus cells (CCs) related to oocyte quality?
SUMMARY ANSWER
Malonate, 5-oxyproline, and erythronate were positively associated with pregnancy potential.
WHAT IS KNOWN ALREADY
CCs are removed and discarded prior to ICSI, thereby constituting an interesting biological material on which to perform molecular analysis aimed to predict oocyte developmental competence. Mitochondrial DNA content and transcriptional analyses in CC have been shown to provide a poor predictive value of oocyte competence, but the untargeted analysis of biochemical compounds (metabolomics) has been unexplored.
STUDY DESIGN, SIZE, DURATION
CCs were obtained from three groups of cumulus–oocyte complexes (COCs) of known developmental potential: oocytes not developing to blastocyst following ICSI (Bl−); oocytes developing to blastocyst but failing to establish pregnancy following embryo transfer (P−); and oocytes developing to blastocyst able to establish a pregnancy (P+). Metabolomics analyses were performed on 12 samples per group, each sample comprising the CC recovered from a single COC.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Human CC samples were obtained from IVF treatments. Only unfrozen oocytes and embryos not submitted to preimplantation genetic testing were included in the analysis. Metabolomics analysis was performed by ultra-high performance liquid chromatography-tandem mass spectroscopy.
MAIN RESULTS AND THE ROLE OF CHANCE
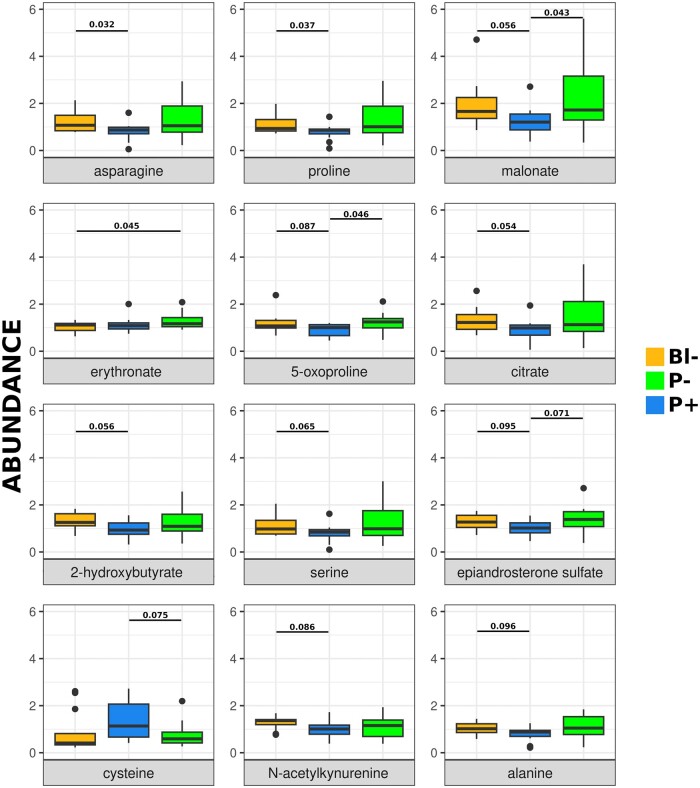
The analysis identified 98 compounds, five of which were differentially abundant (P < 0.05) between groups: asparagine, proline, and malonate were less abundant in P− compared to Bl−, malonate and 5-oxoproline were less abundant in P− group compared to P+, and erythronate was less abundant in Bl− group compared to P+. No significant association between the abundance of the compounds identified and donor age or BMI was noted.
LIMITATIONS, REASONS FOR CAUTION
Data dispersion and the lack of coherence between developmental groups preclude the direct use of metabolic markers in clinical practice, where the uterine environment plays a major role in pregnancy outcome. The abundance of other compounds not detected by the analysis may be associated with oocyte competence. As donors were lean (only two with BMI > 30 kg/m2) and young (<34 years old), a possible effect of obesity or advanced age on the CC metabolome could not be determined.
WIDER IMPLICATIONS OF THE FINDINGS
The abundance of malonate, 5-oxyproline, and erythronate in CC was significantly higher in COCs ultimately establishing pregnancy, providing clues on the pathways required for oocyte competence. The untargeted analysis uncovered the presence of compounds that were not expected in CC, such as β-citrylglutamate and the neurotransmitter N-acetyl-aspartyl-glutamate, which may play roles in chromatin remodeling and signaling, respectively.
STUDY FUNDING/COMPETING INTEREST(S)
Research was supported by the Industrial Doctorate Project IND2017/BIO-7748 funded by Madrid Region Government. The authors declare no competing interest.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: cumulus cells, oocyte quality, metabolomics, metabolome, cumulus signaling, malonate, oxyproline, erythronate, NAAG, β-citrylglutamate
Introduction
Oocyte growth and maturation occur alongside the adjacent somatic cells, called cumulus cells (CC), which communicate directly with the oocyte through transzonal projections that allow the transfer of regulatory factors and metabolites to the oocyte, forming an interconnected structure termed the cumulus–oocyte complex (COC). Within the COC, the oocyte and the surrounding CC are co-dependent in terms of both metabolism and signaling pathways: oocytes fail to progress in meiosis when they are devoid of CC (De La Fuente and Eppig, 2001; Fatehi et al., 2002), and CC fail to proliferate and expand (Vanderhyden et al., 1990), and show an altered metabolism when they are separated from the oocyte (Sugiura et al., 2005). Given that these supporting cells are discarded prior to ICSI, analysis of the biochemical compounds present in this easily accessible biological material may serve to shed light on the metabolic and signaling pathways used by the COC and to uncover molecular proxies to assist oocyte or embryo selection.
