Highlights
-
•
Different models recapitulate different aspects of human SVD – the right model should be used to answer the right question.
-
•
Successful translation requires close-working relationship between preclinical and clinical researchers to identify bridging points linking the basic science to the clinics.
-
•
Co-ordination of more rigorous, robust, and detailed preclinical evaluation of animal models is required through the concept of pRCTs.
-
•
The UK DRI vascular Theme, alongside DPUK experimental medicine Incubator, are working towards building a community to boost VCI preclinical and clinical research.
Keywords: Vascular cognitive impairment, Vascular dementia, Small vessel disease, Cerebral vascular disease, Dementia
Abstract
Although dementia research has been dominated by Alzheimer's disease (AD), most dementia in older people is now recognised to be due to mixed pathologies, usually combining vascular and AD brain pathology. Vascular cognitive impairment (VCI), which encompasses vascular dementia (VaD) is the second most common type of dementia. Models of VCI have been delayed by limited understanding of the underlying aetiology and pathogenesis. This review by a multidisciplinary, diverse (in terms of sex, geography and career stage), cross-institute team provides a perspective on limitations to current VCI models and recommendations for improving translation and reproducibility. We discuss reproducibility, clinical features of VCI and corresponding assessments in models, human pathology, bioinformatics approaches, and data sharing. We offer recommendations for future research, particularly focusing on small vessel disease as a main underpinning disorder.
1. Introduction
Dementia is a major global public health problem, with about 55 million people worldwide [1] thought to be living with dementia, although this figure may be an underestimate with dementia being under diagnosed, particularly where healthcare provision is thinly spread. Dementia prevention, identification and treatment is now a priority for many governments however research spend remains below that of other major non-communicable diseases [2].
Vascular cognitive impairment (VCI), which encompasses vascular dementia (VaD) is the second most common type of dementia, caused as a result of vascular injury to the brain [3]. Although dementia research has been dominated for decades by Alzheimer's disease (AD), most dementias in older people are now recognised to be due to mixed pathologies, usually combining vascular and AD brain pathology [4]. ‘Vascular contributions to cognitive impairment and dementia’ (VCID) is used when considering wider effects of vascular disease in mixed dementias, and with VCI, are now preferred terms to VaD.
Historically, VCI was considered to occur after a stroke and to have a step-like clinical course as new strokes occurred [5]. Stroke is a risk factor for dementia [6]. While VCI can result from haemorrhagic or ischaemic stroke, the commonest cause is now recognised to be subcortical microvascular disease also known as small vessel disease (SVD) [7].
Models of VCI, including SVD, have been delayed by limited understanding of the underlying aetiology and pathogenesis. To address this issue, in January 2017 we convened a workshop to discuss ‘Small vessels, dementia and chronic diseases – molecular mechanisms and pathophysiology’ [8], supported by Dementias Platform UK (DPUK-1), British Heart Foundation (BHF) and Royal Society of Edinburgh. This multidisciplinary workshop, and subsequent review paper [9], identified a range of potential models and mechanisms that mimicked some or all of the epidemiological or histopathological features of human SVD. The workshop also highlighted limitations and implications for future research that were necessary to bridge major gaps in knowledge [9]. Some of these were addressed in a subsequent meeting addressing assessment of cognition in preclinical models [10].
To assess progress in the field in modelling SVD and VCI, identify priorities for immediate future research, and recognising the major additional Government investments in dementia research in the UK, we reconvened the workshop in March 2022, organised and supported by UK Dementia Research Institute (UK DRI), DPUK-2 and BHF. The workshop brought together key experts from multidisciplinary, diverse (sex, geography and career stage), cross-institute groups, drawn from as many UK labs working on preclinical VCI and clinical experts as possible. It addressed important points on other vascular models, reproducibility, clinical features of VCI and corresponding assessments in models, human pathology, bioinformatics approaches, and data sharing. There were several focused break out discussions, with feedback and discussion by the whole group. In this report, we summarise the key points raised by experts and outputs of the focused group discussions, including recommendations for future research, particularly focusing on SVD as a main underpinning disorder.
2. What have we learned since the first workshop in 2017 [9]?
Since the workshop in 2017, there has been progress in understanding human SVD mechanisms [11,12] and symptoms [13], in harmonising methods to translate between preclinical and clinical SVDs studies [[11], [14]] and in reverse translation to unpick SVD mechanisms in preclinical models. For example, systematic reviews had identified Spontaneously Hypertensive Stroke Prone (SHRSP) rats as a potentially relevant rodent model of sporadic SVD [15,16]. The SHRSP model develops hypertension reliably in adolescence, superseded by endothelial cell (EC) autonomous dysfunction [17], rendering it vulnerable to vessel and tissue damage from hypertension in later life. This EC dysfunction includes impaired tight junction formation, impaired nitric oxide (NO) production, microglial activation and blocking of oligodendrocyte precursor (OPC) maturation [17]. This EC dysfunction has now been associated with gene ATP11B [17] and subsequent development of the ATP11B knockout rat showed the same cellular, histopathological and cognitive-behavioural abnormalities as the SHRSP, in the absence of hypertension [18]. This demonstrates that an EC autonomous dysfunction can cause SVD, without hypertension, consistent with clinical observations [[17], [18]], and clinical trial data showing that a) antihypertensive therapy, even intensive antihypertensive therapy, has modest effect on preventing SVD progression [19], and b) drugs which restore EC function (replace NO, unblock OPC maturation block) reduce recurrent stroke, cognitive impairment and dependency long term after small vessel (lacunar) stroke [20].
For monogenic SVDs, there are also more reliable models of CADASIL [21], COL4A1/COL4A2 [22,23], CARASIL [24], TREX [25]; and while each might start with a different gene-protein abnormality, the consequences at the glio-vascular unit and for the neuron, are similar – altered basement membranes, inflammation, impaired vascular function and secondary tissue damage. Additional models that explore effects of hypertension and diet in sporadic SVD have been developed [26].
As a further example, the carotid coil model, which is thought to mimic some brain microvascular and tissue changes of sporadic SVD via hypoperfusion [27], may instead be acting mainly through increased carotid (and intracranial) vascular stiffness, as shown by increasing data from human epidemiology studies [28]. Furthermore, an early event after coil application is short term blood brain barrier (BBB) leak [29], suggesting that ‘generic’ pathophysiological processes that damage vessels and tissue can arise from a range of triggers. However, many gaps remain (Table 1). The following sections describe the present Workshop participants’ proposals for translational approaches to accelerate from understanding to effective prevention and treatment of VCI.
Table 1.
Gaps in knowledge and requirements to advance knowledge of human cerebral small vessel disease.
| Feature | Requirements for early advances in knowledge | Gap in knowledge or resource |
|---|---|---|
| General |
|
|
| Vessels |
|
|
| Vascular function |
|
|
| Glia–oligodendrocyte, astrocyte, microglia |
|
|
| Inflammation |
|
|
| Fluids, waste clearance |
|
|
| Cognitive-behavioural relevance |
|
|
| Interventions |
|
|
3. Co-morbidities, inflammation and cerebrovascular disease
3.1. Co-morbidities
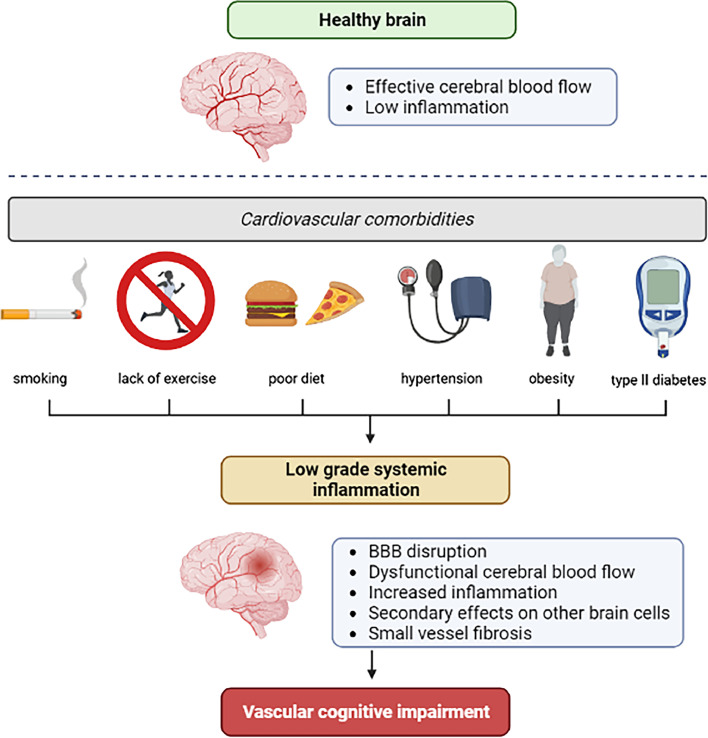
Most SVD is sporadic and commonly associated with comorbidities that are also vascular risk factors (e.g. hypertension, diabetes, stroke, AD; Fig. 1). This heterogeneity is not replicated in preclinical studies, and experimental models seldom include common risk factors for cerebrovascular disease and dementia [30], despite the fact that they clearly influence disease pathology [31]. Studies typically concentrate on modelling the genetic form of SVD such as CADASIL [21] or one potential aspect of sporadic SVD, such as hypertension [15], to enhance reproducibility in a controlled setting, rather than modelling diversity to improve translation. It is possible that sporadic SVD is a combination of comorbidities or environmental factors in addition to SVD-associated pathways or genes, which are below the threshold to develop SVD in isolation and therefore overlooked. Several rodent models are currently used in comorbidity research fields (see Table 2) [32,33] and there is the possibility of combining these in future research, although a consensus of which models are relevant for SVD and VCI will need to be reached.
Fig. 1.
Hypothesised links between co-morbidities and vascular cognitive impairment, potentially mediated by low grade inflammation. Figure kindly provided by Josephine Thomas.
Table 2.
