Abstract
Background
travellers’ diarrhoea (TD) is frequently reported with incidence up to 40% in high-risk destinations. Previous studies showed that the number of loose stools alone is inadequate to holistically predict the severity of TD. To improve the prediction of prognosis and to optimize treatments, a simple risk-based clinical severity classification has been developed.
Methods
pooled baseline data of signs and symptoms and number of loose stools from 1098 subjects enrolled in two double-blind Phase 3 trials of rifamycin-SV were analyzed with correlation, multiple correspondence analyses, prognostic factor criteria, and Contal and O’Quigley method to generate a TD severity classification (mild, moderate and severe). The relative importance of this classification on resolution of TD was assessed by Cox proportional model hazard model on the time to last unformed stool (TLUS).
Results
the analysis showed that TLUS were longer for the severe [hazard ratio (HR) 0.24; P < 0.001; n = 173] and moderate (HR 0.54; P = 0.0272; n = 912) vs mild. Additionally, when the treatment assigned in the studies was investigated in the severity classification, the results yielded that rifamycin-SV significantly shortened TLUS vs placebo for all subjects (HR 1.9; P = 0.0006), severe (HR 5.9; P = 0.0232) and moderate (HR 1.7; P = 0.0078) groups and was as equally efficacious as ciprofloxacin for all subjects, moderate and severe groups (HRs: 0.962, 0.9, 1.2; all P = NS, respectively). When reassessed by this classification, rifamycin-SV showed consistent efficacy with the Phase 3 studies.
Conclusions
this newly developed TD clinical severity classification demonstrated strong prognostic value and clinical utility by combining patients’ multiple signs and symptoms of enteric infection and number of loose stools to provide a holistic assessment of TD. By expanding on the current classification by incorporating patient reported outcomes in addition to TLUS, a classification like the one developed, may help optimize patient selection for future clinical studies.
Keywords: clinical trials, Travellers’ diarrhoea
Introduction
The 2019 COVID-19 pandemic halted international travel with lockdowns and travel restrictions. However, spending on international travel has increased from $40 B in 2021 to $141 B in 2022, and travel volume is forecasted to reach pre-pandemic levels by 2024.1 Historically, travellers’ diarrhoea (TD) is the most common illness experienced by international travellers originating in industrialized countries and visiting resource-challenged regions and low-income countries. Incidence rates for TD are generally in the range of 20–40% in high-risk countries including Asia, Africa and Latin America.2–4
There are several challenges associated with TD management. Epidemiologic and clinical studies showed that bacterial enteropathogens accounted for 80–90% of cases and that antimicrobial resistance to fluroquinolones and azithromycin has increased in high-risk destination countries.2–8 Additionally, systemic antibiotics for treating TD are associated with increased acquisition (8.8–80.0%) and transmission of the extended-spectrum beta-lactamase–producing Enterobacteriaceae (ESBL-PE).7,9–13 Non-absorbable antibiotics, such as rifamycin-SV, for treating non-dysenteric TD are associated with significantly lower ESBL-PE acquisition than ciprofloxacin.5,6,8 To better understand TD prognosis, there is a need for a standardized disease severity classification that objectively assesses the frequency of stools, and in addition, integrates patient reported signs or symptoms of disease. This approach aims to better capture the patient experience. Furthermore, clinically practical severity assessments may increase identification of appropriate patients for treatment and promote antibiotic stewardship.
Older disease severity definitions in TD studies used to focus on stool consistency or frequency and do not account for severity of signs and symptoms.14 The International Society of Travel Medicine (ISTM) consensus conference recommended using functional impact to assess TD severity and TD treatment options.3 A recent study utilized data from TD in active-duty military personnel to develop a TD severity scale using classification regression trees and multiple correspondence analysis (MCA).15 Other studies have used MCA, receiver-operator curves and hierarchical cluster analysis on human challenge models with enterotoxigenic Escherichia coli and Shigella to develop disease severity scoring classification.14,16 A TD clinical severity classification incorporating multiple aspects of baseline TD disease characteristics has not been developed based on current TD treatment modalities for a broad population of international travellers on short-term (<2 weeks) business or leisure travel to common destinations. This study aims to build on prior literature to develop a TD clinical severity classification incorporating severity of loose stools and signs and symptoms of disease based on baseline characteristics of a broad population of patients in a clinical trial. This classification offers a simple risk-based assessment of severity through intuitive patient-centric scoring of TD signs and symptoms and is a potential tool for objective assessment of TD severity in future studies. In addition, we validate the clinical performance of this newly developed TD clinical severity classification based on the US Food and Drug Administration (FDA) defined TD treatment endpoint from the rifamycin-SV Phase 3 studies.6,8
Methods
Individual patient-level data for this study were obtained from two rifamycin-SV double-blind Phase 3 multicentre studies (n = 1098).6,8 The studies were conducted in accordance with good clinical practice, the Declaration of Helsinki, all applicable national laws and regulations, and were approved by independent ethics committees at each of the centres prior to starting recruitment.