Current knowledge of COC metabolic and signaling pathways has been gathered from pioneer experiments that evaluated the developmental effects of the addition of specific metabolites or activators/inhibitors of specific metabolic or signaling routes to the media where COC develops (Biggers et al., 1967; Barbehenn et al., 1974; Herrick et al., 2006). This information, combined with the analysis of metabolites in the spent medium where COC developed, the expression of key metabolic enzymes, transporters or signaling receptors, and the unequivocal answers provided by gene ablation approaches (Cetica et al., 2002; Bermejo-Alvarez et al., 2010; Sutton-McDowall et al., 2010; Lamas-Toranzo et al., 2018) has served to uncover the details of COC metabolism and signaling pathways required for oocyte competence (Sobinoff et al., 2013; Richani et al., 2021). High-throughput metabolomics analysis provides an alternative, and largely unexplored, means to gain novel insights into metabolic and signaling pathways. Such analysis allows an untargeted search of biochemical compounds and has been applied to different reproductive fluids and tissues, such as human follicular fluid (de la Barca et al., 2017; Castiglione Morelli et al., 2019; Huang et al., 2022), but, to our knowledge, a global untargeted analysis of biochemical compounds has not been conducted in human CC.
Beyond the interest in providing basic knowledge on COC metabolism and signaling pathways, the analysis of the biochemical compounds present in CC could provide predictive markers of oocyte or embryo developmental competence. These markers could assist in embryo selection to maximize clinical pregnancy rate, which remains around 35% (European IVF-monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE), 2020). Unfortunately, although significant efforts have been devoted to uncover useful markers of developmental competence, embryo selection based on morphology still constitutes the only undisputed tool to improve pregnancy rate (Gardner and Balaban, 2016). Metabolomics analyses in the medium where the embryo develops (spent medium) have yielded promising preclinical markers to infer prospective pregnancy success, but the markers identified by independent groups vary and final clinical performance has been poor (reviewed by Hernandez-Vargas et al., 2020). Focusing on the search for molecular markers that could predict pregnancy potential in CC, global transcriptomics analysis (Green et al., 2018; Martinez-Moro et al., 2023) or the amount of mitochondrial DNA (Kumar et al., 2021; Liu et al., 2021; Martinez-Moro et al., 2022a,b) have been reported to provide a poor predictive value of pregnancy outcome. Compared to these analyses, metabolomics provides a downstream view of CC function, integrating possible differences between competent and non-competent COCs occurring at upstream levels, such as gene expression, post-transcriptional modification, or enzymatic activity.
The objective of this study was to investigate the biochemical compounds present in human CC and to determine if they could be used as predictors of subsequent embryo development. CCs were collected from individual COCs and, once the developmental outcome of each oocyte was known, the stored samples were allocated to one of three groups according to the oocyte’s developmental potential: oocytes not developing to blastocyst (Bl−); oocytes developing to blastocyst but not establishing pregnancy (P−); or oocytes ultimately establishing a pregnancy (P+).
Materials and methods
Sample collection
CCs were obtained from donor cycles at IVF Spain Madrid between June 2018 and June 2020. All patients agreed to participate in the study (providing written informed consent), which was approved by the Ethical Committee from La Princesa University Hospital (Madrid). Inclusion criteria were the following: absence of uterine abnormalities; donor age ≤37 years; recipient age ≤50 years; and normal male sperm count (>2 millions spermatozoa/ml in ejaculate). BMI, endometrial thickness, and donor age were similar between experimental groups (Supplementary Table S1), although recipient age was higher in P+ group (Table 1). Only unfrozen oocytes and embryos not submitted to preimplantation genetic testing were included in the analysis.
Table 1.
Characteristics of patients and donors in a study of the metabolome of cumulus cells obtained from cumulus–oocyte complexes.
| Bl− | P− | P+ | |
|---|---|---|---|
| Age patients (years) | – | 39.25 ± 1.95a | 44.50 ± 0.75b |
| BMI patients (kg/m2) | – | 25.18 ± 1.83 | 21.92 ± 1.03 |
| Smoker (patient) | – | 3/12 | 2/12 |
| Endometrial thickness (mm) | – | 9.16 ± 0.42 | 8.80 ± 0.45 |
| Age donors (years) | 27.33 ± 1.17 | 25.92 ± 1.37 | 27.33 ± 1.16 |
| BMI donors (kg/m2) | 22.59 ± 1.12 | 21.36 ± 0.89 | 22.64 ± 1.24 |
| Smoker (donors) | 6/12 | 4/12 | 5/12 |
Data are shown as mean ± SEM (n = 12/group), significant differences are indicated by different letters (ANOVA; P < 0.05).
Patients and donors were stimulated using recombinant FSH (corifollitropin alfa, Elonva®, MSD Biotech, Ireland) starting on the second menstruation day and ovulation was induced with GnRH analog (ganirelix, Orgalutran®, MSD Biotech, Ireland) analog when three or more follicles larger than 17 mm were present (Melo et al., 2009). Ovum pick-up was performed 36 h after hCG analog (triptorelin, Decapeptyl®, Tecnofarma, Chile) injection by transvaginal ultrasound guidance. Endometrium stimulation treatment in recipients consisted of 6 mg/day of estradiol (Meriestra®, Sandoz, Spain) and embryo transfer was performed only if the endometrium measured 7–13 mm (Prapas et al., 1998). Luteal support started on the night of oocyte retrieval with 400 mg of progesterone administered every 12 h until a pregnancy test (Edwards et al., 1984).
COCs were retrieved following follicular aspiration, washed, and individually cultured in G-IVF Plus Media (Vitrolife, Sweden) (6.3% CO2, 5% O2, 88.7% N2 at 37 °C). Denudation was performed individually 2 h after COC recovery, using hyaluronidase solution (Irvine). CCs detached from oocytes were collected from denudation medium, pelleted by centrifugation at 1500×g for 10 min at room temperature, snap frozen in liquid nitrogen, and stored at −80°C until analysis. Oocytes were fertilized by intracytoplasmic sperm injection (ICSI) using G-MOPS Plus (Vitrolife, Sweden) and spermatozoa were allocated in 7% polyvinylpyrrolidone solution to slow their movement. Following ICSI, the presumptive zygotes were individually cultured in Continuous Single Culture SAGE 1-StepTM (Irvine, CA, USA) for 6 days, up to the blastocyst stage. Morphological score was used to select blastocysts for embryo transfer (Gardner et al., 2015). Pregnancy was assessed at the fourth or fifth week post-fertilization by fetal heart rate detection by ultrasound echography. Once the embryo development was known, the previously stored CCs were allocated into three groups according to the oocyte’s developmental potential: oocytes not developing to blastocyst (Bl−); oocytes developing to blastocyst but failing to establish pregnancy following embryo transfer (P−); or oocytes developing to blastocyst able to establish pregnancy (P+).
