Abstract
The influence of reduced water activity (aw) on lactose metabolism by Lactococcus lactis subsp. cremoris 2254 and 2272 was studied at different pH values. In control incubations (aw, 0.99) with nongrowing cells in pH-controlled phosphate buffer, the levels of carbon recovered as l-(+)-lactate were 92% at pH 6.1 and 5.3 and 78% at pH 4.5. However, the levels of recovery decreased to ∼50% at all pH values tested when the aw was 0.88 (with glycerol as the humectant). When growing cells in broth controlled at pH 6.3 were used, a reduction in the aw from 0.99 to 0.96 resulted in a decrease in the level of lactose carbon recovered as l-(+)-lactate from 100 to 71%. Low levels of l-(+)-lactate carbon recovery (<50%) were also observed with cells resuspended in pH-uncontrolled reconstituted skim milk at aw values of 0.99 and 0.87 and in young cheese curds. The missing lactose carbon could not be accounted for by acetate, ethanol, formate, acetaldehyde, or pyruvate. Attempts were made to determine where the missing lactose carbon was diverted to under the stress conditions used. Some of the missing lactose carbon was recovered as galactose (0.1 to 2.5 mM) in culture supernatants. Decreasing either the aw or the pH resulted in increased galactose accumulation by nongrowing cells; adjusting both environmental factors together potentiated the effect. The sensitivities of the two lactococcal strains tested were different; strain 2272 was more prone to accumulate galactose under stress conditions. A methyl pentose(s) and additional galactose were found in acid-hydrolyzed supernatants from cultures containing both growing and nongrowing cells, indicating that a saccharide(s) rich in these components was formed by lactococci under low-aw and low-pH stress conditions.
Water activity (aw) affects the growth, physiology, and metabolism of microorganisms and their resistance to inimical agents (22, 54). A number of microorganisms respond to a low-aw environment by intracellular accumulation of low-molecular-weight compatible solutes, such as amino acids, amino acid derivatives, trehalose, and polyols (6, 12). These compatible solutes restore turgor pressure and membrane tension to levels very similar to those that occur before osmotic upshift (12), preserve enzyme activity and protein stability, and maintain the integrity and stability of membranes and nucleic acids (6).
There have been several reports concerning the accumulation of compatible solutes in lactic acid bacteria. Hutkins et al. (26) found that betaine was accumulated by an osmotolerant strain of Lactobacillus acidophilus, while Molenaar et al. (38) reported that Lactococcus lactis subsp. lactis ML3 contained high levels of proline or betaine when it was grown under osmotic stress conditions in complex media. Lactobacillus plantarum, however, accumulated not only betaine and proline, but also carnitine, glutamic acid, and trehalose when it was cultured in a complex medium having a reduced aw (29, 30, 36). The accumulation of betaine enhanced the survival of several lactic acid bacteria subjected to drying conditions (28, 30).
The influence of reduced aw on substrate metabolism and product formation by lactic acid bacteria has received little attention. Optimal production of lactic acid by some dairy lactic acid bacteria occurs at aw values of 1.0 to 0.95, and production declines dramatically as the aw is decreased, which is consistent with growth inhibition, whereas diacetyl production by some lactic acid bacteria increases with decreasing aw and optimal diacetyl production occurs at aw values of 0.95 to 0.97 (51, 52). Bassit et al. (1) studied the influence of aw on the metabolism of Streptococcus diacetylactis (now Lactococcus lactis subsp. lactis var. diacetylactis) and found that decreasing the aw decreased lactose consumption and lactic acid formation and inhibited growth. Blickstad (3) observed no significant variations in the formation of end products by two meat lactobacilli at different aw values (0.99 to 0.94).
Lactose is the major carbohydrate present in milk and young cheeses, and metabolism of lactose by starters during curd manufacture and early ripening is important for acid development. The cheese aw decreases during manufacture and ripening as a result of dehydration, salting, and the production of water-soluble solutes from glycolysis, proteolysis, and lipolysis; the cheese aw values range from 0.70 for extrahard cheeses to 0.99 for fresh, soft cheeses, such as cottage cheese, while semihard cheeses have aw values of around 0.90 (33, 41). The cheese pH also decreases during manufacture and ripening (19). We describe here the influence of low aw values on lactose metabolism by both growing and nongrowing cells of Lactococcus lactis subsp. cremoris at different pH values in buffer, broth, and reconstituted skim milk (RSM) with and without pH control. Cheese experiments to determine lactose utilization during ripening were also linked with our in vitro studies.
MATERIALS AND METHODS
Chemicals and organisms.
All of the chemicals and biochemicals used were of analytical grade and were purchased from BDH (Poole, England), Sigma Chemical Co. (St. Louis, Mo.), or Boehringer (Mannheim, Germany). Lactococcus lactis subsp. cremoris 2254, 2260, and 2272, which were used for cheese manufacturing, were obtained from the culture collection of the New Zealand Dairy Research Institute, Palmerston North, New Zealand.
Anaerobic preparation of cell suspensions.
The oxygen content of each solution was minimized by applying a vacuum or by sparging with nitrogen. Nitrogen was passed over heated copper turnings to remove any trace of oxygen.
Lactococcal cells grown overnight (20 h) at 22°C in M17 broth (Oxoid) were harvested by centrifugation (7,000 × g, 10 min, 4°C) of 250-ml cultures. Pelleted cells were washed once in 50 mM potassium phosphate buffer (pH 6.3) and were resuspended by turbulent sparging with nitrogen. Cells were finally resuspended by nitrogen sparging in the phosphate buffer at the desired pH values with or without prior adjustment of the aw, which was measured with a model CX-2 aw meter (Decagon, Pullman, Wash.).
Cell incubation: nongrowing cells without pH control.
