Abstract
BACKGROUND
Modern lifestyle has led to an increase in the age at conception. Advanced age is one of the critical risk factors for female-related infertility. It is well known that maternal age positively correlates with the deterioration of oocyte quality and chromosomal abnormalities in oocytes and embryos. The effect of age on endometrial function may be an equally important factor influencing implantation rate, pregnancy rate, and overall female fertility. However, there are only a few published studies on this topic, suggesting that this area has been under-explored. Improving our knowledge of endometrial aging from the biological (cellular, molecular, histological) and clinical perspectives would broaden our understanding of the risks of age-related female infertility.
OBJECTIVE AND RATIONALE
The objective of this narrative review is to critically evaluate the existing literature on endometrial aging with a focus on synthesizing the evidence for the impact of endometrial aging on conception and pregnancy success. This would provide insights into existing gaps in the clinical application of research findings and promote the development of treatment options in this field.
SEARCH METHODS
The review was prepared using PubMed (Medline) until February 2023 with the keywords such as ‘endometrial aging’, ‘receptivity’, ‘decidualization’, ‘hormone’, ‘senescence’, ‘cellular’, ‘molecular’, ‘methylation’, ‘biological age’, ‘epigenetic’, ‘oocyte recipient’, ‘oocyte donation’, ‘embryo transfer’, and ‘pregnancy rate’. Articles in a language other than English were excluded.
OUTCOMES
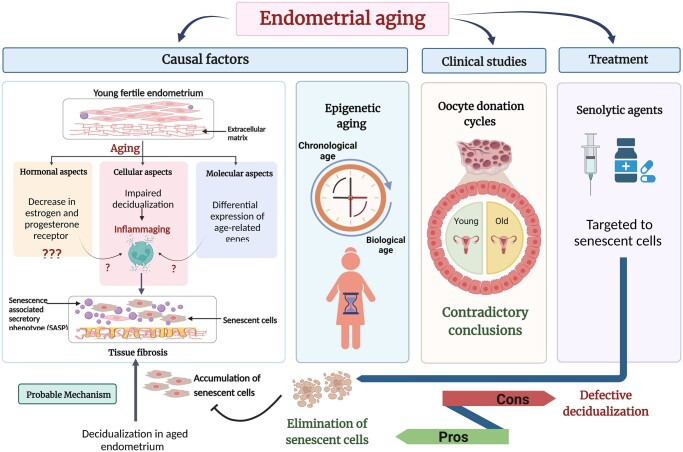
In the aging endometrium, alterations occur at the molecular, cellular, and histological levels suggesting that aging has a negative effect on endometrial biology and may impair endometrial receptivity. Additionally, advanced age influences cellular senescence, which plays an important role during the initial phase of implantation and is a major obstacle in the development of suitable senolytic agents for endometrial aging. Aging is also accountable for chronic conditions associated with inflammaging, which eventually can lead to increased pro-inflammation and tissue fibrosis. Furthermore, advanced age influences epigenetic regulation in the endometrium, thus altering the relation between its epigenetic and chronological age. The studies in oocyte donation cycles to determine the effect of age on endometrial receptivity with respect to the rates of implantation, clinical pregnancy, miscarriage, and live birth have revealed contradictory inferences indicating the need for future research on the mechanisms and corresponding causal effects of women’s age on endometrial receptivity.
WIDER IMPLICATIONS
Increasing age can be accountable for female infertility and IVF failures. Based on the complied observations and synthesized conclusions in this review, advanced age has been shown to have a negative impact on endometrial functioning. This information can provide recommendations for future research focusing on molecular mechanisms of age-related cellular senescence, cellular composition, and transcriptomic changes in relation to endometrial aging. Additionally, further prospective research is needed to explore newly emerging therapeutic options, such as the senolytic agents that can target endometrial aging without affecting decidualization. Moreover, clinical trial protocols, focusing on oocyte donation cycles, would be beneficial in understanding the direct clinical implications of endometrial aging on pregnancy outcomes.
Keywords: endometrial aging, endometrial receptivity, cellular senescence, decidualization, oocyte donation, epigenetics
Graphical Abstract
Advanced age may negatively affect the endometrial functioning, therefore further research is needed to understand the exact mechanisms involved and impact on receptivity and pregnancy outcomes Created with BioRender.com (https://biorender.com/).
Introduction
Today, the average maternal age at childbirth has risen owing to the wider use of contraception and changes in various socio-economic and lifestyle-related factors, such as the educational and professional growth of women, housing and economic uncertainty, unmarried cohabitation before the birth of a first child, improved gender equity, etc. (Mills et al., 2011). Additionally, the advent of novel and more effective ART has encouraged women to postpone childbearing. Statistically, the average childbearing age has increased by ∼1 year every decade since 1970 across the Organisation for Economic Co-operation and Development countries, with the average years of postponement ranging from 1.5 to 5 years (Mills et al., 2011). During the preceding decade, advanced maternal age was considered as being over 35 years of age; however, based on the literature, the threshold has been lifted to 40 years, beyond which a steep decrease in fertility occurs even in IVF cycles (Shapiro et al., 2016; Bouzaglou et al., 2020). Different definitions have been used in the literature to describe advanced maternal age; however, because of the heterogeneity of study designs and the failure to control for potential confounders, the exact age indicating a decline in female fertility is still elusive. Nevertheless, age-related female infertility was reported to be clinically relevant from the age of 35–40 years (Devesa-Peiro et al., 2022). Traditionally, the depletion of female fecundity with advanced age has been attributed to its evident association with ovarian dysfunction with a decrease in ovarian reserve leading to poor quality oocytes, eventually resulting in non-implantable embryos and even chromosomal abnormalities (Cimadomo et al., 2018). However, age-related decline in fertility is not always limited to ovarian aspects. The currently available ART-related advances, such as oocyte donation and the selection of competent embryos by screening for chromosomal abnormalities using preimplantation genetic testing, have overcome the ovary-related aspects caused by advanced age to some extent. However, other factors, most importantly the aging of the endometrium, also influence the implantation rate, clinical pregnancy rate, and live birth rate in women of advanced age. This has been predominantly investigated in IVF studies using oocyte donation cycles. However, the variability in study design, selection and characteristics of oocyte donors, the clinical and demographic background of recipients, as well as the effect of other confounding factors, have led to controversial inferences about the effectiveness of oocyte donation in advanced-age patients. Moreover, several reports on endometrial aging have been published in discrete areas using animal models and human studies to determine the effect of uterine aging on the endometrial ultrasound parameters such as thickness, histological and cellular senescence in endometrial tissue, levels of steroid hormones, distribution of their receptors in endometrial tissue, and the molecular determinants of endometrial decidualization and receptivity. A recently published review postulates the significance of age-related epigenetic alterations and future implementation of the epigenetic clock to predict the biological age of endometrium by virtue of related disorders and infertility (Deryabin and Borodkina, 2023). Another recently published review article summarizes the overall functional and structural alterations, with probable mechanisms, involved in uterine aging (Wu et al., 2023). However, the current review is intended to compile the available literature on endometrial aging with the prime focus on synthesizing evidence regarding the impact of aging on the success of conception and establishment of a pregnancy. This aspect of endometrial aging has been comprehensively covered in our review, supported by different types of clinical studies with oocyte donation cycles. Thus, we emphasize the clinical significance of endometrial aging, which contribute to the uniqueness of our review. Besides, the review describes those hormonal, cellular, molecular, and epigenetic factors causing an age-related impact on endometrial receptivity. Lastly, the challenges in the implementation of anti-aging therapies, such as senolytic agents, to reverse age-associated endometrial impairments are discussed.
Methods
Search strategy
A comprehensive narrative literature review was conducted based on a search of articles in PubMed (Medline) until February 2023. An overview of the cellular, hormonal, molecular, and epigenetic aspects of healthy endometrium and the associated alterations as an effect of endometrial aging was acquired from the available research studies using animal models as well as human patients and samples. Clinical studies were included to understand the impact of aging on endometrial receptivity among oocyte donation cycles. These studies were segregated into three groups based on the study design and selection criteria for cases and controls: oocytes donated by healthy oocyte donors which were transferred to recipients of younger or advanced age; a single pool of oocytes donated by a young woman and distributed among the recipients with young and advanced age; and infertile patients undergoing IVF who had shared their oocytes with other recipients of advanced age, i.e. standard IVF with own oocytes versus IVF with donated oocytes. Further, knowledge regarding senolytic agents and the associated challenges involved in the implementation of these therapies in alleviating age-related endometrial dysfunction was highlighted using available scientific reports, primarily on animal model studies. The keyword endometrial/uterine aging was searched alone and in combination with ‘receptivity’ and other terms: ‘decidualization’, ‘hormone’, ‘senescence’, ‘cellular’, ‘molecular’, ‘methylation’, ‘biological age’, ‘epigenetic’, ‘oocyte recipient’, ‘oocyte donation’, ‘embryo transfer’, and ‘pregnancy rate’. Duplicate articles were eliminated from consideration.
Data screening and eligibility criteria
From the primary search, retrospective, prospective, and cohort clinical studies published in English only were included. Publications with animal studies and human cases were included wherein the age groups corresponding to reproductive age were compared to indicate their impact on endometrial receptivity. The age groups outside the reproductive years were not considered in this review and were excluded using the keywords ‘post-menopause’, ‘menarche’ and ‘age over 50’, ‘age over 60’. Additionally, since the current review focuses on the effect of aging on pregnancy outcomes and is restricted to conception as the outcome measure, those articles having pregnancy-related complications as the primary outcome measures were also excluded. To ensure an unbiased evaluation of the impact of advanced age on endometrial receptivity, articles concerning cancer, endometriosis, and other endometrial disorders were excluded. Relevant citations and cross-references were also screened and examined.
Data extraction and study selection
The screening of articles was performed independently by two authors by evaluating the titles and abstracts. Any discrepancies were resolved by discussion among other authors. Irrelevant articles were eliminated using the Newcastle-Ottawa Quality Assessment Scale (Lo et al., 2014). When an article was considered suitable for inclusion, the full-text version was referred to. Animal model studies complying with Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines were considered for this review (Kilkenny et al., 2010). The quality of clinical studies was evaluated using the Appraisal Tool for Cross-Sectional Studies (AXIS) guidelines (Downes et al., 2016). For each publication, the data were manually extracted, including study type, publication date, aspect of aging, the origin of samples used, sample size, age groups, comparison groups, outcome measures, and the effect(s) of aging on endometrial biology. In the case of oocyte donation studies, the articles in each group were segregated as per the negative or no impact of endometrial aging based on clinical pregnancy, implantation, and delivery rates. Owing to the absence of sufficient data for a meta-analysis, the studies are summarized here in the text and in tables.
Results
A total of 1734 articles were identified after the primary search, of which 993 were retained after eliminating duplicates. On screening the abstracts for eligibility, 688 articles were evaluated. Further, 515 publications corresponding to the main objectives of ‘cancer’, ‘endometriosis’, ‘disorders’, and ‘menopause’ were removed, and a total of 173 relevant manuscripts were selected and studied for the current review, of which 14 were animal model studies and 24 were clinical studies of oocyte donations. Randomized controlled trials were not available on this subject. The selection flow chart is shown in Fig. 1.
Figure 1.
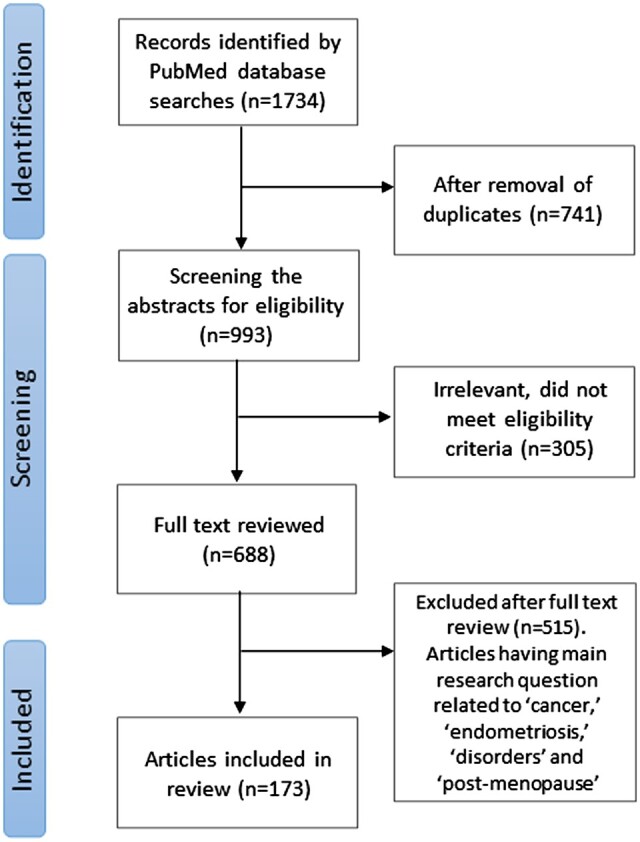
Method flow chart illustrating the identification and screening of literature for this narrative review of aging and the endometrium.
Functioning of healthy endometrium during the reproductive years
The endometrium is a very complex tissue consisting of different cell types, e.g. stromal, epithelial, vascular, and immune cells. The endometrium is functionally divided into two layers—‘functionalis’ and ‘basalis’. Functionalis is the superficial layer of endometrium that undergoes cyclic changes as per the different phases of the menstrual cycle, which are predominantly regulated by the ovarian steroid hormones, estradiol (E2), and progesterone (P4). Histologically, this layer consists of a simple columnar endometrial surface epithelium called the luminal epithelium. The glandular epithelium forms several tubular glands and is surrounded by thick vascular stroma comprising fibroblast-like stromal cells and spiral arteries, which recruit the population of fluctuating innate immune cells. Endometrium functionalis sheds off during menstruation, regenerates during the proliferative phase, and matures during the secretory phase in order to achieve receptive potential for the implanting embryo (Jiménez-Ayala and Jiménez-Ayala Portillo, 2008). In the absence of fertilization, in response to the withdrawal of P4, it undergoes the successive cycle of menstruation.
Endometrium basalis is a thin basal layer that adjoins the myometrium and is located beneath the functionalis layer. In contrast to functionalis, it consists of permanent stroma and endometrial glands, which do not tend to undergo regular cyclic changes (Deryabin et al., 2020). The stroma in the basal layer is denser with an increased nucleocytoplasmic ratio and thicker-walled arteries than the endometrium functionalis. In humans, the basalis layer exhibits the presence of progenitor stem cells, which can differentiate into stromal and epithelial cells (Critchley et al., 2020). Thus, it performs a vital role in maintaining endometrial integrity across every menstrual cycle (Jiménez-Ayala and Jiménez-Ayala Portillo, 2008).