Metabolomics analysis
Metabolomics analyses were performed on 12 samples per group, which were shipped in dry ice to Metabolon Inc. (Durham, NC, USA). Samples were prepared using the automated MicroLab STAR® system from Hamilton Company. Several recovery standards were added prior to the first step in the extraction process for quality control (QC) purposes. To remove protein, dissociate small molecules bound to protein or trapped in the precipitated protein matrix, and to recover chemically diverse metabolites, proteins were precipitated with methanol under vigorous shaking for 2 min followed by centrifugation at 1493×g for 10 min at RT. The resulting extract was divided into five fractions: two for analysis by two separate reversed-phase ultra-high performance liquid chromatography-mass spectrometry reverse phase methods [(RP)/UPLC-MS/MS] with positive ion model electrospray ionization (ESI), one for analysis by RP/UPLC-MS/MS with negative ion mode ESI, one for analysis by hidrophilic interaction chromatography (HILIC)/UPLC-MS/MS with negative ion mode ESI, and one sample was reserved for backup. Samples were placed briefly in TurboVap® (Zymark) to remove the organic solvent. The sample extracts were stored overnight under passive nitrogen gas before preparation for analysis.
Several types of controls were analyzed in concert with the experimental samples: a pooled matrix sample generated by taking a small volume of each experimental sample (or alternatively, use of a pool of well-characterized human plasma) served as a technical replicate throughout the data set; extracted water samples served as process blanks; and a cocktail of QC standards, carefully chosen not to interfere with the measurement of endogenous compounds, were spiked into every analyzed sample, that allowed instrument performance monitoring and aided chromatographic alignment. Instrument variability was determined by calculating the median relative standard deviation (RSD) for the standards that were added to each sample prior to injection into the mass spectrometers. Overall process variability was determined by calculating the median RSD for all endogenous metabolites (i.e. non-instrument standards) present in 100% of the pooled matrix samples. Experimental samples were randomized across the platform run with QC samples spaced evenly among the injections.
Ultrahigh Performance Liquid Chromatography-Tandem Mass Spectroscopy (UPLC-MS/MS) was performed on a Waters ACQUITY UPLC and a Thermo Scientific Q-Exactive high resolution/accurate mass spectrometer interfaced with a heated electrospray ionization (HESI-II) source and Orbitrap mass analyser operated at 35 000 mass resolution (ThermoFisher, MS, USA). The sample extract was dried then reconstituted in solvents compatible to each of the four methods. Each reconstitution solvent contained a series of standards at fixed concentrations to ensure injection and chromatographic consistency. One aliquot was analyzed using acidic positive ion conditions, chromatographically optimized for more hydrophilic compounds. In this method, the extract was gradient eluted from a C18 column (Waters UPLC BEH C18-2.1 × 100 mm, 1.7 µm) using water and methanol, containing 0.05% perfluoropentanoic acid (PFPA) and 0.1% formic acid (FA). Another aliquot was also analyzed using acidic positive ion conditions, but it was chromatographically optimized for more hydrophobic compounds. In this method, the extract was gradient eluted from a C18 column (Waters UPLC BEH C18-2.1 × 100 mm, 1.7 µm) using methanol, acetonitrile, water, 0.05% PFPA, and 0.01% FA and was operated at an overall higher organic content. Another aliquot was analyzed using basic negative ion optimized conditions using methanol and water with 6.5 mM ammonium bicarbonate at pH8. The fourth aliquot was analyzed via negative ionization following elution from a HILIC column (Waters UPLC BEH Amide 2.1 × 150 mm, 1.7 µm) using a gradient consisting of water and acetonitrile with 10 mM ammonium formate, pH 10.8. The MS analysis alternated between MS and data-dependent MSn scan using dynamic exclusion. The scan range varied slighted between methods but covered 70–100 m/z.
Raw data were extracted, peak identified, and QC processed using Metabolon’s hardware and software (Metabolon, NC, USA). Compounds were identified by comparison to library entries of purified standards. More than 3300 standard compounds are registered for analysis. Biochemical identifications are based on three criteria: retention index within a narrow retention time/index window of the proposed identification, accurate mass match to the library±10 parts per million, and the MS/MS forward and reverse scores between the experimental data and authentic standards. Peaks were quantified using area-under-the-curve. A matrix with the normalized peak intensities was processed in an in-house R script, making use of the metabolomics package omu (Tiffany and Baumler, 2019). Pairwise differential analysis was performed through a Welch test implemented within the omu_summary function applying the Benjamini–Hochberg correction. Principal component analysis (PCA) and boxplots were obtained from ggplot2 package and heatmaps with hierarchical clustering were drawn by the package ComplexHeatmap (Gu et al., 2016).
Results
Metabolomics analysis identified 98 compounds of known identity in human CC (excluding xenobiotics: Supplementary Table S2). The list of compounds comprised 30 amino acid-related compounds, including amino acids involved in protein synthesis and amino acids and related compounds involved in other processes, such as glutathione, taurine, creatine, spermidine, β-citrylglutamate, and N-acetyl-aspartyl-glutamate (NAAG). Nine carbohydrates were detected, including five involved in glycolysis (glucose, 3-phosphoglycerate, pyruvate, lactate, and glycerate), and others, such as 6-phosphogluconate (involved in pentose phosphate pathway), fructose, mannitol/sorbitol, and the aminosugar erythronate. Compounds of the tricarboxylic acid (TCA) cycle (citrate, aconitate, alpha-ketoglutarate, and malate), phosphate (involved in oxidative phosphorylation), nucleotides (including AMP/cAMP), and vitamins and cofactors—such as threonate, nicotinamide, and alpha-tocopherol—were also identified in human CC. Lipid constituted the largest group of compounds detected. A large proportion of the 45 lipid-related compounds detected were phospholipids, including 20 glycerophospholipids and nine sphyngolipids. Other lipid-related compounds detected were steroid hormones and precursors, fatty acids, and myo-inositol.