Rodent models used within the comorbidity research field.
| co-morbidity | model | Refs. |
|---|---|---|
| Ageing | samp8 (senescence-accelerated mouse) | [34] |
| Environmental stress models | [35] | |
| Hypertension | SHRSP (spontaneously hypertensive stroke-prone rats) | [36] |
| SHR (spontaneously hypertensive rats) | [37] | |
| Dahl salt-sensitive rats | [38] | |
| Angiotensin II-induced hypertension | [39] | |
| Salt diet-induced hypertension | [40] | |
| Diabetes Mellitus/ Hyperglycaemia | db/db mice (obese type 2 diabetes mellitus) | [41] |
| ob/ob mice (obese type 2 diabetes mellitus) | [41] | |
| Zucker rats (obese type 2 diabetes mellitus) | [42] | |
| Goto-Kakizaki Rat (non-obese type 2 diabetes mellitus) | [43] | |
| Streptozotocin- induced type 1 diabetes mellitus | [44] | |
| High-fat diet induced obesity | [45] | |
| Hyperlipidaemia | ApoE KO (apolipoprotein E knock-out) mice | [46] |
| Low-density lipoprotein receptor (Ldlr−/−) knockout mice | [47] | |
The importance of multimorbidities typical of ageing or lifestyle factors is illustrated by the common co-occurrence of multimorbidities and cognitive decline and the increasing epidemiological evidence suggesting that older adults who maintain an active lifestyle involving a healthy diet, mental, social and physical activities are protected, to a certain degree, against cognitive decline or dementia [48]. The European Stroke Organisation (ESO) Guideline Working Group on covert SVD found few randomised trials but strong observational evidence to support adoption of a healthy lifestyle including diet, exercise, avoidance of smoking, and control of hypertension to prevent progression of covert SVD into clinical outcomes of stroke and dementia [19]. Although these largely observational findings have yet to be translated into strong evidence, nonetheless they are sensible public health measures and support the inclusion of co-morbidities in the design of rodent models of SVD and VCI.
3.2. Inflammation
Both systemic and peripheral inflammation are recognised as an important contributors to the pathophysiology and outcome of stroke and SVD [[49], [50], [51]], although whether they are causal or secondary to the disease process still remains to be determined. Sources of inflammation that affect the brain are not restricted to hallmark neuroinflammatory changes in the brain, such as certain forms of microglial and astrocyte reactivity, BBB breakdown and leucocyte recruitment, but also include systemic inflammatory disorders [52]. Stroke is well known to provoke systemic inflammatory responses which correlate with stroke severity [53] and in turn the risk of post stroke cognitive impairment, and SVD has been associated with reprogramming of the peripheral immune system into a proinflammatory state [54,55]. Further, in both animal models and humans, common vascular risk factors can lead to vascular neuroinflammation, and eventually neuroinflammation (Fig. 1) [56].
Thrombo-inflammation refers to the contribution of platelets and coagulation pathways to disease, and is important in stroke [57]. It is much less studied in dementia, though recent studies suggest a role for activation of the VWF/ADAMTS13 (von Willebrand factor/ADAM metallopeptidase with thrombospondin type 1 motif 13) axis [58,59]. A novel constitutively active variant of ADAMTS13 was recently reported in acute stroke [60], and can be used as a tool model, alongside other sophisticated tools, such as biodegradable and ultrasensitive microprobes to track immune response [61] and investigate the contribution of inflammation in models of VCI.
4. Translating model data to human tissues - The Neuropathological viewpoint
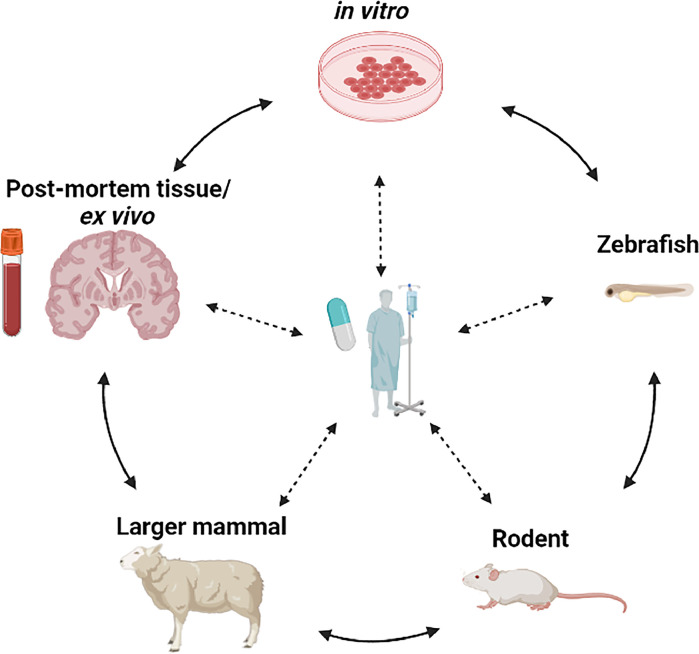
There are fundamental differences between rodents and humans that limit the formers’ relevance to understanding of human disease pathophysiology. If animal models are to play an important role in pathophysiological discovery, then they need to replicate features and underlying causal mechanisms seen in the human brain, from the primary vascular changes through to the secondary parenchymal changes, highlighting the exceptional importance of close-working between preclinical and clinical researchers in SVD, to drive relevant bi-directional translation (Fig. 2).
Fig. 2.
Bi-directional translation within the clinical and preclinical fields are essential in furthering our understanding of VCI. Figure adapted from [62], and kindly provided by Josephine Thomas. [62].
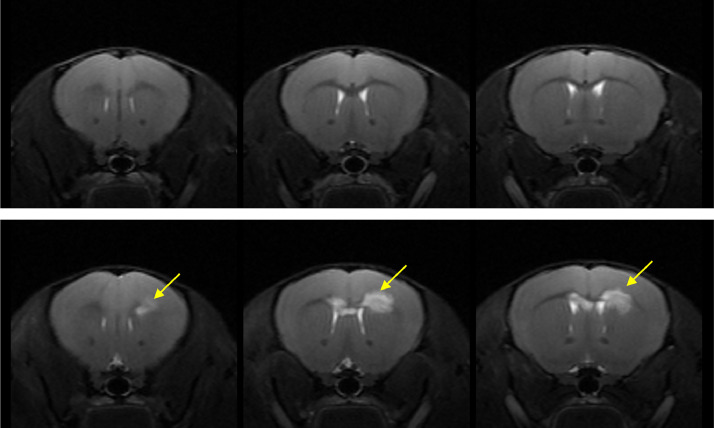
One of the hallmarks of SVD is the presence of diffuse white matter lesions, seen as white matter hyperintensities (WMH). There are distinct differences between the structures of a rodent and a human brain, including gyrencephalic versus lissencephalic cortical structure and differences in the organisation of subcortical regions [63,64]. Further, while it is possible to observe acute ischaemic lesions in the white matter of rodents (Fig. 3), diffuse white matter changes resembling clinical WMH are not commonly observed in rodents [65]. Recent advances in the regional mapping of rodent and human brains, by comparative transcriptomic [66] and functional methods [67], can be used to systematically understand the limitations of the rodent brain to avoid mis-interpretation of preclinical research.
Fig. 3.
T2-weighted images from mice undergoing bilateral carotid artery stenosis (BCAS) induced hypoperfusion, the bottom panel highlights white matter lesions (yellow arrows). Figure kindly provided by Dr Tracy D Farr.
Though mice are more generally used for genetic manipulation studies, there are recent transgenic rat models [[18], [68]]. Rats offer some advantages over mice for behavioural testing and for white matter MRI [[10], [69]]. Large experimental species (primates, canines, sheep etc.) offer more human-like brain structure, with extensive white matter. These are not amenable to high volume drug screening. Rather they are likely to be of value in mechanistic studies and focused dose finding studies prior to human use [70].
Panel 1 - Neuropathologist's viewpoint of in vivo models
| Be clear about what the model is actually modelling. What aspect of the human spectrum of SVD is being assessed? |
| Where possible, compare or relate animal model tissue-level observations to human tissues. Is the animal model observation relevant to the human disease? |
| SVD – even quite severe SVD – can be clinically silent |
| A “good” model need not have cognitive phenotypes |
| A SVD model needs some vascular pathology |
5. Translating animal model data to clinical trials - The clinical viewpoint
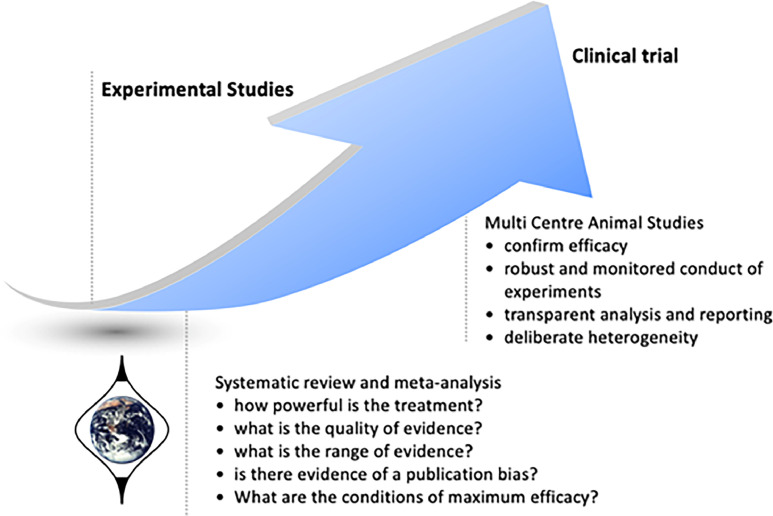
Multi-centre randomized controlled trials (RCTs) are the standard for clinical evidence on therapies, often involving large sample sizes at phase 3 to increase generalisability. In the case of preclinical studies, multicentre approaches using methods adapted from clinical trials (Fig. 4), such as Multi-PART (Multicentre Preclinical Animal Research Team; https://cordis.europa.eu/project/id/603043/reporting), could help overcome poor inter-laboratory replication.