In the normal menstrual cycle of reproductive-aged women, the proliferative phase is characterized by the proliferation of endometrial cells synchronized with follicular growth in response to increasing levels of E2. The action of E2 is mediated by genomic and non-genomic E2 signalling pathways via their corresponding nuclear E2 receptors (ERs, ERα, and ERβ) and membrane-associated ERs (ERα36 and G protein-coupled E2 receptor (GPER)), respectively (Pagano et al., 2020; Yu et al., 2022). During this phase, the luminal epithelium is thinner and consists of tall columnar cells. Endometrial glands are straight and narrow and become torturous towards the end of the proliferative phase. Glands exhibit signs of mitosis and pseudo-stratification of glandular epithelium. Stroma is compact and dense, having small stromal cells with a comparatively large nucleus and scanty cytoplasm undergoing mitosis (Noyes et al., 1950). After ovulation, the E2 is opposed by elevated P4 levels produced by the corpus luteum, which promotes the differentiation of endometrial cells forming thick, mature, and blood vessel-rich tissue defining the secretory phase of the menstrual cycle. Secretory endometrium is characterized by larger, more tortuous glands lined by low columnar cells, an absence of mitosis, and pseudo-stratification (Noyes et al., 1950). The nuclei of glandular epithelium are observed to be shifted at the base of cells with the presence of subnuclear cytoplasmic glycogen vacuoles contributing to secretion into the lumen of glands, causing enlargement. The secretory phase is presented with stromal oedema underlying the luminal epithelium with the diffused appearance of the walls of spiral arteries. During the mid-secretory phase, under the influence of ovarian P4 and cyclic AMP, the endometrial stromal cells undergo a cellular differentiation and transformation into the decidual stromal cells accompanied by changes of the cellular population in the endometrial tissue; the process is characterized as decidualization (Okada et al., 2018). Decidualization is associated with the recruitment of leukocytes and immune cells at the decidua, which collectively secretes cytokines, chemokines, and several growth factors, making the endometrium receptive to an implanting embryo (Ng et al., 2020). Decidualization is responsible for opening the window of implantation (WOI), which is the self-limiting period that typically occurs 6–7 days following the LH surge and continues for approximately 4 days, within which the event of embryo implantation may take place (Achache and Revel, 2006; Koot et al., 2012). The transformation from early-secretory to mid-secretory endometrium is regulated at the molecular level resulting in alterations in gene expression (Altmäe et al., 2017). Further, with the diminishing level of P4, the endometrium shows degeneration of glandular cells and displacement of secretory glands and stromal aggregates causing tissue haemorrhage, and the superficial functional layer separates from the basal layer, causing menstruation. Thus, the endometrium exhibits cellular and histological changes in response to fluctuations in the levels of hormones across the menstrual cycle (Noyes et al., 1950; Gellersen and Brosens, 2014; Garcia-Alonso et al., 2021).
Effect of endometrial aging: hormonal, cellular, and molecular aspects
Effect on endometrial aging: hormonal aspects
During the normal menstrual cycle, two distinct peaks of serum E2 levels are observed. The first peak occurs during the proliferative phase and is associated with the increased expression of endometrial E2 receptors ERα, ERβ, and GPER inducing mitogenic activity (Giudice, 2006; Yu et al., 2022). The progesterone dominated secretory phase is accompanied by lower levels of estradiol and ERα than the proliferative phase (Dockery and Rogers, 1989; Critchley et al., 2020; Yu et al., 2022). During the mid-secretory phase, the second peak of E2 coincides with the WOI and increasing levels of P4. The action of P4 stimulates the decidualization process that enables the receptivity status of the endometrium (Yu et al., 2022). In the case of successful embryo implantation, the pregnancy is maintained in response to the higher levels of E2 and P4. In the absence of implantation, decreased levels of E2 and P4 lead to the initiation of the menstrual phase (Ruiz-Alonso et al., 2012; Critchley et al., 2020).
The serum concentration of E2 across the ovulatory menstrual cycles is variable among women of advanced age. According to the studies performed, the average serum E2 concentrations decreased with age from 42–45 to 52–55 years, with a steep decline towards higher ages (Burger et al., 1999; Randolph et al., 2004). However, another cross-sectional study showed similar follicular E2 levels among women in early and mid-reproductive age (21–45 years) as well as for the age group of >45 years (Hale et al., 2007). Conversely, a separate study (Burger et al., 2008) during ovulatory cycles revealed that the serum E2 levels in the follicular phase were elevated in older women (>45 years) compared to younger women (21–35 years). This age-related unexpected increase in E2 levels in the follicular phase were explained by the hypothesis that the feedback mechanism operating via inhibin B secretion is affected earlier than E2 secretion. As follicle numbers decrease with age, it first affects the inhibin B secretion instead of E2, leading to declined levels of inhibin B, which is associated with the consequent increase in FSH levels. This, eventually, can result in elevating E2 production by granulosa cells (Burger et al., 2008). Thus, the evidence implies that advanced age affects the hypothalamic pituitary–ovarian axis, with fluctuating levels of E2 in the follicular phase of ovulatory cycles in the late-reproductive stage compared to younger women. The close correlation between E2 levels and endometrial proliferation suggests that abnormal E2 concentrations in older women may have a detrimental effect on endometrial growth and thickness. Moreover, during the secretory phase, serum levels of E2 and P4 decrease progressively with age (MacNaughton et al., 1992), which further can influence cellular differentiation during endometrial receptivity.
The age-dependent variation in E2 and P4 levels may have a considerable impact on the endometrium by altering the endometrial expression of their corresponding cellular receptors. Furthermore, the age-related endometrial downregulation of ER and P4 receptors (PR) has been demonstrated in the endometrial tissue. Additionally, the decreased capacity of the endometrium to take up these steroids (Larson et al., 1972; Blaha and Leavitt, 1974; Saiduddin and Zassenhaus, 1979) was associated with increased collagen and fibrosis in endometrial tissue (Ohta, 1987; Nelson et al., 2013). The mouse model study also exhibited impaired hormonal responsiveness in aged mice as around 50% of de-regulated genes in aged decidua were associated with ERα and/or PR. Additionally, expression of ER and PR was more variable in aged mice showing mosaic staining patterns in luminal epithelium versus homogenous expression in young mice (Woods et al., 2017). In human endometrium, the expression of cytosolic ER has been reported to change with age, being highest in the 30–39 years age group compared to the 20–29 years and 40–55 years age groups. On the other hand, the nuclear ER and PR showed an age-dependent decrease in the endometrium of women aged 20–29, 30–39, 40–49, and above 50 years (Bergqvist and Ferno, 1993). Furthermore, the staining of epithelial cell nuclei positive for ERα was 100% on the day of oocyte retrieval among healthy oocyte donors aged <30 years, which significantly declined to 90% staining among women >30 years of age, showing an age-related decline in the expression of ERα (Klonos et al., 2020). In contrast, another study showed similar expression of ER and PR in both age groups of women <30 years and >40 years, indicating that there was no effect of age on the endometrial function until menstruation is present (Noci et al., 1995). Similarly, functionally agonadal women in the different age groups from 25 to 60 years showed a similar effect of hormone replacement therapy on histologic and ultrasonographic characteristics of the endometrium, as well as expression of ER and PR, regardless of their age (Sauer et al., 1993). Thus, more detailed age-dependent studies on the levels and distributions of steroid hormone receptors are needed to determine their causal effect on endometrial aging. Nevertheless, empirical evidence suggests abnormal levels of ovarian hormones and their receptors in older women, indicating the direct influence on endometrial proliferation, receptivity, and overall functioning.
Endometrial thickness and aging
E2 and P4 are responsible for achieving the desired endometrial thickness, which is a pre-requisite for the implanting embryo. Endometrial morphology can be assessed using ultrasonographic techniques to measure the thickness and study the echogenic pattern of the endometrium, both of which vary during each phase of the natural menstrual cycle in healthy fertile women (Fitzgerald et al., 1994). Although there is no consensus about the minimum endometrial thickness that is favourable for the implantation of the embryo, clinical pregnancy has been achieved in women with endometrial thickness as low as 4 mm and as high as 16 mm (Ugwu et al., 2022). On the other hand, an endometrial thickness of 7 mm has been reported to be a pre-requisite for implantation (Tomic et al., 2020; Ugwu et al., 2022), followed by an increase in implantation rate when the endometrium increases from 8 to 11 mm, whereas when the endometrial thickness increases to >14 mm, it showed a dramatic decrease in implantation rate (Eftekhar et al., 2019; Ugwu et al., 2022). The assessment of endometrial thickness in different age groups of healthy women revealed that the endometrium was significantly thicker during the mid-secretory phase compared to ovulation day in all the age groups. Further, it was observed that in women aged 32–36 years and 37–45 years, the maximum thickness reached during the secretory phase was 15.3 and 15.9 mm, respectively, which was significantly higher than in younger age groups of 21–25 and 26–31 years showing a maximum thickness of 12.1 and 13.4 mm, respectively (Fitzgerald et al., 1994). However, the extrapolation of this age-related difference in endometrial thickness and its effect on pregnancy rate has not been established. In IVF cycles, studies have reported a positive correlation of endometrial thickness with pregnancy rate (Richter et al., 2007; Yang et al., 2018) whereas other studies did not find any significant association between these parameters (Žáčková et al., 2009; Gingold et al., 2015).
Some studies suggest that endometrial patterns, such as triple-line configuration, patterns of a hyperechoic endometrium, and the presence of a central echogenic line detected by ultrasound as well as by the evaluation of endometrial blood flow, have more impact on the implantation potential as compared to endometrial thickness (Gonen and Casper, 1990; Järvelä et al., 2005; Gingold et al., 2015; Navinchandra et al., 2016). Studies have also reported the combined approach of endometrial pattern together with thickness, and a scoring system for the prediction of pregnancy success during IVF (Baruffi et al., 2002; Chen et al., 2010; Gingold et al., 2015; Khan et al., 2016; Navinchandra et al., 2016; Ugwu et al., 2022; Zhang et al., 2022). An IVF study reported that the triple-line endometrial pattern observed on the day of the administration of hCG was associated with an improved pregnancy rate compared to the homogenous, hyperechogenic, or intermediate endometrial pattern (Gingold et al., 2015). However, as for endometrial thickness, there are conflicting conclusions even on the correlation of endometrial pattern with pregnancy rate (Gonen and Casper, 1990; Check et al., 1993b; Järvelä et al., 2005; Potlog-Nahari et al., 2005; Rashidi et al., 2005; Mercé et al., 2008; Ugwu et al., 2022), without the information on age-related shift. The endometrial thickness and pattern can be used as prognostic indicators for endometrial receptivity; however, these features have not been well accepted as sole predictive markers for successful implantation (Navinchandra et al., 2016). Additionally, detailed information regarding age-related alterations in endometrial morphology and thickness is not yet available. Since these parameters have direct clinical implications, further precise research studies and randomized controlled clinical trials evaluating the impact of age on endometrial thickness and echogenic pattern, and their correlation with pregnancy outcome, would prove to be constructive.
Effect on endometrial aging: cellular aspects
Tissue aging and cellular senescence
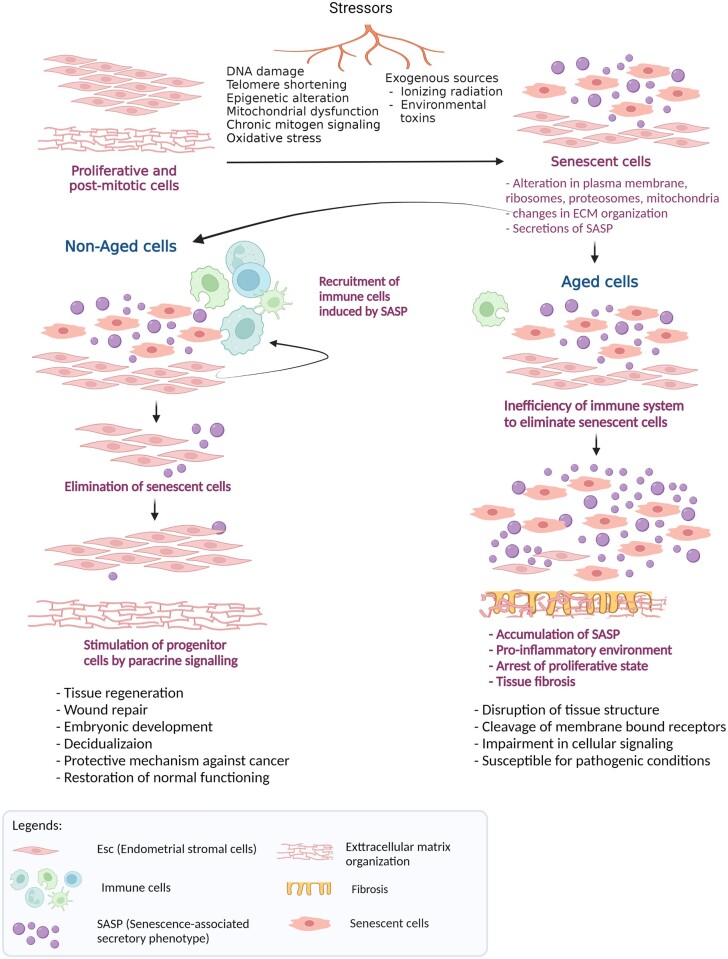
Aging is a complex and multifactorial biological process that promotes time-dependent deterioration of tissue function across multiple organ systems. Aging is a result of cumulative changes caused by several stimuli, such as telomere shortening, epigenetic changes, DNA damage, oxidative stress, chronic mitogen signalling, and mitochondrial dysfunction over a period, which leads to cell proliferation arrest and a decline in cellular function (McHugh and Gil, 2018; Deryabin et al., 2020; Di Micco et al., 2021). Additionally, exogenous sources, including ionizing radiation and environmental toxins, can contribute as stressors resulting in cellular senescence (Fig. 2) (Todhunter et al., 2018; Kumari and Jat, 2021; Yousefzadeh et al., 2021). At the level of a single cell, aging is closely associated with the underlying biological process called cellular senescence. Cellular senescence is the irreversible cell cycle arrest of not only proliferative cells but also post-mitotic cells (Sapieha and Mallette, 2018; Anderson et al., 2019; Maduro et al., 2021). Apart from aging, cellular senescence can also be caused by the response to damage induced by stressors, even in non-aged cells (Todhunter et al., 2018; Deryabin et al., 2020). However, under the influence of an appropriate microenvironment, naturally occurring senescence in healthy cells attracts the immune cells to eliminate them to protect against possible tumour development (Deryabin et al., 2020). Thus, not all senescent cells are aged cells, but cellular senescence is the main hallmark of the aging process.
Figure 2.
Cellular senescence in normal and aged cells. The phenomenon of cellular senescence occurs in different types of human tissues, including endometrium. It involves the transformation of both proliferative and post-mitotic cells into senescent cells in response to various types of stressors. These stimuli can be telomere shortening, epigenetic changes, DNA damage, oxidative stress, chronic mitogen signalling, and mitochondrial dysfunction over a period, which leads to cell proliferation arrest. Additionally, exogenous sources, such as ionizing radiation, ultraviolet light, and environmental toxins, can contribute as stressors resulting in cellular senescence. In normal conditions, the fully senescent cells are targeted by immune cells to be eliminated from the immune system, whereas during circumstances of aging the immune system is unable to clear senescent cells, which leads to the accumulation of senescence-associated secretory phenotype (SASP) and a persistent pro-inflammatory environment. Created with BioRender.com (https://biorender.com/).
