Abstract
Introduction
There have been various clinical studies on the effect of Alpha lipoic acid (ALA) supplementation on blood pressure (BP), but the findings from these are contradictory. Therefore, we performed a systematic review and dose-response meta-analysis to summarize the relation of ALA supplementation and systolic blood pressure (SBP) and diastolic blood pressure (DBP) in adults.
Methods
A comprehensive search was conducted in Medline (PubMed), Embase, Scopus, and ProQuest up to July 2023. Randomized controlled trials (RCTs) evaluating the effect of ALA on SBP and DBP were included. The pooled weighted mean difference (WMD) of included trials was estimated using a random-effects model. The dose-dependent effect was also assessed.
Results and discussion
A total of 11 RCTs with the participation of 674 patients were included. The result of the meta-analysis indicated that using ALA supplementation significantly reduced the SBP (WMD = −5.46 mmHg; 95% CI: −9.27, −1.65; p < 0.001) and DBP (WMD = −3.36 mmHg, 95% CI: −4.99, −1.74; p < 0.001). The ALA administrations significantly reduced SBP and DBP at the dosages of <800 mg/day, when administered for ≤12 weeks. The present meta-analysis revealed that ALA supplementation could exert favorable effects on SBP and DBP. Further well-designed studies with larger samples are needed to ascertain the long-term effects of ALA on BP.
Systematic Review Registration
https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=447658, identifier PROSPERO: CRD42023447658.
Keywords: alpha lipoic acid, blood pressure, diastolic blood pressure, meta-analysis, systolic blood pressure
Introduction
The chronic condition of hypertension (HTN) can lead to serious health complications and is recognized as one of the major significant health challenges on a global scale (1). HTN is a primary risk factor for heart failure, chronic kidney disease, cognitive impairment, dementia, and major cardiovascular events like stroke and heart attack (2). According to the World Health Organization, HTN will affect more than one billion individuals by 2025, with prevalence rates anticipated to rise from 26.4% to 29.2% (3, 4). The improvement of blood pressure (BP) in individuals is crucial from a clinical perspective, as a small reduction in BP could have a substantial impact on mitigating the burden of cardiovascular diseases (CVDs), resulting in significant public health benefits (5, 6). Despite the fact that over 90% of hypertensive cases are idiopathic (7, 8), according to the latest guidelines on the prevention and treatment of HTN, adopting healthy lifestyle habits (smoking cessation, diet and exercise) can delay or prevent the onset of high BP and can reduce the risk of CVDs (9). Furthermore, these lifestyle modifications can enhance the effectiveness of antihypertensive medications (9). The available evidence indicates that a diet with low-fat content and high in fruits and vegetables, combined with reduced sodium intake, has the potential to lower BP (10, 11). Clinical and experimental studies indicate that the incidence of certain CVDs, such as HTN, is linked to a rise in reactive oxygen species production (12). The use of antioxidant supplements taken orally could provide a cost-effective and beneficial alternative for the treatment of high BP (13, 14). Moreover, a Cochrane meta-analysis including 112,059 participants from 79 trials showed that omega-3 fatty acids from fish, which are high in eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA), have beneficial effects on cardiovascular health due to their anti-inflammatory properties (15).
Alpha-lipoic acid (ALA), which is synthesized by the liver and present in both animal and vegetable sources, is a potent mitochondrial antioxidant, functions through multiple pathways to support anti-inflammatory and antithrombotic processes, while also increasing vasodilation through nitric oxide mediation, leading to an improvement in endothelial function and a subsequent decrease in BP (16, 17). Further, ALA is thought to help with weight loss, lipid regulation, insulin sensitization, glucose regulation (18–20), as well as promote wound healing and reduce post-operative complications after cardiac surgery (21–23). Furthermore, ALA plays a crucial role in supporting enzymatic processes that metabolize nutrients into energy (24). It also has anti-inflammatory properties and lowers the risk of CVDs (25). However, the evidence for these uses is not conclusive and more studies are required to establish the effectiveness and safety of ALA supplements.
The effects of ALA supplementation on BP were also inconsistent across different studies; however, the exploration of the reason or mechanism for improving BP was not comprehensive. A number of animal and human studies have explored the impact of ALA on BP, with some suggesting that it may serve as a viable regulator of BP (26–30). According to Mohammadi et al. (31) ALA supplementation in patients with chronic SCI (spinal cord injury) significantly reduced systolic blood pressure (SBP) and diastolic blood pressure (DBP). Also, Pourghasem Gargari et al. (28) showed that supplementation of ALA decreases SBP and DBP in patients with rheumatoid arthritis. In contrast, another study conducted by Bobe et al. (30) has showed significant changes in SBP and DBP after long-term ALA supplementation. The results of another study also demonstrated that no significant change in SBP, and DBP after three months of supplementing with 800 mg of ALA (29). The effect of ALA supplementation on BP was also assessed in a systematic review in 2017. In this study, the effect of ALA supplementation on BP was evaluated similarly to the present study, but a meta-analysis, GRADE assessment, and dose-response analysis were not performed (32). Thus, given the current lack of consensus, we performed a systematic review and meta-analysis of published randomized controlled trials (RCTs) to assess the effects of ALA supplementation on SBP and DBP in adults. Furthermore, the current study used a GRADE assessment and a dose–response analysis, which improved the reliability of the results.
Methods
This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (33) (Supplementary Table S1) and registered in the International Prospective Register of Systematic Reviews (PROSPERO) with the code CRD42023447658. The study protocol was approved by the ethics committee of Isfahan University of Medical Sciences (grant number: 1402161).
Search strategy
The study was designed based on the following PICOS criteria: Population was the human model; intervention was ALA treatment; the comparison was control or placebo; outcomes were SBP and DBP; and study methodology was RCTs. We systematically searched electronic databases including Medline (PubMed), Embase, Scopus, and ProQuest to identify RCTs that examined the effects of ALA supplements on SBP and DBP from inception to July 2023. No limitation was considered for language or publication year. The combination of MeSH and nonMeSH terms were used as follows: “alpha lipoic acid” OR “alpha-lipoic acid” “ALA” OR “α-lipoic acid” OR “α lipoic acid” OR “thioctic acid” AND “systolic blood pressure” OR “systolic blood pressure” OR “SBP” OR “DBP” OR “blood pressure” (Supplementary Table S2). Moreover, manual screening was conducted for the reference lists of eligible articles.
Study selection
The following criteria were used to select studies for inclusion: (1) RCTs with a parallel or crossover design; (2) assessed the effects of ALA supplementation on SBP and DBP. Moreover, all RCTs which supplemented another compound (drugs or supplements) along with ALA in both groups (control and intervention) were included as well; (3) designs that provide enough data to assess outcomes at baseline and after intervention; (4) participants aged 18 years or older. According to the following criteria, studies were excluded: (1) the absence of a control or placebo group; (2) lack of sufficient information for computing the measures; (3) trials involving children or pregnant women; (4) letters, conference abstracts, case reports in vivo and in vitro studies. If another compound or lifestyle intervention along with ALA was supplemented just in the intervention group and was not supplemented in the control group at the same time, and due to the confounding effect and in order to elucidate the definite role of ALA alone, those RCTs were excluded.
