Abstract
This study aimed to demonstrate the role of Zea mays or corn silk (CS) in the treatment of kidney stones after its proven effectiveness in folk medicine. Twenty-four rats were divided into four groups, the first represented the control group (negative control), and the second (positive control), was treated with 75% of ethylene glycol (EG) and 1% of ammonium chloride (AC) to induce stones in the kidneys of experimental animals. The animals of the third and fourth groups were treated with the same proportions of EG and AC, with the addition of extract of CS at a ratio of 200 and 400 mg/kg. After the 28th day, the blood samples were taken from rats. All kidneys of rats from all groups were taken to histological examination. Another ten rats were divided into two groups and took the same time as the original experiment. Group E took a normal diet and served as negative control group whereas the group F took a normal diet with 500 mg/kg of CS to investigate the mechanism of CS as antiurolithiatic treatment. Blood samples were collected on the last day of the experiment to perform the required analyses. The rats were dissected and liver and kidney samples were taken to complete the histological study. The results showed a significant decrease in the CS group in plasma MDA, serum urea, and creatinine. Moreover, the histological study, in the CS rats group appeared to be fewer CaOx crystals. On the other hand, we observed a significant increase in urinary pH, urine volume urinary Mg, and citrate in-group E when compared with the F group. In conclusion, we infer that CS works as an antiurolithiatic drug by increasing urinary pH, diuresis, and its nephroprotective vims. So, we advise its use as an antiurolithiasis treatment but in its pharmaceutical forms.
Keywords: Corn silk, urolithiasis, calcium oxalate, ethylene glycol
Introduction
The Kidneys comb out the toxic substances from the blood and organize the levels of significant substances for body functions as chemicals [1]. Nephrolithiasis (NL), which is known as (kidney stones, urinary stones, urolithiasis, and renal calculi), affects a great number of patients worldwide [2]. Urolithiasis (UL) is the third most spread disorder for the urinary system with a high rate of repetition. Renal calculi creation is one of the most spread urological disorders. Urinary stone disease affects 10-12% of the inhabitance in industrialized countries and it is a spreading disease [3]. Nearly, 80% of stones that composed of calcium oxalate (CaOx) and calcium phosphate, 10% of magnesium ammonium phosphate stones (struvite), 9% of uric acid, as well as the remaining 1% are consisted of cysteine [4].
The stone formation needs supersaturated urine. Moreover, the creation of a kidney stone involves three serious stages including nucleation of (CaOx) crystals then growth and ingathering of crystals [5]. Lately, medical management of UL is quite costly with side effects. Furthermore, invasive procedures for UL may result in dangerous complications as well as a high cost for the patient [6]. EG is an odorless, colorless, and soluble chemical agent. It is converted in life to four organic acids: glycolaldehyde, glycolic acid, and oxalic acid, which cause hyperoxaluria. It is the main action factor for urolithiasis [7].
The three methods that are used in treating nephritic stones: Surgical removal, Drugs, and Extracorporeal Shock Wave Lithotripsy (ESWL) [8]. Alternative treatment using phytotherapy may be a safe remedy. Therefore, many plant species are used as a remedy, and a large number of plants are described in many pharmacopeias worldwide as antiurolithiatic agents [9]. One of these plants is Zea mays (family Poaceae), Maydis stigma (Corn silk) type. Waste material from corn cultivation is CS, which is a cheap medical diet of plants also [10].
For thousands of years, Corn Silk has been used as a folk medicine in the entire world for treating diabetes mellitus, gout, prostatitis cystitis, nephritis, nephritic stones, and edema [11-13]. In addition, treatments for CS include anti-fatigue activity, antidepressant activity, and kaliuretic [14].
Materials and methods
Experimental animals
This study was performed on male Wistar albino rats (Rattus rattus), initially weighing (225±25) g. These animals were reared in the animal house of the Faculty of Science at Sana’a University, Biology Department. Under the same ecological conditions, rats have housed in stainless cages. Rats were permitted an adequate standard diet and given water ad libitum for one week of the adaptation period before the experimental work. In addition, every 48 hrs, the animal cage bedding was changed.
Animals were fed on a diet with a recipe that was perfectly given by the Faculty of Agriculture, Sana’a University of the Animal Production Department.
From Sana’a Governorate in Yemen, Fresh hairs CS were collected. Moreover, the plants were neatly washed with distilled water and dehydrated at room temperature and crushed into powder. Then in airtight polythene bags, the powdered materials were stored for future use. By using the Soxhlet apparatus with 70% methanol, and 30% aqueous, the dried powder was extracted. Then by using a rotary evaporator, the extracts were intensified and put to dry in a freeze dryer until the dry powder was acquired [15]. After that, the percentage yield is 11.50% was found. Finally, the extracts were stored in the refrigerator, until further use.