Aging-associated cellular senescence modulates the cell mechanics and organization of the extracellular matrix (ECM) (Fig. 2). It alters the functioning of intracellular organelles, including the plasma membrane, ribosomes, proteosomes, mitochondria, and lysosomes, changes the morphology of nuclear structures, and dysregulates gene expression as well as epigenetic modifications of DNA and histones (Borodkina et al., 2018; Ogrodnik et al., 2019). Additionally, as a response to DNA damage, the senescent cells secrete several pro-inflammatory cytokines, chemokines, growth factors, and matrix metalloproteinases, which are collectedly termed the ‘senescence-associated secretory phenotype (SASP)’ (Borodkina et al., 2018; Deryabin et al., 2020; Maduro et al., 2021). In a normal healthy scenario, controlled secretion of SASP is reported to be beneficial and allows the clearance of senescent cells by elicitation of the immune response and replacing them with adjacent progenitor cells via paracrine signalling to achieve tissue regeneration (Deryabin et al., 2020; Deryabin and Borodkina, 2022). However, the accumulation of senescent cells in the aged tissues of humans, primates, and rodents has been demonstrated (Herbig et al., 2006; Wang et al., 2009; van Deursen, 2014), characterizing it as chronic senescence. A predominant drawback of this chronic aging process is the inefficiency of the body’s immune system to eliminate senescent cells, for various reasons: an increased rate of senescence with age; age-associated immunodeficiency in the innate and adaptive immune systems, reducing the potential for senescent-cell clearance; and dysfunction in haematopoietic stem cells with age, thus compromising the immune system (Fig. 2) (van Deursen, 2014). In this scenario, the senescent, aged cells continue to be increasingly dependent on the cell cycle checkpoints leading to the arrest of the proliferation state for a longer period, which is associated with the accumulation of SASP secretion (van Deursen, 2014; Deryabin et al., 2020). The accumulated population of senescent cells and SASP results in disruption of the tissue structure, cleavage of membrane-bound receptors, impairment in cellular signalling, and dysfunction of the ECM. Elevated levels of pro-inflammatory cytokines, such as IL-6 and IL-8, stimulate chronic inflammation and the infiltration of macrophages and lymphocytes, and induces tissue fibrosis (Coppé et al., 2008; Campisi, 2013; van Deursen, 2014). Thus, aging exhibits tissue-specific changes, which ultimately influence its functioning.
Association between decidualization and cellular senescence
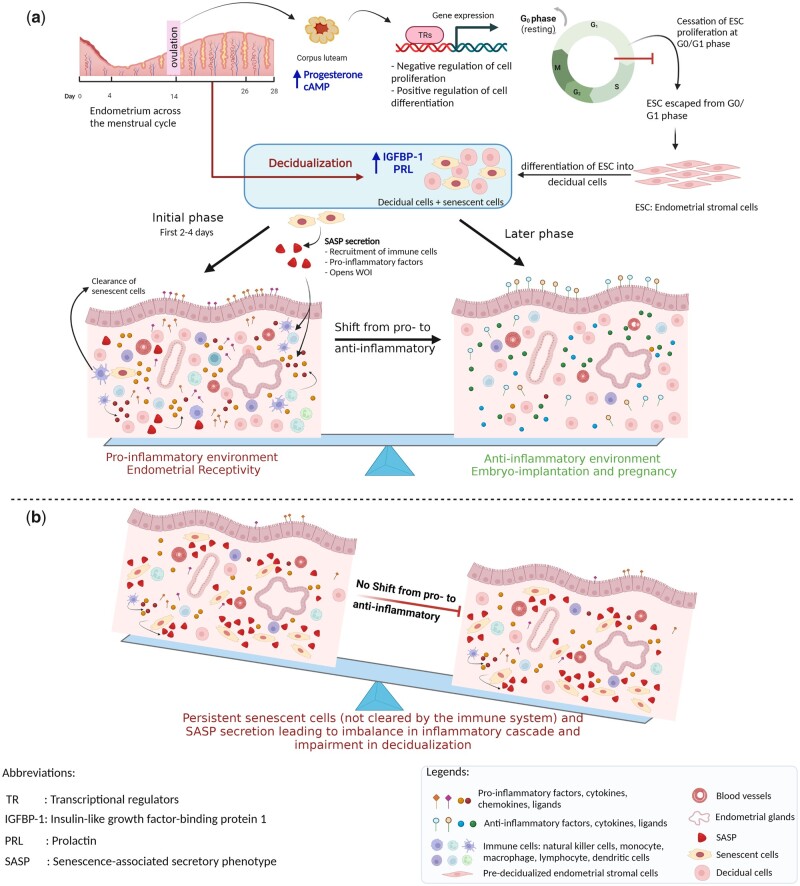
Decidualization involves several biological functions including cell differentiation, immune and inflammatory responses, transcription regulation, negative regulation of cell proliferation, coagulation, complement cascade, ECM organization, vascularization, and tissue remodelling (Okada et al., 2018). In humans, decidualization occurs in every menstrual cycle irrespective of the presence of an embryo (Teklenburg et al., 2010), unlike other species, such as rodents, where the signalling of the blastocyst initiates decidualization (Das, 2010). Thus, in humans, decidualization is solely induced by the maternal system through various transcription factors that mediate negative regulation of endometrial stromal cell (ESC) proliferation by cessation of the cell cycle at the G0/G1 phase (Rytkönen et al., 2019; Deryabin et al., 2020). It induces the endometrial expression of insulin-like growth factor-binding protein-1 (IGFBP-1) and prolactin, which are decidualization markers, and thus stimulates the differentiation of ESCs into decidual cells (Okada et al., 2018) (Fig. 3a). This transformation opens the WOI and is accompanied by the recruitment of monocytes and other immune cells from the circulation including uterine natural killer (uNK) cells (Mori et al., 2016). Endometrial decidual cells and immune cells secrete an array of pro-inflammatory mediators, such as tumour necrosis factor α, IL2, IL12, prokineticin 1, and interferon γ (IFNG), as well as chemokines, and cytokines such as C-X-C motif chemokine ligand 12 (CXCL12), CXCL14, leukemia inhibitory factor, IL1β, IL11, and IL-6. Additionally, other important factors involved in embryo implantation, such as growth factors like epidermal growth factor (EGF), heparin-binding EGF, insulin-like growth factor 1 (IGF1), and morphogenesis proteins Indian hedgehog, Wnt family member 4, and bone morphogenetic protein 2 (BMP2) also play crucial roles (Achache and Revel, 2006; Ruiz-Alonso et al., 2012). Moreover, the nuclear factor kB-dependent signalling pathways via ER are activated to promote cellular inflammation during the WOI (King et al., 2010). Although the proinflammatory milieu in the endometrium during decidualization is essential, it is a time-dependent and well-regulated phase limited to the initiation of WOI. Later, the decidual process shifts from the expression of a pro-inflammatory to an anti-inflammatory profile, to secrete the anti-inflammatory hormone cortisol, other anti-inflammatory factors, and cytokines, such as IL-4, IL-5, and IL-10 (Deryabin et al., 2020), which protect the semi-allogeneic embryo from the maternal immune system (Fig. 3a). An anti-inflammatory condition is thus maintained until delivery in case of a successful pregnancy; otherwise, in the absence of fertilization, a reducing level of P4 triggers the vasoconstriction of blood vessels and induces menstrual bleeding.
Figure 3.
Relation between decidualization and cellular senescence in normal conditions and during the aging process. (a) Normal physiological conditions: in the mid-secretory phase, under the influence of P4 and cAMP, the endometrial stromal cells escape from the cell cycle at the G0/G1 phase and undergo cell differentiation pathways to transform into decidual cells, in the process called decidualization. In the initial phase during decidualization, the subpopulation of senescent cells is present along with the mature decidual cells. Senescence-associated secretory phenotype (SASP) manifested by the endometrial cells is essential for developing an adequate pro-inflammatory response required for the secretion of cytokines, chemokines, and cell adhesion ligands that are pre-requisites for embryo implantation. In the later phase, mature senescent cells are targeted by the immune cells, decreasing the levels of SASP and shifting the endometrial milieu to the anti-inflammatory response that is required to maintain a semi-allogeneic embryo during the pregnancy. (b) During the aging process: in a chronic condition like aging, the immune cells cannot eliminate the mature senescent cells during decidualization. This results in the accumulation of pre-senescent, fully senescent cells and secreted SASP causing persistent unregulated pro-inflammation during mid-secretory endometrium. This fails to maintain the inflammatory balance leading to impaired decidualization and defective endometrial receptivity. Created with BioRender.com (https://biorender.com/).
The induction of successful decidualization is associated with the presence of senescence markers in decidual ESCs (Deryabin et al., 2020). Senescence-specific protein p16 was upregulated in a subpopulation of ESCs, which was characterized as senescent decidual cells because they expressed genes involved in the cellular senescence pathway (Brighton et al., 2017). Furthermore, the study infers that an adequate degree of senescence is responsible for producing a sub-population of senescent decidual cells, which is a pre-requisite for the decidualization process. A recent study using single-cell RNA sequencing confirmed the presence of both mature decidual cells along with a senescent decidual subpopulation that occurred after normal ESC decidualization (Lucas et al., 2020). Thus, the initial pro-inflammatory response during decidualization is the result of the presence of mature, as well as senescent, decidual cells. The intensity of this pro-inflammatory response depends on the degree of the pre-senescent population of ESCs (Deryabin et al., 2020; Lucas et al., 2020). Senescent decidual cells also secret IL15, which is one of the essential decidualization markers, that activates peripheral NK cells and transforms them into tissue-resident uNK cells. Transformed uNK cells are CD54+ and CD16-, without their cytotoxicity property, which are essential for vascularization and spiral artery remodelling during the implantation and placentation process (Jabrane-Ferrat and Siewiera, 2014; Brighton et al., 2017; Sojka et al., 2019; Zhang and Wei, 2021). Furthermore, in the later phase of decidualization, the generated senescent decidual subpopulation was eliminated by uNK cells without affecting mature decidual cells, which are essential for embryo implantation and invasion (Fig. 3a) (Brighton et al., 2017), suggesting that the NK cells play a central role in endometrial cellular senescence.
Recently, the age-dependent depletion of NK cell functions has been demonstrated (Brauning et al., 2022). As a result of this, under the conditions of age-associated alterations, unlike the normal decidualization process, uNK cells lose their ability to eliminate senescent decidual cells and fully senescent ESCs (Deryabin et al., 2020). Thus, this leads to the generation and accumulation of undifferentiated senescent ESCs in the endometrial tissue, which neither proliferate nor have the potential to differentiate into decidual cells. Also, it was shown that the presence of undifferentiated senescent ESCs might negatively affect the emergence of mature and senescent decidual cells subpopulations (Deryabin and Borodkina, 2022). Moreover, the persistence of senescent cells causes prolonged secretion of SASP resulting in an uncontrolled pro-inflammatory milieu during the crucial step of decidualization, which can disturb the secretory cascade of decidual cells and the balance between the pro-inflammatory and anti-inflammatory secretory profiles (Fig. 3b) (Deryabin et al., 2020). SASP produced by senescent ESCs might induce paracrine senescence in the neighbouring normal cells, further reducing the plasticity of endometrial tissue, or might directly impair decidualization of the healthy surroundings, multiplying the negative impact of senescent cells (Griukova et al., 2019; Deryabin and Borodkina, 2022). Moreover, age-associated dysregulation of the cellular senescence phenomenon was also observed in a recent study by our group, which demonstrated the significant upregulation of CDKN2A (p16INK4A) in endometrial epithelium of women aged >45 years compared to younger women of <30 years (Loid et al., 2023). Thus, although senescence is one of the vital factors for the initiation of decidualization, age-related cellular changes can impair the senescence-associated network causing an inadequate pro-inflammatory microenvironment that has a detrimental effect on the further pathways involved in decidualization, ECM organization, and hormonal responsiveness of decidual cells, eventually causing failure in embryo implantation (Deryabin and Borodkina, 2022).
Endometrial inflammaging and immune tolerance in aged endometrium
Endometrial inflammaging
The effect of advanced age causing an aberrant pro-inflammatory response is also referred to as ‘inflammaging’ resulting in hampering the endometrial function (Shirasuna and Iwata, 2017; Hirata et al., 2021). Pertaining to endometrial receptivity, inflammaging can be demonstrated in animal model studies. An in vitro bovine endometrial cell model of aged versus young animals revealed an upregulation of pro-inflammatory biomarkers, such as IL1A, interferon regulatory factor 7, and IFNG, involved in inflammatory and interferon signalling pathways contributing to reproductive dysfunction due to advanced age (Tanikawa et al., 2017). The study also demonstrated deregulation in the cell cycle with activation of G2/M DNA Damage Checkpoint Regulation, thus promoting the age-dependent accumulation of DNA damage and cessation of cell proliferation. The imbalance in the cell cycle was associated with elevated secretion of interferons in endometrial cells, resulting in a pro-inflammatory environment in the endometrium, thus making it less responsive to interferon tau (IFNT) leading to the ‘inflammaging’ phenomenon in the bovine endometrium of advanced age (Tanikawa et al., 2017). Additionally, a study comparing younger mares aged 5–7 years with older mares aged ≥15 years showed an age-dependent increased endometrial inflammatory cell infiltration, higher levels of fibrotic changes, and less dense endometrial glands, altogether causing uterine dysfunction, a decreased pregnancy rate, and increased incidence of pregnancy loss (Carnevale and Ginther, 1992). Nevertheless, our knowledge of endometrial inflammaging in humans is less clear.
Immune tolerance
As discussed above, an adequate and controlled pro-inflammatory response elicited by CD4+T cells to release Th1 and Th17 subsets of cytokines is essential to promote embryo implantation and trophoblastic invasion (Guerin et al., 2009; Wang et al., 2020a; Wu et al., 2022). In parallel to this, regulatory T cells (Treg) (CD4+ CD25+) also play a vital role in regulating immune tolerance at the implantation site to avoid rejection of the semi-allogeneic embryo during the implantation process (Guerin et al., 2009; Murata et al., 2021). Treg cells target the adaptive immune response by inhibiting proliferation and cytokine production in both CD4+ and CD8+ T cells, thereby supressing the functions of B cells, and antigen-presenting cells such as dendritic cells and macrophages (Guerin et al., 2009). Compared to other immune cell types, the proportion of these cells is lowest during the WOI. At the initiation of decidualization, these cells are accumulated in the endometrium along with the recruitment of other immunological cells and increase during the early pregnancy to mid-gestation to bring about an immunomodulatory reaction with trophoblast cells. Further, the decidual levels of CD4+ CD25+ Treg cells tend to decline as the pregnancy progresses towards delivery (Guerin et al., 2009; Monin et al., 2020; Murata et al., 2021). Similarly, the cytotoxic activity by CD8+T cells is suppressed maximally during the implantation events to create a tolerogenic environment (Shen et al., 2021). However, these cells are found to be prominent during the second and third trimesters of pregnancy, to function in the defence mechanisms against pathogens (Wu et al., 2022).
Although direct evidence of Treg cells or CD8+ T cells affecting the embryo implantation process due to endometrial aging is not available, the decreased populations of CD4+T cells and CD4+Th17 cells, and increased CD8+T cell populations in the endometrium of pre-menopausal women have been reported (Rodriguez-Garcia et al., 2014, 2018). The abundance and functionality of CD4+ T cells gradually decrease with age, eventually compromising their functions such as activation, proliferation, cytokine production, and apoptosis signalling. Thus, it leads to defective immunological synapse formation and cytoskeleton signalling (Garcia and Miller, 2011; Lefebvre and Haynes, 2012). These changes might have started taking place much earlier, even before the perimenopausal period, progressing further with advancing age; however, this needs to be explored in the future. Moreover, the imbalance in the population of CD4+ T cells, CD4+ T17 cells, and CD8+ T cells in human decidua may potentially impact embryo implantation, resulting in an increased rate of spontaneous abortions (Murata et al., 2021).
Endometrial fibrosis and aging
The endometrium has been reported to undergo histological changes in response to an imbalance in ovarian hormones caused by advanced age. The ESCs have a considerably lower proliferation rate and become less responsive to decidualization stimuli as age increases (Woods et al., 2017). Moreover, the significant characteristic of aged endometrium is the deposition of collagen in the endometrial stroma with associated tissue fibrosis. Various animal models exhibited age-related increases in uterine fibrosis (Burack et al., 1941; Biggers et al., 1962; Maurer and Foote, 1972; Gosden, 1979; Craig, 1981; Mulholland and Jones, 1993).