Fig. 4.
Evidence based translational medicine using results of systematic reviews and meta-analysis to drive multicentre animal studies. Figure kindly provided by Prof Malcolm Macleod.
Another methodological advance that has not yet been fully implemented in the clinical dementia field is the multi-arm, multi-stage (MAMS) trials platform [71] widely used in cancer, and recently in COVID-19 [72]. This type of ‘rolling’ trial avoids a number of pitfalls associated with ‘single-use’ protocols, enabling faster testing, and where appropriate, rejection of interventions. A number of trial platforms have been developed for testing interventions in dementia [73], although to-date these have been limited to pre-symptomatic prevention trials, or rarer genetic forms of dementia [74,75].
Over a decade ago a group of stroke researchers proposed a multicentre, randomised and blinded preclinical trial (pRCT) to improve translation for novel therapeutics [76]. Such a trial would not replace the curiosity-driven preclinical research which identifies and validates a therapeutic target. Rather, it would be an additional step prior to clinical testing [77]. Such trials are logistically complex, requiring large-scale funding, intense oversight by a steering group and multiple ethical approvals beyond those required for individual research studies. Nevertheless, some have been successfully completed and reported [78,79], most recently, the NIH funded the Stroke Preclinical Assessment Network (SPAN). The aim of SPAN is to conduct a randomized, placebo-controlled, blinded, multi-laboratory trial using a MAMS protocol to identify one or more potential stroke treatments with a high chance of success in human clinical stroke trials [80], recently completing a proof of concept trial that assessed several acute stroke treatments [81]. We could learn from the success of the stroke field by co-ordinating more rigorous, robust, and detailed preclinical evaluation within the VCI field through the concept of pRCTs using MAMS protocols.
6. Current models and how to improve them: consensus from group discussion
A driving factor for the shortfall of translatable interventions in dementia research is uncertainty over disease models that can achieve this, driven by our limited understanding of the causes and progression of VCI. Instead, we could aim to capture key features that accurately reflect clinical SVD. Although the models might potentially only capture one relevant feature or process, a focus on replicating the process as accurately as possible could increase the relevance of the model. To achieve this, clear communication between preclinical and clinical fields is required to identify which features should be modelled and how best to measure them. Table 1 summarises Gaps in Knowledge and requirements to advance knowledge in human VCI and SVD, updated from the first workshop. The following headings were discussed by a multi-disciplinary roundtable, with a range of clinical and non-clinical expertise at all levels of seniority from graduate students through to senior Investigators.
6.1. Limitations of rodent models
Rodent research has its disadvantages (Table 3). There are substantial costs for generating a surgical model or transgenic strain. Furthermore, longitudinal studies often lead to survivor bias or a lack of sufficient power as rodents that display stronger phenotypes may not achieve the most chronic endpoint. Rodent development and ageing follows a different time-course than humans, and this must be considered during experimental design. In most experimental settings, animals have a sedentary life, unlimited access to food, are protected from pathogens and other environmental stresses, and this may also influence ageing. How the processes that underlie human ageing can be better modelled in rodents is highly debated. For example, genetically altered (progeroid) mouse models display premature ageing due to mutations in ageing-related genes, though their relation to typical ageing in humans is unclear [82]. Models of accelerated senescence have also been developed [83,84] as well as environmental stress models (ozone and radiation exposure) that also display features of accelerated ageing and frailty [35].
Table 3.
Strengths and limitations of preclinical models used within the VCI field.
| Model | Strengths | Limitations |
|---|---|---|
| iPSC |
|
|
| Organoid |
|
|
| Zebrafish model |
|
|
| Rodent models |
|
|
| Large mammalian model |
|
|
The value and interpretation of behavioural testing in rodents needs to be considered. While a composite of tests is often used in humans, equivalent tests for rodents should be appropriate to rodent behaviour, function and cognition and not require months of training or food restriction which may confound the mechanism of interest. Moreover, task-relevant sensory, motor and anxiety confounds of behavioural performance should be analysed whenever possible to ensure that poor task performance is not misinterpreted.
6.2. Alternatives to rodent models
There are a variety of in vitro platforms that complement in vivo research (Table 3) and hold promise to replace animals in the future, such as cell cultures derived from induced pluripotent stem cells (iPSCs). Somatic cells can be derived from patients with a genetic predisposition and dedifferentiated to form iPSCs, which can be further differentiated into multiple cell types, for instance into the different cell types in the neurovascular unit, or into organoids or agglomerates [85,86]. This facilitates study of biological processes, such as maintenance of BBB, extracellular matrix maintenance and immune cell signalling within the context of the genetic predisposition or risk factor but within a highly constrained environment. These cell culture platforms can also be used to screen large numbers of drugs, prior to in vivo testing and recent developments such as CRISPR (Clustered Regulatory Interspaced Short Palindromic Repeats) editing can facilitate additional manipulations. However, their limitations are important: the constrained environment within the cell preparation that may lack diversity in the native cell population, the artificial environment in relation to the integrated physiology of the whole organ or animal, the lack of vasculature, and these developmental cells could have limitations when modelling age associated diseases, or anything resembling cognitive outcome measures.
Other animal models are also currently being explored (Table 3). Zebrafish have the advantages of prolific reproduction rates and larval transparency allowing for live imaging, coupled with numerous genetic reporter lines [87]. Higher in the evolutionary tree, larger mammals (e.g. sheep, dogs, pigs or primates) have more white matter, closer in proportion and structure to that seen in humans, and more human-like vasculature [[88], [89], [90], [91]]. An interesting alternative approach to laboratory studies is the use of companion animals (dogs, felines) for studies of common disorders like VCI or SVD, including relevant lifestyles, and assessment of animal behaviour, cognition and brain pathology.
6.3. Bridging points between preclinical and clinical studies
Successful translation of preclinical studies requires bridging points linking the basic science to the clinics (Fig. 2). For example, Magnetic Resonance Imaging (MRI) can be performed in animals, using equivalent sequences as in clinical scans, and thus provide translational information on structural changes and vascular function [14]. Therefore findings in both species, such as enlarged perivascular spaces [92], dysfunctional BBB or cerebrovascular reactivity can be compared and provide reassurance that the model or intervention is relevant to human disease [14]. Similarly, molecular and cellular level association between the disease model and human disease through, for example, -omics-based cell profiling and fluid biomarker measures would enable fairly objective “species-bridging” measures.
Cognitive function in patients can be assessed with multiple tasks covering a large range of cognitive domains. Rodent behaviour is well understood but requires more research to develop tests of cognition that are relevant to VCI by mapping onto human cognitive domains affected in VCI [[10], [93]]. A UK consensus on assessment in preclinical studies of VCI has already been published and should be more widely followed [10].
There are a number of innovations from research in other conditions that may potentially transform how we design future trials in the field of VCI. Current dementia trials still rely on relatively dated outcome measures, such as ADAS-Cog (Alzheimer's Disease Assessment Scale, cognitive subscale) and CDR (Clinical Dementia Rating) [94], which are often performed at infrequent intervals. Wearable technologies and other technical devices make it increasingly possible for researchers to access granular information about daily activities, from walking and sleeping to device interaction (e.g. sleep mat to monitor sleep patterns, gait speed and laterality monitoring devices). These can potentially provide a far more thorough understanding of treatment effect, as well as allowing for better detection of adverse events and side effects [95]. Such detailed datasets can also potentially be combined with a ‘n of 1′ approach, allowing researchers to evaluate the effects of interventions on an individual basis [96]. By analogy, outcomes in preclinical studies should aim to capture cognition, function, mobility and activities, for example via 24/7 cage-monitoring technology [97], to provide a more comprehensive profile of the animal's status.
6.4. Bedside-to-bench approach
In stroke, most current treatments were developed through clinical research testing drugs repurposed from other vascular disease – e.g. aspirin for secondary prevention, thrombolytic agents to remove occlusive thrombus –not from drugs or mechanisms identified in preclinical models. The pharmaceutical industry was subsequently able to develop more effective antiplatelet (e.g. Clopidogrel) and thrombolytic agents (e.g. Alteplase, Tenecteplase) following testing in preclinical models. This contrasts with the perceived ‘conventional’ route by which drugs are developed and tested from research at the ‘bench’ and translated to the ‘bedside’.
This ‘bedside-to-bench’ approach could work well in VCI by testing repurposed drugs from other diseases that have potentially relevant modes of action on the proposed mechanisms in SVD. This repurposing approach is not commonly followed, especially by the pharmaceutical industry, and existing viable drugs might be dismissed [98]. Preclinical ‘platform’ trials, including MAMS trials described earlier, would offer a valuable complementary approach to drug testing to help determine potential modes of action of repurposed drugs that showed promise in clinical trials, and could help design better compounds.
The process of bedside-to-bench can also be informed by analyses of electronic health records. This approach was used for COVID-19 by the UK Longitudinal Linkage Collaboration (https://ukllc.ac.uk/) and in AD [99]. We can also use large longitudinal research registry datasets for VCI research, associating the outcomes of the diseases for at-risk individuals with potential factors such as lifestyle, risk factors or medication (see Panel 2 for useful resources). However, caution is required when interpreting effects of medication in electronic health records or research registry data since the allocation of medication is not randomised and many sources of bias are likely to exist in the data. However, the findings might broaden the understanding of the disease and reveal potential (alternative) therapeutic targets that have been overlooked by the conventional view.
Moving forward, better links between academia and industry, including large pharmaceutical companies, small and medium enterprises, contract research organisations, and start-ups, will facilitate multicentre collaborations and more rapid progression in finding new treatments for VCI. Finally, ‘industry bootcamps’ would educate academics on how to approach industry with an idea, how to put together a research package to present to industry, and how to start and maintain a mutually beneficially relationship with industry stakeholders.