Stone creation
By the administration (0.75%) v/v of EG and (1%) v/w of AC in drinking water for the first seven days, hyperoxaluria was motivated in rats according to [16,17].
Dose preparation
The process was done by dissolving the methanolic extract of CS at a dose of mg/kg of body weight inside filtered water then shacked until disappeared perfectly.
Experimental animals
In this study, 24 male rats were divided randomly into 4 groups; each group consisted of six rats. Group A: fed with a standard diet, which served as a negative control. Group B: took a normal diet with the amount of EG (0.75%) and AC (1%) for 28 days and served as a positive control. Groups C and D: were fed with the same materials as group B with the amount of 200 mg/kg and 400 mg/kg from CS respectively for 28 days and served as treated groups.
Assessment of antiurolithiatic activity
Collection and analysis of blood
At the end of the study period, all rats were fasted overnight and sacrificed under ether anesthesia, and blood was taken from orbital veins. Serum was analyzed after being separated from blood by centrifugation at 3,000 r.p.m for 15 mins. Then blood plasma was separated by centrifugation at 3,000 r.p.m for 15 mins.
Estimation of lipid peroxidation (LPO)
Malondialdehyde (MDA) was determined according to the method of [18].
Biochemical analysis
By kinetic UV assay colorimetric methods, the serum levels of creatinine and urea were measured using fixture tools through Roche diagnosis existing in Roche/Hitachi analyzer by using different biochemical estimations.
Histopathological study
In this study, Kidneys were weighed, then put in formalin 10% and processed by alcohol and xylene. After that, they were inserted in paraffin, divided at 5 µm, and colored with hematoxylin and eosin for histopathological exam under a light microscope.
Investigate the mechanism of antiurolithiasis of CS
Additional 10 male rats were randomly divided into 2 groups; each of 5 rats were used to investigate the mechanism of CS as antiurolithiatic substance. Group E: was given a normal diet, and then left as a negative control group whereas, group F: took a normal diet with 500 mg/kg of CS and was allowed free access to food and drinking water [19]. These groups were determined by measuring water intake, urine volume and PH of urine, Mg in urine, and citrate.
Determination of water intake, urine volume, and PH
The rats were kept separately in metabolic cages. Water intake was measured and 24 hrs and urine samples were collected. A concentrated hydrochloric acid drop was put into the urine before saving at 4°C, to measure its urine volume. Then from all rats, the pH of the fresh urine samples was measured with a calibrated pH meter (Model: WTW-Series pH-720) [20].
Determination of urine magnesium and citrate
The determination of the Mg concentrations and citrate in urine samples were performed by the colorimetric procedure.
Antibacterial activity
For this study, five types of bacteria were used as Gram-positive bacteria, which included Bacillus cereus, and Staphylococcus aureus. Gram-negative bacteria included Escherichia coli, Proteus and Kelibsella pneumoniae. All of these tested strains were local isolated in the Faculty of Science, Sana’a University, the Department of Biology, Division of Microbiology. These bacteria were used for pathogens’ antibacterial vigor checking.
From every selected plant extraction, three different concentrations (50, 100, and 150 mg/ml) were dissolved in 10% dimethyl-sulfoxide (DMSO), and in filtered water used in the antibacterial vigor exam. Just before carrying out the test, extract solutions were prepared. Then by the agar well diffusion method, the antibacterial vigor of the extracts was determined.
In petri dishes plates, the bacterial suspensions containing 106 CFU/ml of bacteria were spread with a sterile swab wet with the bacterial suspension. Then 5 wells were severed using a standard corn borer (7 mm) in each of these plates. Almost 60 μl of each extract was put into different wells (duplicate each concentricity). As a negative control, DMSO was used, and a positive control antibiotic wells were placed in the plate. For 24 hrs at 37°C, all the plates were incubated. After that, bioactivity was estimated by measuring the inhibition zone. This test was done by 2 antibiotics criterion Gentamycin (10 mcg), which was used as a reference to determine each bacterial species sensitivity tested and used like a control positive. By the agar diffusion method, the antibacterial vigor of CS extract was determined according to [21].
Statistical analysis
In this study, GraphPad prism was used for data analysis. The data were collected and expressed as mean ± SE. Moreover, by using a one-way analysis of variance (ANOVA), the statistical significance among groups was analyzed. A p-value <0.05 was considered significant.