Although the mechanism of fibrosis in tissues of older mammals remains elusive, collagen deposition in the endometrium has been reported to be induced by E2 (Mulholland and Jones, 1993; Sharma et al., 2002). This was demonstrated in a study where young ovariectomized rats showed accumulation of collagen after administration of E2 treatment; however, it did not cause an increase in the total uterine collagen, suggesting that the collagen was parallelly degraded as it was synthesized in young rats (Dyer et al., 1980). Similarly, the large bundles of collagen fibrils were present even after the decidualization by aged stromal cells whereas, in young stromal cells, the collagen was observed to have almost disintegrated (Mulholland and Jones, 1993). Thus, collagen deposition in older rats indicates a decline in the potential of collagen degradation as age increases. One of the underlying reasons may be the higher levels of hydroxyproline exhibited by uterine collagen in advanced-age women (Woessner, 1963) and golden hamsters (Rahima and Soderwall, 1977), which affects the degree of crosslinking and thus impairs the degradation potential of collagenases (Mulholland and Jones, 1993). As endometrial aging is associated with inflammation, this can be another important reason for collagen deposition involving various biological functions such as chronic inflammation, oxidative stress, accumulation of senescent cells, caspases, growth factors, etc. (Wynn, 2008). Moreover, the increased level of P4 in rodents at an older age inhibits the action of collagenases in endometrial cells and explant cultures (Jeffrey et al., 1971; Jeffrey, 1981). A micromolar concentration of P4 and glucocorticoids has been shown to impair the synthesis of collagenase in uterine smooth muscle in cell culture (Jeffrey et al., 1990; Mulholland and Jones, 1993). This confirms the notion that the function of collagenases and other proteases decreases with increased age, even in the uterine cells (Maurer and Foote, 1972). The inability of aged endometrium to degrade the collagen also suggests an impairment in the capacity of stromal cells to remodel the ECM organization, which further affects the formation of junctional complexes hampering autocrine and paracrine cell-to-cell signalling events during decidualization (Mulholland and Jones, 1993; Wynn, 2008). Thus, the advanced age and increased fibrosis can be associated with a dysregulation in inflammatory pathways causing pregnancy loss (Hirata et al., 2021). On the contrary, the study by Noci et al. (1995) has demonstrated no significant difference in the histology of endometrium with respect to stromal cells and collagen accumulation between younger women <30 years versus older women >40 years. However, older women included in this study group ranged between 40 and 46 years, which may explain the insignificant difference in fibrosis among older women. The correlation of age and collagen accumulation cannot be ruled out in women of more advanced age (>45 years) because most of the notable age-related changes have a negative impact on endometrial biology after 45 years of age. To summarize, aging aggravates collagen deposition along with a decrease in collagen degradation via different mechanisms that ultimately affect various functions, such as ECM organization, decidualization, and uterine senescence, all of which play an important role in female fertility.
Alterations in endometrial epithelium and aging
Furthermore, the alterations in endometrial epithelium related to advanced age have been shown using various animal models and even human endometrium. Regarding the animal model studies, the most predominant feature highlighted in aged mice endometrium was variation in number, shape, and distribution of microvilli on the surface epithelium. In aged endometrium, they were of irregular shape, variable in length with an abnormal distribution ranging from tightly clustered to sparsely distributed, giving the appearance of ‘bald’ endometrial cells (Mulholland and Jones, 1993). The cytoplasm was observed to be devoid of organelles with tufts of fine filaments composed of proteins from collapsed microvilli. Intra-epithelial leukocytes, primarily lymphocytes, were observed to be elevated in old rats compared to younger rats (Mulholland and Jones, 1993).
Moreover, the endometrial laminar and glandular epithelium contain ciliated cells, which are believed to aid in the transportation of glandular secretions. They also help the gamete and embryo to move along the uterine lining. However, their exact role in this context remains unclear (Newman et al., 2023). The populations of ciliated epithelial cells in the endometrium exhibit significant variation in numbers during the menstrual cycle and they also have distinct transcriptomic profiles as compared to other endometrial cell types (Wang et al., 2020b; Garcia-Alonso et al., 2021; Loid et al., 2023). It is unclear whether a higher quantity of ciliated epithelial cells creates a favourable environment or, conversely, hinders trophoblast invasion. Also, as multiciliated cells possess sensory functions through hormone and IL receptors on their surface (Nutu et al., 2009; Shao et al., 2009), compromised cilial development may interfere with embryo-uterine communication.
Effect on endometrial aging: molecular aspects
Several genomic studies using animal models have identified the impact of aging on endometrial receptivity and implantation. The study in the murine model revealed upregulation of the pro-inflammatory cytokine il17rb, and chemokines such as cxcl12, and cxcl14 in aged versus young wild-type mice (Kawamura et al., 2021). Additionally, altered expression of genes associated with mitotic division, angiogenesis, cell migration, immune response, and inflammation was observed in aged mice (Kawamura et al., 2021). Another in vitro transcriptome study in mice endometrium showed delayed decidualization of stromal cells and impairment in the progression of decidual differentiation with significant downregulation of key regulatory genes like Prl8a2 (Dtprp), Bmp2, Hand2, Hoxa10, Nr2f2 (Coup-tf II), Igfbp5, Sfrp5, Ltf, Muc1 and Cdh1 in aged females (Woods et al., 2017).
Moreover, the recent in-vitro candidate gene study in human primary stromal cells (PSC) reported an age-dependent decrease in the proliferative capacity of stromal cells with a significant decrease in mRNA and protein expression of BMP3 and STAT3 in women aged ≥36 years (Berdiaki et al., 2022). Furthermore, in vitro induction of decidualization of PSC in aged women showed significant mRNA downregulation of the decidualization markers IGFBP1 and PRL, indicating that the age-related dysfunction in stromal cell proliferation can potentially eventually affect decidualization, endometrial receptivity, endometrial thickness, and embryo implantation (Berdiaki et al., 2022).
Based on the available literature, there are very limited data on endometrial aging and its effect on receptivity in humans using a genome-wide approach. Intracellular and molecular interactions in mid-secretory endometrium of healthy women and infertile patients have been studied using advanced omics technologies (Messaoudi et al., 2019; Ruiz-Alonso et al., 2021). These studies have led to the development of molecular diagnostic tools for the assessment of endometrial receptivity and to pinpoint the exact day for embryo transfer in patients suffering from recurrent implantation failure. However, in these studies, the factor of ‘advanced age’ has not been explored; thus, the molecular mechanism of endometrial aging and its impact on receptivity potential has not been well established.
Recently, in silico tools have been used to retrospectively analyse the published gene expression datasets based on the ‘age’ factor varying from 23 to 43 years (Devesa-Peiro et al., 2022). The study revealed a total of 5778 differentially expressed age-related genes involved in the dysregulation of biological functions, such as inhibition of epithelial cell proliferation, vascular endothelial growth factor signalling, angiogenesis, insulin signalling, and aging hallmark processes, such as cell cycle arrest and telomer protection, among women 35 years or older (Devesa-Peiro et al., 2022). A recent study by our group demonstrated that, in addition to general cell cycle and immune pathways dysregulated in women over 45 years, a potent senescence marker CDKN2A (also known as p16INK4A) is significantly upregulated in the endometrial epithelium of advanced age women. The accumulation of p16-positive cells may lead to specific changes in endometrial epithelial development through interference with extracellular signal-regulated kinase (ERK), Notch and Hedgehog pathways (Loid et al., 2023).
Additionally, as reported by Devesa-Peiro et al (2022), the majority of differentially expressed genes were associated with ciliary processes, cilia motility, and ciliogenesis. Similar findings were obtained by Loid et al. (2023) wherein a large number of cilia-associated genes was up-regulated in endometrial samples corresponding to the WOI among advanced age women (>45 years) compared to younger women (<30 years). The study also identified a larger population of ciliated epithelial cells in women of advanced age (Loid et al., 2023). Likewise, age-related differences have also been reported in a recent study performed on microRNAs. According to this study, the levels of miR-449c.1 and its precursor were downregulated in the mid-secretory endometrium of a 49-year-old woman compared to a 34-year-old woman of reproductive age. The miR-449c.1 belongs to the miR-34/449 family, which is involved in the regulation of cilia formation that is a prerequisite for the establishment of endometrial receptivity (Naydenov et al., 2022). Endometrial ciliated cells are transcriptionally distinct epithelial cells with varying numbers of cell populations across the menstrual cycle (Wang et al., 2020b; Garcia-Alonso et al., 2021), and therefore, their functions and optimal number need to be investigated further with respect to endometrial receptivity and chronological aging.
Embryo implantation is a result of the successful dialogue between receptive endometrium and the competent embryo. In other words, endometrial receptivity is also modulated by the embryo itself. The interactions between endometrium and embryo have been explained by the concept of exosome biogenesis (Ntostis et al., 2021). Extracellular exosomes secreted by the receptive endometrium, as well as by the competent embryo, encapsulate essential signals for the implantation process. At a younger age and under a favourable microenvironment created by steroid hormones, anti-apoptosis, and energy generation pathways, these exosomes exchange signals, which induce cell adhesion and cell migration, to achieve synchronized embryo-endometrial co-ordination. Trophectoderm derived from the embryos of younger women showed upregulation of genes associated with extracellular exosome biogenesis, reduction of oxidative stress, mitochondrial ATP synthesis, and cholesterol biosynthesis compared to women of advanced age. Thus, in the implantation process, exosome-related genes derived from the trophectoderm are altered in women of advanced age compared to younger women, leading to unsuccessful embryo-endometrial competency (Ntostis et al., 2021). Moreover, maternal age-related asynchrony between embryo and endometrium has also been demonstrated in the stimulated IVF cycles wherein the 50% chance of asynchrony in women younger than 35 years of age is elevated to 65% in women of advanced age (Shapiro et al., 2016). Still, although recent studies have supported the hypothesis that age affects endometrial function, the exact mechanism(s) and integrated associated factors involved needs to be further elucidated to generate substantial evidence.
Endometrial epigenetic aging
The term aging is generally applied to the chronological age of the individual. However, the concept of biological age has been introduced recently, according to which each individual, or more specifically each tissue type, has a biological age that can differ from the chronological age (Franceschi et al., 2018). The biological age of an individual can be assessed by virtue of the ‘epigenetic clock’ or ‘methylation age’ (Horvath, 2013) as well as the ‘telomer clock’ (Shammas, 2011). However, the literature associated with the biological age of the endometrium is more prevalently reported in terms of epigenetic alterations.
Epigenetics has a vital role in several biological processes including all crucial stages of reproduction (Retis-Resendiz et al., 2021). DNA methylation is a widely studied aspect of epigenetic regulation that shows age-related alterations characterized by hypo- or hypermethylation of CpG sites in the genes (Horvath, 2013). The age-associated deviation in the methylation pattern varies with tissue type, which enables us to define the biological age of that particular tissue. Based on this approach, for the first time, ‘Horvath’s epigenetic clock’ was developed in multiple tissues, which aimed to predict the biological age of the tissues and may even predict the attributed risk for disorders such as cancer (Horvath, 2013). Horvath’s epigenetic clock, which was based on the DNA methylation status of 353 CpG sites and was tested across 51 human tissues, however, showed a marginal correlation of chronological age with endometrial epigenetic age (R = 0.55). After the Horvath’s clock, a ‘DNA methylation Pheno-Age clock’ was developed using age-associated significant biomarkers from whole blood to predict the phenotypic age and then correlated with multi-tissues and different cell types (Levine et al., 2018). However, the correlation of chronological age and epigenetic age of human endometrium was not accurately predicted by the Pheno-Age clock (R = 0.39) (Levine et al., 2018; Deryabin and Borodkina, 2023). Another predictor of methylation age, with improved precision, was developed called ‘Zhang clock’ which was primarily based on blood but was tested across non-blood tissues (Zhang et al., 2019). The Zhang clock showed higher accuracy in prediction of the epigenetic age of endometrium (R = 0.77) than the original Horvath’s clock. Recently, the ‘AltumAge’ epigenetic clock (de Lima Camillo et al., 2022) was recalibrated in multi-tissues with higher prediction accuracy using machine learning models (Vijayakumar and Cho, 2022), showing improved performance, for the correlation of chronological age and endometrial age (R = 0.78). Thus, the last two epigenetic predictors; Zhang clock and ‘AltumAge’ epigenetic clock, as well as Horvath’s clock after the menstrual phase correction were more accurate and precise predictor tools for endometrial age, with decreased mean absolute error (Deryabin and Borodkina, 2023).
The endometrium is a very dynamic tissue that undergoes cellular and biochemical alterations during different phases of the menstrual cycle associated with transcriptomic alterations regulating the molecular milieu (Wang et al., 2020b). The differential gene expression across the menstrual cycle is regulated temporally and spatially to some extent by epigenetic mechanisms such as DNA methylation (Retis-Resendiz et al., 2021). The DNA methylation profile in the endometrium of healthy fertile women changes as it shifts from a pre-receptive to receptive endometrium and correlates with alterations in the gene expression profile across the menstrual cycle (Houshdaran et al., 2014; Kukushkina et al., 2017), having a unique methylation profile during the WOI (Kukushkina et al., 2017). This suggests that age can influence the endometrial DNA methylation pattern based on the menstrual phase. This can cause significant changes in the methylation levels of CpG sites of the epigenetic clock and eventually deviate the biological age of the endometrium (Olesen et al., 2018). This hypothesis was further confirmed by evaluation of endometrial age by Horvath’s epigenetic clock using menstrual phase correction in healthy women at the mid-secretory phase having a regular menstrual cycle. After the menstrual phase correction, Horvath’s clock showed an improved correlation between chronological age and endometrial epigenetic age (from R = 0.55 versus R = 0.80), with a reduction in mean absolute error from 11 to 4.4 years. Thus, the endometrium collected at the specific time-point was more accurately synchronized with the chronological age, with a higher correlation (Olesen et al., 2018). This correlation was also inferred to be constant, even for the second endometrial biopsy in the consecutive menstrual cycle. Thus, the approach of standardized sampling time in combination with the highly precise epigenetic clock can be used to adjust the epigenetic clock for the accurate prediction of endometrial age (Deryabin and Borodkina, 2023).
Since an aberrant endometrial DNA methylation profile has been correlated with disorders such as endometriosis (Xue et al., 2007; Saare et al., 2016) and cancer (Multinu et al., 2020), biological age can also be used as a predictive biomarker for pathology progression. A recent study showed that the methylation pattern of the PTENP1 pseudogene (regulator of tumour suppressor gene PTEN) in the human endometrium is also age-dependent (Kovalenko et al., 2021). The level of methylation and associated expression of the endometrial PTENP1 pseudogene was elevated in women aged 45 years or older. Interestingly, the age-specific alteration in methylation and subsequent expression of PTENP1 pseudogene was hypothesized to serve as a protective mechanism against endometrial malignancies among older women heading towards menopause (Kovalenko et al., 2021).
As postulated in a recent review, epigenetic alterations can be reversed by modifications in environmental and dietary factors, administration of drugs targeting epigenetic pathways and genetic reprogramming using OSKM transcriptional factors (OCT4, SOX2, KLF4, and C-MYC) (Deryabin and Borodkina, 2023). Implementation of these approaches can be used to slow down or reverse the affected epigenetic clock and can be considered to diminish the age-related impairment in the endometrium (Deryabin and Borodkina, 2023).