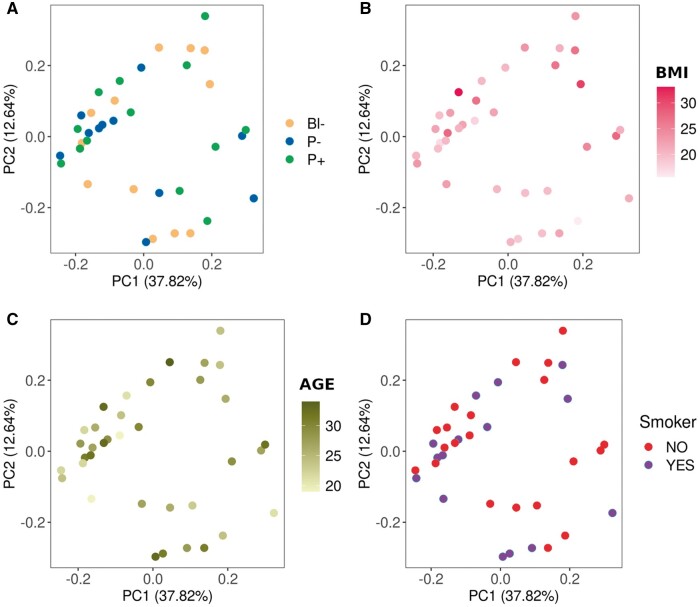
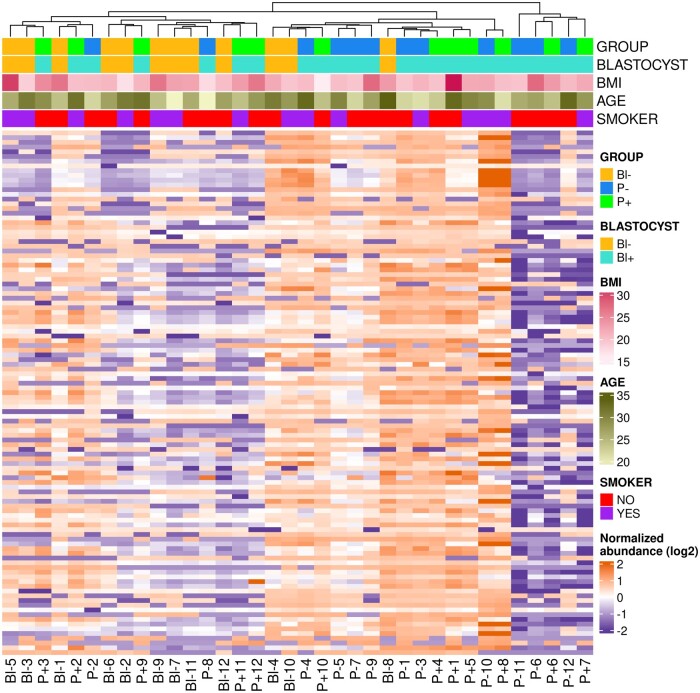
No evident association was observed between the abundance of all biochemical compounds identified in CC and the developmental competence of the enclosed oocyte, as PCA and hierarchical clustering failed to group samples according to subsequent embryo development (Figs 1A and 2). There was also no association between the abundance of all biochemicals detected and donor weight, age, or smoking status (Figs 1B–D and 2). The abundance of five individual compounds showed statistically significant differences between the groups that differed in developmental ability (Fig. 3). In particular, asparagine, proline, and malonate were less abundant in P− compared to Bl−, malonate, and 5-oxoproline were less abundant in P− group compared to P+, and erythronate was less abundant in Bl− group compared to P+. Tendencies (0.05 < P < 0.1) were noted for another seven compounds: citrate, 2-hydroxybutyrate, serine, epiandrosterone sulfate, cysteine, N-acetylkynurenine, and alanine (Fig. 2). When data were analyzed depending on the ability to reach the blastocyst stage (i.e. Bl− versus Bl+, the latter composed by P− and P+ samples), no statistically significant differences were found, although trends (P 0.07–0.08) were found for erythronate—more abundant in Bl+ group- and N-acetylkynurenine—less abundant in Bl− group- (Supplementary Fig. S1), and Bl− samples roughly clustered together when all biochemicals detected were considered in an hierarchical clustering (Fig. 2).
Figure 1.
Principal component analysis of the metabolomics data obtained from the cumulus cells of 36 single human cumulus–oocyte complexes. The analyses are presented according to developmental ability (A), donor BMI (kg/m2) (B), age (years) (C), or smoking status (D). (A) Cumulus cells were classified into three groups depending on the developmental ability of their corresponding oocyte: oocytes unable to develop to blastocysts (Bl−, orange), able to develop to blastocyst but not establishing pregnancy (P−, blue) or able to establish pregnancy (P+, green). (B and C) Color scales were employed for numerical variables: BMI (light to dark red) and age (light to dark green). (D) Smoking status is depicted as red for non-smokers and purple for smokers. PC, principal component.
Figure 2.
Hierarchical clustering of the metabolomics data obtained from the cumulus cells of 36 single human cumulus–oocyte complexes. Upper color bars indicate developmental ability and donor characteristics. The uppermost color bar indicates developmental ability: oocytes unable to develop to blastocysts (Bl−, orange), able to develop to blastocyst but not establishing pregnancy (P−, blue) or able to establish pregnancy (P+, green). The next bar down distinguishes oocytes unable to develop to blastocysts (Bl−, orange) and those able to develop to blastocyst (Bl+, turquoise). The next two color bars indicate donor BMI (light to dark red) and age (light to dark green). The lowest color bar indicates smoking status, red for non-smokers, and purple for smokers.
Figure 3.
Relative abundance of biochemical compounds in cumulus cells surrounding human oocytes of different developmental ability. Oocytes unable to develop to blastocysts (Bl−, orange), able to develop to blastocyst but not establishing pregnancy (P−, blue) or able to establish pregnancy (P+, green). Compounds above the black line (asparagine, proline, malonate, erythronate, and 5-oxyproline) were differentially abundant between groups based on Welch test, P-values are indicated for each compound and statistically significant comparison. Individual points indicate samples diverging greatly from the group (included in the analysis). The seven compounds below the black line tended to differ (0.05 < P < 0.01) between groups.