Results
Effect of CS on plasma MDA level
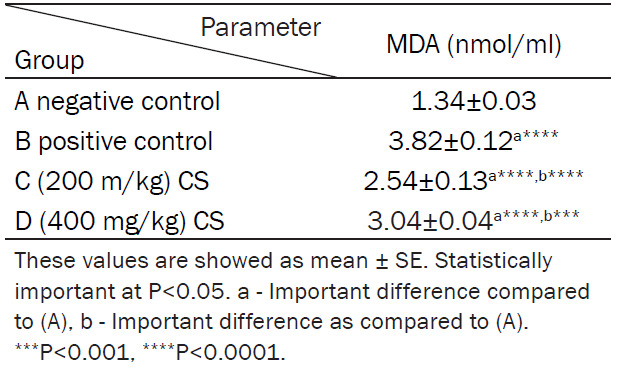
The mean levels of MDA were (1.34±0.03) nmol/ml, (3.82±0.12) nmol/ml, (2.54±0.13) nmol/ml, and (3.04±0.04) nmol/ml in groups A, B, C and D, respectively (Table 1; Figure 1).
Table 1.
Effect of CS on plasma MDA level
|
Figure 1.
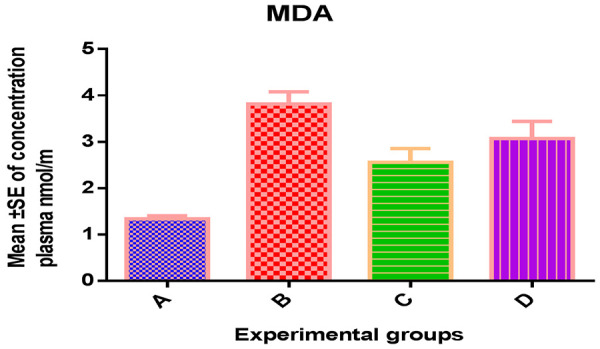
Effect of CS on plasma MDA level.
Impact of CS extract impact on creatinine and urea
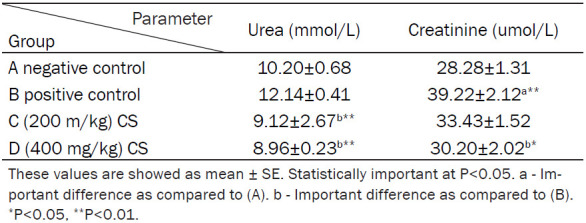
The mean levels of urea were (10.20±0.68) nmol/ml, (12.14±0.41) nmol/ml, (9.12±2.67) nmol/ml, and (8.96±0.23) nmol/ml, while the mean levels of creatinine were (28.28±1.31) nmol/ml, (39.22±2.12) nmol/ml, (33.43±1.52) nmol/ml, and (30.20±2.02) nmol/ml in groups A, B, C and D, respectively (Table 2; Figure 2).
Table 2.
Effect of CS on the serum level of creatinine and urea
|
Figure 2.
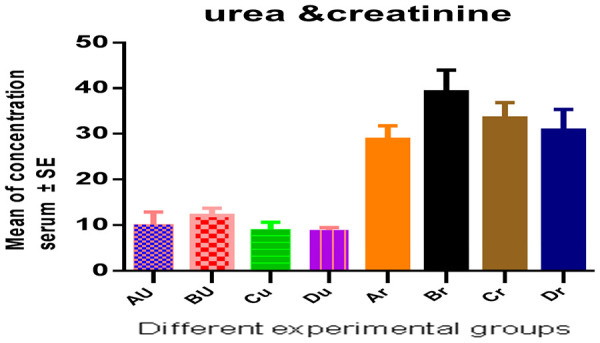
Serum urea and creatinine.
Effects of methanolic extract of CS on water intake, urine volume, PH, magnesium and citrate
A significant increase in water intake, urine volume, pH, urinary volume, magnesium and citrate were observed in group F. However, there were no significant differences observed in comparison to Group E (Table 3; Figure 3). Table 4 and Figure 4 showed a significant decrease appeared of kidney weight in CS groups when compared with positive control group.
Table 3.
Effect of methanolic extract of CS on Water intake, urine volume and PH, urinary Mg and citrate
Figure 3.
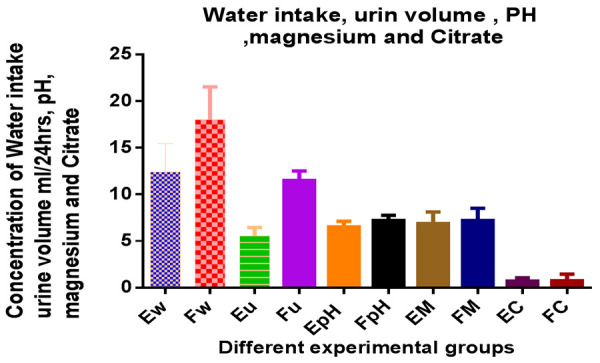
Water intake, urine volume, urinary PH, Mg and citrate in CS treated rats.
Table 4.