Human models to study the clinical outcome of endometrial aging
Oocyte donation cycles and the effect of aging on endometrial receptivity
Advanced age is one of the major obstacles in achieving a clinical pregnancy and live birth. The gathered evidence has proven that advanced age can affect oocyte quantity, oocyte quality and, eventually, embryo quality, which can be accountable for pregnancy loss, decreased implantation rate, and complications in pregnancy outcome (Cimadomo et al., 2018; Zhang et al., 2020). This can be resolved to some extent with the transfer of embryos generated by fertilizing donor oocytes retrieved from younger women (Seshadri et al., 2021). The success of oocyte donation can be influenced by various factors, for example, the age of the oocyte donor and recipient, clinical indication for oocyte donation, embryo quality, endometrial thickness and blood flow in the recipient (Moomjy et al., 1999; Noyes et al., 2001; Huang et al., 2008; Braga et al., 2020). Although the donor’s age can be controlled in oocyte donation, the effect of the recipient’s age on the endometrial receptivity can be a critical factor in the success of oocyte donation cycles and thus cannot be overlooked. In human studies, oocyte donation may therefore serve as a good model to evaluate the endometrial-associated factors independent of oocyte quality in women of advanced age.
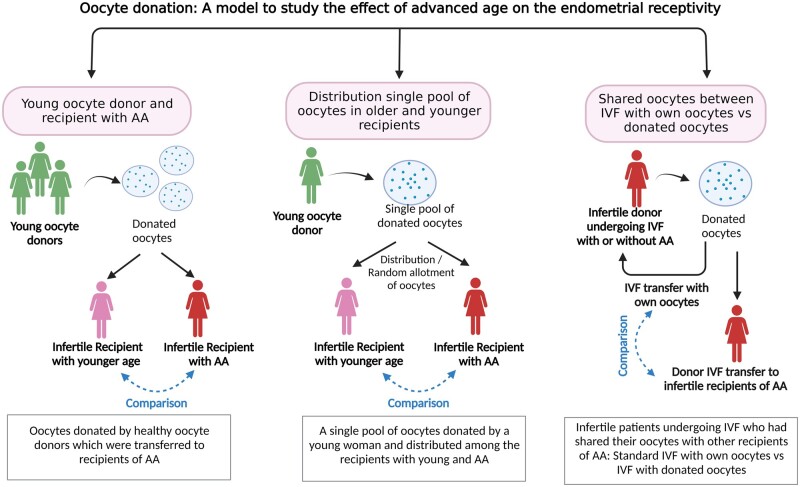
The respective studies can be divided into three main groups based on their inclusion criteria and study design (Fig. 4 and Table 1): studies including healthy, younger oocyte donors and recipients with advanced age to deduce the effect of recipient’s age on endometrial receptivity; studies in which the oocytes donated from single young and healthy woman were distributed amongst the recipients of younger and advanced age; and studies undertaken wherein the infertile patients undergoing IVF had shared their oocytes with other recipients of advanced age, thus indicating the effect of age on endometrial receptivity between IVF cycles using own oocyte and donated oocytes using the same pool of oocytes retrieved from same women.
Figure 4.
Oocyte donation: a model to study recipient aging and her endometrial receptivity. The reported studies have been divided into three arms: oocytes donated by healthy oocyte donors which were transferred to younger recipients or those of advanced age (left); a single pool of oocytes donated by a young woman and distributed among young and advanced age recipients (middle); and infertile patients undergoing IVF who had shared their oocytes with other recipients of advanced age: standard IVF with own oocytes versus IVF with donated oocytes (right). AA, advance age. Created with BioRender.com (https://biorender.com/).
Table 1.
Summary of studies performed in oocyte donation IVF cycles to determine the impact of advanced age on endometrial receptivity.
| Study design |
Reference studies that suggest AA
1 has an impact on ER2 |
Reference studies that suggest AA has no impact on ER | |
|---|---|---|---|
| First author, year | Detailed conclusion if any | First author, year | |
| Oocytes donated by healthy oocyte donors which were transferred to recipients of AA | Abdalla et al. (1990) | – | Balmaceda et al. (1994) |
| Meldrum (1993) | The impact of age on ER can be reversed | Sauer et al. (1994) | |
| Weckstein et al. (1993) | The impact of age on ER can be reversed | Legro et al. (1995) | |
| Yaron et al. (1998) | – | Remohi et al. (1997) | |
| Moomjy et al. (1999) | Impact of age on IR3 but not on PR4 and miscarriage rate | Paulson et al. (1997) | |
| Toner et al. (2002) | Impact of age on ER after 40 years of age | Mirkin et al. (2003) | |
| Soares et al. (2005) | Impact of age on ER after 45 years of age | Wang et al. (2012) | |
| Yeh et al. (2014) | Impact of age on ER after 45 years of age | – | |
| A single pool of oocytes donated by a young woman and distributed among the recipients with young and AA | Cano et al. (1995) | – | Navot et al. (1994) |
| Harris et al. (2002) | AA has a negative impact on the live birth rate but not on PR | Noyes et al. (2001) | |
| Infertile patients undergoing IVF who had shared their oocytes with other recipients of advanced age: standard IVF with own oocytes versus IVF with donated oocytes | Levran et al. (1991) | – | Navot et al. (1991) |
| Check et al. (1993a) | – | Check et al. (1994) | |
| Borini et al. (1996) | – | ||
| Braga et al. (2020) | – | – | |
AA: advanced age.
ER: endometrial receptivity.
IR: implantation rate.
PR: pregnancy rate.
Young oocyte donors and recipients with advanced age
In this group, young oocyte donors had donated their oocytes to the recipients of advanced age (Table 1 and Fig. 4; Supplementary Table S1). Several studies in this group have reported that the recipient’s age had no impact on endometrial receptivity (Balmaceda et al., 1994; Sauer et al., 1994; Legro et al., 1995; Paulson et al., 1997; Mirkin et al., 2003; Wang et al., 2012). The studies, including both fresh and frozen embryo transfer cycles with the recipient age groups ranging from 23 to 54 years, revealed no significant differences in endometrial thickness, pregnancy rate, miscarriage rate, and implantation rate among the different age groups of recipients (Balmaceda et al., 1994; Mirkin et al., 2003). However, this result applies only to patients having endometrial thickness ≥8 mm because the pregnancy rate was observed to drop from 45% to 17% in the recipients with an endometrial thickness of 4–8 mm (Mirkin et al., 2003). Comparable findings were reported in the studies where the recipient’s age had no impact even on the cumulative pregnancy rate and cumulative live birth rate after four consecutive cycles (Paulson et al., 1997; Remohi et al., 1997).
On the other hand, a decreased pregnancy rate was observed with increasing age of recipients (Yaron et al., 1998). Another study demonstrated a drastic decline in endometrial receptivity, with a reduction in the delivery rate from 46% to 21% and an elevation of the abortion rate from 17% to 41% as the recipient’s age increased over 40 years (Meldrum, 1993). This study postulated that the distribution of ER in endometrium decreases with age, which further causes the downregulation of PR leading to impairment in the endometrial microenvironment, making it unfavourable for implantation. The endometrial receptivity was restored, as shown by an increase in the delivery rate from 21% to 54%, by administering a higher dose of exogenous P4 from 50 to 100 mg in advanced-age recipients (Meldrum, 1993). Similarly, the implementation of additional P4 improved the clinical pregnancy rate significantly from 24% to 66% in recipients aged 40 years and above (Weckstein et al., 1993). However, one study (Abdalla et al., 1990) reported a steady decline in pregnancy rate from 50% to 9.7% among the group of recipients aged 25–29 and 45–49 years, respectively, even after prescribing a sufficiently higher dose of P4. Still, these studies collectively highlight that the aged endometrium with altered uterine vasculature and dysregulated PR can potentially restore the receptive potential upon supplementation of additional P4.
Furthermore, an analysis of national data on oocyte donation IVF cycles of the US registry in 2002 (Toner et al., 2002) revealed that the implantation rate, pregnancy rate, and delivery rate were stable for the recipient’s age group ranging from 25 to 40 years, whereas a decline in fecundity was reported in women of advanced age, in their late 40s. In 2014 (Yeh et al., 2014), the US national registry inferred a cut-off of 45 years for the decline in female fertility; more specifically it showed that the age group of 45–49 years can be designated as a transitional period for recipients, which indicates the gradual decrease in pregnancy success. A similar cut-off point of 45 years that affects female fertility was found in a study with a large sample size of 3089 donation cycles (Soares et al., 2005). However, a clinical diagnosis of the recipient may also determine the effect of the recipient’s age on endometrial receptivity. For example, a direct correlation of a decline in implantation rate with increasing age has been found in recipients with tubal disease (Moomjy et al., 1999).
A single pool of oocytes donated by a young woman and distributed among young and advanced-age recipients
Furthermore, to increase the specificity and precision, the oocytes from a single pool donated by a young and healthy woman were distributed among recipients of advanced age and of younger ages (Table 1 and Fig. 4; Supplementary Table S2). In such types of studies, the effect of endometrial aging can be assessed precisely as the parameter of oocyte quality remains constant. One such study reported no significant difference in implantation rate and delivery rate among the recipients aged <40 and ≥40 years, for an even and random distribution of oocytes (Navot et al., 1994). The study by Noyes et al. (2001) had similar findings of showing no effect of the recipient’s age on the live birth rate; however, the live birth rate significantly lowered as the endometrial thickness was <8 mm.
In another study, the oocytes donated by fertile as well as by infertile women undergoing IVF were divided evenly between younger and older recipients, with an age limit of 40 years (Cano et al., 1995). Although the implantation rate between the two groups of recipients did not show any significant difference, the abortion rate was significantly higher in older recipients owing to impaired uterine vascularization and late secretion of E2 and P4 by the placenta, reducing its functional efficacy.
As the factors associated with oocyte donors can be controlled, the importance of the careful selection of donors to improve the success rate of IVF needs to be considered (Harris et al., 2002). Moreover, it has been observed that the donor’s parity and the history of miscarriage can have a causal effect on the probability of pregnancy in their corresponding recipients (Harris et al., 2002).
Infertile patients undergoing IVF who had shared their oocytes with other recipients of advanced age: standard IVF with own oocytes versus IVF with donated oocytes
Some studies have been undertaken wherein the infertile patients undergoing IVF shared their oocytes with recipients of advanced age, thus comparing standard IVF cycles using women’s own oocytes and IVF cycles with donated oocytes (Table 1 and Fig. 4; Supplementary Table S3). Using this study design, both oocyte aging and endometrial aging can be studied simultaneously. Such studies, wherein the donors and advanced-aged recipients had received the unselected oocytes from the same cohort, revealed a similar clinical pregnancy rate in recipients and donors irrespective of age (Navot et al., 1991; Check et al., 1994).
On the contrary, other reports with a similar study design have shown an age-related negative impact on endometrial aging. These reports suggest a decline in endometrial receptivity among older recipients compared with younger donors who have undergone the embryo transfer with their own oocyte (Levran et al., 1991; Check et al., 1993a; Borini et al., 1996; Braga et al., 2020). Overall, the literature on treatment options for oocyte donation in women of advanced age showed very ambiguous inferences based on endometrial aging.
Anti-aging therapies and endometrial receptivity
The treatment option for endometrial aging in patients with advanced age is to treat the cellular senescence process associated with endometrial aging. Scientific advances have led to the development of drug therapies which can target senescent cells and prevent their detrimental effect causing pathogenic conditions, including age-related diseases. Such drugs are referred to as ‘senolytics’, ‘senomorphics’, or ‘senostatics’, which cause apoptosis of the senescent cells or inhibit the function of senescent cells and their secretions in terms of SASP (Deryabin et al., 2020; Secomandi et al., 2022). There are several senolytic drugs, using different interventions or modes of action, to target the senescent cells, such as attenuation of the pro-survival pathway, anti-apoptotic proteins, targeting cellular metabolism pathway of senescent cells, and stimulation of the immune system for the targeted clearance of senescent cells (Secomandi et al., 2022).
Various senolytic therapies have been used in animal models for several age-associated pathological conditions, such as idiopathic pulmonary fibrosis, kidney diseases, and atherosclerosis as well as ovarian dysfunction, and can even restore the pregnancy rate and oocyte competence (Sugiyama et al., 2015; Bertoldo et al., 2020; Secomandi et al., 2022). However, the implementation of senolytic therapies to improve endometrial receptivity is extremely challenging. As discussed above, the phenomenon of cellular senescence is essential in the initial phase of decidualization to elicit the necessary pro-inflammatory response to create a favourable microenvironment and to open the temporal window for successful implantation. Aging may cause inability of the immune system to eliminate senescent ESCs, which may interfere with the inflammatory balance during the decidualization process (Brighton et al., 2017). Thus, advanced age can alter the normal senescence phenomenon that is essential for endometrial receptivity. Senolytic therapy targeted to the senescent cells can affect the pre-senescent ESC and senescent decidualized cells; thus, there is a possibility of destroying either or both of the cellular populations, which could have been contributing to endometrial receptivity (Deryabin et al., 2020; Secomandi et al., 2022). To resolve this problem, the senolytic agent dasatinib, a selective tyrosine kinase receptor inhibitor that inhibits the cellular apoptosis resistance pathway causing an apoptotic effect on senescent cells, has been investigated. A study with ESC culture was conducted involving the targeted elimination of pre-senescent ESCs using dasatinib treatment, which resulted in a significant reduction in pro-inflammatory secretion during decidualization (Brighton et al., 2017). Furthermore, a combination of dasatinib and quercin was used as the inhibitor of cellular pro-survival pathways in the murine uterus in vitro, which showed upregulation of the p53 pathway and suggested an anti-fibrotic uterine effect. However, the results could not be replicated in vivo (Cavalcante et al., 2020). Additionally, senomorphic agents such as rapamycin or resveratrol, used for the disruption of undifferentiated senescence cells, showed impairment of the decidualization process (Brighton et al., 2017; Ochiai et al., 2019). Resveratrol treatment induces dysfunction of a retinoic acid signalling pathway in decidualized ESCs, which has a detrimental effect on decidualization causing a reduction in implantation rate and increasing the risk of miscarriage when used in vivo (Ochiai et al., 2019). At the same time, a recent study revealed that pre-treatment of senescent ESCs with senomorphic agents such as metformin or rapamycin might improve further decidualization and blastocyst implantation, at least in vitro (Deryabin and Borodkina, 2022). It is likely that negative or beneficial outcomes of senomorphics might be related to the phase of the menstrual cycle. Indeed, if resveratrol is added during the WOI, it would further negatively affect embryo implantation (Brighton et al., 2017; Ochiai et al., 2019). However, if resveratrol supplementation is restricted to the proliferative phase, it would not adversely impact embryo implantation or ESCs decidualization (Kuroda et al., 2020). Therefore, the use of senolytic therapy to improve decidualization and endometrial receptivity needs to be considered with extreme caution. For this purpose, the factors such as time period, dosage, phase of the menstrual cycle, adverse effects and potential risk for generation of inappropriate inflammatory response should be critically evaluated by means of precise experimental studies and appropriately designed clinical trials.