6.5. Improving standardisation and reporting of data
There is a growing requirement to standardise research through reproducible protocols and standardisation between models. Lessons can be learnt from the success of the preclinical stroke field, that has come a long way in the pathophysiological understanding of stroke. Over the last twenty years, attempts have been made to refine experimental methods used in preclinical stroke research, improve reproducibility and reduce the number of animals used largely via the publication of guidelines. The best-known guidelines in preclinical stroke research are the Stroke Therapy Academic Industry Roundtable (STAIR) guidelines [100,101]. Further guidelines are aimed at the use of stem cells in preclinical stroke models [102], methodological approaches to improve animal welfare and scientific outcomes via the IMPROVE (Ischaemia Models: Procedural Refinements Of in vivo Experiments) guidelines [103] and merging of previously published guidelines into a more rigorous approach via the RIGOUR guidelines [104]. The same principles can apply to the VCI field, including having a central database of standardised protocols for behavioural testing, surgical procedure, and ex vivo experiments which would permit consistency of protocols across institutes, and facilitate meta-analyses.
To address the issue of transparent reporting, and facilitate reproducibility, the ARRIVE criteria (Animal Research: Reporting of in vivo Experiments) were published in 2010 [105] and updated in 2020 [106]. This includes careful definition of the independent experimental unit in the study (e.g. the animal/cage) and the study design including the control groups included. Defining the sample size required for the principal outcome measures prior to the experiment, using experimentally determined standard deviations and effect sizes to ensure sufficient experimental power whilst minimising the number of animals in the study. Ensuring that randomisation and blinding are used during both data acquisition and analysis avoids bias. Full reporting of the model used to include recognised nomenclatures and reference numbers, age, sex, experimental procedures, husbandry conditions and all other associated-meta- data is also critical. As well as the reporting of drop-out and any exclusion criteria (e.g. because of technical failure or welfare issue) and full reporting and justification of statistical analysis methods. Pre-registration of the study protocol including the above points improves research reliability. Ensuring complete adherence to the ARRIVE 2.0 essential 10 checklist will significantly enhance the translational value of preclinical research and researchers are encouraged to use them to increase the benefit of their research output and its long-time impact on patients.
Alongside guidelines for conducting and reporting preclinical research, a number of initiatives from the wider biosciences community including clinical research may serve to promote reproducibility, including open access practices [107], study preregistration [108] and resources to improve experimental design and analysis [109]. Within clinical research it is routine to conduct a systematic review to assess treatment effectiveness, and to routinely publish negative or neutral studies, however both are less common practices in preclinical research. Systematic reviews are an essential tool for obtaining an objective view of all the available evidence on a topic (thus helping to avoid repeating research that does not need to be repeated), and identifying potential disease mechanisms, or therapeutic targets for further investigation in larger, even multicentre, in vivo studies, prior to clinical testing. This approach has been highly effective in identifying (and excluding) potential SVD models [[15], [16], [110]], SVD pathology [111,112], and potential drugs to treat SVD in preclinical studies [113] and clinical trials [[20], [98], [114]], leading to promising results improving outcomes in SVD [20]. The extent to which the results of systematic reviews might be biased due to missing unpublished negative or neutral studies can be assessed through techniques such as funnel plots and by approaching authors for unpublished data, and should not preclude the use of systematic reviews as a highly valuable research tool when conducted properly.
Whilst academia benefits from an environment that allows freedom of thought, a lot can be learnt from the ‘fail-fast’ industry approach. The industry model is designed to rapidly test reproducibility and validity, with no negative implications for failed compounds or targets. A shift in culture is needed toward reporting on approaches that lack efficacy, and to know when to abandon them rather than continuing a flawed premise or pathway.
6.6. Need for wider multidisciplinary approaches
A key strategy to accelerate the field could be to diversify interdisciplinary collaboration to areas not typically involved in vascular or neurodegenerative brain research. For example, mathematicians and informaticians can model animal and human neurovasculature and blood flow, which may provide insights into disease mechanisms. Furthermore, engineers and physicists are essential to develop novel MRI and microscopic imaging techniques, alongside computational neuroimaging [115]. An additional benefit of utilising expertise from non-traditional biological backgrounds, is that they typically do not require animal models and therefore support the 3Rs mission of replacement, reduction and refinement of animals used in research [116].
6.7. Building a SVD community forum
Perhaps a disconnect between clinical and preclinical research in SVD and VCI is contributing to the failure to translate between ‘bench and bedside’. It would help to share practical expertise (Standard Operating Protocols and experiences) as well as fundamental knowledge and standardised definitions of preclinical and clinical terminology. Efforts in this direction are now being implemented in the UK through the UK DRI Vascular Theme and DPUK Experimental Medicine Incubator, plus BHF research initiatives and regional clinically-orientated brain health initiatives. Furthermore, local research-to-clinic initiatives such as the Geoffrey Jefferson Brain Research Centre in Manchester (https://www.gjbrc.org) and the Row Fogo Centre for Research into Ageing and the Brain in Edinburgh (https://www.ed.ac.uk/clinical-brain-sciences/research/row-fogo-centre/about) are providing hubs of researchers to boost activity and awareness in the UK. The ESO Guidelines on SVD, part 1 Covert SVD [19] and part 2 Lacunar Ischaemic Stroke (in prep, publication expected autumn 2023), are providing a much needed benchmark to guide current best clinical practice. The nascent SVDs Clinical Services Collaboration will improve clinical services for patients with SVD as well as research infrastructure. The NIH-funded MarkVCID (Biomarkers for Vascular Contributions to Cognitive Impairment and Dementia) initiative in the USA has given a major boost to VCI and SVDs preclinical and clinical research and awareness of the condition in the USA – a similar national initiative would greatly accelerate research and improved clinical services in the UK.
There is a growing need for a centralised database of information on SVD models. For example, one such database is Alzheimer Research Forum (https://www.alzforum.org/), an online community resource of specific knowledge to promote communication, research, collaborative and multidisciplinary interactions [117]. No such database existed for SVD/VCI at the time of the workshop, but has now been started by the UK DRI (see Panel 2). It so far includes 14 models, and will become a very valuable resource for research into vascular contributions to neurodegeneration. Interested researchers are invited to submit data on animals not yet represented in the database (contact Sarmi Sri, s.sri@ukdri.ucl.ac.uk).
7. Summary
The UK DRI-DPUK-BHF workshop provided an opportunity to share knowledge, technical skills, facility access, funding opportunities and create collaborations. The establishment of vascular disease and dementia consortia, both nationally and internationally, needs to be community-driven and include researchers from different centres, disciplines, and backgrounds. Inclusion of ECR days to consortium meetings cultivates the next generation of VCI researchers, and has been promoted in the UK by the UK DRI Vascular Theme for all interested ECRs. Panel 2 highlights some important resources for researchers within the UK vascular community.
Panel 2 – Useful resources to highlight to the VCI community
| UK DRI Vascular theme (https://ukdri.ac.uk/research-themes) |
| DPUK portal (https://portal.dementiasplatform.uk/) |
| Vascular ECR community (https://ukdri.ac.uk/news-and-events/from-bench-to-bedside-bridging-the-gap-between-discovery-research-and-the-clinic-in-vascular-research) |
| Vascular models database (to be launched early 2024) |
| VISTA Cognition (https://www.virtualtrialsarchives.org/vista-cognition/) |
| StrokeCOG consortium (http://www.strokecog.ie/) |
| MultiPART (Multicentre Preclinical Animal Research Team) (https://cordis.europa.eu/project/id/603043/reporting) |
Declaration of Competing Interest
No conflict of Interest.
Acknowledgments
Funding
The workshop upon which this paper was based was supported by the UK Dementia Research Institute, Dementia Platforms UK (DPUK1: MRC grant MR/L023784/2: UK Dementias Research Platform; DPUK2: MRC grant MR/T033371/1: MICA: Dementias Platform UK 2 – Integrated Dementia Experimental Medicine), British Heart Foundation and Guarantors of Brain. The UK Dementia Research Institute receives its funding from UK DRI Ltd, funded by the UK Medical Research Council, Alzheimer’s Society and Alzheimer’s Research UK.
References
- 1.WHO 2023. World health organisation dementia fact sheet. 2023 published online 15 March https://www.who.int/news-room/fact-sheets/detail/dementia#:~:text=Key%20facts,injuries%20that%20affect%20the%20brain (accessed 22 May 2023).