Kidney weight in different groups of CS
| Group | Kidney weight |
|---|---|
| A negative control | 2.12±0.08 |
| B positive control | 3.10±0.197a **** |
| C (200 mg/kg CS) | 2.45±0.08b **** |
| D (400 mg/kg CS) | 2.72±0.11a *,b **** |
Important difference as compared to the negative control.
Important difference as compared to the positive control.
P<0.05;
P<0.0001.
Figure 4.
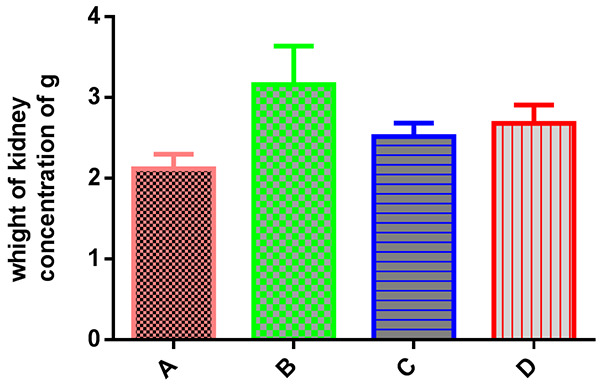
Kidney weight in comparison to control groups.
Effect of methanolic extract of CS as antibacterial
CS extract had antibacterial activity on all bacteria tested, which was dose dependent. This activity was better than gentamycin for Klebsiella Pneumonia and proteus (Table 5).
Table 5.
Antibacterial activity of methanolic extract of CS
| Type of Bacteria | Inhibitory zone in mm | ||||
|---|---|---|---|---|---|
|
| |||||
| CS extract concentration mg | Gentamycin | Placebo | |||
|
| |||||
| 50 mg | 100 mg | 150 mg | |||
| E. Coli | 00 | 8 mm | 9 mm | 19 mm | 0 |
| Bacillus cereus | 12 mm | 13 mm | 15 mm | 23 mm | 0 |
| S. aureus | 12 mm | 14 mm | 17 mm | 25 mm | 0 |
| Klebsiella pneumoniae | 12 mm | 15 mm | 22 mm | 16 mm | 0 |
| Proteus | 12 mm | 15 mm | 18 mm | 15 mm | 0 |
Effect of methanolic extract of CS on calcium oxalate density and integrity of kidney
Histological examination of the kidneys of different groups was performed. The results revealed a complete absence of oxalate crystals in groups A, C, but few crystals in the D group. The positive control group (group B) shows crystals in rats treated with EG/AC, CaOx crystals were plentiful and large. This group also shows adaptive responses in their kidneys. These responses include massive dilation of Bowman’s space, hypercellularity of the glomeruli and dilatation of renal tubules. These are blocked by oxalate crystals. The casts appeared intratubular and extra tubular with interstitial hemorrhage (Figures 5, 6, 7).
Figure 5.
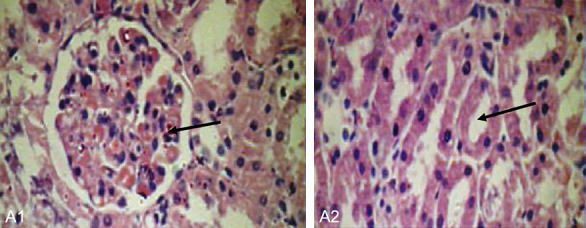
Normal kidney (group A/negative control). A1: Normal glomerulous (40 × 400). A2: Normal tubule free of cryastals (40 × 400).
Figure 6.
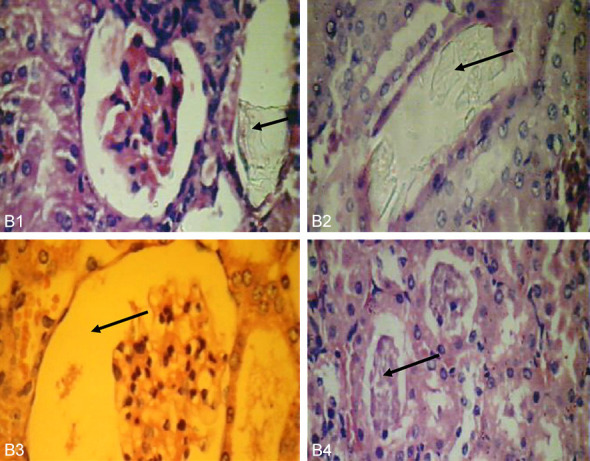
Histological changes in positive control group (group B). B1: (positive control) oxalate crystals in cortical tubules near glomerulous (40 × 400). B2: Tubules mostly blocked with crystals with desquamated epithelia (40 × 400). B3: Wide urinary space of Bowman’s capsule (40 × 400). B4: Chronic pylonephritis with thyro-dizationor necrotic tissue (10 × 100).