Discussion
CCs constitute a readily available material on which to perform analyses aimed at inferring the developmental ability of the oocyte they nourished (Kordus and LaVoie, 2017). Mitochondrial DNA and transcriptomics analyses of CC have failed to provide reliable and clinically useful markers of the embryo potential to establish pregnancy (Green et al., 2018; Kumar et al., 2021; Liu et al., 2021; Martinez-Moro et al., 2022a,b, 2023), but metabolomics has remained largely unexplored, probably owing to the technical difficulties of performing such analysis of the limited amount of cells present in a COC. Herein, using an improved MS/MS protocol, more sensitive than nuclear magnetic resonance approaches (Emwas, 2015), we were able to quantify the relative abundance of 98 compounds.
Despite the high number of compounds analyzed, the comparison between the three groups differing in their developmental ability failed to yield clear biochemical markers of oocyte competence. Although some metabolites showed statistically significant differences between groups, such differences did not follow a logical trend. A reliable marker to infer the oocyte’s ability to reach the blastocyst stage should display differences in both Bl− versus P− and Bl− versus P+ comparisons (i.e. the group showing impaired preimplantation development compared to either group successfully developing to blastocysts). Conversely, a dependable proxy of the ability to establish pregnancy would be expected to display significant differences in both Bl− versus P+ and P− versus P+ comparisons (i.e. the group establishing pregnancy compared to the groups arresting their development earlier), although in this case the value of P+ versus Bl− is less informative. None of the five compounds showing statistically significant differences between groups fulfilled those stringent conditions and the Bl− versus Bl+ comparison also failed to yield significant differences. However, malonate and 5-oxyproline were more abundant in P+ compared to P, and erythronate was more abundant in P+ compared to Bl− and numerically higher in P+ versus P−.
Malonate inhibits succinate dehydrogenase (Pardee and Potter, 1949), a component of the TCA cycle and complex II of the mitochondrial electron transport chain. The increased malonate content in P+ group may indicate a diminished TCA activity in more competent COCs. Such reduction in TCA activity could reduce oxidative stress, shifting metabolism to anaerobic routes (Bermejo-Alvarez et al., 2010). 5-Oxyproline (pyroglutamic acid) is a precursor of glutamate, an amino acid suggested to play signaling roles in oocytes and preimplantation embryos (Spirkova et al., 2022). Glutamate is also one of the three amino acids forming glutathione, together with cysteine and glycine, all detected in CC by the analysis. Glutathione constitutes the major non-enzymatic anti-oxidant (Meister and Anderson, 1983) and has been linked to oocyte quality, as mice unable to synthesize glutathione (Glmc KO) show an accelerated age-related decline in female fertility that is associated with preimplantation embryonic mortality (Nakamura et al., 2011), and glutathione content in the oocyte has been positively associated with oocyte competence (Perreault et al., 1988). Paradoxically, 5-oxyproline content in CC was elevated in women with polycystic ovary syndrome, an increase suggested to be caused by a reduced capacity to synthesize glutathione (Turathum et al., 2022). Despite the differences observed in 5-oxyproline levels, our analysis found no differences in glutamate or glutathione content in CC between groups exhibiting different developmental ability. From this perspective, the increased amount of 5-oxyproline in the P+ group might be indicative of an available surplus after the synthesis of glutamate and/or glutathione, which could have been transported from the CC into the oocyte before cumulus expansion. Finally, erythronic acid is formed by the oxidation of N-acetyl-D-glucosamine (GlcNAc), a constituent of hyaluronic acid (HA). HA is a linear glycosaminoglycan, consisting of alternating units of glucuronic acid and GlNAc that plays an essential role during CC expansion (Chen et al., 1990; Salustri et al., 1992). The increased content in erythronate may therefore be indicative of a greater or earlier CC expansion, which has been positively associated with oocyte quality in animal models (Qian et al., 2003; Martinez-Moro et al., 2022a,b).
Despite the large number of lipid compounds detected, their amounts were not associated with developmental ability. In contrast, a prior lipidomics analysis conducted on human CC comparing groups equivalent to P− and P+ here suggested a higher abundance of phosphatidylcholine in P+ and a higher abundance of phosphatidylethanolamine, phosphatidylserine, and phosphatidylinositol in P− (Montani et al., 2012). Unfortunately, these compounds were not among the 98 compounds identified by our analysis, although 11 compounds related to phosphatidylcholine and four related to phosphatidylethanolamine were detected and did not show differences between groups. A possible explanation for the discrepancies between studies may be the different methods used for lipid detection and characterization, but given that no relative amount data or fold-change was provided on the lipidomics study (Montani et al., 2012), data analysis constitutes a major difference between the studies. Donor BMI and age have been found to exert an effect on different molecular markers in CC (reviewed by Richani et al. (2021), Babayev and Duncan (2022)), but their effects on the CC metabolome were unexplored. Here, we did not observe a significant correlation between donor BMI and age and the CC metabolome but the population analyzed does not allow us to draw solid conclusions, as donors were mostly lean (only 2/30 with BMI > 30 kg/m2) and young (the oldest being 34 years old).
Beyond the search for proxies of oocyte competence, the untargeted analysis of biochemical compounds present in CC provides a novel means to uncover signaling pathways. The metabolomics analysis identified amino acid-related compounds whose presence in CC was unexpected. Among them, β-citrylglutamate is a pseudodipeptide abundant in several neonatal tissues whose concentration declines rapidly after birth, with the exception of testes, where it increases with age coinciding with the development of late spermatocytes into early spermatids (Montani et al., 1982). The presence of this compound in CC was, therefore, unexpected and its role in follicular development is unknown. In mice, the β-citrylglutamate synthase Rimklb is expressed in Leydig cells and its ablation has been observed to result in male infertility (Maekura et al., 2021) or subfertility caused by impaired histone to protamine exchange and retention of transition protein 2 during spermiogenesis (Wang-Eckhardt et al., 2022). Despite the remarkable differences in chromatin compaction between male and female mammalian gametogenesis, β-citrylglutamate could intervene in chromatin condensation during oogenesis. As Rimklb knock-out (KO) mice do not show female infertility, β-citrylglutamate role may not be required for female gametogenesis, but it may be possible that KO COCs are not devoid of β-citrylglutamate, as another β-citrylglutamate synthase may be acting during oogenesis.