Samples (30 ml) were taken from 250-ml cultures grown to the stationary phase, and the cells were harvested and washed as described above. Pelleted cells were resuspended in 9 ml of 50 mM phosphate buffer (pH 5.0) with and without prior aw adjustment with either glycerol, polyethylene glycol 200 (PEG 200), sodium chloride, or sucrose. The aw range used is described below. A lactose solution (1 ml, 50 mM) was then added to each cell suspension, and the preparations were mixed thoroughly. In experiments in which the buffer aw was decreased, the cell suspensions were incubated at room temperature for 15 min before the lactose solution was added. All cell suspensions were sparged with nitrogen at all times. After lactose was added, the cell suspensions were incubated at 22°C for 10 h under nitrogen. The samples used for subsequent analysis were taken immediately after lactose was added and at the end of the incubation period.
Cell incubation: nongrowing cells with pH control.
Samples (250 ml) were taken and cells were harvested and washed as described above. Pelleted cells were resuspended in 90 ml of 50 mM potassium phosphate buffer at pH 6.1, 5.3, or 4.5 and at an aw of 0.99 (control, no glycerol added) or 0.88 (40% [wt/vol] glycerol added), and lactose (10 ml of a 100 mM solution) was added. An adaptation period of 15 min was included before lactose was added to the cell suspensions with decreased aw. Cell suspensions were incubated at 22°C for 10 h under nitrogen, and the pH was maintained at the appropriate levels by titration with 4 M NaOH by using a pH stat. Samples were taken immediately after lactose was added and at the end of the incubation period.
Cell incubation: growing cells without pH control.
Lactococci were cultured in anaerobic tubes containing 10 ml of M17 broth. The aw of the medium (without lactose) was decreased with glycerol or sodium chloride to the values given below before the medium was dispensed into anaerobic tubes and autoclaved. An appropriate amount of a lactose solution (0.5 ml) was then added anaerobically to the autoclaved medium (9.5 ml) to give a final lactose concentration of 14 mM. The pH of the complete medium at each aw was 6.3 ± 0.1. Lactococcal cultures were inoculated (1%, vol/vol) and incubated anaerobically at 22°C. The growth of cultures was monitored at 600 nm. Samples were taken immediately after inoculation and when incubation was terminated.
Cell incubation: growing cells with pH control.
Lactococcus lactis subsp. cremoris 2254 was cultured anaerobically in 2 liters of M17 broth having an initial pH of 6.5 in a fermentation vessel (series III fermentor; LH Engineering, Stoke Poges, England) at 22°C. The inoculum used was a 1% (vol/vol) inoculum in the case of the control (aw, 0.99) and a 10% (vol/vol) inoculum when the aw was 0.96 (adjusted with glycerol). The culture pH was maintained at 6.3 by titration with 4 M NaOH by using a pH stat under nitrogen. The control fermentation preparation was incubated for 15 h, and the fermentation preparation with the lower aw was incubated for 60 h. Growth was monitored at 600 nm, and samples were taken aseptically immediately after inoculation and when fermentation was terminated.
Cell incubation: cells in RSM without pH control.
Cultures of lactococcal strains 2254 and 2272 were each grown at 30°C overnight in 1 liter of 10% (wt/vol) RSM. At the end of the incubation period, 60 ml of 25% trisodium citrate was added to each culture, and the pH was adjusted to 7.0 with NaOH. Each clarified milk culture was then strained through nylon cloth prior to centrifugation at 7,000 × g and 4°C for 10 min. The cell pellets were resuspended in 10 ml of 50 mM potassium phosphate buffer (pH 6.3) and added to 100 ml of 10% RSM at pH 6.3, which had an aw value of either 0.99 (control) or 0.87 (preadjusted preparation with glycerol). The cell suspensions having aw values of 0.99 and 0.87 were incubated statically at 22°C for 2 and 5 h, respectively. Samples were taken immediately after RSM was added and when incubation was terminated.
Cheesemaking.
Cheddar cheese was manufactured from 10-liter vats of pasteurized milk. Cook, cut, and stir were controlled with a programmable MS Windows-based PC control system (Innovative Engineering Limited, Cambridge, New Zealand). The procedures used for manufacture, sampling, and analysis of experimental cheeses were similar to those described for large vats (360 liters) by Crow et al. (11), as follows: a 1.9% (vol/vol) starter inoculum, a set temperature of 32°C, a set-to-drain time of 3.33 h (drain pH, 6.15), and a drain-to-salt time of 2.25 h (curd pH at salting, 5.25). The rennet level was 0.15 ml/liter of milk. The drained curd was transferred to a covered draining screen and placed in a 37°C incubator until the pH reached 5.25. Following salting and pressing overnight, the curd was divided into equal (150-g) lots, vacuum sealed in plastic cheese bags (thickness, 120 μm), and ripened at 13°C in an anaerobic container. The cheese had a fat content of 36.0% (wt/wt), a pH of 5.3, and a salt-in-moisture content of 6.6%. The cheese aw was not determined.
Sample treatment and analysis of substrate and metabolites.