- 2.Hachinski V., Einhäupl K., Ganten D., Alladi S., Brayne C., Stephan B.C.M., Sweeney M.D., Zlokovic B., Iturria-Medina Y., Iadecola C., Nishimura N., Schaffer C.B., Whitehead S.N., Black S.E., Østergaard L., Wardlaw J., Greenberg S., Friberg L., Norrving B., Rowe B., Joanette Y., Hacke W., Kuller L., Dichgans M., Endres M., Khachaturian Z.S. Preventing dementia by preventing stroke: the Berlin Manifesto. Alzheimers Dement. 2019;15:961–984. doi: 10.1016/j.jalz.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bir S.C., Khan M.W., Javalkar V., Toledo E.G., Kelley R.E. Emerging concepts in vascular dementia: a review. J. Stroke Cerebrovasc. Dis. 2021:30. doi: 10.1016/j.jstrokecerebrovasdis.2021.105864. [DOI] [PubMed] [Google Scholar]
- 4.Mehta R.I., Schneider J.A. What is 'Alzheimer's disease'? The neuropathological heterogeneity of clinically defined Alzheimer's dementia. Curr. Opin. Neurol. 2021;34:237–245. doi: 10.1097/WCO.0000000000000912. [DOI] [PubMed] [Google Scholar]
- 5.O'brien J.T., Thomas A. Vascular dementia. Lancet. 2015;386:1698–1706. doi: 10.1016/S0140-6736(15)00463-8. [DOI] [PubMed] [Google Scholar]
- 6.Kuźma E., Lourida I., Moore S.F., Levine D.A., Ukoumunne O.C., Llewellyn D.J. Stroke and dementia risk: a systematic review and meta-analysis. Alzheimers Dement. 2018;14:1416–1426. doi: 10.1016/j.jalz.2018.06.3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verdelho A., Biessels G.J., Chabriat H., Charidimou A., Duering M., Godefroy O., Pantoni L., Pavlovic A., Wardlaw J. Cerebrovascular disease in patients with cognitive impairment: a white paper from the ESO dementia committee – A practical point of view with suggestions for the management of cerebrovascular diseases in memory clinics. Eur. Stroke J. 2021;6:111–119. doi: 10.1177/2396987321994294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wardlaw J.M., Horsburgh K. Small vessels, dementia and chronic diseases-molecular mechanisms and pathophysiology. Clin. Sci. 2016;130:1875–1879. doi: 10.1042/CS20160376. (Lond.) [DOI] [PubMed] [Google Scholar]
- 9.Horsburgh K., Wardlaw J.M., Van Agtmael T., Allan S.M., Ashford M.L.J., Bath PM., Brown R., Berwick J., Cader M.Z., Carare RO., Davis JB., Duncombe J., Farr TD., Fowler J.H., Goense J., Granata A., Hall C.N., Hainsworth A.H., Harvey A., Hawkes C.A., Joutel A., Kalaria R.N., Kehoe PG., Lawrence C.B., Lockhart A., Love S., Macleod M.R., Macrae I.M., Markus HS., Mccabe C., Mccoll BW., Meakin PJ., Miller A., Nedergaard M., O'sullivan M., Quinn T.J., Rajani R., Saksida L.M., Smith C., Smith KJ., Touyz R.M., Trueman RC., Wang T., Williams A., Williams S.C.R., Work L.M. Small vessels, dementia and chronic diseases – molecular mechanisms and pathophysiology. Clin. Sci. 2018;132:851–868. doi: 10.1042/CS20171620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mcfall A., Hietamies T.M., Bernard A., Aimable M., Allan S.M., Bath P.M., Brezzo G., Carare R.O., Carswell H.V., Clarkson A.N., Currie G., Farr T.D., Fowler J.H., Good M., Hainsworth A.H., Hall C., Horsburgh K., Kalaria R., Kehoe P., Lawrence C., Macleod M., Mccoll B.W., Mcneilly A., Miller A.A., Miners S., Mok V., O'sullivan M., Platt B., Sena E.S., Sharp M., Strangward P., Szymkowiak S., Touyz R.M., Trueman R.C., White C., Mccabe C., Work L.M., Quinn T.J. UK consensus on pre-clinical vascular cognitive impairment functional outcomes assessment: questionnaire and workshop proceedings. J. Cereb. Blood Flow Metab. 2020;40:1402–1414. doi: 10.1177/0271678X20910552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wardlaw J.M., Benveniste H., Williams A. Cerebral vascular dysfunctions detected in human small vessel disease and implications for preclinical studies. Annu. Rev. Physiol. 2022;84:409–434. doi: 10.1146/annurev-physiol-060821-014521. [DOI] [PubMed] [Google Scholar]
- 12.Wardlaw J.M., Smith C., Dichgans M. Small vessel disease: mechanisms and clinical implications. Lancet Neurol. 2019;18:684–696. doi: 10.1016/S1474-4422(19)30079-1. [DOI] [PubMed] [Google Scholar]
- 13.Clancy U., Gilmartin D., Jochems A.C.C., Knox L., Doubal F.N., Wardlaw J.M. Neuropsychiatric symptoms associated with cerebral small vessel disease: a systematic review and meta-analysis. Lancet Psychiatry. 2021;8:225–236. doi: 10.1016/S2215-0366(20)30431-4. [DOI] [PubMed] [Google Scholar]
- 14.Stringer M.S., Lee H., Huuskonen M.T., Macintosh B.J., Brown R., Montagne A., Atwi S., Ramirez J., Jansen M.A., Marshall I., Black S.E., Zlokovic B.V., Benveniste H., Wardlaw J.M. A review of translational magnetic resonance imaging in human and rodent experimental models of small vessel disease. Transl. Stroke Res. 2021;12:15–30. doi: 10.1007/s12975-020-00843-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bailey E.L., Smith C., Sudlow C.L.M., Wardlaw J.M. Is the spontaneously hypertensive stroke prone rat a pertinent model of sub cortical ischemic Stroke? A systematic review. Int. J. Stroke. 2011;6:434–444. doi: 10.1111/j.1747-4949.2011.00659.x. [DOI] [PubMed] [Google Scholar]
- 16.Hainsworth A.H., Markus H.S. Do in vivo experimental models reflect human cerebral small vessel disease? A systematic review. J. Cereb. Blood Flow Metab. 2008;28:1877–1891. doi: 10.1038/jcbfm.2008.91. [DOI] [PubMed] [Google Scholar]
- 17.Rajani R.M., Quick S., Ruigrok S.R., Graham D., Harris S.E., Verhaaren B.F.J., Fornage M., Seshadri S., Atanur S.S., Dominiczak A.F., Smith C., Wardlaw J.M., Williams A. Reversal of endothelial dysfunction reduces white matter vulnerability in cerebral small vessel disease in rats. Sci. Transl. Med. 2018;10:eaam9507. doi: 10.1126/scitranslmed.aam9507. [DOI] [PubMed] [Google Scholar]
- 18.Quick S., Procter T.V., Moss J., Seeker L., Walton M., Lawson A., Baker S., Beletski A., Garcia D.J., Mohammad M., Mungall W., Onishi A., Tobola Z., Stringer M., Jansen M.A., Vallatos A., Giarratano Y., Bernabeu M.O., Wardlaw J.M., Williams A. Loss of the heterogeneous expression of flippase ATP11B leads to cerebral small vessel disease in a normotensive rat model. Acta Neuropathol. 2022;144:283–303. doi: 10.1007/s00401-022-02441-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wardlaw J.M., Debette S., Jokinen H., De Leeuw F.E., Pantoni L., Chabriat H., Staals J., Doubal F., Rudilosso S., Eppinger S., Schilling S., Ornello R., Enzinger C., Cordonnier C., Taylor-Rowan M., Lindgren A.G. ESO Guideline on covert cerebral small vessel disease. European Stroke Journal. 2021;6:CXI–CLXII. doi: 10.1177/23969873211012132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wardlaw J.M., Woodhouse L.J., Mhlanga, Oatey K., Heye A.K., Bamford J., Cvoro V., Doubal F.N., England T., Hassan A., Montgomery A., O’Brien J.T., Roffe C., Sprigg N., Werring D.J, Bath P.M. Lacunar Intervention Trial-2 (LACI-2) Investigator Group. Isosorbide Mononitrate and Cilostazol Treatment in Patients With Symptomatic Cerebral Small Vessel Disease: The Lacunar Intervention Trial-2 (LACI-2) Randomized Clinical Trial. JAMA Neurol. 2023;80:682–692. doi: 10.1001/jamaneurol.2023.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joutel A., Monet-Leprêtre M., Gosele C., Baron-Menguy C., Hammes A., Schmidt S., Lemaire-Carrette B., Domenga V., Schedl A., Lacombe P., Hubner N. Cerebrovascular dysfunction and microcirculation rarefaction precede white matter lesions in a mouse genetic model of cerebral ischemic small vessel disease. J. Clin. Invest. 2010;120:433–445. doi: 10.1172/JCI39733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gould D.B., Phalan F.C., Breedveld G.J., Van Mil S.E., Smith R.S., Schimenti J.C., Aguglia U., Van Der Knaap M.S., Heutink P., John S.W.M. Mutations in Col4a1cause perinatal cerebral hemorrhage and porencephaly. Science. 2005;308:1167–1171. doi: 10.1126/science.1109418. [DOI] [PubMed] [Google Scholar]
- 23.Kuo D.S., Labelle-Dumais C., Gould D.B. COL4A1 and COL4A2 mutations and disease: insights into pathogenic mechanisms and potential therapeutic targets. Hum. Mol. Genet. 2012;21:R97–R110. doi: 10.1093/hmg/dds346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chuanfen L., Xiaoling W., Wen J., Bingzhen C., Min W. HtrA1L364P leads to cognitive dysfunction and vascular destruction through TGF-β/Smad signaling pathway in CARASIL model mice. Brain Behav. 2022;12:e2691. doi: 10.1002/brb3.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mulder I.A., Rubio-Beltran E., Ibrahimi K., Dzyubachyk O., Khmelinskii A., Hoehn M., Terwindt G.M., Wermer M.J.H., Maassenvandenbrink A., Maagdenberg A.M.J.M.V.D. Increased mortality and vascular phenotype in a knock-in mouse model of retinal vasculopathy with cerebral leukoencephalopathy and systemic manifestations. Stroke. 2020;51:300–307. doi: 10.1161/STROKEAHA.119.025176. [DOI] [PubMed] [Google Scholar]