Evaluation of participants
Eligibility criteria were briefly summarized as follows: men and women of least 18 years who arrived within the past 4 weeks from an industrialized country were eligible if they had acute moderate to severe TD, defined as at least three unformed, watery or soft stools accompanied by symptoms within 24 h preceding randomization with duration of illness ≤ 72 h. The intensity of the following TD symptoms was documented as none, mild, moderate or severe: gas or flatulence, nausea, vomiting, abdominal cramps or pain, rectal tenesmus and defecation urgency. Symptoms were considered moderate if they interfered with planned activities and as severe if they completely prevented planned activities; fever (>100.4°F or 38°C) was documented as yes or no; number of stools was recorded and consistency was documented as formed, soft or watery. Patients were treated empirically with rifabutin-SV or placebo17 or comparator8 at the time of randomization; further details of the studies are provided in these publications.
The study protocols excluded patients who were residents of any country with high incidence rates of diarrhoea, fever (>38.0°C), passage of grossly bloody stools, known or suspected infection with a non-bacterial pathogen (e.g. HIV or viral hepatitis), moderate or severe dehydration, history of inflammatory bowel disease or celiac disease. Safety and efficacy were assessed at Visit 2 (Day 2), Visit 3 (Day 4 or 5) and the final visit (Day 6).6,8,18 Studies were conducted during the timeframe of in 2010–16; they were conducted at a total of 27 non-military clinical research sites located in India, Mexico, Guatemala and Ecuador.6,8
Statistical methods
Baseline clinical information was analyzed for all subjects, regardless of treatment, for both loose stools and for signs and symptoms of enteric infection. The scores were obtained from patients’ baseline characteristics (symptom severity and loose stool frequency) during the clinical trial, as defined by the study protocols.
MCA was utilized to identify underlying associations between signs or symptoms of enteric infection via a set of nominal categorical data utilizing Euclidean distances in the baseline (i.e. pre-treatment) data.19 Pairwise data were tabulated in a K-by-K table and were visually graphed on a 2D graph: the more proximal the variables, the more similar their distribution and the better formation of clusters.
The severity categorization of loose stool frequency was based on previously published TD prognostic factor studies,16 which defined mild as (one to two loose stools), moderate (three to five loose stools) and severe (greater than equal to six loose stools). Scoring values for the grading of loose and watery stools were also based on previously published literature14,16: defining score of 0 for no loose stools, 1 for one to two loose stools, 2 for three to five loose stools and 3 for greater than equal to six loose stools. The ordinal proximity distribution of enteric signs and symptoms groupings, the overlap in symptom severity and the correlations of each symptom were evaluated to construct the final signs and symptoms of enteric infection score values. Score values ranged ordinally from 0 to 3 for each categorical parameter based on severity.
The scores from the signs and symptoms of enteric infection and the frequency of loose stools were added to calculate the total disease severity score for each patient from the clinical studies. The resulting total disease severity score was then categorized into three-tier levels: mild, moderate or severe. To adopt this three-tier categorization of TD severity, the Contal and O’Quigley20,21 method was utilized to identify the optimal total disease severity score cut-points based on the clinically relevant outcome, time to last unformed stool (TLUS). In the rifamycin-SV studies, TLUS was defined as the interval (hours) between the first dose of study drug and the time the last unformed stool (watery or soft) was passed before achieving clinical cure. Clinical cure during the 120-h data collection period was defined as either two or fewer soft stools, no watery stools, no fever and no symptoms of enteric infection (except mild flatulence) during a 24-h interval, or no stools or only formed stools, and no fever during a 48-h interval, with or without symptoms of enteric infection. The Contal and O’Quigley method identified threshold values that significantly separate patients into mild, moderate and severe categories with respect TLUS in the pooled clinical data sets (regardless of treatment groups assignment). Prognostic value for this new TD Severity Classification was determined by assessing the distributions of TLUS based on severity categories utilizing the Kaplan–Meier product limit method and a difference in distributions was assessed using the Cox proportional model to generate hazard ratios (HRs). Furthermore, the clinical relevance of this new TD Severity Classification was assessed by utilizing this severity classification to re-analyze the efficacy of rifamycin-SV placebo and ciprofloxacin in the respective Phase 3 trials.