Conclusion
Advanced age of the woman can be one of the significant risk factors for the impaired cellular senescence phenomenon and defective endometrial receptivity, which may contribute to infertility. Published studies using animal models have uncovered the potential risk of tissue fibrosis with changes in the tissue histology, dysregulation of the decidualization process, and immune and inflammatory imbalance, causing deterioration of endometrial receptivity as age increases. However, limited publications in this area indicate that the effect of increasing age, specifically on human endometrium, has been inadvertently underestimated. In the past two decades, the molecular mechanism, a network of biological pathways, and biomarkers of endometrial receptivity have been elucidated in the literature with the main focus being on the development of diagnostic tools for its assessment (Ruiz-Alonso et al., 2021). However, information on the correlation between advanced age and the molecular aspects of endometrial receptivity in these studies is mostly lacking. A recent study based on transcriptome datasets of endometrium in different reproductive age groups revealed that endometrial gene expression was altered after the age of 35 years (Devesa-Peiro et al., 2022); although the design of this study was retrospective, it is the only published study that provides endometrial data determining the role of maternal age using the whole-genome approach. Owing to the paucity of evidence in this domain, it is difficult to draw a concise conclusion pertaining to the cut-off age for the physiological initiation of endometrial aging. Therefore, there is a need for prospective research studies with appropriate designs to detect the influence of age on various aspects of endometrial functioning. Such studies can be aimed at detecting the age-related distribution of hormone receptors and immunological biomarkers in human endometrium, and to evaluate endometrial profiling along with cellular heterogeneity in the ciliated epithelial cell populations among older versus younger women to understand the exact mechanism(s) affecting endometrial receptivity. Additionally, investigating the correlation between endometrial thickness and ultrasound pattern with advanced age using clinical trials would further contribute to the clinical implications associated with pregnancy outcomes. Moreover, future studies can be conducted considering the hypothesis of establishing a personalized age threshold at which the endometrium starts displaying adverse effects of aging.
Furthermore, oocyte donation is one of the best models to study the effect of age on the recipient’s endometrium by using the oocytes from younger and healthy donors. Although different study designs and variables have been used in the literature on oocyte donation cycles, the resulting data have contradictory inferences about the impact of advanced age on implantation or pregnancy rate in recipients. In the future, randomized controlled clinical studies evaluating the impact of using oocytes from younger donors on the endometrium of advanced-aged recipients, using clinical pregnancy rate and live birth rate as the main outcome measures, would be promising. Looking at the perspective of treatment options to restore endometrial receptivity in older women, several senolytic therapies have been used in animal models (Secomandi et al., 2022). However, because cellular senescence is essential during decidualization, implementing these senolytic agents to restore endometrial receptivity without hampering the decidualization is extremely challenging.
Thus, in this review, we have attempted to cover most of the aspects related to endometrial receptivity that can be influenced by age and that would exert an impact on the implantation of the embryo. This review was restricted to conception and the implantation process, and the subsequent features related to miscarriage and pregnancy-associated complications have not been covered.
In summary, although the exact mechanisms involved in the effects of advanced age on endometrial receptivity are still elusive, the outcomes of recent publications are suggestive of the negative effect of aging on endometrial biology. However, cumulative scientific literature indicates a dearth of research studies conducted in this area, that results in a knowledge gap concerning the underlying causes and associated effects. To validate these findings, future research is needed, focusing on the molecular mechanisms underlying the effect of age on cellular senescence, cellular compositions, and transcriptomic changes with regard to endometrial receptivity. This research can utilize advanced techniques, such as single-cell sequencing, to gain a comprehensive and deeper understanding of these processes. Additionally, there is a need for continued research to support the development of treatment options that address the age-related decline in endometrial receptivity. Exploring the use of senolytic agents, which can selectively eliminate senescent cells, to restore endometrial receptivity without compromising the decidualization process would be a promising avenue to investigate. In parallel, in the future, studying the model of oocyte donation cycles with an unbiased study design using clinical trial protocols would be constructive in order to understand the direct clinical implications of endometrial aging on pregnancy outcomes.
Supplementary Material
Contributor Information
Amruta D S Pathare, Department of Obstetrics and Gynaecology, Institute of Clinical Medicine, University of Tartu, Tartu, Estonia.
Marina Loid, Department of Obstetrics and Gynaecology, Institute of Clinical Medicine, University of Tartu, Tartu, Estonia; Competence Centre on Health Technologies, Tartu, Estonia.
Merli Saare, Department of Obstetrics and Gynaecology, Institute of Clinical Medicine, University of Tartu, Tartu, Estonia; Competence Centre on Health Technologies, Tartu, Estonia.
Sebastian Brusell Gidlöf, Division of Obstetrics and Gynaecology, Department of Clinical Science, Intervention and Technology (CLINTEC), Karolinska Institute and Karolinska University Hospital, Stockholm, Sweden; Department of Gynecology and Reproductive Medicine, Karolinska University Hospital, Stockholm, Sweden.
Masoud Zamani Esteki, Division of Obstetrics and Gynaecology, Department of Clinical Science, Intervention and Technology (CLINTEC), Karolinska Institute and Karolinska University Hospital, Stockholm, Sweden; Department of Clinical Genetics, Maastricht University Medical Centre+, Maastricht, The Netherlands; Department of Genetics and Cell Biology, GROW School for Oncology and Reproduction, Maastricht University, Maastricht, The Netherlands.
Ganesh Acharya, Division of Obstetrics and Gynaecology, Department of Clinical Science, Intervention and Technology (CLINTEC), Karolinska Institute and Karolinska University Hospital, Stockholm, Sweden; Department of Clinical Medicine, Women’s Health and Perinatology Research Group, UiT The Arctic University of Norway, Tromsø, Norway.
Maire Peters, Department of Obstetrics and Gynaecology, Institute of Clinical Medicine, University of Tartu, Tartu, Estonia; Competence Centre on Health Technologies, Tartu, Estonia.
Andres Salumets, Department of Obstetrics and Gynaecology, Institute of Clinical Medicine, University of Tartu, Tartu, Estonia; Competence Centre on Health Technologies, Tartu, Estonia; Division of Obstetrics and Gynaecology, Department of Clinical Science, Intervention and Technology (CLINTEC), Karolinska Institute and Karolinska University Hospital, Stockholm, Sweden.
Supplementary data
Supplementary data are available at Human Reproduction Update online.
Data availability
No new data were generated or analysed in support of this research.
Authors’ roles
A.S., S.B.G., G.A., and A.D.S.P. participated in the conception and designing of the review article. A.D.S.P. contributed to literature search and screening of articles. A.D.S.P and M.L. prepared the first draft of the manuscript. M.P., M.S., S.B.G., M.Z.E., G.A., and A.S. critically reviewed each version of the manuscript, and all the authors confirmed the final version.
Funding
The preparation of the current review was supported by the Estonian Research Council (grants PRG1076 and MOBJD1056) and Horizon 2020 innovation grant (ERIN, grant no. EU952516).
Conflict of interest
All the authors declare no conflict of interest.
References
- Abdalla HI, Baber R, Kirkland A, Leonard T, Power M, Studd JWW. A report on 100 cycles of oocyte donation; factors affecting the outcome. Hum Reprod 1990;5:1018–1022. [DOI] [PubMed] [Google Scholar]
- Achache H, Revel A. Endometrial receptivity markers, the journey to successful embryo implantation. Hum Reprod Update 2006;12:731–746. [DOI] [PubMed] [Google Scholar]
- Altmäe S, Koel M, Võsa U, Adler P, Suhorutšenko M, Laisk-Podar T, Kukushkina V, Saare M, Velthut-Meikas A, Krjutškov K et al. Meta-signature of human endometrial receptivity: a meta-analysis and validation study of transcriptomic biomarkers. Sci Rep 2017;7:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R, Lagnado A, Maggiorani D, Walaszczyk A, Dookun E, Chapman J, Birch J, Salmonowicz H, Ogrodnik M, Jurk D et al. Length‐independent telomere damage drives post‐mitotic cardiomyocyte senescence. EMBO J 2019;38:e100492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmaceda JP, Bernardini L, Ciuffardi I, Felix C, Ord T, Sueldo CE, Asch RH. Oocyte donation in humans: a model to study the effect of age on embryo implantation rate. Hum Reprod 1994;9:2160–2163. [DOI] [PubMed] [Google Scholar]
- Baruffi RLR, Contart P, Mauri AL, Petersen C, Felipe V, Garbellini E, Franco JGJ. A uterine ultrasonographic scoring system as a method for the prognosis of embryo implantation. J Assist Reprod Genet 2002;19:99–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdiaki A, Zafeiropoulou S, Makrygiannakis F, Drakopoulos P, Gurgan T, Makrigiannakis A. Ageing, a modulator of human endometrial stromal cell proliferation and decidualization: a role for implantation? Reprod Biomed 2022;45:202–210. [DOI] [PubMed] [Google Scholar]
- Bergqvist A, Ferno M. Oestrogen and progesterone receptors in endometriotic tissue and endometrium: comparison of different cycle phases and ages. Hum Reprod 1993;8:2211–2217. [DOI] [PubMed] [Google Scholar]
- Bertoldo MJ, Listijono DR, Ho W-HJ, Riepsamen AH, Goss DM, Richani D, Jin XL, Mahbub S, Campbell JM, Habibalahi A et al. NAD+ repletion rescues female fertility during reproductive aging. Cell Rep 2020;30:1670–1681.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggers JD, Finn CA, McLAREN A. Long-term reproductive performance of female mice. I. Effect of removing one ovary. J Reprod Fertil 1962;3:303–312. [DOI] [PubMed] [Google Scholar]
- Blaha GC, Leavitt WW. Ovarian steroid dehydrogenase histochemistry and circulating progesterone in aged golden hamsters during the estrous cycle and pregnancy. Biol Reprod 1974;11:153–161. [DOI] [PubMed] [Google Scholar]
- Borini A, Bianchi L, Violini F, Maccolini A, Cattoli M, Flamigni C. Oocyte donation program: pregnancy and implantation rates in women of different ages sharing oocytes from single donor. Fertil Steril 1996;65:94–97. [DOI] [PubMed] [Google Scholar]
- Borodkina A, Deryabin P, Giukova A, Nikolsky N. “Social life” of senescent cells: what is SASP and why study it? Acta Naturae 2018;10:4–14. [PMC free article] [PubMed] [Google Scholar]
- Bouzaglou A, Aubenas I, Abbou H, Rouanet S, Carbonnel M, Pirtea P, Ayoubi JMB. Pregnancy at 40 years old and above: obstetrical, fetal, and neonatal outcomes. Is age an independent risk factor for those complications? Front Med (Lausanne) 2020;7:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga DPAF, Setti AS, Iaconelli A, Borges E. Predictive factors for successful pregnancy in an egg-sharing donation program. J Bras Reprod Assist 2020;24:163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauning A, Rae M, Zhu G, Fulton E, Admasu TD, Stolzing A, Sharma A. Aging of the immune system: focus on natural killer cells phenotype and functions. Cells 2022;11:1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brighton PJ, Maruyama Y, Fishwick K, Vrljicak P, Tewary S, Fujihara R, Muter J, Lucas ES, Yamada T, Woods L et al. Clearance of senescent decidual cells by uterine natural killer cells in cycling human endometrium. Elife 2017;6:e31274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burack E, Wolfe JM, Lansing W, Wright AW. The effect of age upon the connective tissue of the uterus, cervix, and vagina of the rat. Cancer Res 1941;1:227–235. [Google Scholar]
- Burger HG, Dudley EC, Hopper JL, Groome N, Guthrie JR, Green A, Dennerstein L. Prospectively measured levels of serum follicle-stimulating hormone, estradiol, and the dimeric inhibins during the menopausal transition in a population-based cohort of women. J Clin Endocrinol Metab 1999;84:4025–4030. [DOI] [PubMed] [Google Scholar]
- Burger HG, Hale GE, Dennerstein L, Robertson DM. Cycle and hormone changes during perimenopause. Menopause 2008;15:603–612. [DOI] [PubMed] [Google Scholar]
- Carnevale EM, Ginther OJ. Relationships of age to uterine function and reproductive efficiency in mares. Theriogenology 1992;37:1101–1115. [DOI] [PubMed] [Google Scholar]
- Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol 2013;75:685–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano F, Simon C, Remohi J, Pellicer A. Effect of aging on the female reproductive system: evidence for a role of uterine senescence in the decline in female fecundity. Fertil Steril 1995;64:584–589. [DOI] [PubMed] [Google Scholar]
- Cavalcante MB, Saccon TD, Nunes ADC, Kirkland JL, Tchkonia T, Schneider A, Masternak MM. Dasatinib plus quercetin prevents uterine age-related dysfunction and fibrosis in mice. Aging (Albany NY) 2020;12:2711–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Check JH, Askari HA, Choe J, Baker A, Adelson HG. The effect of the age of the recipients on pregnancy rates following donor-oocyte replacement. J Assist Reprod Genet 1993a;10:137–140. [DOI] [PubMed] [Google Scholar]
- Check JH, Askari HA, Fisher C, Vanaman L. The use of a shared donor oocyte program to evaluate the effect of uterine senescence. Fertil Steril 1994;61:252–256. [DOI] [PubMed] [Google Scholar]
- Check JH, Lurie D, Dietterich C, Callan C, Baker A. Pregnancy: adverse effect of a homogeneous hyperechogenic endometrial sonographic pattern, despite adequate endometrial thickness on pregnancy rates following in-vitro fertilization. Hum Reprod 1993b;8:1293–1296. [DOI] [PubMed] [Google Scholar]
- Chen S-L, Wu F-R, Luo C, Chen X, Shi X-Y, Zheng H-Y, Ni Y-P. Combined analysis of endometrial thickness and pattern in predicting outcome of in vitro fertilization and embryo transfer: a retrospective cohort study. Reprod Biol Endocrinol 2010;8:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimadomo D, Fabozzi G, Vaiarelli A, Ubaldi N, Ubaldi FM, Rienzi L. Impact of maternal age on oocyte and embryo competence. Front Endocrinol (Lausanne) 2018;9:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppé J-P, Patil CK, Rodier F, Sun Y, Muñoz DP, Goldstein J, Nelson PS, Desprez P-Y, Campisi J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol 2008;6:e301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig SS. Effect of age upon uterine response to deciduagenic stimulus. Acta Anat (Basel) 1981;110:146–158. [DOI] [PubMed] [Google Scholar]
- Critchley HOD, Maybin JA, Armstrong GM, Williams ARW. Physiology of the endometrium and regulation of menstruation. Physiol Rev 2020;100:1149–1179. [DOI] [PubMed] [Google Scholar]