Figure 7.
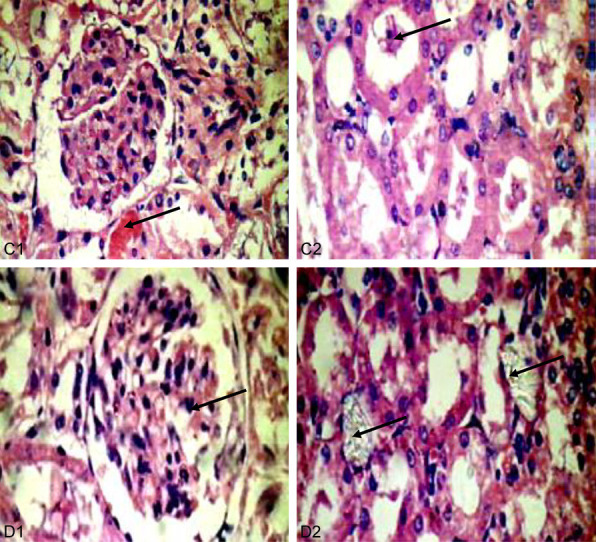
Kidneys of CS treated groups (C: 200 mg/kg, D: 400 mg/kg). C1: Little intratubular bleeding and hemorrhage (40 × 400). C2: No crystals but with intratubular casts in renal tubules (40 × 400). D1: Kidney with multi lobulated glomerulus (40 × 400). D2: CS less crystals, and less intratubuler casts in renal tubules (40 × 400).
Discussion
In this study, CS impact on nephrotic stones creation inside male rats with experimentally motivate urolithiasis and the biochemical changes in the liver. In this research, it was important to note that, CS administration to cure rats displayed statistically important changes in all measured variable factors when compared to the normal control group.
Urolithiasis (UL) is one of the oldest famous diseases Worldwide. At the same time, in urinary tubules, CaOx crystals can lead to damage in the epithelial cells (ECs). On the other hand, ECs harm and crystal detention in the nephron that is considered needed for stone creation by CaOx crystals, and can bind to ECs [22]. In addition, hyperoxaluria created by EG is a far more important risk factor in the pathogenesis of renal stones than hypercalciuria [23].
In addition, ammonium chloride (AC) ingestion induces urinary acidification. Therefore, AC promotes the deposition of CaOx crystals in rat kidneys [16].
Our study investigated the CS extract’s impact on the administration of renal stones genesis and decay. In urinary tubules, CaOx crystals can make damages the ECs. In addition, the free radicals, which are created by EG cause, damage to side urinary tract cells [24]. Moreover, oxalate is known to damage the renal cellular membrane safety probably by motivating lipid peroxidation (LPO), which can make renal epithelial injury, that super fats the areas available for crystal attachment and eventual retention in the kidney due to produce the free radicals [25,26].
Corn silk (CS) has enormous phenolic compounds set like flavonoids and alkaloids, which act probably similar to antioxidants scavenging reactive oxygen kinds (ROS) and damping lipid peroxidation [27].
The current works revealed an important increase in the plasma TBARS levels, in the urolithiasis rats group. The growing levels of plasma LPO products noticed in EG-induced urolithiasis are in general due to some morbid changes in the tissues, which super fat the production and emancipation of LPO into the blood circulation. This result agreed with [9]. On the contrary, the decreased levels of the plasma TBARS observed in the treated group with CS index are rich with antioxidant components (i.e., flavonoids, polyphenols, alkaloids, and tannins) and in agreement with [28,29].
Different groups of creatinine and urea in serum
Ethylene Glycol poisoning enables to make acute nephritic failure and is distinguished by proximal tubular necrosis and gathering of CaOx monohydrate crystals within the urine and kidney tissues [30]. The serum concentricity estimation of protein metabolism end outputs (urea and creatinine), gives us an image of the viability of nephritic tissue [31]. Our study displayed that renal obstruction by CaOx leads to an increase of serum creatinine and urea in urolithiasis group B when compared to negative control A. Moreover, the raise in serum scale of these parameters in-group B agreed with [32].
On the other hand, it has been believed that certain medicinal plants can prevent the growth and adhesion of pathogens [33]. Furthermore, the importance minimizes in this test among group B from CS in the creatinine and urea, which may lead to prevent crystal sedimentation in the nephritic tubules that prevent motivating injury in nephritic tubules. Therefore, these outcomes were in assent with the detection of [34,35].