Samples from buffer preparations and broth cultures were immediately centrifuged in an Eppendorf centrifuge for 10 min, and the supernatants were stored frozen at −20°C prior to analysis. Samples from milk cultures were also stored at −20°C. Before analysis, milk samples (0.4 ml) were treated with 0.2 ml of barium hydroxide (1.8%, wt/vol) and 0.2 ml of zinc sulfate (2.0%, wt/vol), and the precipitated protein was removed by centrifugation (53). In the case of cheese samples, 20 g of cheese was grated and mixed, and then a portion (0.6 g) was homogenized for 2 min in 4.0 ml of distilled water in a ground glass tissue homogenizer (Kontes, Vineland, N.J.) and left at room temperature for 10 min. The homogenate was centrifuged in an Eppendorf centrifuge at 4°C for 10 min, and the supernatant was kept for analysis. Lactose, glucose, galactose, d-(−)-lactate, l-(+)-lactate, acetaldehyde, ethanol, acetate, formate, and pyruvate contents were determined enzymatically with enzyme test kits (Boehringer). Hexose contents were determined by the primary cysteine method (13), and methyl pentose contents were determined by the cysteine-sulfuric acid method with extended heating (13). The carbohydrate contents of some supernatants were determined before and after acid hydrolysis. Hydrolysis involved mixing 0.5 ml of a sample with an equal volume of 8 M HCl, placing the preparation in a boiling water bath for 30 min, cooling it, and adjusting the pH to ∼7.0 with 4 M NaOH. The hydrolyzed samples were analyzed enzymatically to determine free glucose and galactose contents, chemically to determine hexose and methyl pentose contents, and by Dionex LC carbohydrate chromatography (Dionex Corp., Sunnyvale, Calif.) to determine monosaccharide, methyl pentose, and oligosaccharide contents. Cell pellets from some experiments were also resuspended in 4 ml of water, hydrolyzed as described above, and analyzed to determine hexose contents.
RESULTS
Effect of reduced aw on lactose metabolism by nongrowing cells without pH control.
Table 1 shows the amounts of carbon recovered as l-(+)-lactate from lactose utilized by nongrowing cells (with an initial pH of 5.0) of three lactococcal strains at different aw values. No lactococcal heterofermentative products were detectable (i.e., the concentrations of acetate, ethanol, acetaldehyde, formate, and pyruvate were less than 0.1 mM). For strain 2254, 78% of the lactose utilized was recovered as l-(+)-lactate at an aw of 0.99. The amount of carbon recovered as l-(+)-lactate was smaller at lower aw values; the amount recovered at an aw of 0.88 was only 35%. While a similar trend was observed with strains 2260 and 2272, the recovery of carbon as l-(+)-lactate was less affected by the aw, particularly for strain 2260.
TABLE 1.
Recovery of carbon as l-(+)-lactate from lactose utilized by nongrowing cells of lactococci at different aw values without pH controla
| aw | Carbon recovery (%)b
|
||
|---|---|---|---|
| Strain 2254 | Strain 2260 | Strain 2272 | |
| 0.99c | 78 | 69 | 93 |
| 0.94 | 61 | 78 | 76 |
| 0.92 | 53 | 67 | 70 |
| 0.90 | 56 | 64 | 70 |
| 0.88 | 35 | 61 | 62 |
Cells of Lactococcus lactis subsp. cremoris strains harvested from broth cultures were washed and resuspended in phosphate buffer having an initial pH of 5.0 with or without prior aw adjustment with glycerol. The cell suspensions were incubated statically at 22°C for 10 h under nitrogen. The biomasses of the cell suspensions ranged from 1.3 to 2.6 mg (dry weight) per ml, and the biomass was the same at each aw for a given strain.
Carbon recovery values were based on the theoretical conversion of 1 mol of lactose to 4 mol of l-(+)-lactate. The concentrations of lactose used during the incubation period varied from 3 to 5 mM depending on the strain and the aw (the initial lactose concentration was 5 mM). The results are the averages of values from duplicate determinations.
Control (no glycerol added).
Strain 2272 was also tested at a range of aw values (aw values of 0.99 to 0.96) with NaCl, sucrose, and PEG 200 as humectants. No correlation was found between the amount of carbon recovered as l-(+)-lactate and aw with either NaCl or sucrose (data not shown). When PEG 200 was the humectant, however, smaller amounts of carbon were recovered as l-(+)-lactate at the lower aw values (data not shown). These results suggest that the effects of reductions in aw on the lactose metabolism of lactococci vary with the humectant used, and the reasons for this are not known.
Effect of reduced aw on lactose metabolism by nongrowing cells with pH control.
When lactococcal strain 2254 was incubated at an aw of 0.99 with pH control, the amount of carbon recovered as l-(+)-lactate was 92% at pH 6.1 and 5.3 but only 78% at pH 4.5 (data not shown). This suggests that a low pH per se decreases the amount of carbon recovered as l-(+)-lactate during lactose metabolism by lactococci. In contrast, at an aw of 0.88, only 60, 53, and 48% of the carbon from the lactose utilized was recovered as l-(+)-lactate at pH 6.1, 5.3, and 4.5, respectively. This finding demonstrated that decreasing the aw also reduced the proportion of carbon recovered from lactose as l-(+)-lactate and supports the findings presented in Table 1. With strain 2272 under the same conditions, the amounts of carbon recovered as l-(+)-lactate at an aw of 0.99 were 92 and 81% at pH 6.1 and 5.3, respectively, but were only 64 and 61%, respectively, when the aw was 0.88 (data not shown). Acetate, ethanol, acetaldehyde, formate, and pyruvate were not detected (concentrations, <0.1 mM) with either strain 2254 or strain 2272.
Effect of reduced aw on lactose metabolism by growing cells without pH control.
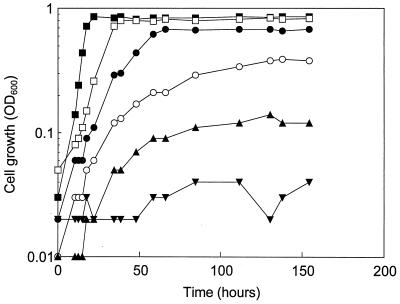
Figure 1 shows the growth of lactococcal strain 2260 in M17 broth at different aw values. A low aw resulted in a longer lag phase and decreased growth rate and cell yield. Growth was insignificant (optical density at 600 nm, <0.1) at aw values of 0.95 and below. Similar responses were obtained with strains 2254 and 2272.