- Das SK. Regional development of uterine decidualization: molecular signaling by Hoxa-10. Mol Reprod Dev 2010;77:387–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lima Camillo LP, Lapierre LR, Singh R. A pan-tissue DNA-methylation epigenetic clock based on deep learning. Npj Aging 2022;8:4. [Google Scholar]
- Deryabin P, Griukova A, Nikolsky N, Borodkina A. The link between endometrial stromal cell senescence and decidualization in female fertility: the art of balance. Cell Mol Life Sci 2020;77:1357–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deryabin PI, Borodkina AV. Stromal cell senescence contributes to impaired endometrial decidualization and defective interaction with trophoblast cells. Hum Reprod 2022;37:1505–1524. [DOI] [PubMed] [Google Scholar]
- Deryabin PI, Borodkina AV. Epigenetic clocks provide clues to the mystery of uterine ageing. Hum Reprod Update 2023;29:259–271. [DOI] [PubMed] [Google Scholar]
- Devesa-Peiro A, Sebastian-Leon P, Parraga-Leo A, Pellicer A, Diaz-Gimeno P. Breaking the ageing paradigm in endometrium: endometrial gene expression related to cilia and ageing hallmarks in women over 35 years. Human Reproduction 2022;37:762–776. [DOI] [PubMed] [Google Scholar]
- Dockery P, Rogers AW. The effects of steroids on the fine structure of the endometrium. Baillieres Clin Obstet Gynaecol 1989;3:227–248. [DOI] [PubMed] [Google Scholar]
- Downes MJ, Brennan ML, Williams HC, Dean RS. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS). BMJ Open 2016;6:e011458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer RF, Sodek J, Heersche JN. The effect of 17 beta-estradiol on collagen and noncollagenous protein synthesis in the uterus and some periodontal tissues. Endocrinology 1980;107:1014–1021. [DOI] [PubMed] [Google Scholar]
- Eftekhar M, Mehrjardi SZ, Molaei B, Taheri F, Mangoli E. The correlation between endometrial thickness and pregnancy outcomes in fresh ART cycles with different age groups: a retrospective study. Middle East Fertil Soc J 2019;24:16–20. [Google Scholar]
- Fitzgerald CT, Fellow R, Seif MW, Killick SR, Bennett DA. Age related changes in the female reproductive cycle. Br J Obstet Gynaecol 1994;101:229–233. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A. Inflammaging: a new immune–metabolic viewpoint for age-related diseases. Nat Rev Endocrinol 2018;14:576–590. [DOI] [PubMed] [Google Scholar]
- Garcia GG, Miller RA. Age-related defects in the cytoskeleton signaling pathways of CD4 T cells. Ageing Res Rev 2011;10:26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Alonso L, Handfield L-F, Roberts K, Nikolakopoulou K, Fernando RC, Gardner L, Woodhams B, Arutyunyan A, Polanski K, Hoo R et al. Mapping the temporal and spatial dynamics of the human endometrium in vivo and in vitro. Nat Genet 2021;53:1698–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellersen B, Brosens JJ. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr Rev 2014;35:851–905. [DOI] [PubMed] [Google Scholar]
- Gingold JA, Lee JA, Rodriguez-Purata J, Whitehouse MC, Sandler B, Grunfeld L, Mukherjee T, Copperman AB. Endometrial pattern, but not endometrial thickness, affects implantation rates in euploid embryo transfers. Fertil Steril 2015;104:620–628.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giudice LC. Endometrium in PCOS: implantation and predisposition to endocrine CA. Best Pract Res Clin Endocrinol Metab 2006;20:235–244. [DOI] [PubMed] [Google Scholar]
- Gonen Y, Casper RF. Prediction of implantation by the sonographic appearance of the endometrium during controlled ovarian stimulation for in vitro fertilization (IVF). J in Vitro Fert Embryo Transf 1990;7:146–152. [DOI] [PubMed] [Google Scholar]
- Gosden RG. Effects of age and parity on the breeding potential of mice with one or two ovaries. J Reprod Fertil 1979;57:477–487. [DOI] [PubMed] [Google Scholar]
- Griukova A, Deryabin P, Shatrova A, Burova E, Severino V, Farina A, Nikolsky N, Borodkina A. Molecular basis of senescence transmitting in the population of human endometrial stromal cells. Aging (Albany NY) 2019;11:9912–9931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerin LR, Prins JR, Robertson SA. Regulatory T-cells and immune tolerance in pregnancy: a new target for infertility treatment? Hum Reprod Update 2009;15:517–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale GE, Zhao X, Hughes CL, Burger HG, Robertson DM, Fraser IS. Endocrine features of menstrual cycles in middle and late reproductive age and the menopausal transition classified according to the staging of reproductive aging workshop (STRAW) staging system. J Clin Endocrinol Metab 2007;92:3060–3067. [DOI] [PubMed] [Google Scholar]
- Harris SE, Faddy M, Levett S, Sharma V, Gosden R. Analysis of donor heterogeneity as a factor affecting the clinical outcome of oocyte donation. Hum Fertil (Camb) 2002;5:193–198. [DOI] [PubMed] [Google Scholar]
- Herbig U, Ferreira M, Condel L, Carey D, Sedivy JM. Cellular senescence in aging primates. Science 2006;311:1257. [DOI] [PubMed] [Google Scholar]
- Hirata Y, Katsukura Y, Henmi Y, Ozawa R, Shimazaki S, Kurosawa A, Torii Y, Takahashi H, Iwata H, Kuwayama T et al. Advanced maternal age induces fetal growth restriction through decreased placental inflammatory cytokine expression and immune cell accumulation in mice. J Reprod Dev 2021;67:257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S. DNA methylation age of human tissues and cell types. Genome Biol 2013;14:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houshdaran S, Zelenko Z, Irwin JC, Giudice LC. Human endometrial DNA methylome is cycle-dependent and is associated with gene expression regulation. Mol Endocrinol 2014;28:1118–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L-S, Lee M-S, Cheng E-H, Lee T-H, Liu C-H, Lee M-C, Chou M-C. Recipient age and pulsatility index affect uterine receptivity in oocyte donation programmes. Reprod Biomed Online 2008;17:94–100. [DOI] [PubMed] [Google Scholar]
- Jabrane-Ferrat N, Siewiera J. The up side of decidual natural killer cells: new developments in immunology of pregnancy. Immunology 2014;141:490–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järvelä IY, Sladkevicius P, Kelly S, Ojha K, Campbell S, Nargund G. Evaluation of endometrial receptivity during in-vitro fertilization using three-dimensional power Doppler ultrasound. Ultrasound Obstet Gynecol 2005;26:765–769. [DOI] [PubMed] [Google Scholar]
- Jeffrey JJ. Collagen synthesis and degradation in the uterine deciduoma: regulation of collagenase activity by progesterone. Coll Relat Res 1981;1:257–268. [DOI] [PubMed] [Google Scholar]
- Jeffrey JJ, Coffey RJ, Eisen AZ. Studies on uterine collagenase in tissue culture. I. Relationship of enzyme production to collagen metabolism. Biochim Biophys Acta 1971;252:136–142. [DOI] [PubMed] [Google Scholar]
- Jeffrey JJ, Roswit WT, Ehlich LS. Regulation of collagenase production by steroids in uterine smooth muscle cells: an enzymatic and immunologic study. J Cell Physiol 1990;143:396–403. [DOI] [PubMed] [Google Scholar]
- Jiménez-Ayala M, Jiménez-Ayala Portillo B. Cytology of the normal endometrium-cycling and postmenopausal. In: Endometrial Adenocarcinoma: Prevention and Early Diagnosis: Including contributions by Iglesias Goy, E. (Madrid); Rios Vallejo, M. (Madrid), S.Karger AG, 2008, 32–39. [DOI] [PubMed]
- Kawamura T, Tomari H, Onoyama I, Araki H, Yasunaga M, Lin C, Kawamura K, Yokota N, Yoshida S, Yagi H et al. Identification of genes associated with endometrial cell ageing. Mol Hum Reprod 2021;27:gaaa078. [DOI] [PubMed] [Google Scholar]
- Khan MS, Shaikh A, Ratnani R. Ultrasonography and Doppler study to predict uterine receptivity in infertile patients undergoing embryo transfer. J Obstet Gynaecol India 2016;66:377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 2010;8:e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AE, Collins F, Klonisch T, Sallenave JM, Critchley HOD, Saunders PTK. An additive interaction between the NFB and estrogen receptor signalling pathways in human endometrial epithelial cells. Hum Reprod 2010;25:510–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klonos E, Katopodis P, Karteris E, Papanikolaou E, Tarlatzis B, Pados G. Endometrial changes in estrogen and progesterone receptor expression during implantation in an oocyte donation program. Exp Ther Med 2020;20:1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koot YEM, Teklenburg G, Salker MS, Brosens JJ, Macklon NS. Molecular aspects of implantation failure. Biochim Biophys Acta 2012;1822:1943–1950. [DOI] [PubMed] [Google Scholar]
- Kovalenko TF, Morozova K V, Pavlyukov MS, Anufrieva KS, Bobrov MY, Gamisoniya AM, Ozolinya LA, Dobrokhotova YE, Shakhparonov MI, Patrushev LI. Methylation of the PTENP1 pseudogene as potential epigenetic marker of age-related changes in human endometrium. PLoS One 2021;16:e0243093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukushkina V, Modhukur V, Suhorutšenko M, Peters M, Mägi R, Rahmioglu N, Velthut-Meikas A, Altmäe S, Esteban FJ, Vilo J et al. DNA methylation changes in endometrium and correlation with gene expression during the transition from pre-receptive to receptive phase. Sci Rep 2017;7:3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari R, Jat P. Mechanisms of cellular senescence: cell cycle arrest and senescence associated secretory phenotype. Front Cell Dev Biol 2021;9:645593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda K, Ochiai A, Brosens JJ. The actions of resveratrol in decidualizing endometrium: acceleration or inhibition? Biol Reprod 2020;103:1152–1156. [DOI] [PubMed] [Google Scholar]
- Larson LL, Spilman CH, Foote RH. Uterine uptake of progesterone and estradiol in young and aged rabbits. Proc Soc Exp Biol Med 1972;141:463–466. [DOI] [PubMed] [Google Scholar]
- Lefebvre JS, Haynes L. Aging of the CD4 t cell compartment. Open Longev Sci 2012;6:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legro RS, Wong IL, Paulson RJ, Lobo RA, Sauer MV. Recipient’s age does not adversely affect pregnancy outcome after oocyte donation. Am J Obstet Gynecol 1995;172:96–100. [DOI] [PubMed] [Google Scholar]
- Levine ME, Lu AT, Quach A, Chen BH, Assimes TL, Bandinelli S, Hou L, Baccarelli AA, Stewart JD, Li Y et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany NY) 2018;10:573–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levran D, Ben-Shlomo I, Dor J, Ben-Rafael Z, Nebel L, Mashiach S. Aging of endometrium and oocytes: observations on conception and abortion rates in an egg donation model. Fertil Steril 1991;56:1091–1094. [DOI] [PubMed] [Google Scholar]
- Lo CK-L, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol 2014;14:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loid M, Obukhova D, Kask K, Meltsov A, Derks KWJ, Altmäe S, Saare M, Peters M, Minajeva A, Adler P et al. Aging affects ciliated cells development in the human endometrial epithelium. medRxiv. doi: 10.1101/2023.05.22.23290333, preprint. [DOI]
- Lucas ES, Vrljicak P, Muter J, Diniz-da-Costa MM, Brighton PJ, Kong C-S, Lipecki J, Fishwick KJ, Odendaal J, Ewington LJ et al. Recurrent pregnancy loss is associated with a pro-senescent decidual response during the peri-implantation window. Commun Biol 2020;3:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNaughton J, Banah M, McCloud P, Hee J, Burger H. Age related changes in follicle stimulating hormone, luteinizing hormone, oestradiol and immunoreactive inhibin in women of reproductive age. Clin Endocrinol (Oxf) 1992;36:339–345. [DOI] [PubMed] [Google Scholar]
- Maduro AT, Luís C, Soares R. Ageing, cellular senescence and the impact of diet: an overview. Porto Biomed J 2021;6:e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer RR, Foote RH. Uterine collagenase and collagen in young and ageing rabbits. J Reprod Fertil 1972;30:301–304. [DOI] [PubMed] [Google Scholar]
- McHugh D, Gil J. Senescence and aging: causes, consequences, and therapeutic avenues. J Cell Biol 2018;217:65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldrum DR. Female reproductive aging—ovarian and uterine factors. Fertil Steril 1993;59:1–5. [DOI] [PubMed] [Google Scholar]
- Mercé LT, Barco MJ, Bau S, Troyano J. Are endometrial parameters by three-dimensional ultrasound and power Doppler angiography related to in vitro fertilization/embryo transfer outcome? Fertil Steril 2008;89:111–117. [DOI] [PubMed] [Google Scholar]
- Messaoudi S, el Kasmi I, Bourdiec A, Crespo K, Bissonnette L, Le Saint C, Bissonnette F, Kadoch I-J. 15 years of transcriptomic analysis on endometrial receptivity: what have we learnt? Fertil Res Pract 2019;5:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Micco R, Krizhanovsky V, Baker D, d'Adda di Fagagna F. Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat Rev Mol Cell Biol 2021;22:75–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills M, Rindfuss RR, McDonald P, Te Velde E; ESHRE Reproduction and Society Task Force Why do people postpone parenthood? Reasons and social policy incentives. Hum Reprod Update 2011;17:848–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirkin S, Gimeno TG, Bovea C, Stadtmauer L, Gibbons WE, Oehninger S. Factors associated with an optimal pregnancy outcome in an oocyte donation program. J Assist Reprod Genet 2003;20:400–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monin L, Whettlock EM, Male V. Immune responses in the human female reproductive tract. Immunology 2020;160:106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moomjy M, Cholst I, Mangieri R, Rosenwaks Z. Oocyte donation: insights into implantation. Fertil Steril 1999;71:15–21. [DOI] [PubMed] [Google Scholar]
- Mori M, Bogdan A, Balassa T, Csabai T, Szekeres-Bartho J. The decidua—the maternal bed embracing the embryo—maintains the pregnancy. Semin Immunopathol 2016;38:635–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland J, Jones CJP. Characteristics of uterine aging. Microsc Res Tech 1993;25:148–168. [DOI] [PubMed] [Google Scholar]
- Multinu F, Chen J, Madison JD, Torres M, Casarin J, Visscher D, Shridhar V, Bakkum-Gamez J, Sherman M, Wentzensen N et al. Analysis of DNA methylation in endometrial biopsies to predict risk of endometrial cancer. Gynecol Oncol 2020;156:682–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata H, Tanaka S, Okada H. Immune tolerance of the human decidua. J Clin Med 2021;10:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navinchandra R, Shankar S, Kavitha D, Kamath M, Devdas S, Vineela P. Relationship between uterine scoring system for reproduction and pregnancy in controlled ovarian stimulation-intrauterine insemination cycles. IVF Lite 2016;3:115. [Google Scholar]
- Navot D, Bergh RA, Williams MA, Garrisi GJ, Guzman I, Sandler B, Grunfeld L. Poor oocyte quality rather than implantation failure as a cause of age-related decline in female fertility. Lancet 1991;337:1375–1377. [DOI] [PubMed] [Google Scholar]
- Navot D, Drews MR, Bergh PA, Guzman I, Karstaedt A, Scott RT, Garrisi GJ, Hofmann GE. Age-related decline in female fertility is not due to diminished capacity of the uterus to sustain embryo implantation. Fertil Steril 1994;61:97–101. [DOI] [PubMed] [Google Scholar]
- Naydenov M, Nikolova M, Apostolov A, Glogovitis I, Salumets A, Baev V, Yahubyan G. The dynamics of miR-449a/c expression during uterine cycles are associated with endometrial development. Biology (Basel) 2022;12:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SM, Telfer EE, Anderson RA. The ageing ovary and uterus: new biological insights. Hum Reprod Update 2013;19:67–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman L, Chopra J, Dossett C, Shepherd E, Bercusson A, Carroll M, Walker W, Lucas JS, Cheong Y. The impact of primary ciliary dyskinesia on female and male fertility: a narrative review. Hum Reprod Update 2023;29:347–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng SW, Norwitz GA, Pavlicev M, Tilburgs T, Simón C, Norwitz ER. Endometrial decidualization: the primary driver of pregnancy health. Int J Mol Sci 2020;21:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noci I, Borr P, Chieffi O, Scarselli G, Biagiott R, Moncini D, Paglierani M, Taddei G. I. Aging of the human endometrium: a basic morphological and immunohistochemical study. Eur J Obstet Gynecol Reprod Biol 1995;63:181–185. [DOI] [PubMed] [Google Scholar]