Data extraction
Two authors (MV, NN) extracted the data independently from each of the included articles. A chief investigator (H S-G) evaluated any inconsistencies to reach a consensus. For each eligible study, the following data were collected: authors' first names, publication year, location and design of the study, study duration, ALA dosage, gender, mean age, the sample size in each group, and health conditions of participants.
Quality assessment and certainty of evidence
An assessment of the methodological quality of the included studies was conducted using the Cochrane quality assessment tool in the following domains: (1) random sequence generation; (2) participant and personnel blinding; (3) concealment of allocation sequence; (4) outcome assessment; (5) selective outcome reporting; (6) incomplete outcome data; (7) other possible causes of bias (34). The quality items were divided into three groups: low risk, high risk, or unclear risk, based on their bias risk (34). In order to grade the overall certainty of evidence across the studies, GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) Working Group guidelines were used (35). Evidence quality can be classified into four categories: low, moderate, and high, depending on the evaluation criteria (36).
Statistical analysis
Our meta-analysis was conducted using Stata (StataCorp, College Station, TX, USA) version 14. Based on the means and standard deviations (SDs) reported for the control and intervention groups, we calculated the overall estimates (37). Weighted mean differences (WMD) and 95% confidence intervals (CIs) were used to measure treatment effects (38). The heterogeneity among studies was assessed using Cochrane Q and I-squared (I2) statistics, defining a significant heterogeneity as Cochrane Q <0.10 and/or I2 >50% (34). The fixed-effects model was selected when no significant heterogeneity was observed; otherwise, the random-effects model was applied. A subgroup analysis was conducted to detect heterogeneity based on the duration of intervention, dose of ALA supplementation, gender, health condition of participants, and sample size. An analysis of sensitivity was performed to determine the impact of each study on the overall effect size. Publication bias was evaluated using Egger's and Begg's tests with visual inspection of funnel plots. Moreover, a fractional polynomial model was also used to determine the non-linear effects of ALA dosage and treatment duration on SBP and DBP (39).
Results
Study selection
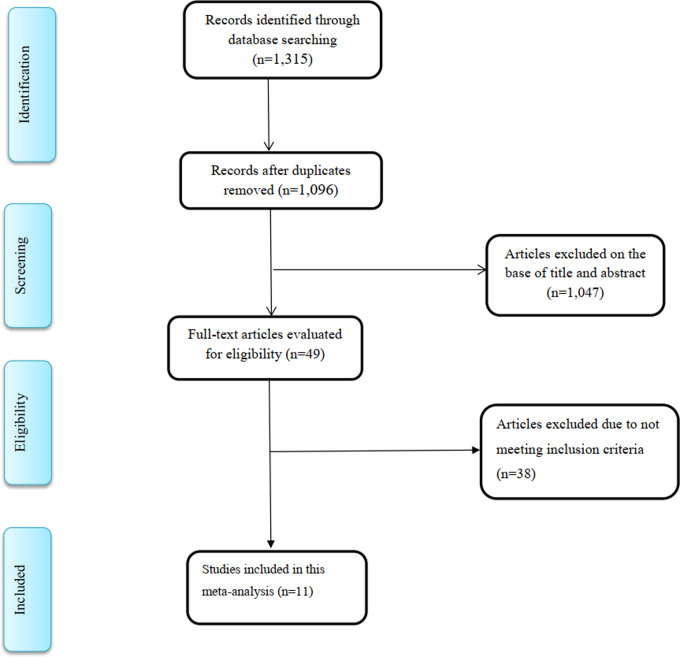
A total of 1,315 articles were recorded from the literature search. After removal of duplicates, 1,096 studies remained, of which 1,047 articles were excluded during the reviewing of titles and abstracts. After comprehensive full-text review of the remaining 49 studies, 38 studies were further omitted because the studies did not include the data necessary to analyze the findings in a meta-analysis. Finally, 11 RCTs (17, 28, 30, 31, 40–46), with 13 intervention arms, were selected for the current meta-analysis (Figure 1).
Figure 1.
Flow diagram of study screening and selection process.
Characteristics of included studies
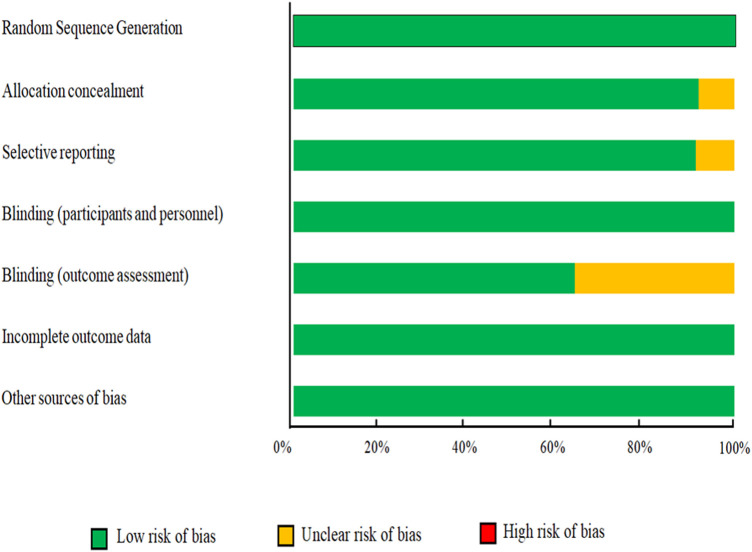
The characteristics of the included trials are summarized in Table 1. There were 674 participants included (cases = 346 and control = 328) in these RCTs. Eligible trials were published between 1997 and 2021. The studies were performed in Iran (28, 31, 40, 42), United States (41, 45, 47), Mexico (17), Italy (43), Korea (44), and Germany (46). The intervention duration of selected RCTs ranged from 8 to 48 weeks, with a sample size ranging from 12 to 155 participants. Of the eleven studies included in this meta-analysis, eight involved participants of both genders, while two focused exclusively on female subjects (28, 43) and one on male subjects (31). ALA supplementation dosage varied between 600 mg/day to 1,800 mg/day. Participants in included studies were patients with rheumatoid arthritis (28), type 2 diabetes mellitus (T2DM) (17, 45), takotsubo cardiomyopathy (43), pre-diabetics (41), stroke (42), non-insulin dependent diabetes mellitus (NIDDM) (46), SCI (31), overweight, and obesity (40, 44, 47). The quality assessment of the included trials according to the Cochrane Collaboration's risk of bias tool is presented in Figure 2. Overall, the included trials had a low risk of bias. Most trials revealed adequate quality for key factors. All of the included studies had a low risk of bias for random sequence generation, blinding (participants and personnel), incomplete outcome data and other sources of bias. Appropriate blinding (outcome assessment) was observed in 64% of included studies. Finally, the risk of selective reporting and allocation concealment was unclear in one study.
Table 1.