Pathological study and mechanism of action as antiurolithiasis remedy
The histopathological study of the kidney sections also supported the physiological results. The urolithiasis group administration of the EG and AC resulted in increased crystal, CaOx crystals in the renal tubules, renal dilatation of Bowman space, tubular damage and tubular dilatation when compared to the kidneys of the negative control group. This result was in agreement with the results obtained by [36]. Furthermore, in our results, the preventive group showed no crystal deposit characters.
Our results in histological part reveled less crystal in CS treated group when compared with the normal control. The preventive results coincided with the finding of [37,38]. The exact mechanism of this protection was unclear. However, the following are possible mechanisms: CS methanol extract reduces the LPO level thus preventing CaOx crystal attachment and subsequent development of kidney stones. It possesses a high amount of antioxidant and phenolic content prevent calcium and oxalate deposition [39].
The mechanism of such urolithiolytic effect of CS was also, investigated in our study.
There were significant increases in water intake, urine volume, urinary pH, urinary Mg and citrate in-group E in comparison to group F. The urine volume reduces the supersaturating of urine by CaOx and this is consistent with others [40]. In addition, the diuretic effect of CS may be due to the inhibition of the Angiotensin Converting Enzyme and aldosterone [41].
For kidney stonegenesis, urinary pH is usually a major determinant; a urine pH of approximately 6 on the pH scale minimizes the risk of kidney stone genesis, however, the risk of uric acid and calcium stone genesis raises gradually at urinary pH<5.5 [42]. Moreover, the slight reduction in pH leads to a raise in urine calcium excretion mediated by reduce in renal tubular calcium reabsorption. In addition, the raise in systemic acidity makes reduce urinary citrate excretion [28]. So, if urinary pH rises, renal citrate production does as well, thus producing a decrease in tubular citrate reabsorption, and increased citrate excretion [43].
In our study, the antiurolithiatic of CS linked to the rise of urinary pH, making it a perfect anti-CaOx remedy. Moreover, Mg combines with oxalate, potentially reducing oxalate absorption in the gastrointestinal tract (GIT) and decreasing CaOx supersaturation in urine [44]. Moreover, Mg has been shown to lower urinary supersaturation of CaOx and increase urinary citrate [45].
Finally, the antibacterial activity of CS was also investigated and we found an acceptable antibacterial effect against some bacteria that may be a predisposing factor for urolithiasis.
In conclusion, CS was good for urolithiasis as a protective remedy. It had diuretic activity, rising urinary pH, and increasing urinary citrate and magnesium. Additionally, it had antibacterial activity, and renal tissues.
Acknowledgements
The authors are thankful to Deanship of Scientific Research and under the supervision of the Scientific and Engineering Research center at Najran University for supporting this work under the Research Centers Funding program grant code (NU/RCP/MRC/12/1).
Disclosure of conflict of interest
None.
References
- 1.Barbas C, García A, Saavedra L, Muros M. Urinary analysis of nephrolithiasis markers. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;781:433–455. doi: 10.1016/s1570-0232(02)00557-3. [DOI] [PubMed] [Google Scholar]
- 2.Praba T, Kingsly A, Essakkypandian G, Antony Duraichi R. Evaluation of in-vitro anti-urolithiasis activity of Nerunjil kudineer. Int J Curr Res Biol Med. 2018;3:93–99. [Google Scholar]
- 3.Shekha MS, Ismail TF, Aziz FM. Anti-urolithiatic and anti-oxidant effects of fenugreek on ethylene glycol-induced kidney calculi in rats. Jordan J Biol Sci. 2015;8:159–163. [Google Scholar]
- 4.Bartoletti R, Cai T, Mondaini N, Melone F, Travaglini F, Carini M, Rizzo M. Epidemiology and risk factors in urolithiasis. Urol Int. 2007;79(Suppl 1):3–7. doi: 10.1159/000104434. [DOI] [PubMed] [Google Scholar]