FIG. 1.
Growth of Lactococcus lactis subsp. cremoris 2260 at 22°C in M17 broth (initial lactose concentration, 14 mM; initial pH, 6.3 ± 0.1) at different aw values (with glycerol as the humectant) without pH control. Symbols: ▪, aw of 0.99 (control, no glycerol added); □, aw of 0.98; •, aw of 0.97; ○, aw of 0.96; ▴, aw of 0.95; ▾, aw of 0.93. OD600, optical density at 600 nm.
Table 2 shows lactose utilization and the amounts of carbon recovered as l-(+)-lactate by strains 2254, 2260, and 2272 growing in M17 broth at different aw values. At aw values of 0.97 and above, on average 80% or more of the lactose carbon was recovered as l-(+)-lactate for all strains. At an aw of 0.96, however, the amount of carbon recovered as l-(+)-lactate was only 41% for strain 2254, while for strains 2260 and 2272 the values were 68 and 64%, respectively. These trends in carbon recovery were comparable to the trends observed with nongrowing cells of the same three strains at an aw of 0.88 (Table 1).
TABLE 2.
Lactose utilization and recovery of carbon as l-(+)-lactate by lactococci growing in broth at different aw values without pH controla
| aw | Strain 2254
|
Strain 2260
|
Strain 2272
|
|||
|---|---|---|---|---|---|---|
| Lactose used (mM) | Carbon recovery (%)b | Lactose used (mM) | Carbon recovery (%)b | Lactose used (mM) | Carbon recovery (%)b | |
| 0.99c | 16.7 | 78 | 14.3 | 80 | 9.6 | 100 |
| 0.98 | 16.8 | 80 | 15.9 | 93 | 17.2 | 81 |
| 0.97 | 13.3 | 81 | 12.7 | 81 | 14.6 | 87 |
| 0.96 | 10.4 | 41 | 12.2 | 68 | 9.4 | 64 |
Strains of Lactococcus lactis subsp. cremoris were inoculated into M17 broth (initial pH, 6.3 ± 0.1) with or without prior aw adjustment with glycerol and were incubated statically at 22°C for ∼160 h under anaerobic conditions.
Carbon recovery values were based on the theoretical conversion of 1 mol of lactose to 4 mol of l-(+)-lactate. The results are the averages of values from duplicate determinations.
Control (no glycerol added).
With NaCl as the humectant, the growth pattern of strain 2272 was similar to the growth pattern obtained with glycerol, although the minimum aw for significant growth was 0.97. The high levels of carbon recovered as l-(+)-lactate (85 to 93%) were not affected by the aw (data not shown). No heterofermentative products, such as acetate, ethanol, acetaldehyde, formate, and pyruvate, were detected (concentrations, <0.1 mM) for any of the strains.
Effect of reduced aw on lactose metabolism by growing cells with pH control.
When strain 2254 was grown in M17 broth under pH-controlled conditions (pH 6.3), the amount of the lactose carbon recovered as l-(+)-lactate was 100% at an aw of 0.99 but only 71% at an aw of 0.96. At both aw values the lactose consumption was ∼14.5 mM lactose (data not shown).
Effect of reduced aw on lactose metabolism by lactococcal cells in RSM without pH control.
For strain 2254 and 2272 cells suspended in RSM, the amounts of carbon recovered from lactose as l-(+)-lactate were low. For strain 2254, the levels of recovery were 44 and 35% at aw values of 0.99 and 0.87, respectively (the lactose concentrations used were 26.1 and 20.1 mM). For strain 2272, the levels of recovery were 36 and 34% at aw values of 0.99 and 0.87, respectively (the lactose concentrations used were 43.8 and 16.4 mM). Galactose was detected at low concentrations (2.1 to 2.5 mM at an aw of 0.99 and 0.5 to 0.6 mM at an aw of 0.87 for both strains). Glucose and acetaldehyde were not found; other products were not studied.
Lactose metabolism and recovery of carbon as l-(+)-lactate in cheese.
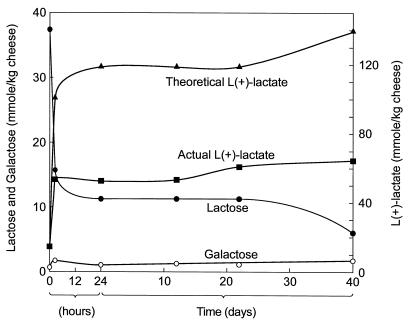
Figure 2 shows the disappearance of lactose and the accumulation of l-(+)-lactate during ripening of a Cheddar cheese made with strains 2254 and 2272. The adventitious bacterial levels were <10 CFU per g of cheese on day 1 and <104 CFU per g of cheese after 40 days, compared with 3 × 108 CFU per g of cheese on day 1 and 107 CFU per g of cheese on day 40 for the starter strains. In the ripening cheese, 22 to 31 mmol of lactose per kg of cheese was utilized, and only 36 to 45% of the lactose carbon was recovered as l-(+)-lactate. Small amounts of galactose (0.7 to 1.7 mmol per kg of cheese) were detected during manufacture and ripening. In addition, acetate (<2 mmol per kg of cheese) and ethanol (<6 mmol per kg of cheese) were produced, but glucose and acetaldehyde were not found. Similar results were obtained for another Cheddar cheese having a similar composition and for two washed curd cheeses (data not shown), all manufactured with the same two starters.
FIG. 2.
Lactose utilization and accumulation of l-(+)-lactate and galactose during manufacture and ripening of a Cheddar cheese (pH 5.3; salt-in-moisture content, 6.6%; fat content, 36% [wt/wt]) made with Lactococcus lactis subsp. cremoris 2254 and 2272.