Characteristics of included studies in the meta-analysis.
| First author | Year, country | Subjects | Participants | Gender | Mean Age | Mean BMI | Design | Supplement | Comparator | Dose (mg/d) | Duration (week) | Baseline mean (SBP/DBP) | Medications | Adjustments | Main results | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Int | Con | Int | Con | Int | Con | Int | Con | ||||||||||||
| Nasiri et al. | 2021, Iran | Overweight | 21 | 21 | B | 34.86 | 34.67 | 27.78 | 27.57 | RDBPC | ALA + probiotic | Probiotic | 600 | 16 | (140.40/91.27) | (140.17/91.28) | – | – | Significant reduction in SBP and DBP in the ALA group |
| Bobe et al. | 2020, United States | Overweight | 31 | 33 | B | 38 | 40 | 34.8 | 34.4 | RDBPC | (R)- ALA | Placebo (Placebo type unclear) | 600 | 24 | (127/75) | (125/74) | – | – | No differences in SBP and DBP between groups |
| Mendoza-Núñez et al. | 2019, Mexico | T2DM | 42 | 38 | B | 63 | 64 | 28.69 | 28.96 | RCT | ALA | Placebo (Microcrystalline cellulose) | 600 | 24 | (124/78) | (126/77) | Glibenclamide/metformin | – | No significant difference in SBP and DBP between groups |
| Gosselin et al. | 2019, United States | pre-diabetics | 12 | 12 | B | 47.1 | 47.1 | 33.4 | 33.4 | RDBPC | ALA | Placebo (cellulose) | 600 | 4 | (130.2/87) | (130.2/87) | – | – | No significant difference in SBP and DBP between groups |
| Mohammadi et al. | 2018, Iran | Stroke | 33 | 34 | B | 62.33 | 64.23 | – | – | RDBPC | ALA | Placebo (wheat flour) | 600 | 12 | (133.18/84.24) | (132.94/86.02) | – | Baseline values, energy intake, and weight | Significant reduction in SBP and DBP in the ALA group compared with the placebo group |
| Raffaele et al. | 2016, Italy | TCM | 22 | 21 | F | 63.7 | 63.9 | 27 | 27.6 | RDBPC | ALA | Placebo | 600 | 48 | (122/81.3) | (123/82.2) | Beta-blockers, ACEi/ARB, Aspirin, and Statin | – | No significant change in SBP and DBP between groups |
| Gargari et al. | 2015, Iran | RA | 33 | 32 | F | 36.09 | 38.28 | 29 | 29.02 | RDBPC | ALA | Placebo (Maltodextrin) | 1,200 | 8 | (121.59/117.65) | (77.04/72.69) | – | Baseline values | Significant reduction in SBP and DBP between groups |
| Mohammadi et al. | 2015, Iran | SCI | 28 | 30 | M | 39 | 36.8 | 27.77 | 28.02 | RDBPC | ALA | Placebo (wheat flour) | 600 | 12 | (126.43/123.50) | (87.85/82.50) | – | Baseline covariates and changes in weight | Significant reduction in SBP and DBP between groups |
| Koh et al. | 2011, Korea | Obesity | 82 | 73 | B | 41.4 | 40.7 | 33.3 | 33.1 | RDBPC | ALA | Placebo (lactose and cellulose) | 1,800 | 20 | (137.48/84.67) | (131.95/79.95) | Antihypertensive/hypoglycemic agents/lipid-lowering medications | – | No significant difference in SBP and DBP between groups |
| Lukaszuk et al. | 2009, United States | T2DM | 13 | 7 | B | 56 | 53.14 | 33.8 | 33.3 | RDBPC | R- ALA | Placebo (Microcellulose) | 600 | 13 | (127.6/78.3) | (123.9/76.3) | Anti-diabetic medication and/or insulin, anti-hypertensives, anti-hyperlipidemic, anti-inflammatories, and… | – | No significant differences in blood pressure between the groups |
| Ziegler et al. | 1997, Germany | NIDDM | 29 | 27 | B | 57.9 | 58.6 | – | – | RDBPC | ALA | Placebo (Placebo type unclear) | 800 | 16 | (144/81.4) | (144/85.3) | Insulin treatment/Oral anti-diabetic agents | Baseline values | No significant difference in SBP and DBP between groups |
ALA, alpha-lipoic acid; BMI, body mass index; DBP, diastolic blood pressure; SCI, spinal cord injury; TCM, Takotsubo cardiomyopathy; RA, Rheumatoid arthritis; RDBPC, randomized double-blind placebo-controlled; RCT, randomized controlled trial; T2DM, SBP, systolic blood pressure; Type 2 Diabetes mellitus, NIDDM, Non-insulin-dependent diabetes mellitus; M, male; F, female; B, both; Int, intervention; Con, control.
Figure 2.
Results of risk of bias assessment for trials included in the current meta-analysis on the effects of ALA supplementation on blood pressure.
GRADE assessment
The overall certainty of the evidence for the effects of ALA supplementation on SBP and DBP is presented in Supplementary Table S3. The quality of the evidence for SBP was graded as “moderate” after being downgraded for publication bias. Meanwhile, the quality of the evidence for DBP was graded as “high”.
Findings from meta-analysis
Effect of ALA supplementation on SBP
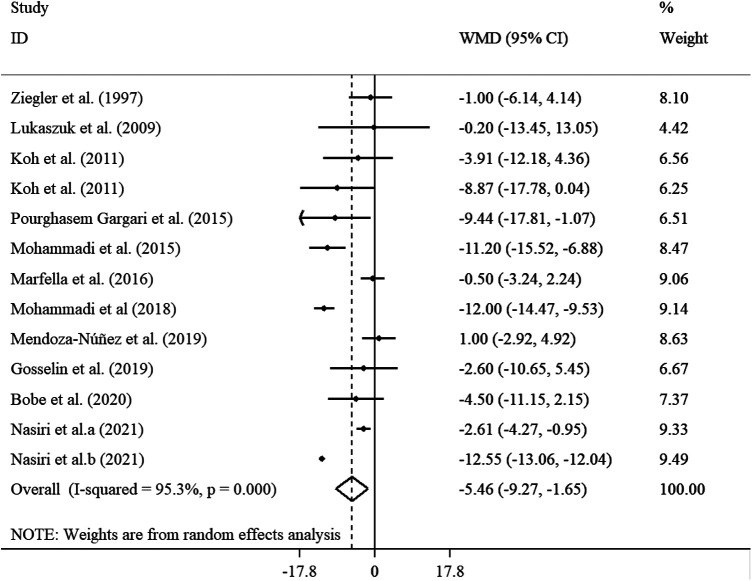
The effects of 11 studies, containing 13 effect sizes that measured SBP levels after ALA supplementation (cases = 346 and control = 328), demonstrated a significant reduction in SBP levels (WMD = −5.46 mmHg; 95% CI: −9.27, −1.65; p < 0.001) (Figure 3). As there was a significant heterogeneity (I2 = 95.3%; p < 0.001), we performed subgroup analysis and found that sample size, supplementation dosage, baseline BMI, duration of follow-up, participants' mean age, health status, and gender could explain between-study heterogeneity. Reductions in SBP levels were more pronounced in subjects aged <45 years, those with a baseline BMI of 25–29.9 kg/m2, in trials that prescribed <800 mg/day ALA, as well as in studies with ≤12 weeks of duration, in studies with sample size <60, and those performed on subjects with overweight and obesity (Supplementary Table S4).