- 26.Guy R., Volkman R., Wilczynski E., Yagil C., Yagil Y., Findler M., Auriel E., Nevo U., Offen D. A novel rodent model of hypertensive cerebral small vessel disease with white matter hyperintensities and peripheral oxidative stress. Int. J. Mol. Sci. 2022;23:5915. doi: 10.3390/ijms23115915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duncombe J., Kitamura A., Hase Y., Ihara M., Kalaria R.N., Horsburgh K. Chronic cerebral hypoperfusion: a key mechanism leading to vascular cognitive impairment and dementia. Closing the translational gap between rodent models and human vascular cognitive impairment and dementia. Clin. Sci. 2017;131:2451–2468. doi: 10.1042/CS20160727. (Lond.) [DOI] [PubMed] [Google Scholar]
- 28.Vikner T., Karalija N., Eklund A., Malm J., Lundquist A., Gallewicz N., Dahlin M., Lindenberger U., Riklund K., Bäckman L., Nyberg L., Wåhlin A. 5-Year associations among cerebral arterial pulsatility, perivascular space dilation, and white matter Lesions. Ann. Neurol. 2022;92:871–881. doi: 10.1002/ana.26475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Q., Radwanski R., Babadjouni R., Patel A., Hodis D.M., Baumbacher P., Zhao Z., Zlokovic B., Mack W.J. Experimental chronic cerebral hypoperfusion results in decreased pericyte coverage and increased blood-brain barrier permeability in the corpus callosum. J. Cereb. Blood Flow Metab. 2019;39:240–250. doi: 10.1177/0271678X17743670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mccann S.K., Lawrence C.B. Comorbidity and age in the modelling of stroke: are we still failing to consider the characteristics of stroke patients? BMJ Open Sci. 2020;4 doi: 10.1136/bmjos-2019-100013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mccoll B.W., Rose N., Robson F.H., Rothwell N.J., Lawrence C.B. Increased brain microvascular MMP-9 and incidence of haemorrhagic transformation in obese mice after experimental stroke. J. Cereb. Blood Flow Metab. 2010;30:267–272. doi: 10.1038/jcbfm.2009.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Candelario-Jalil E., Paul S. Impact of aging and comorbidities on ischemic stroke outcomes in preclinical animal models: a translational perspective. Exp. Neurol. 2021;335 doi: 10.1016/j.expneurol.2020.113494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cho S., Yang J. What do experimental models teach us about comorbidities in stroke? Stroke. 2018;49:501–507. doi: 10.1161/STROKEAHA.117.017793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yagi H., Irino M., Matsushita T., Katoh S., Umezawa M., Tsuboyama T., Hosokawa M., Akiguchi I., Tokunaga R., Takeda T. Spontaneous spongy degeneration of the brain stem in SAM-P/8 mice, a newly developed memory-deficient strain. J. Neuropathol. Exp. Neurol. 1989;48:577–590. doi: 10.1097/00005072-198909000-00008. [DOI] [PubMed] [Google Scholar]
- 35.Cai N., Wu Y., Huang Y. Induction of accelerated aging in a mouse model. Cells. 2022;11:1418. doi: 10.3390/cells11091418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacob H.J., Lindpaintner K., Lincoln S.E., Kusumi K., Bunker R.K., Mao Y.P., Ganten D., Dzau V.J., Lander E.S. Genetic mapping of a gene causing hypertension in the stroke-prone spontaneously hypertensive rat. Cell. 1991;67:213–224. doi: 10.1016/0092-8674(91)90584-l. [DOI] [PubMed] [Google Scholar]
- 37.Ely D.L., Turner M.E. Hypertension in the spontaneously hypertensive rat is linked to the Y chromosome. Hypertension. 1990;16:277–281. doi: 10.1161/01.hyp.16.3.277. [DOI] [PubMed] [Google Scholar]
- 38.Rapp J.P. Dahl salt-susceptible and salt-resistant rats. A review. Hypertension. 1982;4:753–763. doi: 10.1161/01.hyp.4.6.753. [DOI] [PubMed] [Google Scholar]
- 39.Xue B., Johnson A.K., Hay M. Sex differences in angiotensin II- induced hypertension. Braz. J. Med. Biol. Res. 2007;40:727–734. doi: 10.1590/s0100-879x2007000500018. [DOI] [PubMed] [Google Scholar]
- 40.Basting T., Lazartigues E. DOCA-salt hypertension: an update. Curr. Hypertens. Rep. 2017;19:32. doi: 10.1007/s11906-017-0731-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coleman D.L. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia. 1978;14:141–148. doi: 10.1007/BF00429772. [DOI] [PubMed] [Google Scholar]
- 42.Bray G.A. The Zucker-fatty rat: a review. Fed. Proc. 1977;36:148–153. [PubMed] [Google Scholar]
- 43.Sajid Hamid Akash M., Rehman K., Chen S. Goto-kakizaki rats: its suitability as non-obese diabetic animal model for spontaneous type 2 diabetes mellitus. Curr. Diabetes Rev. 2013;9:387–396. doi: 10.2174/15733998113099990069. [DOI] [PubMed] [Google Scholar]
- 44.Furman B.L. Streptozotocin-induced diabetic models in mice and rats. Curr. Protoc. Pharmacol. 2015;70 doi: 10.1002/0471141755.ph0547s70. 5.47.1-5.47.20. [DOI] [PubMed] [Google Scholar]
- 45.Hariri N., Thibault L. High-fat diet-induced obesity in animal models. Nutr. Res. Rev. 2010;23:270–299. doi: 10.1017/S0954422410000168. [DOI] [PubMed] [Google Scholar]
- 46.Nakashima Y., Plump A.S., Raines E.W., Breslow J.L., Ross R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler. Thromb. 1994;14:133–140. doi: 10.1161/01.atv.14.1.133. A Journal of Vascular Biology. [DOI] [PubMed] [Google Scholar]
- 47.Ishibashi S., Brown M.S., Goldstein J.L., Gerard R.D., Hammer R.E., Herz J. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J. Clin. Invest. 1993;92:883–893. doi: 10.1172/JCI116663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qiu C., Kivipelto M., Von Strauss E. Epidemiology of Alzheimer's disease: occurrence, determinants, and strategies toward intervention. Dialogues Clin Neurosci. 2009;11:111–128. doi: 10.31887/DCNS.2009.11.2/cqiu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Endres M., Moro M.A., Nolte C.H., Dames C., Buckwalter M.S., Meisel A. Immune pathways in etiology, acute phase, and chronic sequelae of ischemic stroke. Circ. Res. 2022;130:1167–1186. doi: 10.1161/CIRCRESAHA.121.319994. [DOI] [PubMed] [Google Scholar]
- 50.Mun K.T., Hinman J.D. Inflammation and the link to vascular brain health: timing is brain. Stroke. 2022;53:427–436. doi: 10.1161/STROKEAHA.121.032613. [DOI] [PubMed] [Google Scholar]
- 51.Walsh J., Tozer D.J., Sari H., Hong Y.T., Drazyk A., Williams G., Shah N.J., O'brien J.T., Aigbirhio F.I., Rosenberg G., Fryer T.D., Markus H.S. Microglial activation and blood-brain barrier permeability in cerebral small vessel disease. Brain. 2021;144:1361–1371. doi: 10.1093/brain/awab003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wiseman S.J., Ralston S.H., Wardlaw J.M. Cerebrovascular disease in rheumatic diseases. Stroke. 2016;47:943–950. doi: 10.1161/STROKEAHA.115.012052. [DOI] [PubMed] [Google Scholar]
- 53.Whiteley W., Jackson C., Lewis S., Lowe G., Rumley A., Sandercock P., Wardlaw J., Dennis M., Sudlow C. Inflammatory markers and poor outcome after stroke: a prospective cohort study and systematic review of interleukin-6. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Noz M.P., Ter Telgte A., Wiegertjes K., Joosten L.A.B., Netea M.G., De Leeuw F.E., Riksen N.P. Trained immunity characteristics are associated with progressive cerebral small vessel disease. Stroke. 2018;49:2910–2917. doi: 10.1161/STROKEAHA.118.023192. [DOI] [PubMed] [Google Scholar]
- 55.Noz M.P., Ter Telgte A., Wiegertjes K., Tuladhar A.M., Kaffa C., Kersten S., Bekkering S., Van Der Heijden C., Hoischen A., Joosten L.A.B., Netea M.G., Duering M., De Leeuw F.E., Riksen N.P. Pro-inflammatory monocyte phenotype during acute progression of cerebral small vessel disease. Front. Cardiovasc. Med. 2021;8 doi: 10.3389/fcvm.2021.639361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Drake C., Boutin H., Jones M.S., Denes A., Mccoll B.W., Selvarajah J.R., Hulme S., Georgiou R.F., Hinz R., Gerhard A., Vail A., Prenant C., Julyan P., Maroy R., Brown G., Smigova A., Herholz K., Kassiou M., Crossman D., Francis S., Proctor S.D., Russell J.C., Hopkins S.J., Tyrrell P.J., Rothwell N.J., Allan S.M. Brain inflammation is induced by co-morbidities and risk factors for stroke. Brain Behav. Immun. 2011;25:1113–1122. doi: 10.1016/j.bbi.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stoll G., Nieswandt B. Thrombo-inflammation in acute ischaemic stroke — Implications for treatment. Nat. Rev. Neurol. 2019;15:473–481. doi: 10.1038/s41582-019-0221-1. [DOI] [PubMed] [Google Scholar]
- 58.Hanas J.S., Hocker J.R.S., Vannarath C.A., Lerner M.R., Blair S.G., Lightfoot S.A., Hanas R.J., Couch J.R., Hershey L.A. Distinguishing alzheimer's disease patients and biochemical phenotype analysis using a novel serum profiling platform: potential involvement of the VWF/ADAMTS13 axis. Brain Sci. 2021;11:583. doi: 10.3390/brainsci11050583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wolters F.J., Boender J., De Vries P.S., Sonneveld M.A., Koudstaal P.J., De Maat M.P., Franco O.H., Ikram M.K., Leebeek F.W., Ikram M.A. Von Willebrand factor and ADAMTS13 activity in relation to risk of dementia: a population-based study. Sci. Rep. 2018;8:5474. doi: 10.1038/s41598-018-23865-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.South K., Saleh O., Lemarchand E., Coutts G., Smith C.J., Schiessl I., Allan S.M. Robust thrombolytic and anti-inflammatory action of a constitutively active ADAMTS13 variant in murine stroke models. Blood. 2022;139:1575–1587. doi: 10.1182/blood.2021012787. [DOI] [PubMed] [Google Scholar]