- Noyes N, Hampton BS, Berkeley A, Licciardi F, Grifo J, Krey L. Factors useful in predicting the success of oocyte donation: a 3-year retrospective analysis. Fertil Steril 2001;76:92–97. [DOI] [PubMed] [Google Scholar]
- Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Fertil Steril 1950;1:3–25. [DOI] [PubMed] [Google Scholar]
- Ntostis P, Swanson G, Kokkali G, Iles D, Huntriss J, Pantou A, Tzetis M, Pantos K, Picton HM, Krawetz SA et al. The effects of aging on molecular modulators of human embryo implantation. iScience 2021;24:102751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutu M, Weijdegård B, Thomas P, Thurin-Kjellberg A, Billig H, Larsson DJ. Distribution and hormonal regulation of membrane progesterone receptors β and γ in ciliated epithelial cells of mouse and human fallopian tubes. Reprod Biol Endocrinol 2009;7:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochiai A, Kuroda K, Ozaki R, Ikemoto Y, Murakami K, Muter J, Matsumoto A, Itakura A, Brosens JJ, Takeda S. Resveratrol inhibits decidualization by accelerating downregulation of the CRABP2-RAR pathway in differentiating human endometrial stromal cells. Cell Death Dis 2019;10:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogrodnik M, Salmonowicz H, Gladyshev VN. Integrating cellular senescence with the concept of damage accumulation in aging: Relevance for clearance of senescent cells. Aging Cell 2019;18:e12841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta Y. Age-related decline in deciduogenic ability of the rat uterus’. Biol Reprod 1987;37:779–785. [DOI] [PubMed] [Google Scholar]
- Okada H, Tsuzuki T, Murata H. Decidualization of the human endometrium. Reprod Med Biol 2018;17:220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen MS, Starnawska A, Bybjerg-Grauholm J, Bielfeld AP, Agerholm I, Forman A, Overgaard MT, Nyegaard M. Biological age of the endometrium using DNA methylation. Reproduction 2018;155:167–172. [DOI] [PubMed] [Google Scholar]
- Pagano MT, Ortona E, Dupuis ML. A role for estrogen receptor alpha36 in cancer progression. Front Endocrinol (Lausanne) 2020;11:506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson RJ, Hatch IE, Lobo RA, Sauer MV. Cumulative conception and live birth rates after oocyte donation: implications regarding endometrial receptivity. Hum Reprod 1997;12:835–839. [DOI] [PubMed] [Google Scholar]
- Potlog-Nahari C, Catherino WH, McKeeby JL, Wesley R, Segars JH. A suboptimal endometrial pattern is associated with a reduced likelihood of pregnancy after a day 5 embryo transfer. Fertil Steril 2005;83:235–237. [DOI] [PubMed] [Google Scholar]
- Rahima A, Soderwall AL. Uterine collagen content in young and senescent pregnant golden hamsters. J Reprod Fertil 1977;49:161–162. [DOI] [PubMed] [Google Scholar]
- Randolph JF, Sowers M, Bondarenko IV, Harlow SD, Luborsky JL, Little RJ. Change in estradiol and follicle-stimulating hormone across the early menopausal transition: effects of ethnicity and age. J Clin Endocrinol Metab 2004;89:1555–1561. [DOI] [PubMed] [Google Scholar]
- Rashidi BH, Sadeghi M, Jafarabadi M, Tehrani Nejad ES. Relationships between pregnancy rates following in vitro fertilization or intracytoplasmic sperm injection and endometrial thickness and pattern. Eur J Obstet Gynecol Reprod Biol 2005;120:179–184. [DOI] [PubMed] [Google Scholar]
- Remohi J, Yalil S, Gartner B, Simon C, Gallardo E, Pellicer A. Pregnancy and birth rates after oocyte donation. Fertil Steril 1997;67:717–723. [DOI] [PubMed] [Google Scholar]
- Retis-Resendiz AM, González-García IN, León-Juárez M, Camacho-Arroyo I, Cerbón M, Vázquez-Martínez ER. The role of epigenetic mechanisms in the regulation of gene expression in the cyclical endometrium. Clin Epigenetics 2021;13:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter KS, Bugge KR, Bromer JG, Levy MJ. Relationship between endometrial thickness and embryo implantation, based on 1,294 cycles of in vitro fertilization with transfer of two blastocyst-stage embryos. Fertil Steril 2007;87:53–59. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Garcia M, Barr FD, Crist SG, Fahey J V, Wira CR. Phenotype and susceptibility to HIV infection of CD4+ Th17 cells in the human female reproductive tract. Mucosal Immunol 2014;7:1375–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Garcia M, Fortier JM, Barr FD, Wira CR. Aging impacts CD103 + CD8 + T cell presence and induction by dendritic cells in the genital tract. Aging Cell 2018;17:e12733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Alonso M, Blesa D, Simón C. The genomics of the human endometrium. Biochim Biophys Acta 2012;1822:1931–1942. [DOI] [PubMed] [Google Scholar]
- Ruiz-Alonso M, Valbuena D, Gomez C, Cuzzi J, Simon C. Endometrial receptivity analysis (ERA): data versus opinions. Hum Reprod Open 2021;2021:hoab011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rytkönen KT, Erkenbrack EM, Poutanen M, Elo LL, Pavlicev M, Wagner GP. Decidualization of human endometrial stromal fibroblasts is a multiphasic process involving distinct transcriptional programs. Reprod Sci 2019;26:323–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saare M, Modhukur V, Suhorutshenko M, Rajashekar B, Rekker K, Sõritsa D, Karro H, Soplepmann P, Sõritsa A, Lindgren CM et al. The influence of menstrual cycle and endometriosis on endometrial methylome. Clin Epigenetics 2016;8:2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiduddin S, Zassenhaus HP. Estrous cycles, decidual cell response and uterine estrogen and progesterone receptor in Fischer 344 virgin aging rats. Proc Soc Exp Biol Med 1979;161:119–122. [DOI] [PubMed] [Google Scholar]
- Sapieha P, Mallette FA. Cellular senescence in postmitotic cells: beyond growth arrest. Trends Cell Biol 2018;28:595–607. [DOI] [PubMed] [Google Scholar]
- Sauer MV, Miles RA, Dahmoush L, Paulson RJ, Press M, Moyer D. Evaluating the effect of age responsiveness to hormone histologic ultrasonographic, on endometrial replacement therapy: a and tissue receptor analysis. J Assist Reprod Genet 1993;10:47–52. [DOI] [PubMed] [Google Scholar]
- Sauer MV, Paulson RJ, Ary BA, Lobo RA. Three hundred cycles of oocyte donation at the University of Southern California: assessing the effect of age and infertility diagnosis on pregnancy and implantation rates. J Assist Reprod Genet 1994;11:92–96. [DOI] [PubMed] [Google Scholar]
- Secomandi L, Borghesan M, Velarde M, Demaria M. The role of cellular senescence in female reproductive aging and the potential for senotherapeutic interventions. Hum Reprod Update 2022;28:172–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshadri S, Morris G, Serhal P, Saab W. Assisted conception in women of advanced maternal age. Best Pract Res Clin Obstet Gynaecol 2021;70:10–20. [DOI] [PubMed] [Google Scholar]
- Shammas MA. Telomeres, lifestyle, cancer, and aging. Curr Opin Clin Nutr Metab Care 2011;14:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao R, Nutu M, Karlsson-Lindahl L, Benrick A, Weijdegård B, Lager S, Egecioglu E, Fernandez-Rodriguez J, Gemzell-Danielsson K, Ohlsson C et al. Downregulation of cilia-localized Il-6Rα by 17β-estradiol in mouse and human fallopian tubes. Am J Physiol Cell Physiol 2009;297:C140–C151. [DOI] [PubMed] [Google Scholar]
- Shapiro BS, Daneshmand ST, Desai J, Garner FC, Aguirre M, Hudson C. The risk of embryo-endometrium asynchrony increases with maternal age after ovarian stimulation and IVF. Reprod Biomed Online 2016;33:50–55. [DOI] [PubMed] [Google Scholar]
- Sharma R, Srivastava S, Bajpai VK, Balapure AK. Histological and ultrastructural regulation in rabbit endometrial explants by estrogen in serum-free culture. In Vitro Cell Dev Biol Anim 2002;38:293. [DOI] [PubMed] [Google Scholar]
- Shen Z, Rodriguez-Garcia M, Patel M V, Wira CR. Direct and Indirect endocrine-mediated suppression of human endometrial CD8+T cell cytotoxicity. Sci Rep 2021;11:1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasuna K, Iwata H. Effect of aging on the female reproductive function. Contracept Reprod Med 2017;2:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares SR, Troncoso C, Bosch E, Serra V, Simón C, Remohí J, Pellicer A. Age and uterine receptiveness: predicting the outcome of oocyte donation cycles. J Clin Endocrinol Metab 2005;90:4399–4404. [DOI] [PubMed] [Google Scholar]
- Sojka DK, Yang L, Yokoyama WM. Uterine natural killer cells. Front Immunol 2019;10:960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama M, Kawahara-Miki R, Kawana H, Shirasuna K, Kuwayama T, Iwata H. Resveratrol-induced mitochondrial synthesis and autophagy in oocytes derived from early antral follicles of aged cows. J Reprod Dev 2015;61:251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanikawa N, Ohtsu A, Kawahara-Miki R, Kimura K, Matsuyama S, Iwata H, Kuwayama T, Shirasuna K. Age-associated mRNA expression changes in bovine endometrial cells in vitro. Reprod Biol Endocrinol 2017;15:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teklenburg G, Salker M, Molokhia M, Lavery S, Trew G, Aojanepong T, Mardon HJ, Lokugamage AU, Rai R, Landles C et al. Natural selection of human embryos: decidualizing endometrial stromal cells serve as sensors of embryo quality upon implantation. PLoS One 2010;5:e10258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todhunter ME, Sayaman RW, Miyano M, LaBarge MA. Tissue aging: the integration of collective and variant responses of cells to entropic forces over time. Curr Opin Cell Biol 2018;54:121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomic V, Kasum M, Vucic K. Impact of embryo quality and endometrial thickness on implantation in natural cycle IVF. Arch Gynecol Obstet 2020;301:1325–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toner JP, Grainger DA, Frazier LM. Clinical outcomes among recipients of donated eggs: an analysis of the U.S. national experience, 1996-1998. Fertil Steril 2002;78:1038–1045. [DOI] [PubMed] [Google Scholar]
- Ugwu HC, Onwuzu SWI, Agbo JA, Abonyi OE, Agwu KK. Sonographic prediction of successful embryonic implantation in in-vitro fertilization and embryo transfer cycle procedures, using a multi-parameter approach. Radiography (Lond) 2022;28:473–479. [DOI] [PubMed] [Google Scholar]
- van Deursen JM. The role of senescent cells in ageing. Nature 2014;509:439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayakumar KA, Cho G. Pan-tissue methylation aging clock: recalibrated and a method to analyze and interpret the selected features. Mech Ageing Dev 2022;204:111676. [DOI] [PubMed] [Google Scholar]
- Wang C, Jurk D, Maddick M, Nelson G, Martin-Ruiz C, von Zglinicki T. DNA damage response and cellular senescence in tissues of aging mice. Aging Cell 2009;8:311–323. [DOI] [PubMed] [Google Scholar]
- Wang W, Sung N, Gilman-Sachs A, Kwak-Kim J. T Helper (Th) cell profiles in pregnancy and recurrent pregnancy losses: Th1/Th2/Th9/Th17/Th22/Tfh cells. Front Immunol 2020a;11:2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Vilella F, Alama P, Moreno I, Mignardi M, Isakova A, Pan W, Simon C, Quake SR. Single-cell transcriptomic atlas of the human endometrium during the menstrual cycle. Nat Med 2020b;26:1644–1653. [DOI] [PubMed] [Google Scholar]
- Wang YA, Farquhar C, Sullivan EA. Donor age is a major determinant of success of oocyte donation/recipient programme. Hum Reprod 2012;27:118–125. [DOI] [PubMed] [Google Scholar]
- Weckstein LN, Jacobson A, Galen D, Hampton K, Ivani K, Andres J. Improvement of pregnancy rates with oocyte donation in older recipients with the addition of progesterone vaginal suppositories. Fertil Steril 1993;60:573–575. [DOI] [PubMed] [Google Scholar]
- Woessner JF. Age-related changes of the human uterus and its connective tissue framework. J Gerontol 1963;18:220–226. [DOI] [PubMed] [Google Scholar]
- Woods L, Perez-Garcia V, Kieckbusch J, Wang X, Demayo F, Colucci F, Hemberger M. Decidualisation and placentation defects are a major cause of age-related reproductive decline. Nat Commun 2017;8:352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H-M, Chen L-H, Hsu L-T, Lai C-H. Immune tolerance of embryo implantation and pregnancy: the role of human decidual stromal cell- and embryonic-derived extracellular vesicles. IJMS 2022;23:13382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Li M, Zhang J, Wang S. Unveiling uterine aging: much more to learn. Ageing Res Rev 2023;86:101879. [DOI] [PubMed] [Google Scholar]
- Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol 2008;214:199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Q, Lin Z, Cheng Y-H, Huang C-C, Marsh E, Yin P, Milad MP, Confino E, Reierstad S, Innes J et al. Promoter methylation regulates estrogen receptor 2 in human endometrium and endometriosis. Biol Reprod 2007;77:681–687. [DOI] [PubMed] [Google Scholar]
- Yang W, Zhang T, Li Z, Ren X, Huang B, Zhu G, Jin L. Combined analysis of endometrial thickness and pattern in predicting clinical outcomes of frozen embryo transfer cycles with morphological good-quality blastocyst. Medicine (United States) 2018;97:e9577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaron Y, Ochshorn Y, Amit A, Kogosowski A, Yovel I, Lessing JB. Oocyte donation in Israel: a study of 1001 initiated treatment cycles. Hum Reprod 1998;13:1819–1824. [DOI] [PubMed] [Google Scholar]
- Yeh JS, Steward RG, Dude AM, Shah AA, Goldfarb JM, Muasher SJ. Pregnancy outcomes decline in recipients over age 44: an analysis of 27,959 fresh donor oocyte in vitro fertilization cycles from the society for assisted reproductive technology. Fertil Steril 2014;101:1331–1336. [DOI] [PubMed] [Google Scholar]
- Yousefzadeh M, Henpita C, Vyas R, Soto-Palma C, Robbins P, Niedernhofer L. DNA damage—how and why we age? Elife 2021;10:e62852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K, Huang ZY, Xu XL, Li J, Fu XW, Deng SL. Estrogen receptor function: impact on the human endometrium. Front Endocrinol (Lausanne) 2022;13:827724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Žáčková T, Järvelä IY, Tapanainen JS, Feyereisl J. Assessment of endometrial and ovarian characteristics using three dimensional power Doppler ultrasound to predict response in frozen embryo transfer cycles. Reprod Biol Endocrinol 2009;7:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Chen C, Wang J, Wang Y, Wen S, Cao Y, Qian W. An endometrial receptivity scoring system basing on the endometrial thickness, volume, echo, peristalsis, and blood flow evaluated by ultrasonography. Front Endocrinol (Lausanne) 2022;13:907874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J-J, Liu X, Chen L, Zhang S, Zhang X, Hao C, Miao Y-L. Advanced maternal age alters expression of maternal effect genes that are essential for human oocyte quality. Aging (Albany NY) 2020;12:3950–3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Vallerga CL, Walker RM, Lin T, Henders AK, Montgomery GW, He J, Fan D, Fowdar J, Kennedy M et al. Improved precision of epigenetic clock estimates across tissues and its implication for biological ageing. Genome Med 2019;11:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wei H. Role of decidual natural killer cells in human pregnancy and related pregnancy complications. Front Immunol 2021;12:728291. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new data were generated or analysed in support of this research.