Figure 3.
Forest plot illustrating weighted mean difference and 95% confidence intervals for the impact of ALA on SBP.
Effect of ALA supplementation on DBP
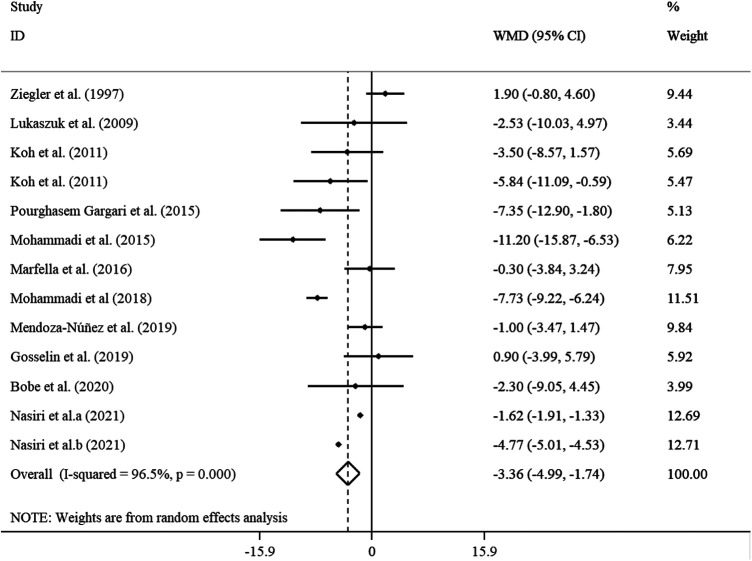
The analysis of the 11 studies with 13 effect sizes (cases = 346 and control = 328) revealed a significant reduction in DBP (WMD = −3.36 mmHg, 95% CI: −4.99, −1.74; p < 0.001), with high heterogeneity (I2 = 96.5%; p < 0.001), after ALA supplementation (Figure 4). In the subgroup analysis, we found that the sample size, supplementation dosage, baseline BMI, duration of follow-up, participants' mean age, health status, and gender explained this heterogeneity. Reductions in DBP levels were more pronounced in subjects aged ≥45 years, those with a baseline BMI of 25–29.9 kg/m2, in trials that prescribed <800 mg/day ALA, as well as in studies with ≤12 weeks of duration, in studies with sample size ≥60, and those performed on both genders (Supplementary Table S5).
Figure 4.
Forest plot illustrating weighted mean difference and 95% confidence intervals for the impact of ALA on DBP.
Meta-regressions and non-linear dose-response meta-analyses
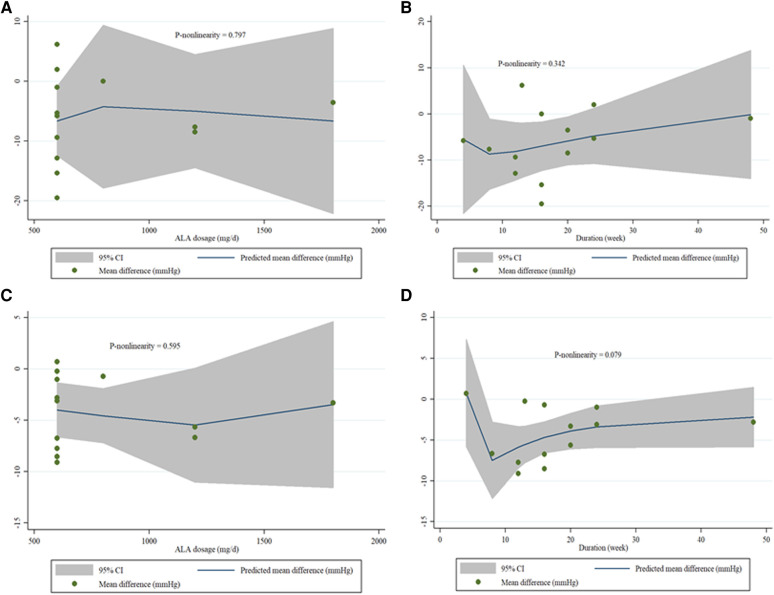
A non-linear dose-response analysis was carried out between the dose and duration of ALA supplementation for SBP and DBP. In the non-linear dose-response analysis, we did not observe a significant effect of dose and duration of ALA supplementation on SBP (P non-linearity = 0.797; 0.342), and DBP (P non-linearity = 0.595; 0.079) (Figures 5A–D). We performed a meta-regression analysis to examine the potential relationship between a decrease in SBP and DBP levels with dose and duration of ALA supplementation. The analysis did not show any significant association between the dose and duration of intervention with changes in SBP and DBP (Supplementary Figures S1A–D).
Figure 5.
(A–D). Dose-response relations between and ALA dosage (mg/d) and duration of treatment (week) and mean difference in SBP (A,B), and DBP (C,D).
Sensitivity analysis
The sensitivity analysis was also performed to evaluate the effect of each individual trial on the pooled effect size by removing each trial in turn. The sensitivity analysis demonstrated that the calculated overall effect sizes for SBP and DBP were not substantially changed after removing each study individually (Supplementary Figures S2A,B).
Publication bias
Visual inspection of the funnel plot revealed no evidence of publication bias in studies assessing the effect of ALA supplementation on SBP and DBP (Supplementary Figures S3A,B). There was no evidence of publication bias for studies examining the effect of ALA on DBP (Begg's test: p = 0.669 and Egger's test: p = 0.910). Nevertheless, result of Egger's test showed a significant publication bias in studies that reported the effect of ALA on SBP (Begg's test: p = 0.502; and Egger's test: p = 0.023). However, the trim-and-fill analysis yielded findings similar to the original.
Discussion
This systematic review and meta-analysis comprehensively evaluated the effects of supplementation of ALA on BP in adults. The results support the positive effect of ALA administration on lowering SBP and DBP levels compared with the control group. Sensitivity analysis shows that the calculated overall effect sizes for SBP and DBP did not change significantly after each individual study was removed, suggesting that the study results are robust and reliable, and do not depend on a single study.