Another unexpected compound detected in human CC was NAAG, a dipeptide that consists of glutamate and N-acetylaspartate (both also detected in CCs by the analysis) coupled via a peptide bond. NAAG acts as neurotransmitter through activation of the presynaptic metabotropic receptor 3 (GRM3, also known as mGluR3) (Wroblewska et al., 1997), which is almost exclusively expressed in brain (Fagerberg et al., 2014) and is undetectable in CC from humans and close mammalian models (Martinez-Moro et al., 2022a,b, 2023). The presence of NAAG in CC was therefore unexpected and its role is unknown, but it may mediate signaling roles through NMDA (N-methyl-D-aspartate) receptors, as observed in neurons (Khacho et al., 2016), given that multiple NMDA receptors (GRINA, GRIN2A GRIN2C, GRIN2D, and GRIN3B) are expressed in human CC (Martinez-Moro et al., 2022a,b). Other amino acid-related compounds detected, such as taurine, creatine, and spermidine, have been previously observed in COCs or follicular fluid. Taurine has been previously detected in CC (Lobo et al., 2001), where it probably plays anti-oxidant and biological membrane stabilization roles (Goberdhan, 2010). Creatine has been suggested to act as a probable modulator of ATP concentration in the oocyte (Scantland et al., 2014), and spermidine is a polyamine involved in post-translational modifications (Pegg, 2016) that has been reported to be more abundant in follicular fluid from women with diminished ovarian reserve (Gokce et al., 2023).
In conclusion, the abundance of the 98 compounds identified in CC was largely unrelated to the developmental competence of the enclosed oocyte, although malonate, 5-oxyproline, and erythronate were positively associated with pregnancy potential. However, given data dispersion and the lack of coherence between the three groups analyzed, the predictive value in clinical practice is poor. Two unexpected amino acid-related compounds, β-citrylglutamate and NAAG, were detected in CC, where they could play chromatin remodeling and signaling roles, respectively.
Supplementary Material
Acknowledgements
The authors want to acknowledge the patients and donors who agree to participate in the study.
Contributor Information
Álvaro Martínez-Moro, Animal Reproduction Department, INIA, CSIC, Madrid, Spain; IVF Spain, Madrid, Spain.
Leopoldo González-Brusi, Animal Reproduction Department, INIA, CSIC, Madrid, Spain.
Ana Querejeta-Fernández, Departamento de Química Inorgánica, Facultad de Ciencias Químicas, Universidad Complutense, Madrid, Spain.
Ester Padilla-Ruiz, IVF Spain, Madrid, Spain.
Javier García-Blanco, IVF Spain, Madrid, Spain.
Pablo Bermejo-Álvarez, Animal Reproduction Department, INIA, CSIC, Madrid, Spain.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Authors’ roles
Human samples were collected by A.M.-M., J.G-B., and E.P.-R. L.G.-B. performed data analysis. Data interpretation was performed by A.M.-M., L.G.-B., A.Q.-F., and P.B.-A. A.M.-M. and P.B.-A. wrote the article, being supervised by all authors.
Funding
The research was supported by the Industrial Doctorate Project IND2017/BIO-7748.
Conflict of interest
The authors declare no conflict of interest.
References
- Babayev E, Duncan FE.. Age-associated changes in cumulus cells and follicular fluid: the local oocyte microenvironment as a determinant of gamete quality. Biol Reprod 2022;106:351–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbehenn EK, Wales RG, Lowry OH.. The explanation for the blockade of glycolysis in early mouse embryos. Proc Natl Acad Sci USA 1974;71:1056–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermejo-Alvarez P, Lonergan P, Rizos D, Gutierrez-Adan A.. Low oxygen tension during IVM improves bovine oocyte competence and enhances anaerobic glycolysis. Reprod Biomed Online 2010;20:341–349. [DOI] [PubMed] [Google Scholar]
- Biggers J, Whittingham D, Donahue R.. The pattern of energy metabolism in the mouse oocyte and zygote. Proc Natl Acad Sci USA 1967;58:560–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castiglione Morelli MA, Iuliano A, Schettini SCA, Petruzzi D, Ferri A, Colucci P, Viggiani L, Cuviello F, Ostuni A.. NMR metabolic profiling of follicular fluid for investigating the different causes of female infertility: a pilot study. Metabolomics 2019;15:19. [DOI] [PubMed] [Google Scholar]
- Cetica P, Pintos L, Dalvit G, Beconi M.. Activity of key enzymes involved in glucose and triglyceride catabolism during bovine oocyte maturation in vitro. Reproduction 2002;124:675–681. [PubMed] [Google Scholar]
- Chen L, Wert SE, Hendrix EM, Russell PT, Cannon M, Larsen WJ.. Hyaluronic acid synthesis and gap junction endocytosis are necessary for normal expansion of the cumulus mass. Mol Reprod Dev 1990;26:236–247. [DOI] [PubMed] [Google Scholar]
- de la Barca JMC, Boueilh T, Simard G, Boucret L, Ferre-L’Hotellier V, Tessier L, Gadras C, Bouet PE, Descamps P, Procaccio V. et al. Targeted metabolomics reveals reduced levels of polyunsaturated choline plasmalogens and a smaller dimethylarginine/arginine ratio in the follicular fluid of patients with a diminished ovarian reserve. Hum Reprod 2017;32:2269–2278. [DOI] [PubMed] [Google Scholar]
- De La Fuente R, Eppig JJ.. Transcriptional activity of the mouse oocyte genome: companion granulosa cells modulate transcription and chromatin remodeling. Dev Biol 2001;229:224–236. [DOI] [PubMed] [Google Scholar]