Balance of lactose metabolism at different aw and pH values.
Table 3 shows the balance of lactose metabolism by growing and nongrowing cells of strain 2254 at different aw values and under controlled pH conditions. At an aw of 0.99, glucose and galactose did not accumulate in suspensions of nongrowing cells at pH 6.1 or 5.3 or in broth cultures containing growing cells at pH 6.3. However, a significant concentration (2.5 mM) of galactose, accounting for ∼50% of the unaccounted-for lactose carbon, was detected in suspensions of nongrowing cells at pH 4.5 and an aw of 0.99. Galactose was also detected following incubation of nongrowing cells at an aw of 0.88 at all pH values tested, but this galactose accounted for only 10 to 21% of the missing lactose carbon. Following growth in broth at pH 6.3 and an aw of 0.96, galactose accounted for only 1% of the missing lactose carbon. Suspensions of nongrowing strain 2272 cells also produced galactose (0.15 to 2.1 mM) at aw values of 0.99 and 0.88 at all pH values tested (data not shown). For both strains, more galactose per mole of lactose utilized was detected at reduced aw and/or low pH values.
TABLE 3.
Balance of lactose metabolism by growing and nongrowing cells of Lactococcus lactis subsp. cremoris 2254 under different conditions
| Conditions
|
Initial lactose concn (mM) | Residual lactose concn (mM) | Galactose concn before hydrolysis (mM) | Monosaccharide concn after hydrolysis (mM)
|
Lactate carbon recovery (%)d | Sugar-lactate carbon recovery (%)e | |||
|---|---|---|---|---|---|---|---|---|---|
| pH | aw | Glucosec | Galactose | Methyl pentose | |||||
| 6.1 | 0.99a | 9.7 | <0.1 | <0.1 | <0.1 | 0.2 | 0.8 | 92 | 97 |
| 6.1 | 0.88a | 10.6 | 2.1 | 0.8 | 1.7 | 4.9 | 1.2 | 60 | 84 |
| 5.3 | 0.99a | 9.7 | <0.1 | <0.1 | <0.1 | 0.3 | 0.7 | 92 | 97 |
| 5.3 | 0.88a | 8.9 | 3.5 | 0.9 | 2.8 | 5.1 | 1.4 | 53 | 81 |
| 4.5 | 0.99a | 9.7 | <0.1 | 2.5 | <0.1 | 2.8 | 2.2 | 78 | 104 |
| 4.5 | 0.88a | 9.8 | 4.7 | 1.0 | 3.8 | 5.5 | 2.6 | 48 | 81 |
| 6.3 | 0.99b | 14.9 | <0.1 | <0.1 | 2.3 | 1.8 | <0.1 | 100 | 114 |
| 6.3 | 0.96b | 13.9 | <0.1 | 0.1 | 2.0 | 7.0 | 1.0 | 71 | 107 |
Nongrowing cells were incubated in buffer with an aw of 0.99 or 0.88 with pH controlled at three values (see text for details).
Growing cells were incubated in broth with an aw of 0.99 or 0.96 with pH controlled at 6.3 (see text for details).
Glucose was analyzed before acid hydrolysis of supernatants but was not detected (concentration, <0.1 mM) in any sample.
Amount of carbon recovered as l-(+)-lactate (see text for details).
Amount of carbon recovered as l-(+)-lactate plus methyl pentose(s) and galactose detected following hydrolysis of supernatants and correction for any residual lactose.
Hydrolyzed supernatants from nongrowing cell suspensions of strain 2254 yielded galactose in addition to the free galactose, which did not arise from the residual lactose. In addition, 0.7 to 2.6 mM methyl pentose(s) was detected in seven of the eight preparations. No methyl pentose(s) was detected in a broth culture grown at pH 6.3 and an aw of 0.99, but the culture supernatant was glucose enriched. This finding, along with the level of carbon recovery (114%), suggests that the extra glucose originated from the medium. In growth studies performed at the lower aw values, the level of carbon recovery decreased from 107 to 93% when values were corrected for the carbohydrates that originated from the media. The data strongly suggested that a saccharide(s) rich in galactose and methyl pentose(s) was formed by lactococci under stress conditions (reduced aw and low pH).
DISCUSSION
Lactococci are classified as homofermenters based on the fact that they convert 1 mol of glucose into 2 mol of l-(+)-lactate and thus theoretically produce 4 mol of l-(+)-lactate per mol of lactose metabolized (9, 19). In practice, however, only >90% of the glucose or lactose sugar is converted into l-(+)-lactate (43, 46, 53). When these organisms are grown on galactose, low levels of glucose, or lactose, other significant products of pyruvate metabolism in addition to l-(+)-lactate (e.g., formate, acetate, and ethanol) are produced (18, 46, 47). The influence of pH on lactose fermentation has not been examined in detail in lactococci, although in the homofermentative organism Streptococcus bovis grown in a pH-controlled glucose-limited chemostat, the amount of heterolactic products decreased with increases in the amount of lactate as the pH decreased below 6.5 (17). Also, in the homofermentative organism Lactobacillus plantarum grown in an aerated, glucose-limited continuous culture, production of acetate and l-lactate decreased but the levels of d-(−)-lactate increased as the pH decreased under acidic conditions (4). In the present study, the low levels of carbon recovered as l-(+)-lactate at low pH values were not due to diversion of carbon to traditional lactococcal heterofermentative products (acetate, ethanol, acetaldehyde, formate, and pyruvate) but were due to production of other saccharides. While production of polysaccharide(s) was not measured directly, the formation of additional monosaccharides upon acid hydrolysis of culture supernatants implicates formation of saccharides which may include oligosaccharides and polysaccharides. However, it should be pointed out that other possible metabolic products, such as diacetyl, acetoin, and 2,3-butanediol, were not examined in this study.