Results
The pooled data set consisted of 1098 subjects (n = 619 for rifamycin-SV, n = 414 for ciprofloxacin, n = 65 for placebo). The mean age was 28 years (range, 18–87 years) with 49.5% female and 83.4% Caucasian. At baseline, 43.16% patients experienced onset of symptoms < 24 h and 39% in 24 h to <48 h. TD symptoms included abdominal cramps and pain (95%), faecal urgency (76.5%), rectal tenesmus (64.3%), nausea (63.2%), excess flatulence (48.5%) and vomiting (36.2%). About 98% of subjects had three or more unformed stools (loose stools) within 24 h of baseline; the median number of unformed stools was 4 (range, 2–20) (Supplementary Tables 1 and 2); 39.1% of patients rated unformed stool as severe. Distribution of symptom severity is shown in Supplementary Table 3. Because most patients (96.7%) were afebrile (36 patients had body temperature, 38–39.5°C; no patients had temperature > 39.5°C), fever was not included in the MCA analysis.
MCA analysis illustrated a clear separation of no/mild, moderate and severe symptom clusters as well as the ordinal relationship of those clusters (Figure 1). Severe signs and symptoms occupied the upper right dimension. The moderate symptoms cluster with close proximity includes nausea, tenesmus, vomiting. Moderate signs and symptoms generally clustered and occupied a distinct dimension from the No and Mild cluster. The respective ordinal scores of the symptom severity clusters (score 0–2) and degree of severity based on number of loose stools (score 0–3) are shown in Figure 2.
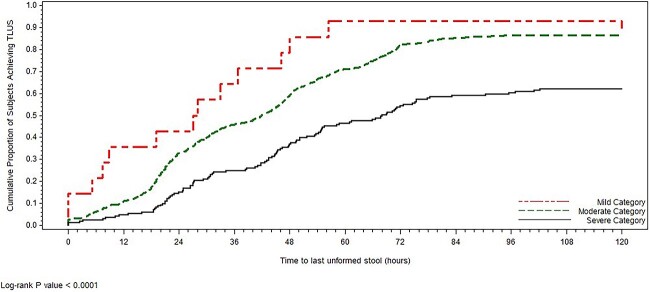
Figure 1.
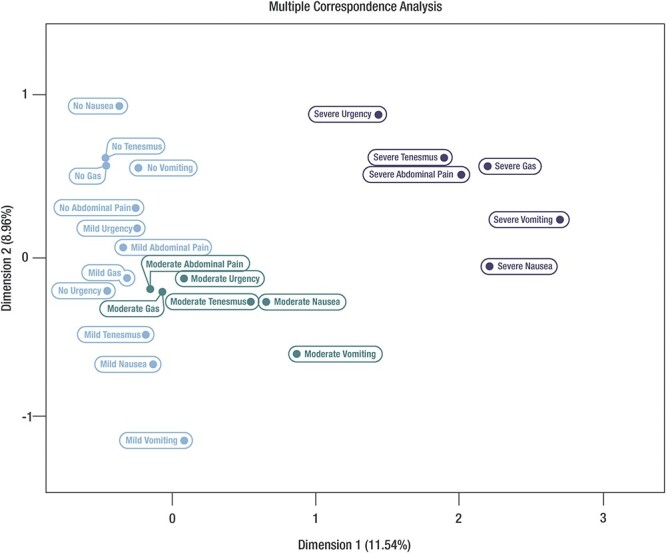
MCA: association and clusters (pooled rifamycin-SV Studies 1 and 2, ITT population)
Figure 2.