Subgroup analyses showed that the direction of the effects of ALA was generally the same in the subgroups based on sample size, study duration, participant age, and initial BMI for SBP and DBP. However, we found a significant subgroup difference in participants' health status, the results shows that ALA supplementation was more effective in lowering SBP and DBP in obese and overweight participants and had no significant effect on prediabetes patients. Previous studies indicated that obesity may affect BP through several mechanisms. For example, obesity has been associated with high angiotensin levels II, increased aldosterone secretion, increased renal tubule sodium uptake, and consequently, increased BP (48). Thus, the reason why supplementation of ALA was more effective in obese and overweight patients may be due to the weight-reducing effects of ALA in addition to the antihypertensive effect of ALA (49). In regards to the doses of supplementation of ALA, although both doses of ALA (more than and less than 800 mg/day) were found to lower SBP, the results were more significant at the dose of less than 800 mg/day. As for DBP, only a dose of less than 800 mg/day significantly lowered DBP. However, the credibility of the dose-based subgroup should be interpreted with caution due to the small number of studies conducted with doses greater than 800 mg/day. It is recommended that further studies be conducted with different doses to make these results clearer and more reliable. In addition, the results of our meta-regression showed no significant relationship between dose and duration of ALA supplementation with changes in SBP and DBP. The difference between findings of subgroup analysis and meta-regression can be explained by the fact that in the subgroup analysis, the dose and duration of ALA supplementation were entered in classified forms and analyzed, while in meta-regression analysis, dose and duration of ALA supplementation were entered in continuous form.
In this meta-analysis, ALA reduced SBP by 5.46 mmHg and DBP by 3.36 mmHg. It is an antioxidant that may help with CVDs, diabetes, and inflammation (21–23). However, the evidence for its effectiveness is inconclusive. Although ALA is known for its antioxidant effects, it may not be very beneficial for reducing BP. Moreover, it also has some potential drawbacks, such as gastrointestinal, urological, nervous, or allergic complications or skin rashes in some individuals who use it (50, 51). Consequently, the benefits of ALA are not well-established and may vary depending on the duration, dose, and individual factors. Moreover, there was controversy in previous meta-analyses investigating the effect of antioxidant supplementation on BP (52–55). A study conducted by Guan et al. (52) reported that vitamin C supplementation significantly reduced SBP and DBP in patients with essential HTN. In contrast, resveratrol administration had no significant effect on SBP or DBP in a systematic review and meta-analysis, although significant results were observed in studies with resveratrol dosages of more than 300 mg daily and in diabetics (54). In a meta-analysis by Emami et al. (55) vitamin E supplements were found to reduce only SBP and not DBP. In the context of cardioprotection, a majority of studies have indicated that a higher body status of omega-3 fatty acids, particularly eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA), can significantly reduce the risk of CVDs (56, 57). However, it's worth noting that some studies have reported only a marginal reduction in coronary heart disease (CHD) deaths and CHD events with increased intake of EPA and DHA. Furthermore, an increment in the intake of α-linolenic acid has been associated with a slightly reduced risk of cardiovascular events and arrhythmia (15). A lower risk of CVD has also been associated with higher intakes of omega-3 fatty acids, EPA, and DHA (15, 58) by modulating CVD risk factors, such as BP (59, 60). According to a dose-response meta-analysis, omega-3 fatty acids lower BP in doses between 2 and 3 grams per day. In groups at high cardiovascular risk, omega-3 fatty acid intakes above the recommended 3 g/d may be associated with additional benefits (61). Therefore, while the benefits are clear, the extent to which these antioxidants contribute to cardioprotection may vary.
The effect of ALA supplementation on lowering SBP and DBP was previously investigated in a review study. However, meta-analysis and dose-response analyses were not performed in this review. It was concluded in this review that since most of the included studies did not show a significant effect of ALA supplementation on BP, associations between ALA and BP might not be significant (32). A variety of mechanisms may be involved in the action of ALA supplementation on SBP and DBP, for example, ALA may help reduce BP by inhibiting the reduction of SIRT3 (sirtuin 3), hyperacetylation of superoxide dismutase 2 (SOD2), and overproduction of reactive oxygen species (ROS) in mitochondria (62). It also increases endothelial nitric oxide and decreases angiotensin-converting enzyme activity (59, 63). The suppression of the renin–angiotensin system may be related to the activation of Peroxisome proliferator- activated receptor gamma (PPAR-γ) (64). Activation of PPAR-γ inhibits adhesion cascades and harmful inflammatory events in the vasculature. Thus, HTN may be treated by direct regulation of endothelial function and anti-inflammatory mechanisms in the vasculature triggered by PPAR-γ activation in humans (65, 66). Another mechanism for lowering BP is related to matrix metalloproteinases (MMPs) (67). MMPs alter smooth muscle tone, which can lead to a vicious cycle as BP increases (68, 69). However, further research should be conducted to elucidate the other mechanisms associated with the antihypertensive effect of ALA.
Implications for practice
In this meta-analysis, ALA supplementation improved SBP and DBP. However, clinical targets are point to note in RCTs. Although the threshold for a clinically significant reduction in BP depends on individual circumstances, such as the patient's age and health status, one study reported that such a significant reduction can be considered to be at least 5 to 10 mm Hg for SBP compared to baseline or 3 to 5 mm Hg for DBP (70). In addition, this reduction may be associated with a lower risk of CVD and other health problems. In this meta-analysis, the researchers observed a reduction of −5.46 mm Hg and −3.36 mm Hg for SBP and DBP, respectively, clearly demonstrating the clinically significant effects of the study. As this supplement has not yet been approved by the FDA, further, larger, and longer studies are necessary to reach a more conclusive conclusion. In addition, these results cannot be applied to other health conditions that were not included in this study.
Implications for research
Future studies should be conducted with a homogeneous population on a large scale. It is also imperative that confounding factors like absorption capacity, dietary adherence, lifestyle factors, and storage be taken into account when designing an appropriate RCT. The cost-benefit effects of ALA supplementation on improving BP should also be considered. Lastly, for determining the appropriate dosage of ALA, dose-escalation studies are also needed.
Strengths and limitations
A number of strengths were identified in this meta-analysis: first, subgroup analyses were conducted based on several confounders; second, sensitivity analyses were carried out to ensure the results were robust; and third, the GRADE method was used to determine the degree of certainty. Nevertheless, some limitations must be considered. It was found that the included studies differed in terms of dosage, study duration, participants' age, and health status. Secondly, the studies included were heterogeneous. To identify the source of heterogeneity, we conducted subgroup analyses based on different variables. Third, it was not possible to consider the baseline levels of ALA in determining the effects of ALA on BP. Fourth, since our study included individuals with various health conditions, we could not identify which type of high BP (primary or secondary) is better treated with ALA. The effects of ALA supplementation on patients with primary and secondary HTN should be examined separately in future studies.
Conclusion
A significant reduction in SBP and DBP levels was observed after ALA consumption. Further clinical studies are needed to evaluate the effects of ALA supplementation on different health conditions, in different doses, in different countries, and over longer periods of time, to confirm our findings.
Acknowledgments
We are thankful to the Student Research Committee, Isfahan University of Medical Sciences for their financial support. The present study has been performed by a grant from the Student Research Committee, Isfahan University of Medical Sciences (grant number: 1402161).