- 61.Martinez De Lizarrondo S., Jacqmarcq C., Naveau M., Navarro-Oviedo M., Pedron S., Adam A., Freis B., Allouche S., Goux D., Razafindrakoto S., Gazeau F., Mertz D., Vivien D., Bonnard T., Gauberti M. Tracking the immune response by MRI using biodegradable and ultrasensitive microprobes. Sci. Adv. 2022;8:eabm3596. doi: 10.1126/sciadv.abm3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Withers S.E., Parry-Jones A.R., Allan S.M., Kasher P.R. A multi-model pipeline for translational intracerebral haemorrhage research. Transl. Stroke Res. 2020;11:1229–1242. doi: 10.1007/s12975-020-00830-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kwiecien T.D., Sy C., Ding Y. Rodent models of ischemic stroke lack translational relevance… are baboon models the answer? Neurol. Res. 2014;36:417–422. doi: 10.1179/1743132814Y.0000000358. [DOI] [PubMed] [Google Scholar]
- 64.Preuss T.M. Do rats have prefrontal cortex? The rose-woolsey-akert program reconsidered. J. Cogn. Neurosci. 1995;7:1–24. doi: 10.1162/jocn.1995.7.1.1. [DOI] [PubMed] [Google Scholar]
- 65.Madigan J.B., Wilcock D.M., Hainsworth A.H. Vascular contributions to cognitive impairment and dementia: topical review of animal models. Stroke. 2016;47:1953–1959. doi: 10.1161/STROKEAHA.116.012066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Beauchamp A., Yee Y., Darwin B.C., Raznahan A., Mars R.B., Lerch J.P. Whole-brain comparison of rodent and human brains using spatial transcriptomics. eLife. 2022;11:e79418. doi: 10.7554/eLife.79418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Balsters J.H., Zerbi V., Sallet J., Wenderoth N., Mars R.B. Primate homologs of mouse cortico-striatal circuits. eLife. 2020:9. doi: 10.7554/eLife.53680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Davis J., Xu F., Zhu X., Van Nostrand W.E. rTg-D: a novel transgenic rat model of cerebral amyloid angiopathy Type-2. Cereb. Circ. Cogn. Behav. 2022;3 doi: 10.1016/j.cccb.2022.100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brittain J.F., Mccabe C., Khatun H., Kaushal N., Bridges L.R., Holmes W.M., Barrick T.R., Graham D., Dominiczak A.F., Mhairi Macrae I., Hainsworth A.H. An MRI-histological study of white matter in stroke-free SHRSP. J. Cereb. Blood Flow Metab. 2013;33:760–763. doi: 10.1038/jcbfm.2013.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Snyder H.M., Shineman D.W., Friedman L.G., Hendrix J.A., Khachaturian A., Le Guillou I., Pickett J., Refolo L., Sancho R.M., Ridley S.H. Guidelines to improve animal study design and reproducibility for Alzheimer's disease and related dementias: for funders and researchers. Alzheimers Dement. 2016;12:1177–1185. doi: 10.1016/j.jalz.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 71.Noor N.M., Love S.B., Isaacs T., Kaplan R., Parmar M.K.B., Sydes M.R. Uptake of the multi-arm multi-stage (MAMS) adaptive platform approach: a trial-registry review of late-phase randomised clinical trials. BMJ Open. 2022;12 doi: 10.1136/bmjopen-2021-055615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Noor N.M., Pett S.L., Esmail H., Crook A.M., Vale C.L., Sydes M.R., Parmar M.K.B. Adaptive platform trials using multi-arm, multi-stage protocols: getting fast answers in pandemic settings. F1000Res. 2020;9:1109. doi: 10.12688/f1000research.26253.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aisen P.S., Bateman R.J., Carrillo M., Doody R., Johnson K., Sims J.R., Sperling R., Vellas B. Platform trials to expedite drug development in alzheimer's disease: a report from the EU/US CTAD task force. J. Prev. Alzheimers Dis. 2021;8:306–312. doi: 10.14283/jpad.2021.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mehta A.R., Pal S., Chataway J., Carpenter J.R., Parmar M.K.B., Chandran S. Smarter adaptive platform clinical trials in neurology: a showcase for UK innovation. Brain. 2022;145:e64–e65. doi: 10.1093/brain/awac169. [DOI] [PubMed] [Google Scholar]
- 75.Wong C., Dakin R.S., Williamson J., Newton J., Steven M., Colville S., Stavrou M., Gregory J.M., Elliott E., Mehta A.R., Chataway J., Swingler R.J., Parker R.A., Weir C.J., Stallard N., Parmar M.K.B., Macleod M.R., Pal S., Chandran S. Motor Neuron disease systematic multi-arm adaptive randomised trial (MND-SMART): a multi-arm, multi-stage, adaptive, platform, phase III randomised, double-blind, placebo-controlled trial of repurposed drugs in motor neuron disease. BMJ Open. 2022;12 doi: 10.1136/bmjopen-2022-064173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bath P.M.W., Macleod M.R., Green A.R. Emulating multicentre clinical stroke trials: a new paradigm for studying novel interventions in experimental models of stroke. Int. J. Stroke. 2009;4:471–479. doi: 10.1111/j.1747-4949.2009.00386.x. [DOI] [PubMed] [Google Scholar]
- 77.Dirnagl U., Fisher M. International, multicenter randomized preclinical trials in translational stroke research: it's time to act. J. Cereb. Blood Flow Metab. 2012;32:933–935. doi: 10.1038/jcbfm.2012.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Llovera G., Hofmann K., Roth S., Salas-Pérdomo A., Ferrer-Ferrer M., Perego C., Zanier E.R., Mamrak U., Rex A., Party H., Agin V., Fauchon C., Orset C., Haelewyn B., De Simoni M.G., Dirnagl U., Grittner U., Planas A.M., Plesnila N., Vivien D., Liesz A. Results of a preclinical randomized controlled multicenter trial (pRCT): anti-CD49d treatment for acute brain ischemia. Sci. Transl. Med. 2015;7:299ra121. doi: 10.1126/scitranslmed.aaa9853. [DOI] [PubMed] [Google Scholar]
- 79.Maysami S., Wong R., Pradillo J.M., Denes A., Dhungana H., Malm T., Koistinaho J., Orset C., Rahman M., Rubio M., Schwaninger M., Vivien D., Bath P.M., Rothwell N.J., Allan S.M. A cross-laboratory preclinical study on the effectiveness of interleukin-1 receptor antagonist in stroke. J. Cereb. Blood Flow Metab. 2016;36:596–605. doi: 10.1177/0271678X15606714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lyden P.D., Bosetti F., Diniz M.A., Rogatko A., Koenig J.I., Lamb J., Nagarkatti K.A., Cabeen R.P., Hess D.C., Kamat P.K., Khan M.B., Wood K., Dhandapani K., Arbab A.S., Leira E.C., Chauhan A.K., Dhanesha N., Patel R.B., Kumskova M., Thedens D., Morais A., Imai T., Qin T., Ayata C., Boisserand L.S.B., Herman A.L., Beatty H.E., Velazquez S.E., Diaz-Perez S., Sanganahalli B.G., Mihailovic J.M., Hyder F., Sansing L.H., Koehler R.C., Lannon S., Shi Y., Karuppagounder S.S., Bibic A., Akhter K., Aronowski J., Mccullough L.D., Chauhan A., Goh A., Siddiqui S., Sheth K., Matouk C., Cruz C.D., Zhou J., Dawson V.L., Dawson T.M., Liang J., Zijl P.C.M.V., Zeiler S.R., Kimberly W.T., Erdogan T., Yu L., Mandeville J., Whittier J.P.W. The Stroke preclinical assessment network: rationale, design, feasibility, and stage 1 results. Stroke. 2022;53:1802–1812. doi: 10.1161/STROKEAHA.121.038047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Morais A., Locascio J.J., Sansing L.H., Lamb J., Nagarkatti K., Imai T., Van Leyen K., Aronowski J., Koenig J.I., Bosetti F., Lyden P., Ayata C. Embracing heterogeneity in the multicenter stroke preclinical assessment network (SPAN) trial. Stroke. 2023;54:620–631. doi: 10.1161/STROKEAHA.122.040638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Harkema L., Youssef S.A., De Bruin A. Pathology of mouse models of accelerated aging. Vet. Pathol. 2016;53:366–389. doi: 10.1177/0300985815625169. [DOI] [PubMed] [Google Scholar]
- 83.Higuchi K. Genetic characterization of senescence-accelerated mouse (SAM) Exp. Gerontol. 1997;32:129–138. doi: 10.1016/s0531-5565(96)00060-5. [DOI] [PubMed] [Google Scholar]
- 84.Takeda T., Hosokawa M., Higuchi K. Senescence-accelerated mouse (SAM): a novel murine model of senescence. Exp. Gerontol. 1997;32:105–109. doi: 10.1016/s0531-5565(96)00036-8. [DOI] [PubMed] [Google Scholar]
- 85.Birey F., Andersen J., Makinson C.D., Islam S., Wei W., Huber N., Fan H.C., Metzler K.R.C., Panagiotakos G., Thom N., O'rourke N.A., Steinmetz L.M., Bernstein J.A., Hallmayer J., Huguenard J.R., Paşca S.P. Assembly of functionally integrated human forebrain spheroids. Nature. 2017;545:54–59. doi: 10.1038/nature22330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Granata A. Functional genomics in stroke: current and future applications of iPSCs and gene editing to dissect the function of risk variants. BMC Cardiovasc. Disord. 2023:23. doi: 10.1186/s12872-023-03227-6. http://europepmc.org/abstract/MED/37120540 [Online]Available. europepmc.org/articles/PMC10148993?pdf=render [Accessed 2023/04//] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Crilly S., Njegic A., Parry-Jones A.R., Allan S.M., Kasher P.R. Using zebrafish larvae to study the pathological consequences of hemorrhagic stroke. J. Vis. Exp. 2019 doi: 10.3791/59716. http://europepmc.org/abstract/MED/31233021 [Online]. Available. [Accessed 2019/06//] [DOI] [PubMed] [Google Scholar]