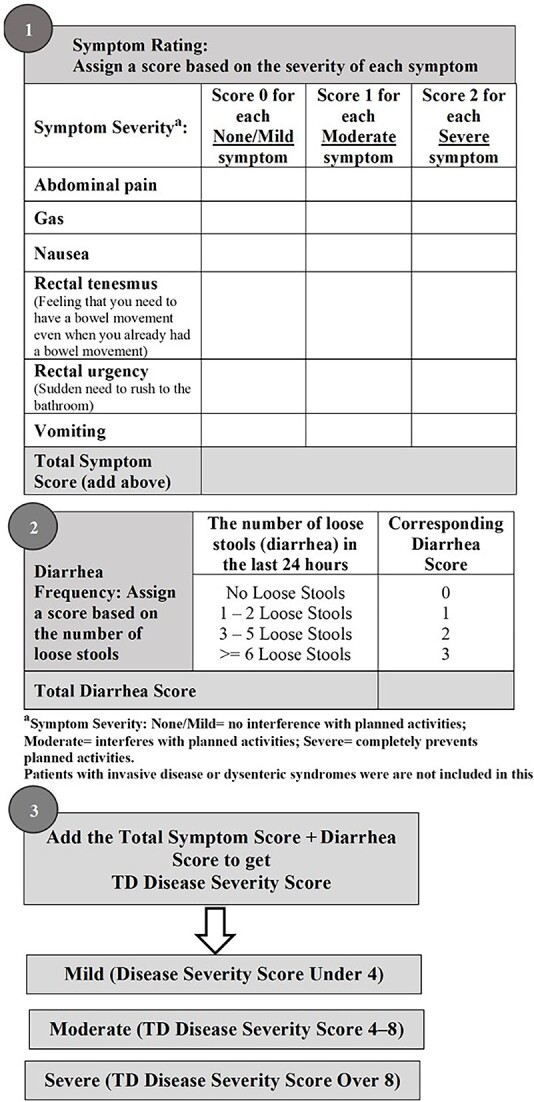
TD clinical severity classification scoring schematic
By combining the signs and symptoms and number of loose stools scores, a disease severity score was generated for each individual patient. The disease severity score was then categorized into mild (score < 4; TD that causes no change in activity level), moderate (score 4–8; TD that causes change in activity level) or severe (score > 8; TD that renders subject disabled). Most patients had moderate illness (82.9%, n = 912) and severe illness (15.7%, n = 173). Only 14 patients (1.3%) had mild illness. The median disease severity scores were 7 (range, 2–13), respectively. The prognostic validity of each TD severity category was confirmed through utilizing the rifamycin-SV data set and the outcomes of TLUS. KM curves showed a clear separation of the proportion of patients in each TD severity category (Figure 3). HR analysis that incorporates the proportion of patients achieving resolution as well as time to achieve resolution showed: moderate (HR 0.54, 95% CI 0.31–0.93; P = 0.0272) and severe illness (HR 0.24, 95% CI 0.13–0.43; P < 0.0001) groups compared with the mild illness group. The median TLUS were 27.5, 42 and 68 h for mild, moderate and severe illness groups, respectively (Table 1).
Figure 3.
Assessment of TLUS based on The TD clinical severity category (pooled rifamycin-SV Studies 1 and 2, ITT population)
Table 1.
TLUS (h) by TD clinical severity classification (ITT population)a
| Clinical severity categoryb | |||
|---|---|---|---|
| Parameters | Mild (n = 14) | Moderate (n = 912) | Severe (n = 173) |
| TLUS (h), median (95% CI) | 27.5 (5.1–44.8) |
42.0 (38.0–44.8) |
68.0 (54.8–75.5) |
| HRc | NA | Moderate vs mild 0.54 (0.31–0.93) |
Severe vs mild 0.24 (0.13–0.43) |
| P-value | NA | 0.0272 | <0.0001 |
aPooled data from NCT01142089: C2009-0201 and NCT1209922: RIT-1/AID regardless of treatment assignment.
bMild illness = disease severity score < 4; moderate illness = disease severity score of 4–8; severe illness = disease severity score > 8.
cThe HR is a comparative measure of TLUS experienced over the entire trial period.