At a low aw, particularly at a low pH, the growth and lactose utilization rates decreased and lactose fermentation to l-(+)-lactate switched to a pathway involving nontraditional saccharide products rather than the traditional lactococcal heterofermentative products. These findings have not been obtained previously, although a decline in lactate production by dairy lactic acid bacteria at aw values below 0.95 commensurate with growth inhibition has been reported (1, 51, 52). However, the previous authors did not describe the stoichiometry of lactose fermentation.
Given the stress conditions (low aw and/or low pH), it is possible that the extracellular galactose found in the present study might have originated from dephosphorylation of galactose 6-phosphate and subsequent excretion from the cells, a known mechanism of detoxification in lactococci (2, 40, 48, 49). In comparison, Streptococcus thermophilus (25, 44) and homofermentative lactobacilli (24) excrete nonutilizable galactose resulting from lactose hydrolysis. The low-aw and low-pH conditions which favor galactose production in these defined laboratory studies are also the conditions prevalent in cheese; the cheese curd pH reaches 4.5 to 5.2, depending on the variety (19), and the aw of most semihard cheeses is around 0.90 (33, 41). The results of this study and other studies (14, 27, 37, 45) confirm that lactococcal starters produce galactose from lactose in cheese, which may provide a growth substrate for adventitious cheese microflora and thereby influence cheese quality.
The production of a galactose-rich saccharide(s) containing a methyl pentose(s) by the lactococcal strains does not fully account for the missing lactose carbon in all cases. For three incubation conditions (Table 3) the level of carbon recovery was still ∼80% when saccharide production was accounted for, which suggests that atypical fermentation products, different monosaccharide components, or other carbon products, such as betaine associated with adaptation to low-aw conditions, were formed. An investigation of monosaccharide components besides glucose, galactose, and methyl pentose(s) following hydrolysis with Dionex LC carbohydrate chromatography revealed that glucosamine and galactosamine were also present. Hydrolysis of cell pellets from buffer preparations containing nongrowing cells and broth cultures containing growing cells revealed the presence of only trace amounts of hexoses. Intracellular compatible solutes, such as amino acids, were not studied.
The mechanism of saccharide and galactose formation by lactococci at low aw and/or low pH values is not known, nor is the biosynthetic potential of the phospho-β-galactosidase present in most lactococci. The β-galactosidase activity of some lactococci (10, 15, 16, 42) may have the potential to catalyze biosynthetic reactions (transgalactosylation of lactose) and make galactose-rich polysaccharides (20, 50). It is probable that a reduced aw value favors the biosynthetic reaction (23), but further work is required to confirm this. Alternatively, enzymes required for the formation of lactococcal cell wall polysaccharides (21) may be responsible. In either case, the ability of lactococci to synthesize a saccharide(s) under low-aw and low-pH conditions could protect the cells against an adverse environment or may only be an incidental consequence of enzyme kinetics in a stressed environment.
While polysaccharide formation has been observed in some lactic acid bacteria used for dairy fermentations (7, 32), including strains of Lactococcus lactis subsp. cremoris (8, 31, 34, 39), the conditions that affect polysaccharide formation are not well understood. From the results presented here, it appears that lactococci may convert approximately one-half of the lactose utilized to a polysaccharide(s) under the stress conditions used (low aw and/or low pH).
Accumulating polysaccharide may facilitate the adhesion of starter bacteria to the cheese curd matrix and therefore minimize the expulsion of the bacterial cells with the whey during the initial stages of cheese syneresis (5). The carbon and energy sources used for growth by adventitious microorganisms in cheese, particularly the nonstarter lactic acid bacteria, have not been established (35). The concentrations of saccharides or polysaccharides formed by starters in cheeses may be sufficient (∼5 to 10 mmol per kg of cheese) to influence growth of the adventitious microorganisms and thus to have an impact on the quality of the ripened cheese. Because of the potential importance of polysaccharides, further investigation to define the nature of the polysaccharides and the mechanisms of polysaccharide formation is warranted.