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
The financial support for conception, design, data analysis and manuscript drafting comes from Student Research Committee, Isfahan University of Medical Sciences, Isfahan, Iran. (grant number: 1402161)
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
MV: Conceptualization, Formal Analysis, Investigation, Writing – original draft, Writing – review & editing. NN: Writing – original draft, Writing – review & editing. SH: Writing – original draft, Writing – review & editing. AB: Conceptualization, Writing – original draft, Writing – review & editing. GA: Conceptualization, Data curation, Investigation, Writing – original draft, Writing – review & editing. HS-G: Conceptualization, Data curation, Writing review & editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer IR-J declared a shared affiliation with the author AB to the handling editor at the time of the review.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1272837/full#supplementary-material
References
- 1.Whelton PK, Carey R, Aronow W, Casey D, Collins K, Dennison Himmelfarb C, et al. Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Hypertension. (2018) 71(6):1269–324. 10.1161/HYP.0000000000000066 [DOI] [PubMed] [Google Scholar]
- 2.Iadecola C, Yaffe K, Biller J, Bratzke LC, Faraci FM, Gorelick PB, et al. Impact of hypertension on cognitive function: a scientific statement from the American heart association. Hypertension. (2016) 68(6):e67–94. 10.1161/HYP.0000000000000053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet. (2012) 380(9859):2224–60. 10.1016/S0140-6736(12)61766-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.James WPT. The epidemiology of obesity: the size of the problem. J Intern Med. (2008) 263(4):336–52. 10.1111/j.1365-2796.2008.01922.x [DOI] [PubMed] [Google Scholar]
- 5.Entezari MH, Hadi A, Kafeshani M. Effects of dietary approaches to stop hypertension diet versus usual dietary advice on glycemic indices in women at risk for cardiovascular disease; a randomized controlled clinical trial. J Renal Inj Prev. (2016) 6(3):205–9. 10.15171/jrip.2017.39 [DOI] [Google Scholar]
- 6.Lee JS, Chang P-Y, Zhang Y, Kizer JR, Best LG, Howard BV. Triglyceride and HDL-C dyslipidemia and risks of coronary heart disease and ischemic stroke by glycemic dysregulation status: the strong heart study. Diabetes Care. (2017) 40(4):529–37. 10.2337/dc16-1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Villegas R, Kearney PM, Perry IJ. The cumulative effect of core lifestyle behaviours on the prevalence of hypertension and dyslipidemia. BMC Public Health. (2008) 8(1):1–7. 10.1186/1471-2458-8-210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forman JP, Stampfer MJ, Curhan GC. Diet and lifestyle risk factors associated with incident hypertension in women. Jama. (2009) 302(4):401–11. 10.1001/jama.2009.1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, et al. International society of hypertension global hypertension practice guidelines. Hypertension. (2020) 75(6):1334–57. 10.1161/HYPERTENSIONAHA.120.15026 [DOI] [PubMed] [Google Scholar]
- 10.Whelton SP, Hyre AD, Pedersen B, Yi Y, Whelton PK, He J. Effect of dietary fiber intake on blood pressure: a meta-analysis of randomized, controlled clinical trials. LWW. (2005) 23(3):475–81. 10.1097/01.hjh.0000160199.51158.cf [DOI] [PubMed] [Google Scholar]
- 11.Appel LJ, Brands MW, Daniels SR, Karanja N, Elmer PJ, Sacks FM. Dietary approaches to prevent and treat hypertension: a scientific statement from the American heart association. Hypertension. (2006) 47(2):296–308. 10.1161/01.HYP.0000202568.01167.B6 [DOI] [PubMed] [Google Scholar]
- 12.Allison SJ. Oxidative stress and immune activation in hypertension. Nat Rev Nephrol. (2016) 12(1):4. 10.1038/nrneph.2015.200 [DOI] [PubMed] [Google Scholar]
- 13.Campese VM, Ye S. A vitamin-E-fortified diet reduces oxidative stress, sympathetic nerve activity, and hypertension in the phenol-renal injury model in rats. J Am Soc Hypertens. (2007) 1(4):242–50. 10.1016/j.jash.2007.04.003 [DOI] [PubMed] [Google Scholar]
- 14.Kuwabara A, Nakade M, Tamai H, Tsuboyama-Kasaoka N, Tanaka K. The association between vitamin E intake and hypertension: results from the re-analysis of the national health and nutrition survey. J Nutr Sci Vitaminol. (2014) 60(4):239–45. 10.3177/jnsv.60.239 [DOI] [PubMed] [Google Scholar]
- 15.Abdelhamid AS, Brown TJ, Brainard JS, Biswas P, Thorpe GC, Moore HJ, et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev. (2020) 3(3):Cd003177. 10.1002/14651858.CD003177.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heitzer T, Finckh B, Albers S, Krohn K, Kohlschütter A, Meinertz T. Beneficial effects of α-lipoic acid and ascorbic acid on endothelium-dependent, nitric oxide-mediated vasodilation in diabetic patients: relation to parameters of oxidative stress. Free Radi Biol Med. (2001) 31(1):53–61. 10.1016/S0891-5849(01)00551-2 [DOI] [PubMed] [Google Scholar]
- 17.Mendoza-Núñez VM, García-Martínez BI, Rosado-Pérez J, Santiago-Osorio E, Pedraza-Chaverri J, Hernández-Abad VJ. The effect of 600 mg alpha-lipoic acid supplementation on oxidative stress, inflammation, and RAGE in older adults with type 2 diabetes mellitus. Oxid Med Cell Longevity. (2019) 12:3276958. 10.1155/2019/3276958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vajdi M, Abbasalizad Farhangi M. Alpha-lipoic acid supplementation significantly reduces the risk of obesity in an updated systematic review and dose response meta-analysis of randomised placebo-controlled clinical trials. Int J Clin Pract. (2020) 74(6):e13493. 10.1111/ijcp.13493 [DOI] [PubMed] [Google Scholar]
- 19.Mahmoudi-Nezhad M, Vajdi M, Farhangi MA. An updated systematic review and dose-response meta-analysis of the effects of α-lipoic acid supplementation on glycemic markers in adults. Nutrition. (2021) 82:111041. 10.1016/j.nut.2020.111041 [DOI] [PubMed] [Google Scholar]
- 20.Ghelani H, Razmovski-Naumovski V, Nammi S. Chronic treatment of (R)-α-lipoic acid reduces blood glucose and lipid levels in high-fat diet and low-dose streptozotocin-induced metabolic syndrome and type 2 diabetes in sprague-dawley rats. Pharmacol Res Perspect. (2017) 5(3):e00306. 10.1002/prp2.306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Badran M, Abuyassin B, Golbidi S, Ayas N, Laher I. Alpha lipoic acid improves endothelial function and oxidative stress in mice exposed to chronic intermittent hypoxia. Oxid Med Cell Longev. (2019) 2019:4093018. 10.1155/2019/4093018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sherif S, Bendas ER, Badawy S. The clinical efficacy of cosmeceutical application of liquid crystalline nanostructured dispersions of alpha lipoic acid as anti-wrinkle. Eur J Pharm Biopharm. (2014) 86(2):251–9. 10.1016/j.ejpb.2013.09.008 [DOI] [PubMed] [Google Scholar]