We next applied the new TD classification to explore treatment outcomes in the two controlled Phase 3 studies (using rifamycin-SV, ciprofloxacin or placebo treatments). In Study 1, when rifamycin-SV was compared with placebo, the reduction in TLUS was nearly 6-fold shorter in the severe illness group (HR 5.9, 95% CI 1.3–27.5; P = 0.0232; median TLUS: 38.7 vs 68.0 h, respectively) and ~2-fold shorter in the moderate illness group (HR 1.7, 95% CI 1.1–2.5; P = 0.0078; median TLUS: 47.2 vs 68.0 h, respectively) (Table 2). Median TLUS was also shortened with rifamycin-SV compared with placebo in the mild illness groups (median TLUS: 6.9 vs 56.4 h, respectively) but the HR was not significant because of small number of patients in each treatment arm (placebo n = 3, rifamycin-SV n = 6) (Table 2). In Study 2, assessing non-inferiority, no statistically significant differences (all P-values > 0.05) were observed in the reduction of TLUS between the rifamycin-SV and ciprofloxacin arms. Median TLUS were similar between rifamycin-SV and ciprofloxacin treatment arms: moderate illness (median TLUS: 40.3 vs 33.7 h, respectively), severe illness (median TLUS: 67.8 vs 73.3 h, respectively) and all subjects (median TLUS: 44.3 vs 40.3 h, respectively). HR was not calculable in mild illness because of small number of patients (n ≤ 3) (Table 2).
Table 2.
Reassessment of rifamycin-SV efficacy in two Phase 3 studies based on TD severity categories: median TLUS [h] by baseline disease severity
| TD Category of severity of illness | Study 1: rifamycin-SV vs placebo (NCT01142089: C2009-0201) |
Study 2: rifamycin-SV vs ciprofloxacin (NCT1209922: RIT-1/AID) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | Rifamycin-SV | Comparison HR | Ciprofloxacin | Rifamycin-SV | Comparison HR | |||||
| n | Median (95% CI) |
n | Median (95% CI) |
HR (95% CI) P-value |
n | Median (95% CI) |
n | Median (95% CI) |
HR (95% CI) P-value |
|
| Mild | 3 | 56.4 (7.4, NC) |
6 | 6.9 (0.0, 46.1) |
11.8 (0.5, 261.8) 0.1186 |
3 | 28.0 (27.0, 33.0) |
2 | 42.4 (36.8, 48.0) |
NC |
| Moderate | 57 | 68.0 (46.7, NC) |
178 | 47.2 (44.6, 56.0) |
1.7 (1.1, 2.5) 0.0078 |
336 | 33.7 (29.9, 40.2) |
341 | 40.3 (32.5, 45.0) |
0.9 (0.8, 1.1) 0.1856 |
| Severe | 5 | 68.0 (48.7, NC) |
15 | 38.7 (3.2, 46.0) |
5.9 (1.3, 27.5) 0.0232 |
76 | 73.3 (54.3, NC) |
77 | 67.8 (53.5, 97.0) |
1.2 (0.8, 1.9) 0.3245 |
| All subjects | 65 | 68.0 [48.7, NC] |
199 | 46.0 (42.8, 50.5) |
1.9 (1.3, 2.7) 0.0006 |
415 | 40.3 (33.5, 44.8) |
420 | 44.3 (40.1, 47.5) |
0.962 (0.8, 1.1) 0.6084 |
Note: NC, not calculable.
Mild illness = disease severity score < 4, moderate illness = disease severity score of 4–8 and severe illness = disease severity score > 8.
HR reflects Kaplan–Meir estimate of time to TLUS event.
Discussion
Although the hallmark symptom of TD is the sudden increase in the frequency of loose stools, international travellers also usually experience other TD-attributable signs and symptoms. Rifamycin-SV data sets and prior similar TD studies showed that the distribution of these TD-attributable signs and symptoms includes abdominal cramps and pain (~90%), faecal urgency (70–80%), rectal tenesmus (25–36%), nausea (53–60%), excess flatulence (50–80%) and vomiting (9–19%).6,8,17,22–25
TD clinical studies typically emphasize the number of loose stools as the primary efficacy endpoint for assessing pharmacological interventions.15 Definitions of TD severity from the FDA based on stool frequency and ISTM consensus conference definition based on the functional ability of the traveller either do not or only indirectly account for other signs and symptoms of TD to objectively and comprehensively assess disease severity. Previous studies showed that the number of loose stools alone is inadequate to holistically predict the severity of TD.14–16 For example, 1.8% patients who had mild diarrhoea also experienced disproportionately higher incidences of mild vomiting (63.8%), followed by nausea (36.7%), rectal tenesmus (35.7%) and urgency (23.5%). In contrast, patients with moderate diarrhoea (58.2%) displayed a different TD sign and symptom profile including high incidences of abdominal cramps and pain (81.2%) and gas/flatulence (74.6%) as well as equally high incidences of urgency, nausea and rectal tenesmus (67.9, 60 and 59.2%, respectively).