- 88.Boltze J., Ferrara F., Hainsworth A.H., Bridges L.R., Zille M., Lobsien D., Barthel H., Mcleod D.D., Grässe F., Pietsch S., Schatzl A.K., Dreyer A.Y., Nitzsche B. Lesional and perilesional tissue characterization by automated image processing in a novel gyrencephalic animal model of peracute intracerebral hemorrhage. J. Cereb. Blood Flow Metab. 2019;39:2521–2535. doi: 10.1177/0271678X18802119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Eaton S.L., Proudfoot C., Lillico S.G., Skehel P., Kline R.A., Hamer K., Rzechorzek N.M., Clutton E., Gregson R., King T., O'neill C.A., Cooper J.D., Thompson G., Whitelaw C.B., Wishart T.M. CRISPR/Cas9 mediated generation of an ovine model for infantile neuronal ceroid lipofuscinosis (CLN1 disease) Sci. Rep. 2019;9:9891. doi: 10.1038/s41598-019-45859-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hainsworth A.H., Allan S.M., Boltze J., Cunningham C., Farris C., Head E., Ihara M., Isaacs J.D., Kalaria R.N., Lesnik Oberstein S.A., Moss M.B., Nitzsche B., Rosenberg G.A., Rutten J.W., Salkovic-Petrisic M., Troen A.M. Translational models for vascular cognitive impairment: a review including larger species. BMC Med. 2017;15:16. doi: 10.1186/s12916-017-0793-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sorby-Adams A.J., Marian O.C., Bilecki I.M., Elms L.E., Camargo J., Hall K., Crowther R.G., Leonard A.V., Wadsworth G.I., Spear J.H., Turner R.J., Jones C.F. Neurological scoring and gait kinematics to assess functional outcome in an ovine model of ischaemic stroke. Front. Neurol. 2023;14 doi: 10.3389/fneur.2023.1071794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Monte B., Constantinou S., Koundal S., Lee H., Dai F., Gursky Z., Van Nostrand W.E., Darbinyan A., Zlokovic B.V., Wardlaw J., Benveniste H. Characterization of perivascular space pathology in a rat model of cerebral small vessel disease by in vivo magnetic resonance imaging. J. Cereb. Blood Flow Metab. 2022;42:1813–1826. doi: 10.1177/0271678X221105668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hietamies T.M., Ostrowski C., Pei Z., Feng L., Mccabe C., Work L.M., Quinn T.J. Variability of functional outcome measures used in animal models of stroke and vascular cognitive impairment – a review of contemporary studies. J. Cereb. Blood Flow Metab. 2018;38:1872–1884. doi: 10.1177/0271678X18799858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Harrison J.K., Noel-Storr A.H., Demeyere N., Reynish E.L., Quinn T.J. Outcomes measures in a decade of dementia and mild cognitive impairment trials. Alzheimers Res. Ther. 2016;8:48. doi: 10.1186/s13195-016-0216-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kaye J., Aisen P., Amariglio R., Au R., Ballard C., Carrillo M., Fillit H., Iwatsubo T., Jimenez-Maggiora G., Lovestone S., Natanegara F., Papp K., Soto M.E., Weiner M., Vellas B. Using digital tools to advance alzheimer's drug trials during a pandemic: the EU/US CTAD task force. J. Prev. Alzheimers Dis. 2021;8:513–519. doi: 10.14283/jpad.2021.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wood F.A., Howard J.P., Finegold J.A., Nowbar A.N., Thompson D.M., Arnold A.D., Rajkumar C.A., Connolly S., Cegla J., Stride C., Sever P., Norton C., Thom S.A.M., Shun-Shin M.J., Francis D.P. N-of-1 trial of a statin, placebo, or no treatment to assess side effects. N. Engl. J. Med. 2020;383:2182–2184. doi: 10.1056/NEJMc2031173. [DOI] [PubMed] [Google Scholar]
- 97.Ho H., Kejzar N., Sasaguri H., Saito T., Saido T.C., De Strooper B., Bauza M., Krupic J. A fully automated home cage for long-term continuous phenotyping of mouse cognition and behavior. Cell Rep. Methods. 2023;3 doi: 10.1016/j.crmeth.2023.100532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bath P.M., Wardlaw J.M. Pharmacological treatment and prevention of cerebral small vessel disease: a review of potential interventions. Int. J. Stroke. 2015;10:469–478. doi: 10.1111/ijs.12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fang J., Zhang P., Zhou Y., Chiang C.W., Tan J., Hou Y., Stauffer S., Li L., Pieper A.A., Cummings J., Cheng F. Endophenotype-based in silico network medicine discovery combined with insurance record data mining identifies sildenafil as a candidate drug for Alzheimer's disease. Nat. Aging. 2021;1:1175–1188. doi: 10.1038/s43587-021-00138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fisher M., Feuerstein G., Howells D.W., Hurn P.D., Kent T.A., Savitz S.I., Lo E.H. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40:2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.STAIR Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999;30:2752–2758. doi: 10.1161/01.str.30.12.2752. [DOI] [PubMed] [Google Scholar]
- 102.STEPS Stem Cell Therapies as an Emerging paradigm in stroke (STEPS): bridging basic and clinical science for cellular and neurogenic factor therapy in treating stroke. Stroke. 2009;40:510–515. doi: 10.1161/STROKEAHA.108.526863. [DOI] [PubMed] [Google Scholar]
- 103.Percie Du Sert N., Alfieri A., Allan S.M., Carswell H.V., Deuchar G.A., Farr T.D., Flecknell P., Gallagher L., Gibson C.L., Haley M.J., Macleod M.R., Mccoll B.W., Mccabe C., Morancho A., Moon L.D., O'neill M.J., Pérez De Puig I., Planas A., Ragan C.I., Rosell A., Roy L.A., Ryder K.O., Simats A., Sena E.S., Sutherland B.A., Tricklebank M.D., Trueman R.C., Whitfield L., Wong R., Macrae I.M. The improve guidelines (ischaemia models: procedural refinements of in vivo experiments) J. Cereb. Blood Flow Metab. 2017;37:3488–3517. doi: 10.1177/0271678X17709185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lapchak P.A., Zhang J.H., Noble-Haeusslein L.J. RIGOR guidelines: escalating STAIR and STEPS for effective translational research. Transl. Stroke Res. 2013;4:279–285. doi: 10.1007/s12975-012-0209-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kilkenny C., Browne W.J., Cuthill I.C., Emerson M., Altman D.G. Improving bioscience research reporting: the arrive guidelines for reporting animal research. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Percie Du Sert N., Ahluwalia A., Alam S., Avey M.T., Baker M., Browne W.J., Clark A., Cuthill I.C., Dirnagl U., Emerson M., Garner P., Holgate S.T., Howells D.W., Hurst V., Karp N.A., Lazic S.E., Lidster K., Maccallum C.J., Macleod M., Pearl E.J., Petersen O.H., Rawle F., Reynolds P., Rooney K., Sena E.S., Silberberg S.D., Steckler T., Würbel H. Reporting animal research: explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020;18 doi: 10.1371/journal.pbio.3000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kidwell M.C., Lazarević L.B., Baranski E., Hardwicke T.E., Piechowski S., Falkenberg L.S., Kennett C., Slowik A., Sonnleitner C., Hess-Holden C., Errington T.M., Fiedler S., Nosek B.A. Badges to acknowledge open practices: a simple, low-cost, effective method for increasing transparency. PLoS Biol. 2016;14 doi: 10.1371/journal.pbio.1002456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nosek B.A., Ebersole C.R., Dehaven A.C., Mellor D.T. The preregistration revolution. Proc. Natl Acad. Sci. 2018;115:2600–2606. doi: 10.1073/pnas.1708274114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Percie Du Sert N., Bamsey I., Bate S.T., Berdoy M., Clark R.A., Cuthill I., Fry D., Karp N.A., Macleod M., Moon L., Stanford S.C., Lings B. The experimental design assistant. PLoS Biol. 2017;15 doi: 10.1371/journal.pbio.2003779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bailey E.L., Mcculloch J., Sudlow C., Wardlaw J.M. Potential animal models of lacunar stroke: a systematic review. Stroke. 2009;40:e451–e458. doi: 10.1161/STROKEAHA.108.528430. [DOI] [PubMed] [Google Scholar]
- 111.Bailey E.L., Smith C., Sudlow C.L., Wardlaw J.M. Pathology of lacunar ischemic stroke in humans–a systematic review. Brain Pathol. 2012;22:583–591. doi: 10.1111/j.1750-3639.2012.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Humphreys C.A., Smith C., Wardlaw J.M. Correlations in post-mortem imaging-histopathology studies of sporadic human cerebral small vessel disease: a systematic review. Neuropathol. Appl. Neurobiol. 2021;47:910–930. doi: 10.1111/nan.12737. [DOI] [PubMed] [Google Scholar]
- 113.Pedder H., Vesterinen H.M., Macleod M.R., Wardlaw J.M. Systematic review and meta-analysis of interventions tested in animal models of lacunar stroke. Stroke. 2014;45:563–570. doi: 10.1161/STROKEAHA.113.003128. [DOI] [PubMed] [Google Scholar]
- 114.Mchutchison C., Blair G.W., Appleton J.P., Chappell F.M., Doubal F., Bath P.M., Wardlaw J.M. Cilostazol for secondary prevention of stroke and cognitive decline: systematic review and meta-analysis. Stroke. 2020;51:2374–2385. doi: 10.1161/STROKEAHA.120.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hall G.R., Boehm-Sturm P., Dirnagl U., Finke C., Foddis M., Harms C., Koch S.P., Kuchling J., Madan C.R., Mueller S., Sassi C., Sotiropoulos S.N., Trueman R.C., Wallis M.D., Yildirim F., Farr T.D. Long-term connectome analysis reveals reshaping of visual, spatial networks in a model with vascular dementia features. Stroke. 2022;53:1735–1745. doi: 10.1161/STROKEAHA.121.036997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Russell W.M.S., Burch R.L. Methuen; 1959. The Principles of Humane Experimental Technique. [Google Scholar]
- 117.Clark T., Kinoshita J. Alzforum and SWAN: the present and future of scientific web communities. Brief Bioinform. 2007;8:163–171. doi: 10.1093/bib/bbm012. [DOI] [PubMed] [Google Scholar]