- 23.Uyar IS, Onal S, Akpinar MB, Gonen I, Sahin V, Uguz AC, et al. Alpha lipoic acid attenuates inflammatory response during extracorporeal circulation. Cardiovasc J Afr. (2013) 24(8):322–6. 10.5830/CVJA-2013-067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tripathi AK, Ray AK, Mishra SK, Bishen SM, Mishra H, Khurana A. Molecular and therapeutic insights of alpha-lipoic acid as a potential molecule for disease prevention. Rev Bras de Farmacogn. (2023) 33(2):272–87. 10.1007/s43450-023-00370-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harding SV, Rideout TC, Jones PJ. Evidence for using alpha-lipoic acid in reducing lipoprotein and inflammatory related atherosclerotic risk. J Diet Suppl. (2012) 9(2):116–27. 10.3109/19390211.2012.683136 [DOI] [PubMed] [Google Scholar]
- 26.Vasdev S, Gill VD, Parai S, Gadag V. Effect of moderately high dietary salt and lipoic acid on blood pressure in wistar-kyoto rats. Exp Clin Cardiol. (2007) 12(2):77. [PMC free article] [PubMed] [Google Scholar]
- 27.Mıcılı SC, Ergur BU, Ozoğul C, Sarıoğlu S, Bağrıyanık HA, Tuğyan K, et al. Effects of lipoic acid in an experimentally induced hypertensive and diabetic rat model. Clin Exp Hypertens. (2013) 35(5):373–81. 10.3109/10641963.2012.732647 [DOI] [PubMed] [Google Scholar]
- 28.Pourghasem Gargari B, Aliasghari F, Kolahi S, Asghari Jafar-abadi M, Mirtaheri E. Effects of alpha-lipoic acid supplementation on blood pressure and some inflammatory factors in women with rheumatoid arthritis. J Arak Univ Med Sci. (2015) 17(12):9–18. [Google Scholar]
- 29.Tromba L, Perla FM, Carbotta G, Chiesa C, Pacifico L. Effect of alpha-lipoic acid supplementation on endothelial function and cardiovascular risk factors in overweight/obese youths: a double-blind, placebo-controlled randomized trial. Nutrients. (2019) 11(2):375. 10.3390/nu11020375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bobe G, Michels AJ, Zhang W-J, Purnell JQ, Woffendin C, Pereira C, et al. A randomized controlled trial of long-term (r)-α-lipoic acid supplementation promotes weight loss in overweight or obese adults without altering baseline elevated plasma triglyceride concentrations. J Nutr. (2020) 150(9):2336–45. 10.1093/jn/nxaa203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohammadi V, Khalili M, Eghtesadi S, Dehghani S, Jazayeri S, Aghababaee S, et al. The effect of alpha-lipoic acid (ALA) supplementation on cardiovascular risk factors in men with chronic spinal cord injury: a clinical trial. Spinal Cord. (2015) 53(8):621–4. 10.1038/sc.2015.35 [DOI] [PubMed] [Google Scholar]
- 32.Mohammadi V, Dehghani S, Askari G. Does alpha-lipoic acid supplement regulate blood pressure? A systematic review of randomized, double-blind placebo-controlled clinical trials. Int J Prev Med. (2017) 8:33. 10.4103/2008-7802.206138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group* t. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. (2009) 151(4):264–9. 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 34.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. Br Med J. (2011) 343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE Guidelines: 1. Introduction—gRADE evidence profiles and summary of findings tables. J Clin Epidemiol. (2011) 64(4):383–94. 10.1016/j.jclinepi.2010.04.026 [DOI] [PubMed] [Google Scholar]
- 36.Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. (2011) 64(4):401–6. 10.1016/j.jclinepi.2010.07.015 [DOI] [PubMed] [Google Scholar]
- 37.Follmann D, Elliott P, Suh IL, Cutler J. Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol. (1992) 45(7):769–73. 10.1016/0895-4356(92)90054-q [DOI] [PubMed] [Google Scholar]
- 38.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. (2007) 28(2):105–14. 10.1016/j.cct.2006.04.004 [DOI] [PubMed] [Google Scholar]
- 39.Xu C, Doi SA. The robust error meta-regression method for dose–response meta-analysis. JBI Evid Implement. (2018) 16(3):138–44. 10.1097/XEB.0000000000000132 [DOI] [PubMed] [Google Scholar]
- 40.Nasiri G, Bastani A, Haji-Aghamohammadi AA, Nooshabadi MR, Shahmirzalou P, Haghighian HK. Effects of probiotic and alpha-lipoic acid supplements, separately or in combination on the anthropometric indicators and maintenance of weight in overweight individuals. Clin Nutr ESPEN. (2021) 41:242–8. 10.1016/j.clnesp.2020.12.007 [DOI] [PubMed] [Google Scholar]
- 41.Gosselin LE, Chrapowitzky L, Rideout TC. Metabolic effects of α-lipoic acid supplementation in pre-diabetics: a randomized, placebo-controlled pilot study. Food Funct. (2019) 10(9):5732–8. 10.1039/C9FO00390H [DOI] [PubMed] [Google Scholar]
- 42.Mohammadi V, Khorvash F, Feizi A, Askari G. Does alpha-lipoic acid supplementation modulate cardiovascular risk factors in patients with stroke? A randomized, double-blind clinical trial. Int J Prev Med. (2018) 9:34. 10.4103/ijpvm.IJPVM_32_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marfella R, Barbieri M, Sardu C, Rizzo MR, Siniscalchi M, Paolisso P, et al. Effects of α-lipoic acid therapy on sympathetic heart innervation in patients with previous experience of transient takotsubo cardiomyopathy. J Cardiol. (2016) 67(2):153–61. 10.1016/j.jjcc.2015.07.012 [DOI] [PubMed] [Google Scholar]
- 44.Koh EH, Lee WJ, Lee SA, Kim EH, Cho EH, Jeong E, et al. Effects of alpha-lipoic acid on body weight in obese subjects. Am J Med. (2011) 124(1):85–e1. 10.1016/j.amjmed.2010.08.005 [DOI] [PubMed] [Google Scholar]
- 45.Lukaszuk JM, Schultz TM, Prawitz AD, Hofmann E. Effects of R-alpha lipoic acid on HbA1c, lipids and blood pressure in type-2 diabetics: a preliminary study. J Complementary Integr Med. (2009) 6(1):1–14. 10.2202/1553-3840.1297 [DOI] [Google Scholar]
- 46.Ziegler D, Schatz H, Conrad F, Gries FA, Ulrich H, Group DS, et al. Effects of treatment with the antioxidant α-lipoic acid on cardiac autonomic neuropathy in NIDDM patients: a 4-month randomized controlled multicenter trial (DEKAN study). Diabetes Care. (1997) 20(3):369–73. 10.2337/diacare.20.3.369 [DOI] [PubMed] [Google Scholar]