To provide a holistic TD severity assessment, we developed a TD disease severity classification tool integrating non-stool, patient-reported clinical symptoms with loose stool frequency. This severity scale was developed by using blinded baseline (i.e. pre-treatment) data from all subjects in Phase 3 trials involving rifamycin-SV. We then applied prognostic factor analysis using randomized outcomes of these trials to verify the clinical relevance of the TD severity scale. We invite others to pursue this approach with new data as we develop principles of therapy for travellers with diarrhoea.
The methodology to develop this classification ensures that appropriate values are assigned to each sign and symptom that the severity categories distinctly align to differing prognosis on the basis of clinically relevant outcome, TLUS. The MCA method confirms there was a clear separation between severity clusters in an ordinal distribution allowing for intuitive sign and severity scoring. The iterative Contal and O’Quigley method allows us to transform the disease severity score into three distinct disease severity categories based on simple cut-points. In addition, we demonstrate the utility of this new TD Severity Classification in evaluating the efficacy of TD treatment by reassessing the efficacy of rifamycin-SV against comparators in the respective Phase 3 trials. The results demonstrated that a reduction in TLUS was ~2-fold in the moderate group, and 6-fold in the severe group when rifamycin-SV was compared with placebo. Additionally, rifamycin-SV was equally efficacious as ciprofloxacin across severity groups.
Previous studies to develop TD-specific disease severity scoring classifications provide a conceptual framework to assess prognosis of TD.14–16 Similar to this classification, the recent Maier 2021 study bases the severity classification on a randomized clinical study (TrEAT) and utilizes baseline disease signs and symptoms (e.g. loose stools, abdominal cramps/pain, etc.) to define disease severity scores that are relevant to TD.15 This work builds on the findings of the TrEAT classification to help travellers understand disease prognosis and provides a simple scale by which individual risk can be inferred, and the reason why treatments maybe be chosen for patients on the basis of risk and forecast of the likely outcome of TD.
While there are many similarities in approach, the clinical context of this classification system differs from the TrEAT TD study in that the patient populations, travel destinations, purpose for travel, and disease signs and symptoms presentation were distinct (Supplementary Table 1). This classification was based on a broad population of 1099 subjects from two large, randomized controlled Phase 3 studies of TD treatment. The clinical data set of the TrEAT TD study data set comprised of 363 subjects, primarily younger (mean age 29 years) male (93%) military personnel, who were deployed for long-term service. The destinations (Kenya, Djibouti, Afghanistan and Honduras) are not commonly visited for leisure or business.15,24 In contrast, this study population, travel destinations (Mexico, Guatemala, India and Ecuador) and bacterial aetiology are largely consistent with epidemiology of TD in short-term business and leisure travel and may be more generalizable to most international travellers. This data set of over 1000 travellers from industrialized countries to Mexico, Guatemala, Ecuador and India over the period of 2010–16 provides a reasonable representation of the clinical characteristics of patients presenting with non-invasive TD. However, it cannot be generalized to all regions or to diarrheal syndromes other than non-invasive TD.
The MCA plots for this classification and the TrEAT TD study were distinct, indicating differing clinical presentation of TD signs and symptoms in these patient groups. The TrEAT TD study MCA had corresponding clusters for which a decision tree was implemented to assign relative values, whereas this MCA indicated clear separation between symptom clusters allowing for simplified ordinal scoring for disease signs and symptoms. International travellers who experience TD could readily assess their own severity of illness before pharmacological therapy is initiated.
Additionally, unlike the TrEAT TD study, the rifamycin-SV clinical data set did not include functional impact assessment in the initial study to understand the correlation between severity score, individual symptoms and functional impact. Nevertheless, the TrEAT TD classification study showed that severe TD signs and symptoms (e.g. nausea, vomiting and abdominal cramps) were strongly associated with functional impact on activities. However, the rifamycin-SV clinical data set had incidence of severe nausea (3.3%), vomiting (1.7%) and abdominal pain (10.2%), which based on the TrEAT TD results would likely result in functional activity impairment (Supplementary Table 3).