- 5.Aggarwal KP, Narula S, Kakkar M, Tandon C. Nephrolithiasis: molecular mechanism of renal stone formation and the critical role played by modulators. Biomed Res Int. 2013;2013:292953. doi: 10.1155/2013/292953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark DL, Connors BA, Evan AP, Handa RK, Gao S. Effect of shock wave number on renal oxidative stress and inflammation. BJU Int. 2011;107:318–322. doi: 10.1111/j.1464-410X.2010.09311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leth PM, Gregersen M. Ethylene glycol poisoning. Forensic Sci Int. 2005;155:179–184. doi: 10.1016/j.forsciint.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Rosa M, Usai P, Miano R, Kim FJ, Finazzi Agrò E, Bove P, Micali S International Translational Research in Uro-Sciences Team (ITRUST) Recent finding and new technologies in nephrolitiasis: a review of the recent literature. BMC Urol. 2013;13:10. doi: 10.1186/1471-2490-13-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aggarwal A, Singla SK, Tandon C. Urolithiasis: phytotherapy as an adjunct therapy. Indian J Exp Biol. 2014;52:103–111. [PubMed] [Google Scholar]
- 10.Ho TY, Li CC, Lo HY, Chen FY, Hsiang CY. Corn silk extract and its bioactive peptide ameliorated lipopolysaccharide-induced inflammation in mice via the nuclear factor-κB signaling pathway. J Agric Food Chem. 2017;65:759–768. doi: 10.1021/acs.jafc.6b03327. [DOI] [PubMed] [Google Scholar]
- 11.Velazquez DV, Xavier HS, Batista JE, de Castro-Chaves C. Zea mays L. extracts modify glomerular function and potassium urinary excretion in conscious rats. Phytomedicine. 2005;12:363–369. doi: 10.1016/j.phymed.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 12.Ebrahimzadeh MA, Pourmorad F, Hafezi S. Antioxidant activities of Iranian corn silk. Turkish J Biol. 2008;32:43–49. [Google Scholar]
- 13.Khan H, Mishra A. The effect of corn silk herbal tea as an immunity booster and its multidisciplinary activity. ASIO-JPHMR. 2020;6:37–41. [Google Scholar]
- 14.Hu QL, Zhang LJ, Li YN, Ding YJ, Li FL. Purification and anti-fatigue activity of flavonoids from corn silk. Int J Phy Sci. 2010;5:321–326. [Google Scholar]
- 15.Sepehri G, Derakhshanfar A, Zadeh FY. Protective effects of corn silk extract administration on gentamicin-induced nephrotoxicity in rat. Comp Clin Pathol. 2011;20:89–94. [Google Scholar]
- 16.Salusjärvi L, Havukainen S, Koivistoinen O, Toivari M. Biotechnological production of glycolic acid and ethylene glycol: current state and perspectives. Appl Microbiol Biotechnol. 2019;103:2525–2535. doi: 10.1007/s00253-019-09640-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan A, Bashir S, Khan SR, Gilani AH. Antiurolithic activity of origanum vulgare is mediated through multiple pathways. BMC Complement Altern Med. 2011;11:96. doi: 10.1186/1472-6882-11-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 19.Kreydiyyeh SI, Usta J. Diuretic effect and mechanism of action of parsley. J Ethnopharmacol. 2002;79:353–357. doi: 10.1016/s0378-8741(01)00408-1. [DOI] [PubMed] [Google Scholar]
- 20.Ntchapda F, Bonabe C, Kemeta Azambou DR, Talla E, Dimo T. Diuretic and antioxidant activities of the aqueous extract of leaves of Vepris heterophylla (Engl.) R. Let (Rutaceae) in rats. BMC Complement Altern Med. 2016;16:516. doi: 10.1186/s12906-016-1439-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma YL, Zhu DY, Thakur K, Wang CH, Wang H, Ren YF, Zhang JG, Wei ZJ. Antioxidant and antibacterial evaluation of polysaccharides sequentially extracted from onion (Allium cepa L.) Int J Biol Macromol. 2018;111:92–101. doi: 10.1016/j.ijbiomac.2017.12.154. [DOI] [PubMed] [Google Scholar]
- 22.Khan SR. Hyperoxaluria-induced oxidative stress and antioxidants for renal protection. Urol Res. 2005;33:349–357. doi: 10.1007/s00240-005-0492-4. [DOI] [PubMed] [Google Scholar]
- 23.Christina AJM, Muthumani P. Phytochemical investigation and anti lithiatic activity of Abelmoschus moschatus medikus. Int J Pharm Pharm Sci. 2013;5:108–113. [Google Scholar]
- 24.Cruzan G, Corley RA, Hard GC, Mertens JJ, McMartin KE, Snellings WM, Gingell R, Deyo JA. Subchronic toxicity of ethylene glycol in Wistar and F-344 rats related to metabolism and clearance of metabolites. Toxicol Sci. 2004;81:502–511. doi: 10.1093/toxsci/kfh206. [DOI] [PubMed] [Google Scholar]
- 25.Khan SR. Crystal/cell interaction and nephrolithiasis. Arch Ital Urol Androl. 2011;83:1–5. [PubMed] [Google Scholar]