REFERENCES
- 1.Bassit N, Cochet N, Lebeault J M. Influence of water activity on Streptococcus diacetylactis metabolism. Appl Microbiol Biotechnol. 1993;40:399–401. [Google Scholar]
- 2.Benthin S, Nielsen J, Villadsen J. Galactose expulsion during lactose metabolism in Lactococcus lactis subsp. cremoris FD1 due to dephosphorylation of intracellular galactose 6-phosphate. Appl Environ Microbiol. 1994;60:1254–1259. doi: 10.1128/aem.60.4.1254-1259.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blickstad E. The effect of water activity on growth and end-product formation of two Lactobacillus spp. and Brochothrix thermosphacta ATCC 11509T. Appl Microbiol Biotechnol. 1984;19:13–17. [Google Scholar]
- 4.Bobillo M, Marshall V M. Effect of acidic pH and salt on acid end-products by Lactobacillus plantarum in aerated, glucose-limited continuous culture. J Appl Bacteriol. 1992;73:67–70. [Google Scholar]
- 5.Brooker B E. Cytochemical observations on the extracellular carbohydrate produced by Streptococcus cremoris. J Dairy Res. 1976;43:283–290. doi: 10.1017/s0022029900015843. [DOI] [PubMed] [Google Scholar]
- 6.Brown A D. Microbial water stress physiology. Chichester, United Kingdom: John Wiley & Sons; 1990. pp. 241–275. [Google Scholar]
- 7.Cerning J. Exocellular polysaccharides produced by lactic acid bacteria. FEMS Microbiol Rev. 1990;87:113–130. doi: 10.1111/j.1574-6968.1990.tb04883.x. [DOI] [PubMed] [Google Scholar]
- 8.Cerning J, Bouillanne C, Landon M, Desmazeaud M. Isolation and characterization of exopolysaccharides from slime-forming mesophilic lactic acid bacteria. J Dairy Sci. 1992;75:692–699. [Google Scholar]
- 9.Cogan T M. Flavor production by dairy starter cultures. J Appl Bacteriol Symp Suppl. 1995;79:49S–64S. [Google Scholar]
- 10.Crow V L, Thomas T D. Properties of a Streptococcus lactis strain that ferments lactose slowly. J Bacteriol. 1984;157:28–34. doi: 10.1128/jb.157.1.28-34.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crow V L, Martley F G, Coolbear T, Roundhill S J. The influence of phage-assisted lysis of Lactococcus lactis subsp. lactis ML8 on Cheddar cheese ripening. Int Dairy J. 1995;5:451–472. [Google Scholar]
- 12.Csonka L N, Hanson A D. Prokaryotic osmoregulation: genetics and physiology. Annu Rev Microbiol. 1991;45:569–606. doi: 10.1146/annurev.mi.45.100191.003033. [DOI] [PubMed] [Google Scholar]
- 13.Dische Z. New color reactions for determination of sugars in polysaccharides. In: Glick D, editor. Methods in biochemical analysis. II. New York, N.Y: Interscience Publishers Inc.; 1955. pp. 313–358. [DOI] [PubMed] [Google Scholar]
- 14.Fagen H J, Stine J B, Hussong R V. The identification of reducing sugars in Cheddar cheese during the early stages of ripening. J Dairy Sci. 1952;35:779–782. [Google Scholar]
- 15.Farrow J A E. Lactose hydrolyzing enzymes in Streptococcus lactis and Streptococcus cremoris and also in some other species of streptococci. J Appl Bacteriol. 1980;49:493–503. doi: 10.1111/j.1365-2672.1980.tb04724.x. [DOI] [PubMed] [Google Scholar]
- 16.Farrow J A E, Garvie E I. Strains of Streptococcus lactis which contain β-galactosidase. J Dairy Res. 1979;46:121–125. [Google Scholar]
- 17.Finlayson H J. The effect of pH on the growth and metabolism of Streptococcus bovis in continuous culture. J Appl Bacteriol. 1986;61:201–208. doi: 10.1111/j.1365-2672.1986.tb04277.x. [DOI] [PubMed] [Google Scholar]
- 18.Fordyce A M, Crow V L, Thomas T D. Regulation of product formation during glucose or lactose limitation in nongrowing cells of Streptococcus lactis. Appl Environ Microbiol. 1984;48:332–337. doi: 10.1128/aem.48.2.332-337.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fox P F, Lucey J A, Cogan T M. Glycolysis and related reactions during cheese manufacture and ripening. Crit Rev Food Sci Nutr. 1990;29:237–253. doi: 10.1080/10408399009527526. [DOI] [PubMed] [Google Scholar]
- 20.Garman J, Coolbear T, Smart J. The effect of cations on the hydrolysis of lactose and the transferase reactions catalysed by β-galactosidase from six strains of lactic acid bacteria. Appl Microbiol Biotechnol. 1996;46:22–27. doi: 10.1007/s002530050778. [DOI] [PubMed] [Google Scholar]
- 21.Gopal P K, Reilly K I. Molecular architecture of the lactococcal cell surface as it relates to important industrial properties. Int Dairy J. 1995;5:1095–1111. [Google Scholar]
- 22.Gould G W. Present state of knowledge of aw effects on microorganisms. In: Simatos D, Multon J L, editors. Properties of water in foods. Dordrecht, The Netherlands: Martinus Nijhoff, Publishers; 1985. pp. 229–245. [Google Scholar]
- 23.Hahn-Hagerdal B. Water activity: a possible external regulator in biotechnical processes. Enzyme Microb Technol. 1986;8:322–327. [Google Scholar]
- 24.Hickey M W, Hillier A J, Jago G R. Transport and metabolism of lactose, glucose and galactose in homofermentative lactobacilli. Appl Environ Microbiol. 1986;51:825–831. doi: 10.1128/aem.51.4.825-831.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hutkins R W, Ponne C. Lactose uptake driven by galactose efflux in Streptococcus thermophilus: evidence for a galactose-lactose antiporter. Appl Environ Microbiol. 1991;57:941–944. doi: 10.1128/aem.57.4.941-944.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hutkins R W, Ellefson W L, Kashket E R. Betaine transport imparts osmotolerance on a strain of Lactobacillus acidophilus. Appl Environ Microbiol. 1987;53:2275–2281. doi: 10.1128/aem.53.10.2275-2281.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jordan K N, Cogan T M. The effect of NSLAB on cheese flavour. In: Cogan T M, editor. 2nd Cheese Symposium. Moorepark, Fermoy, Ireland: National Dairy Products Centre; 1990. pp. 19–22. [Google Scholar]
- 28.Kets E P W, de Bont J A M. Protective effect of betaine on survival of Lactobacillus plantarum subjected to drying. FEMS Microbiol Lett. 1994;116:251–256. [Google Scholar]