This new TD severity classification was limited by small number of patients in the mild illness group. Although our MCA analysis is consistent with the prior study of Porter 2016, of which E. coli is the key pathogen, the MCA distribution is not like prior studies of invasive pathogen such as Shigella.14 Since patients with signs and symptoms of fever and bloody stools (clinically assessed as having non-invasive disease) were excluded from rifamycin-SV Phase 3 studies, the efficacy assessment may not be applicable to patients with TD caused by invasive organisms. Further external validation of this severity classification with other data sets would better optimize this classification system as well as improve our understanding of how and when TD sign and symptoms could affect functional capacity. Finally, approaches such as the one presented here, as well as TrEAT TD endpoints, may be relevant to incorporate into clinical trials given the focus of FDA and EMA on patient-focused drug development.26,27
Conclusion
This newly developed TD disease severity classification incorporates multiple parameters of TD-attributable symptoms in addition to the traditional measure of loose stool frequency. It demonstrated strong prognostic value and clinical utility by combining patients’ multiple signs and symptoms of enteric infection and number of loose stools to provide a holistic assessment of TD. This holistic classification may help optimize patient selection for future clinical studies.28,29
Conflict of interest
June S. Almenoff is an Employee of RedHill Biopharma, which markets rifamycin SV in the US. Mansi Jamindar is a former Employee of RedHill Biopharma. Enoch Bortey, Robert Steffen, and Herbert Dupont have previously served as consultants to RedHill Biopharma.
Data availability
The studies referenced here are available on Clinical clinicaltrials.gov: NCT 01142089 and NCT 011208922.
Supplementary Material
Acknowledgements
This work was funded by RedHill Biopharma, the US manufacturer for Rifamycin SV. The authors acknowledge Phil Yeung (Medical Affairs 360, LLC.) for medical writing support and Kel Sheldon, PhD, for editorial advice and medical writing support.
Contributor Information
Herbert L DuPont, Internal Medicine, University of Texas School of Public Health, Houston, TX 77030, USA.
June S Almenoff, Department of Medical Affairs, Redhill Biopharma Inc., Raleigh, NC 27617, USA.
Mansi S Jamindar, Department of Medical Affairs, Redhill Biopharma Inc., Raleigh, NC 27617, USA.
Enoch Bortey, Pharmaceutical Development Strategies LLC, Chapel Hill, NC 27517, USA.
Robert Steffen, Department of Epidemiology, University of Zurich, 8001 Zurich, Switzerland.
References
- 1. Association UT . Data from: travel forecast summary table. Travel Forecast 2021. https://www.ustravel.org/system/files/media_root/document/Research_Travel-Forecast_Summary-Table.pdf Accessed 8 April 2023. [Google Scholar]
- 2.Connor BA. CDC Yellow Book 2020: Health Information for International Travel, Travelers’ Diarrhea 2(15). New York: Oxford University Press; 2020. https://wwwnc.cdc.gov/travel/yellowbook/2020/preparig-international-travelers/travelers-diarrhea Accessed 10 April 2023. [Google Scholar]
- 3. Riddle MS, Connor BA, Beeching NJ et al. Guidelines for the prevention and treatment of travelers' diarrhea: a graded expert panel report. J Travel Med 2017; 24:S57–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Steffen R, Hill DR, DuPont HL. Traveler's diarrhea: a clinical review. JAMA 2015; 313:71–80. [DOI] [PubMed] [Google Scholar]
- 5. Adler AV, Ciccotti HR, Trivitt SJH, Watson RCJ, Riddle MS. What's new in travellers' diarrhoea: updates on epidemiology, diagnostics, treatment and long-term consequences. J Travel Med 2022; 29:1–10. [DOI] [PubMed] [Google Scholar]
- 6. DuPont HL, Petersen A, Zhao J et al. Targeting of rifamycin SV to the colon for treatment of travelers' diarrhea: a randomized, double-blind, placebo-controlled phase 3 study. J Travel Med 2014; 21:369–76. [DOI] [PubMed] [Google Scholar]
- 7. Lomicronpez-Velez R, Lebens M, Bundy L, Barriga J, Steffen R. Bacterial travellers' diarrhoea: a narrative review of literature published over the past 10 years. Travel Med Infect Dis 2022; 47:102293. 10.1016/j.tmaid.2022.102293. [DOI] [PubMed] [Google Scholar]