- Edwards RG, Fishel SB, Cohen J, Fehilly CB, Purdy JM, Slater JM, Steptoe PC, Webster JM.. Factors influencing the success of in vitro fertilization for alleviating human infertility. J In Vitro Fert Embryo Transf 1984;1:3–23. [DOI] [PubMed] [Google Scholar]
- Emwas AH. The strengths and weaknesses of NMR spectroscopy and mass spectrometry with particular focus on metabolomics research. Methods Mol Biol 2015;1277:161–193. [DOI] [PubMed] [Google Scholar]
- European IVF-monitoring Consortium (EIM)‡ for the European Society of Human Reproduction and Embryology (ESHRE), Wyns C, Bergh C, Calhaz-Jorge C, De Geyter C, Kupka MS, Motrenko T, Rugescu I, Smeenk J, Tandler-Schneider A, Vidakovic S. et al. ART in Europe, 2016: results generated from European registries by ESHRE. Hum Reprod Open 2020;2020:hoaa032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerberg L, Hallstrom BM, Oksvold P, Kampf C, Djureinovic D, Odeberg J, Habuka M, Tahmasebpoor S, Danielsson A, Edlund K. et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteomics 2014;13:397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatehi AN, Zeinstra EC, Kooij RV, Colenbrander B, Bevers MM.. Effect of cumulus cell removal of in vitro matured bovine oocytes prior to in vitro fertilization on subsequent cleavage rate. Theriogenology 2002;57:1347–1355. [DOI] [PubMed] [Google Scholar]
- Gardner DK, Balaban B.. Assessment of human embryo development using morphological criteria in an era of time-lapse, algorithms and ‘OMICS’: is looking good still important? Mol Hum Reprod 2016;22:704–718. [DOI] [PubMed] [Google Scholar]
- Gardner DK, Meseguer M, Rubio C, Treff NR.. Diagnosis of human preimplantation embryo viability. Hum Reprod Update 2015;21:727–747. [DOI] [PubMed] [Google Scholar]
- Goberdhan DC. Intracellular amino acid sensing and mTORC1-regulated growth: new ways to block an old target? Curr Opin Investig Drugs 2010;11:1360–1367. [PMC free article] [PubMed] [Google Scholar]
- Gokce S, Herkiloglu D, Cevik O, Turan V.. Evaluation of intrafollicular syndecan 1, glypican 3, and spermidine levels in women with diminished ovarian reserve. Reprod Sci 2023;30:569–575. [DOI] [PubMed] [Google Scholar]
- Green KA, Franasiak JM, Werner MD, Tao X, Landis JN, Scott RT Jr, Treff NR.. Cumulus cell transcriptome profiling is not predictive of live birth after in vitro fertilization: a paired analysis of euploid sibling blastocysts. Fertil Steril 2018;109:460–466.e2. [DOI] [PubMed] [Google Scholar]
- Gu Z, Eils R, Schlesner M.. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 2016;32:2847–2849. [DOI] [PubMed] [Google Scholar]
- Hernandez-Vargas P, Munoz M, Dominguez F.. Identifying biomarkers for predicting successful embryo implantation: applying single to multi-OMICs to improve reproductive outcomes. Hum Reprod Update 2020;26:264–301. [DOI] [PubMed] [Google Scholar]
- Herrick JR, Brad AM, Krisher RL.. Chemical manipulation of glucose metabolism in porcine oocytes: effects on nuclear and cytoplasmic maturation in vitro. Reproduction 2006;131:289–298. [DOI] [PubMed] [Google Scholar]
- Huang Y, Tu M, Qian Y, Ma J, Chen L, Liu Y, Wu Y, Chen K, Liu J, Ying Y. et al. Age-dependent metabolomic profile of the follicular fluids from women undergoing assisted reproductive technology treatment. Front Endocrinol (Lausanne) 2022;13:818888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khacho P, Wang B, Bergeron R.. The good and bad sides of NAAG. Adv Pharmacol 2016;76:311–349. [DOI] [PubMed] [Google Scholar]
- Kordus RJ, LaVoie HA.. Granulosa cell biomarkers to predict pregnancy in ART: pieces to solve the puzzle. Reproduction 2017;153:R69–R83. [DOI] [PubMed] [Google Scholar]
- Kumar K, Venturas M, Needleman DJ, Racowsky C, Wells D.. Extensive analysis of mitochondrial DNA quantity and sequence variation in human cumulus cells and assisted reproduction outcomes. Hum Reprod 2021;37:66–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamas-Toranzo I, Pericuesta E, Bermejo-Alvarez P.. Mitochondrial and metabolic adjustments during the final phase of follicular development prior to IVM of bovine oocytes. Theriogenology 2018;119:156–162. [DOI] [PubMed] [Google Scholar]
- Liu W, Guo J, Li C, Liao H, Qin Y, Huang G.. Mitochondrial DNA copy number of cumulus cells is not linked to embryo implantation in good prognosis IVF patients. Reprod Biomed Online 2021;42:901–908. [DOI] [PubMed] [Google Scholar]
- Lobo MV, Alonso FJ, Latorre A, del Rio RM.. Immunohistochemical localization of taurine in the rat ovary, oviduct, and uterus. J Histochem Cytochem 2001;49:1133–1142. [DOI] [PubMed] [Google Scholar]
- Maekura K, Tsukamoto S, Hamada-Kanazawa M, Takano M.. Rimklb mutation causes male infertility in mice. Sci Rep 2021;11:4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Moro A, Gonzalez-Brusi L, Lamas-Toranzo I, Gonzalez-Dosal P, Rodriguez-Juarez F, Bermejo-Alvarez P.. The human cumulus cell transcriptome provides poor predictive value for embryo transfer outcome. Reprod Biomed Online 2023;46:783–791. [DOI] [PubMed] [Google Scholar]