- 26.Khan SR. Reactive oxygen species as the molecular modulators of calcium oxalate kidney stone formation: evidence from clinical and experimental investigations. J Urol. 2013;189:803–811. doi: 10.1016/j.juro.2012.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naczk M, Shahidi F. Phenolics in cereals, fruits and vegetables: occurrence, extraction and analysis. J Pharm Biomed Anal. 2006;41:1523–1542. doi: 10.1016/j.jpba.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Hasanudin K, Hashim P, Mustafa S. Corn silk (Stigma Maydis) in healthcare: a phytochemical and pharmacological review. Molecules. 2012;17:9698–9715. doi: 10.3390/molecules17089697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Almadiy AA, Alasbahy AA, Gumaih HS, Nasr ES, Al-Maktari MA. The protective and therapeutic effect of corn silk extract on urolithiatic and hypertensive rats induced by ethylene glycol. Electro J Uni Aden Basic Appl Scis. 2021;2:151–160. [Google Scholar]
- 30.McMartin K. Are calcium oxalate crystals involved in the mechanism of acute renal failure in ethylene glycol poisoning? Clin Toxicol (Phila) 2009;47:859–869. doi: 10.3109/15563650903344793. [DOI] [PubMed] [Google Scholar]
- 31.Ghodkar B. In: Textbook Med. Lab. Technol. 1st edition. Mumbai: Bhalani., Publishing House; 1994. Chemical tests in kidney disease; pp. 118–132. [Google Scholar]
- 32.Karadi RV, Gadge NB, Alagawadi KR, Savadi RV. Effect of Moringa oleifera Lam. Root-wood on ethylene glycol induced urolithiasis in rats. J Ethnopharmacol. 2006;105:306–311. doi: 10.1016/j.jep.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 33.Lüthje P, Dzung DN, Brauner A. Lactuca indica extract interferes with uroepithelial infection by Escherichia coli. J Ethnopharmacol. 2011;135:672–7. doi: 10.1016/j.jep.2011.03.069. [DOI] [PubMed] [Google Scholar]
- 34.Sukandar EY, Sigit JI, Adiwibowo LF. Study of kidney repair mechanisms of corn silk (Zea mays L. Hair)-Binahong (Anredera cordifolia (Ten.) Steenis) leaves combination in rat model of kidney failure. Inter J Pharmacol. 2013;9:12–23. [Google Scholar]
- 35.Gumaih H, Salamah E, AL-Maktari M, AL-Asbahy A. Assess the therapeutic efficacy of cissus rotundifolia as antiurotheliasis and antihypertensive agent herbal. Med. 2021;7:1–5. [Google Scholar]
- 36.Talekar YP, Gund KA, Kale SD, Apte KG, Parab PB. Antiurolithic activity of corn silk extract in mice. Int J Univers Pharm Bio Sci. 2013;2:65–77. [Google Scholar]
- 37.Arokyaraj A, Rathiga G. Evaluation of anti-urolithiasis activity of melia azedarach leaf extract using in-vitro struvite crystal growth inhibition assay. Int J Trans Res Ind Med. 2019;1:29–32. [Google Scholar]
- 38.Al-Yousofy F, Gumaih H, Ibrahim H, Alasbahy A. Parsley! Mechanism as antiurolithiasis remedy. Am J Clin Exp Urol. 2017;5:55–62. [PMC free article] [PubMed] [Google Scholar]
- 39.Bhuvaneshwari M, Sivakami S. Analysis of nutrients and phytochemicals content in corn silk (Zea. Mays) Int J Sci Res. 2019;6:79–81. [Google Scholar]
- 40.Pak CY, Sakhaee K, Crowther C, Brinkley L. Evidence justifying a high fluid intake in treatment of nephrolithiasis. Ann Intern Med. 1980;93:36–39. doi: 10.7326/0003-4819-93-1-36. [DOI] [PubMed] [Google Scholar]
- 41.Sakhaee K. Urinary pH as a risk factor for stone type. Physicochemical and Pathophysiologic Bases of Elevated Urine pH. Ame Inst Phys. 2007;900:74–81. [Google Scholar]
- 42.Bushinsky DA, Grynpas MD, Asplin JR. Effect of acidosis on urine supersaturation and stone formation in genetic hypercalciuric stone-forming rats. Kidney Int. 2001;59:1415–1423. doi: 10.1046/j.1523-1755.2001.0590041415.x. [DOI] [PubMed] [Google Scholar]
- 43.Song Y, Hernandez N, Shoag J, Goldfarb DS, Eisner BH. Potassium citrate decreases urine calcium excretion in patients with hypocitraturic calcium oxalate nephrolithiasis. Urolithiasis. 2016;44:145–8. doi: 10.1007/s00240-015-0819-8. [DOI] [PubMed] [Google Scholar]
- 44.Liebman M, Costa G. Effects of calcium and magnesium on urinary oxalate excretion after oxalate loads. J Urol. 2000;163:1565–1569. [PubMed] [Google Scholar]
- 45.Riley JM, Kim H, Averch TD, Kim HJ. Effect of magnesium on calcium and oxalate ion binding. J Endourol. 2013;27:1487–1492. doi: 10.1089/end.2013.0173. [DOI] [PMC free article] [PubMed] [Google Scholar]