- 8. Steffen R, Jiang ZD, Gracias Garcia ML et al. Rifamycin SV-MMX(R) for treatment of travellers' diarrhea: equally effective as ciprofloxacin and not associated with the acquisition of multi-drug resistant bacteria. J Travel Med 2018; 25:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Buchek G, Mende K, Telu K et al. Travel-associated multidrug-resistant organism acquisition and risk factors among US military personnel. J Travel Med 2021; 28:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Frost I, Van Boeckel TP, Pires J, Craig J, Laxminarayan R. Global geographic trends in antimicrobial resistance: the role of international travel. J Travel Med 2019; 26:1–13. [DOI] [PubMed] [Google Scholar]
- 11. Kantele A, Laaveri T, Mero S et al. Antimicrobials increase travelers' risk of colonization by extended-spectrum betalactamase-producing Enterobacteriaceae. Clin Infect Dis 2015; 60:837–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Riddle MS. Travel, Diarrhea, antibiotics, antimicrobial resistance and practice guidelines - a holistic approach to a health conundrum. Curr Infect Dis Rep 2020; 22:8. [Google Scholar]
- 13. Sridhar S, Turbett SE, Harris JB, LaRocque RC. Antimicrobial-resistant bacteria in international travelers. Curr Opin Infect Dis 2021; 34:423–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Porter CK, Lynen A, Riddle MS et al. Clinical endpoints in the controlled human challenge model for Shigella: a call for standardization and the development of a disease severity score. PloS One 2018; 13:e0194325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maier N, Riddle MS, Gutierrez R et al. A disease severity scale for the evaluation of vaccine and other preventive or therapeutic interventions for travellers' diarrhoea. J Travel Med 2022; 29:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Porter CK, Riddle MS, Alcala AN et al. An evidenced-based scale of disease severity following human challenge with Enteroxigenic Escherichia coli. PloS One 2016; 11:e0149358. 10.1371/journal.pone.0149358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Taylor DN, Bourgeois AL, Ericsson CD et al. A randomized, double-blind, multicenter study of rifaximin compared with placebo and with ciprofloxacin in the treatment of travelers' diarrhea. Am J Trop Med Hyg 2006; 74:1060–6. [PubMed] [Google Scholar]
- 18. Aemcolo Prescribing Information (2019). https://www.aemcolo.com/wp-content/uploads/2021/03/Aemcolo-Master-PI-011720.pdf Accessed 8 April 2023.
- 19. Greenacre M. Correspondence Analysis in Practice, 2nd edn. London, UK: Chapman and Hall/CRC, 2007. [Google Scholar]
- 20. Contal C, O'Quigley J. An application of changepoint methods in studying the effect of age on survival in breast cancer. Comput Stat Data Anal 1999; 30:253–70. [Google Scholar]
- 21. Rothman KJG, Lash TL. Modern Epidemiology, 2nd edn, 1998; Philadelphia PA: Lippincott, Williams and Wilkins.
- 22. DuPont HL, Ericsson CD, Mathewson JJ et al. Rifaximin: a nonabsorbed antimicrobial in the therapy of travelers' diarrhea. Digestion 1998; 59:708–14. [DOI] [PubMed] [Google Scholar]
- 23. DuPont HL, Jiang ZD, Ericsson CD et al. Rifaximin versus ciprofloxacin for the treatment of traveler's diarrhea: a randomized, double-blind clinical trial. Clin Infect Dis 2001; 33:1807–15. [DOI] [PubMed] [Google Scholar]
- 24. Riddle MS, Connor P, Fraser J et al. Trial evaluating ambulatory therapy of travelers' diarrhea (TrEAT TD) study: a randomized controlled trial comparing 3 single-dose antibiotic regimens with Loperamide. Clin Infect Dis 2017; 65:2008–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Steffen R, Sack DA, Riopel L et al. Therapy of travelers' diarrhea with rifaximin on various continents. Am J Gastroenterol 2003; 98:1073–8. [DOI] [PubMed] [Google Scholar]
- 26. Proposed ICH. Guideline Work to Advance Patient Focused Drug Development (EMA). The Netherlands: European Medicines Agency, Domenico Scarlattilaan 6,1083 HS Amsterdam. 2021.
- 27. US FDA . CDER Patient-Focused Drug Development, Silver Springs, MD: US Food and Drug Administration. 2022. .
- 28. Belmares J, Gerding DN, Parada JP, Miskevics S, Weaver F, Johnson S. Outcome of metronidazole therapy for Clostridium difficile disease and correlation with a scoring system. J Infect 2007; 55:495–501. [DOI] [PubMed] [Google Scholar]
- 29. Samaan MA, Mosli MH, Sandborn WJ et al. A systematic review of the measurement of endoscopic healing in ulcerative colitis clinical trials: recommendations and implications for future research. Inflamm Bowel Dis 2014; 20:1465–71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The studies referenced here are available on Clinical clinicaltrials.gov: NCT 01142089 and NCT 011208922.