- Martinez-Moro A, Gonzalez-Brusi L, Lamas-Toranzo I, O’Callaghan E, Esteve-Codina A, Lonergan P, Bermejo-Alvarez P.. RNA-sequencing reveals genes linked with oocyte developmental potential in bovine cumulus cells. Mol Reprod Dev 2022a;89:399–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Moro A, Lamas-Toranzo I, Gonzalez-Brusi L, Perez-Gomez A, Padilla-Ruiz E, Garcia-Blanco J, Bermejo-Alvarez P.. mtDNA content in cumulus cells does not predict development to blastocyst or implantation. Hum Reprod Open 2022b;2022:hoac029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister A, Anderson ME.. Glutathione. Annu Rev Biochem 1983;52:711–760. [DOI] [PubMed] [Google Scholar]
- Nakamura BN, Fielder TJ, Hoang YD, Lim J, McConnachie LA, Kavanagh TJ, Luderer U.. Lack of maternal glutamate cysteine ligase modifier subunit (GCLM) decreases oocyte glutathione concentrations and disrupts preimplantation development in mice. Endocrinology 2011;152:2806–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo M, Busso CE, Bellver J, Alama P, Garrido N, Meseguer M, Pellicer A, Remohi J.. GnRH agonist versus recombinant HCG in an oocyte donation programme: a randomized, prospective, controlled, assessor-blind study. Reprod Biomed Online 2009;19:486–492. [DOI] [PubMed] [Google Scholar]
- Montani DA, Cordeiro FB, Regiani T, Victorino AB, Pilau EJ, Gozzo FC, Miyake M, Kume S, Kakimoto Y.. Correlation of the level of beta-citryl-L-glutamic acid with spermatogenesis in rat testes. Biochim Biophys Acta 1982;719:495–500. [DOI] [PubMed] [Google Scholar]
- Montani DA, Cordeiro FB, Regiani T, Victorino AB, Pilau EJ, Gozzo FC, Ferreira CR, Fraietta R, Lo Turco EG.. The follicular microenviroment as a predictor of pregnancy: MALDI-TOF MS lipid profile in cumulus cells. J Assist Reprod Genet 2012;29:1289–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee AB, Potter VR.. Malonate inhibition of oxidations in the Krebs tricarboxylic acid cycle. J Biol Chem 1949;178:241–250. [PubMed] [Google Scholar]
- Pegg AE. Functions of polyamines in mammals. J Biol Chem 2016;291:14904–14912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault SD, Barbee RR, Slott VL.. Importance of glutathione in the acquisition and maintenance of sperm nuclear decondensing activity in maturing hamster oocytes. Dev Biol 1988;125:181–186. [DOI] [PubMed] [Google Scholar]
- Prapas Y, Prapas N, Jones EE, Duleba AJ, Olive DL, Chatziparasidou A, Vlassis G.. The window for embryo transfer in oocyte donation cycles depends on the duration of progesterone therapy. Hum Reprod 1998;13:720–723. [DOI] [PubMed] [Google Scholar]
- Qian Y, Shi WQ, Ding JT, Sha JH, Fan BQ.. Predictive value of the area of expanded cumulus mass on development of porcine oocytes matured and fertilized in vitro. J Reprod Dev 2003;49:167–174. [DOI] [PubMed] [Google Scholar]
- Richani D, Dunning KR, Thompson JG, Gilchrist RB.. Metabolic co-dependence of the oocyte and cumulus cells: essential role in determining oocyte developmental competence. Hum Reprod Update 2021;27:27–47. [DOI] [PubMed] [Google Scholar]
- Salustri A, Yanagishita M, Underhill CB, Laurent TC, Hascall VC.. Localization and synthesis of hyaluronic acid in the cumulus cells and mural granulosa cells of the preovulatory follicle. Dev Biol 1992;151:541–551. [DOI] [PubMed] [Google Scholar]
- Scantland S, Tessaro I, Macabelli CH, Macaulay AD, Cagnone G, Fournier E, Luciano AM, Robert C.. The adenosine salvage pathway as an alternative to mitochondrial production of ATP in maturing mammalian oocytes. Biol Reprod 2014;91:75. [DOI] [PubMed] [Google Scholar]
- Sobinoff AP, Sutherland JM, McLaughlin EA.. Intracellular signalling during female gametogenesis. Mol Hum Reprod 2013;19:265–278. [DOI] [PubMed] [Google Scholar]
- Spirkova A, Kovarikova V, Sefcikova Z, Pisko J, Ksinanova M, Koppel J, Fabian D, Cikos S.. Glutamate can act as a signaling molecule in mouse preimplantation embryosdagger. Biol Reprod 2022;107:916–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura K, Pendola FL, Eppig JJ.. Oocyte control of metabolic cooperativity between oocytes and companion granulosa cells: energy metabolism. Dev Biol 2005;279:20–30. [DOI] [PubMed] [Google Scholar]
- Sutton-McDowall ML, Gilchrist RB, Thompson JG.. The pivotal role of glucose metabolism in determining oocyte developmental competence. Reproduction 2010;139:685–695. [DOI] [PubMed] [Google Scholar]
- Tiffany CR, Baumler AJ.. omu, a metabolomics count data analysis tool for intuitive figures and convenient metadata collection. Microbiol Resour Announc 2019;8:e00129–e00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turathum B, Gao EM, Yang F, Liu YB, Yang ZY, Liu CC, Xue YJ, Wu MH, Wang L, Grataitong K. et al. Role of pyroglutamic acid in cumulus cells of women with polycystic ovary syndrome. J Assist Reprod Genet 2022;39:2737–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhyden BC, Caron PJ, Buccione R, Eppig JJ.. Developmental pattern of the secretion of cumulus expansion-enabling factor by mouse oocytes and the role of oocytes in promoting granulosa cell differentiation. Dev Biol 1990;140:307–317. [DOI] [PubMed] [Google Scholar]
- Wang-Eckhardt L, Sylvester M, Becker I, Allam JP, Eckhardt M.. Citrylglutamate synthase deficient male mice are subfertile with impaired histone and transition protein 2 removal in late spermatids. Biochem J 2022;479:953–972. [DOI] [PubMed] [Google Scholar]
- Wroblewska B, Wroblewski JT, Pshenichkin S, Surin A, Sullivan SE, Neale JH.. N-acetylaspartylglutamate selectively activates mGluR3 receptors in transfected cells. J Neurochem 1997;69:174–181. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.