- 47.Bobe G, Michels AJ, Zhang WJ, Purnell JQ, Woffendin C, Pereira C, et al. A randomized controlled trial of long-term (R)-α-lipoic acid supplementation promotes weight loss in overweight or obese adults without altering baseline elevated plasma triglyceride concentrations. J Nutr. (2020) 150(9):2336–45. 10.1093/jn/nxaa203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fazliana M, Liyana AZ, Omar A, Ambak R, Mohamad Nor NS, Shamsudin UK, et al. Effects of weight loss intervention on body composition and blood pressure among overweight and obese women: findings from the MyBFF@home study. BMC Women’s Health. (2018) 18(1):93. 10.1186/s12905-018-0592-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kucukgoncu S, Zhou E, Lucas KB, Tek C. Alpha-lipoic acid (ALA) as a supplementation for weight loss: results from a meta-analysis of randomized controlled trials. Obes Rev. (2017) 18(5):594–601. 10.1111/obr.12528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Porasuphatana S, Suddee S, Nartnampong A, Konsil J, Harnwong B, Santaweesuk A. Glycemic and oxidative status of patients with type 2 diabetes mellitus following oral administration of alphalipoic acid: a randomized double-blinded placebocontrolled study. Asia Pac J Clin Nutr. (2012) 21(1):12–21. [PubMed] [Google Scholar]
- 51.Lee SJ, Jeong SJ, Lee YC, Lee YH, Lee JE, Kim CH, et al. Effects of high-dose α-lipoic acid on heart rate variability of type 2 diabetes mellitus patients with cardiac autonomic neuropathy in Korea. Diabetes Metab J. (2017) 41(4):275–83. 10.4093/dmj.2017.41.4.275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guan Y, Dai P, Wang H. Effects of vitamin C supplementation on essential hypertension: a systematic review and meta-analysis. Med (Baltimore). (2020) 99(8):e19274. 10.1097/MD.0000000000019274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hasani H, Arab A, Hadi A, Pourmasoumi M, Ghavami A, Miraghajani M. Does ginger supplementation lower blood pressure? A systematic review and meta-analysis of clinical trials. Phytother Res. (2019) 33(6):1639–47. 10.1002/ptr.6362 [DOI] [PubMed] [Google Scholar]
- 54.Fogacci F, Tocci G, Presta V, Fratter A, Borghi C, Cicero AFG. Effect of resveratrol on blood pressure: a systematic review and meta-analysis of randomized, controlled, clinical trials. Crit Rev Food Sci Nutr. (2019) 59(10):1605–18. 10.1080/10408398.2017.1422480 [DOI] [PubMed] [Google Scholar]
- 55.Emami MR, Safabakhsh M, Alizadeh S, Asbaghi O, Khosroshahi MZ. Effect of vitamin E supplementation on blood pressure: a systematic review and meta-analysis. J Hum Hypertens. (2019) 33(7):499–507. 10.1038/s41371-019-0192-0 [DOI] [PubMed] [Google Scholar]
- 56.Deckelbaum RJ, Calder PC. Is it time to separate EPA from DHA when using omega-3 fatty acids to protect heart and brain? Curr Opin Clin Nutr Metab Care. (2020) 23(2):65–7. 10.1097/MCO.0000000000000632 [DOI] [PubMed] [Google Scholar]
- 57.Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. (2019) 380(1):11–22. 10.1056/NEJMoa1812792 [DOI] [PubMed] [Google Scholar]
- 58.Innes JK, Calder PC. Marine Omega-3 (N-3) fatty acids for cardiovascular health: an update for 2020. Int J Mol Sci. (2020) 21(4):1362. 10.3390/ijms21041362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cabo J, Alonso R, Mata P. Omega-3 fatty acids and blood pressure. Br J Nutr. (2012) 107(S2):S195–200. 10.1017/S0007114512001584 [DOI] [PubMed] [Google Scholar]
- 60.Miller PE, Van Elswyk M, Alexander DD. Long-chain omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid and blood pressure: a meta-analysis of randomized controlled trials. Am J Hypertens. (2014) 27(7):885–96. 10.1093/ajh/hpu024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang X, Ritonja JA, Zhou N, Chen BE, Li X. Omega-3 polyunsaturated fatty acids intake and blood pressure: a dose-response meta-analysis of randomized controlled trials. J Am Heart Assoc. (2022) 11(11):e025071. 10.1161/JAHA.121.025071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li G, Wang X, Yang H, Zhang P, Wu F, Li Y, et al. α-Linolenic acid but not linolenic acid protects against hypertension: critical role of SIRT3 and autophagic flux. Cell Death Dis. (2020) 11(2):83. 10.1038/s41419-020-2277-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cicero AF, Ertek S, Borghi C. Omega-3 polyunsaturated fatty acids: their potential role in blood pressure prevention and management. Curr Vasc Pharmacol. (2009) 7(3):330–7. 10.2174/157016109788340659 [DOI] [PubMed] [Google Scholar]
- 64.Wainstein J, Landau Z, Bar Dayan Y, Jakubowicz D, Grothe T, Perrinjaquet-Moccetti T, et al. Purslane extract and glucose homeostasis in adults with type 2 diabetes: a double-blind, placebo-controlled clinical trial of efficacy and safety. J Med Food. (2016) 19(2):133–40. 10.1089/jmf.2015.0090 [DOI] [PubMed] [Google Scholar]
- 65.Floyd ZE, Stephens JM. Controlling a master switch of adipocyte development and insulin sensitivity: covalent modifications of PPARγ. Biochim Biophys Acta Mol Basis Dis. (2012) 1822(7):1090–5. 10.1016/j.bbadis.2012.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Said RS, Mohamed HA, Kassem DH. Alpha-lipoic acid effectively attenuates ionizing radiation-mediated testicular dysfunction in rats: crosstalk of NF-ĸB, TGF-β, and PPAR-ϒ pathways. Toxicology. (2020) 442:152536. 10.1016/j.tox.2020.152536 [DOI] [PubMed] [Google Scholar]
- 67.Mirtaheri E, Pourghassem Gargari B, Kolahi S, Dehghan P, Asghari-Jafarabadi M, Hajalilou M, et al. Effects of alpha-lipoic acid supplementation on inflammatory biomarkers and matrix metalloproteinase-3 in rheumatoid arthritis patients. J Am Coll Nutr. (2015) 34(4):310–7. 10.1080/07315724.2014.910740 [DOI] [PubMed] [Google Scholar]
- 68.Bisogni V, Cerasari A, Pucci G, Vaudo G. Matrix metalloproteinases and hypertension-mediated organ damage: current insights. Integr Blood Press Control. (2020) 13:157–69. 10.2147/IBPC.S223341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Amraoui F, Spijkers L, Lahsinoui HH, Vogt L, van der Post J, Peters S, et al. SFlt-1 elevates blood pressure by augmenting endothelin-1-mediated vasoconstriction in mice. Ratio (Oxf). (2014) 18:19. 10.1371/journal.pone.0091897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kandzari DE, Mahfoud F, Weber MA, Townsend R, Parati G, Fisher ND, et al. Clinical trial design principles and outcomes definitions for device-based therapies for hypertension: a consensus document from the hypertension academic research consortium. Circulation. (2022) 145(11):847–63. 10.1161/CIRCULATIONAHA.121.057687 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.