- 29.Kets E P W, Galinski E A, de Bont J A M. Carnitine: a novel compatible solute in Lactobacillus plantarum. Arch Microbiol. 1994;162:243–248. [Google Scholar]
- 30.Kets E P W, Teunissen P J M, de Bont J A M. Effect of compatible solutes on survival of lactic acid bacteria subjected to drying. Appl Environ Microbiol. 1996;62:259–261. doi: 10.1128/aem.62.1.259-261.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Macura D, Townsley P M. Scandinavian ropy milk—identification and characterization of endogenous ropy lactic streptococci and their extracellular excretion. J Dairy Sci. 1984;67:735–744. [Google Scholar]
- 32.Malik R K, Prasher R, Mathur D K. Genetics and production of extracellular polysaccharides by lactic acid bacteria—a review. Indian J Dairy Sci. 1994;47:987–994. [Google Scholar]
- 33.Marcos A. Water activity in cheese in relation to composition, stability and safety. In: Fox P F, editor. Cheese: chemistry, physics and microbiology. Vol. 2. London, United Kingdom: Chapman and Hall; 1993. pp. 439–469. [Google Scholar]
- 34.Marshall V M, Cowie E N, Moreton R S. Analysis and production of two exopolysaccharides from Lactococcus lactis subsp. cremoris LC 330. J Dairy Res. 1995;62:621–628. [Google Scholar]
- 35.Martley F G, Crow V L. Interactions between non-starter microorganisms during cheese manufacture and ripening. Int Dairy J. 1993;3:461–483. [Google Scholar]
- 36.Measures J C. Role of amino acids in osmoregulation of non-halophilic bacteria. Nature (London) 1975;257:398–400. doi: 10.1038/257398a0. [DOI] [PubMed] [Google Scholar]
- 37.Miah A H, Reinbold G W, Hartley J C, Vedamuthu E R, Hammond E G. Characteristics of Cheddar cheese cooled at different rates during early curing stages. J Milk Food Technol. 1974;37:47–54. [Google Scholar]
- 38.Molenaar D, Hagting A, Alkema H, Driessen A J M, Konings W N. Characteristics and osmoregulatory roles of uptake systems for proline and glycine betaine in Lactococcus lactis. J Bacteriol. 1993;175:5438–5444. doi: 10.1128/jb.175.17.5438-5444.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakajima H, Toyoda S, Toba T, Itoh T, Mukai T, Kitazawa H, Adachi S. A novel phosphopolysaccharide from slime-forming Lactococcus lactis subsp. cremoris SBT 0495. J Dairy Sci. 1990;73:1472–1477. [Google Scholar]
- 40.Reizer J, Saier M H., Jr Involvement of lactose enzyme II of the phosphotransferase system in rapid expulsion of free galactosides from Streptococcus lactis. J Bacteriol. 1983;156:236–242. doi: 10.1128/jb.156.1.236-242.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruegg M, Blanc B. Influence of water activity on the manufacture and aging of cheese. In: Rockland L B, Stewart G F, editors. Water activity: influences on food quality. New York, N.Y: Academic Press; 1981. pp. 791–811. [Google Scholar]
- 42.Smart J B, Pillidge C J, Garman J H. Growth of lactic acid bacteria and bifidobacteria on lactose and lactose-related mono-, di- and trisaccharides and correlation with distribution of β-galactosidase and phospho-β-galactosidase. J Dairy Res. 1993;60:557–568. [Google Scholar]
- 43.Thomas T D. Regulation of lactose fermentation in group N streptococci. Appl Environ Microbiol. 1976;32:474–478. doi: 10.1128/aem.32.4.474-478.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thomas T D, Crow V L. Selection of galactose-fermenting Streptococcus thermophilus in lactose-limited chemostat cultures. Appl Environ Microbiol. 1984;48:186–191. doi: 10.1128/aem.48.1.186-191.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomas T D, Pearce K N. Influence of salt on lactose fermentation and proteolysis in Cheddar cheese. NZ J Dairy Sci Technol. 1981;16:253–259. [Google Scholar]
- 46.Thomas T D, Ellwood D C, Longyear V M C. Change from homo- to heterolactic fermentation by Streptococcus lactis resulting from glucose limitation in anaerobic chemostat cultures. J Bacteriol. 1979;138:109–117. doi: 10.1128/jb.138.1.109-117.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomas T D, Turner K W, Crow V L. Galactose fermentation by Streptococcus lactis and Streptococcus cremoris: pathways, products, and regulation. J Bacteriol. 1980;144:672–682. doi: 10.1128/jb.144.2.672-682.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thompson J, Saier M H., Jr Regulation of methyl-β-d-thiogalactopyranoside-6-phosphate accumulation in Streptococcus lactis by exclusion and expulsion mechanisms. J Bacteriol. 1981;146:885–894. doi: 10.1128/jb.146.3.885-894.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thompson J, Chassy B M. Novel phosphoenolpyruvate-dependent futile cycle in Streptococcus lactis: 2-deoxy-d-glucose uncouples energy production from growth. J Bacteriol. 1982;151:1454–1465. doi: 10.1128/jb.151.3.1454-1465.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Toba T, Tomita Y, Itoh T, Adachi S. β-Galactosidases of lactic acid bacteria: characterization by oligosaccharides formed during hydrolysis of lactose. J Dairy Sci. 1981;64:185–192. [Google Scholar]
- 51.Troller J A, Christian J H B. Water activity and food. New York, N.Y: Academic Press; 1978. pp. 103–117. [Google Scholar]
- 52.Troller J A, Stinson J V. Moisture requirements for growth and metabolite production by lactic acid bacteria. Appl Environ Microbiol. 1981;42:682–687. doi: 10.1128/aem.42.4.682-687.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Turner K W, Thomas T D. Uncoupling of growth and acid production in lactic streptococci. NZ J Dairy Sci Technol. 1975;10:162–167. [Google Scholar]
- 54.Witter L D, Anderson C B. Osmoregulation by microorganisms at reduced water activity. In: Montville T J, editor. Food microbiology. I. Concepts in physiology and metabolism. Boca Raton, Fla: CRC Press, Inc.; 1987. pp. 1–28. [Google Scholar]